Abstract
IL-22 plays an important role in mucosal epithelial cell homeostasis. Using a dextran sodium sulfate-induced mouse model of acute colitis, we observed an IL-23–dependent up-regulation of IL-22 in the middle and distal colon at the onset of epithelial cell damage. This heightened IL-22 correlated with an influx of innate immune cells, suggesting an important role in colonic epithelial protection. Freshly isolated colon-infiltrating neutrophils produced IL-22 contingent upon IL-23 signaling, and IL-22 production was augmented by TNF-α. Importantly, the depletion of neutrophils resulted in diminished IL-22 levels in the colon, and the transfer of IL-22–competent neutrophils to Il22a-deficient mice protected the colonic epithelium from dextran sodium sulfate-induced damage. In addition, IL-22–producing neutrophils targeted colonic epithelial cells to up-regulate the antimicrobial peptides, RegIIIβ and S100A8. This study establishes a role for neutrophils in providing IL-22–dependent mucosal epithelial support that contributes to the resolution of colitis.
Keywords: interleukin-22, intestinal inflammation, leukocytes
IL-22 is up-regulated in multiple chronic inflammatory diseases including inflammatory bowel disease (IBD), asthma, and psoriasis (1–5). IL-22 acts directly on epithelial and parenchymal cells by binding to a heterodimer receptor complex consisting of IL10 receptor 2 (IL-10R2) and IL22 receptor 1 (IL-22R1; IL-22R) (6). Importantly, IL-22 is considered beneficial for intestinal epithelial barrier function because of increased epithelial cell proliferation, migration, and mucus production (7, 8). In a mouse model of transmissible colitis induced by Citrobacter rodentium, IL-22 was required for increased expression of RegIII antimicrobial proteins, which are thought to promote bacterial clearance through direct microbicidal effects (9). Together these studies indicate an important role for IL-22 in mucosal epithelial antimicrobial defense and integrity.
Polymorphisms in the IL-23 receptor (IL-23R) gene are associated with IBD; therefore, the regulation of signals downstream of the IL-23R such as IL-22 and IL-17 may significantly influence human susceptibility or resistance to intestinal disease (2, 10). Early studies demonstrated that IL-17+CD4+ T cells (Th17 cells) produce IL-22 (1, 11). However, more recent studies demonstrate that several innate lymphoid cell (ILC) populations produce IL-22 (12, 13). Natural killer (NK) subsets that produce IL-22 have been identified in both humans and mice (4, 14–16). In addition, lymphoid tissue inducer cells that are crucial for fetal development of lymphoid tissues and CD11c+ colonic cells stimulated via Toll-like receptor signals secrete IL-22 (9, 14, 17, 18). Furthermore, studies using IL-23R reporter mice showed that γδ+ T cells in the lamina propria of the intestines and some population(s) of CD11b+ cells also express the IL-23R and therefore may contribute importantly to IL-22 production in vivo (19).
IBD (including ulcerative colitis and Crohn’s disease) is characterized by abnormal innate and adaptive immune responses. In IBD and mouse models of colitis, the innate immune response in the colon includes recruited macrophages and granulocytes, which appear to have both proinflammatory and anti-inflammatory roles in colitis (20). Aberrant control of these innate immune cells can result in tissue damage in the colon caused by abundant reactive oxygen species (21–23). However, more recent studies demonstrate that in acute models of colitis, neutrophils play a protective role in the host response (24, 25). Here we show that colon-infiltrating neutrophils are induced by IL-23 to produce IL-22 during acute dextran sodium sulfate (DSS)-induced colitis. In addition, IL-22–competent neutrophils enhance antimicrobial peptide production from colon epithelial cells, and their production of IL-22 is critical to restore the integrity of the epithelium following mucosal injury. These findings define a role for neutrophils in contributing to IL-22–mediated protection of the colonic epithelial barrier.
Results
IL-22 Levels Parallel Influx of Recruited Innate Immune Cells.
Dextran sodium sulfate (DSS) treatment causes direct epithelial cell damage and acute colitis in mice. Significant numbers of CD11b+Ly6C+ innate cells were recruited to the middle and distal colon by the end of the first week of DSS treatment (Fig. 1A). Contained within this population were three distinct innate cell types that could be distinguished by additional cell-surface markers or side-scatter properties, as confirmed by transmission electron microscopy (TEM) of sorted cells. Total colon cells were first gated on CD11b+Ly6C+ cells and then were further divided by Ly6G, F4/80, and side-scatter levels (Fig. 1B and Fig. S1 A and B). Recruited innate cells include a distinct population of neutrophils (CD11b+Ly6CintLy6G+F4/80−) with multilobed nuclei and characteristic granules, eosinophils (CD11b+Ly6Clo/intLy6G−F4/80+SSChi) with distinct cationic granules, and monocytes/macrophages (CD11b+Ly6Cint/hiLy6G−F4/80+SSClo) with characteristic nuclear morphology and phagocytic vesicles (Fig. 1C). Direct epithelial cell damage and signals initiated by an abnormal localization of microbial flora fuel this influx of innate cells (Fig. S2 A and B).
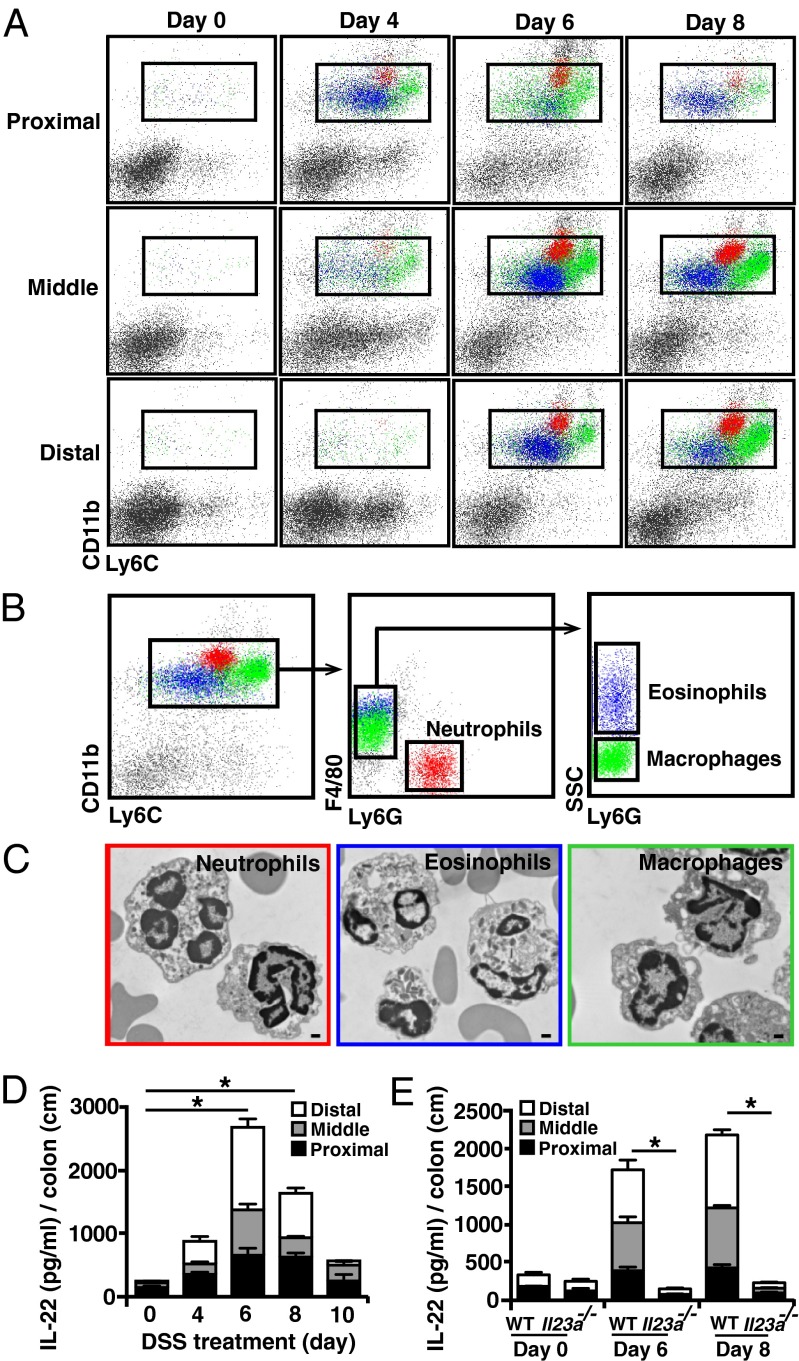
Fig. 1.
Heightened IL-23–dependent IL-22 expression correlates with the influx of macrophages and granulocytes during DSS-induced colitis. (A) Cells isolated from colons of DSS-treated BL/6 mice at various time points were stained with anti-CD11b and anti-Ly6C and were analyzed by flow cytometry. (B and C) CD11b+Ly6C+ colon cells were fractionated further into Ly6G+F4/80− neutrophils (red), Ly6G−F4/80+SSClo macrophages (green), and Ly6G−F4/80+SSChi eosinophils (blue) (B) and were sorted and examined by TEM (C). (Scale bar, 500 nm.) (D and E) Colon tissue from DSS-treated BL/6 mice (D) and Il23a−/− mice (E) was collected at different time points and cultured for 2.5–3 d. Supernatant was analyzed for IL-22 protein by ELISA. Data are representative of three independent experiments with four or five mice per time point. *P < 0.05.
The recruitment of granulocytes and macrophages correlated with a marked increase in IL-22 expression in the middle and distal colon at the end of the first week of DSS treatment (Fig. 1D). IL-22 production at this early stage is crucial to limit epithelial cell damage (Fig. S3A), suggesting that recruited innate cell(s) that express IL-22 might contribute to the maintenance of colonic epithelial integrity. Importantly, IL-23 was required for the enhanced colonic IL-22 expression during DSS-induced colitis (Fig. 1E), because IL-22 production was ablated in IL-23–deficient mice. Furthermore, Myd88−/− mice had severely blunted IL-22 production, indicating that microbial responses contribute to the IL-22 levels in the colon during DSS-induced colitis (Fig. S3B). Together, these data imply that IL-23–dependent IL-22 expression by recruited innate inflammatory cells promotes epithelial protection during acute colitis.
Colonic Neutrophils Respond to IL-23 and TNF-α to Produce IL-22.
Cytokines other than IL-23 can enhance IL-22 production by innate immune cells (1, 26, 27). Moreover, Tnf−/− mice challenged with DSS have enhanced inflammation and colonic epithelial damage in the colon and succumb to DSS-induced colitis (28, 29). Therefore, we questioned whether IL-23 in the presence or absence of TNF-α signals could induce IL-22 production by colonic granulocytes and/or macrophages. Sorted colonic neutrophils stimulated with IL-23 up-regulated IL-22 and IL-17 protein levels, and the addition of TNF-α further enhanced IL-22 production (Fig. 2A). Although TNF-α signals lead to the up-regulation of IL-6 and macrophage inflammatory protein 2 (MIP-2) in colonic eosinophil and macrophage populations, it was not sufficient to induce IL-22 secretion. The expression of IL-22 by colon-infiltrating neutrophils was not unique, because neutrophils isolated from the lungs of asthmatic mice, sorted and then stimulated with IL-23, also secrete IL-22 protein (Fig. S4B).
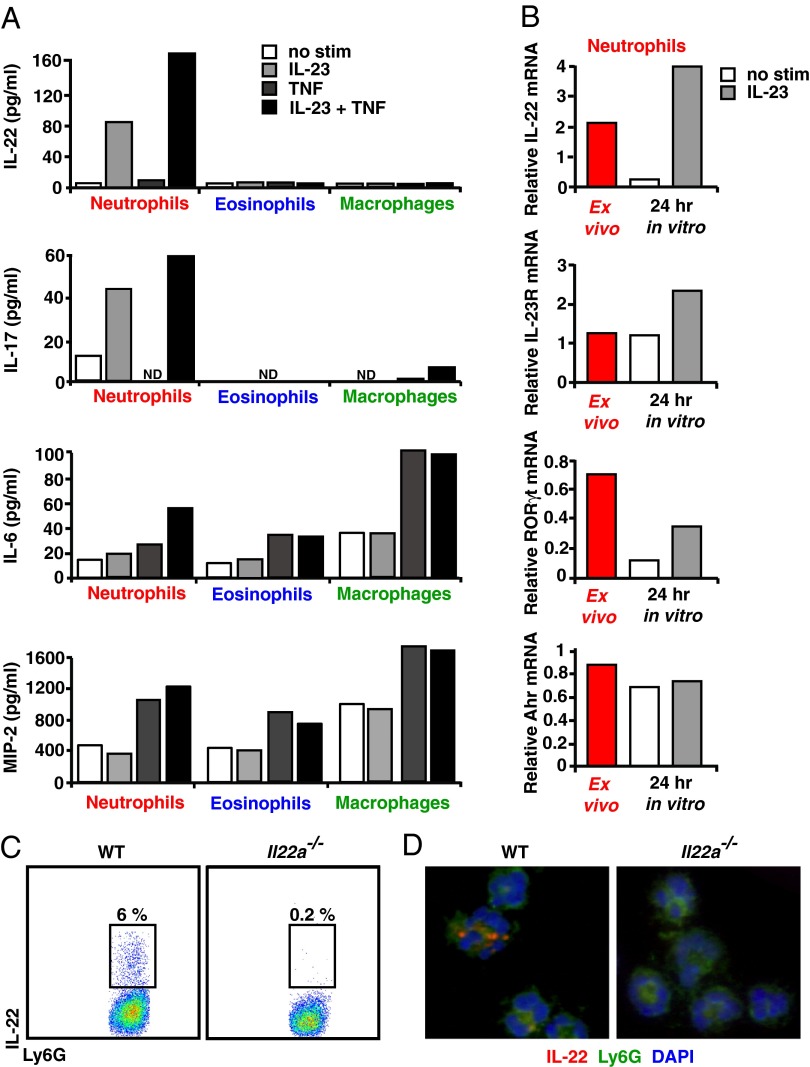
Fig. 2.
IL-23 with TNF-α signals augments IL-22 production from colonic neutrophils. (A) Cells sorted from the colons of DSS-treated mice were cultured for 48–72 h in the presence or absence of IL-23, TNF-α, or IL-23 + TNF-α. Supernatants were analyzed for IL-22, IL-17, IL-6, and MIP-2 protein by ELISA. ND, not detected. (B) Colonic neutrophils from DSS-treated BL/6 mice were sorted, and RNA was collected from cells immediately ex vivo or after in vitro culture in the presence or absence of IL-23. mRNA encoding IL-22, IL-23R, RORγt, or Ahr was quantitated by real-time PCR and was normalized to GAPDH. Data in A and B are representative of three independent experiments in which colon cells from 10–30 DSS-treated mice were pooled and sorted. (C) Colon cells from DSS-treated BL/6 and Il22a−/− mice were cultured with IL-23 for 4 h, stained for intracellular IL-22, and then analyzed by flow cytometry. (D) Colonic neutrophils sorted from DSS-treated BL/6 and Il22a−/− mice were cultured with IL-23 for 4 h, cytospun, and then stained with anti–IL-22 (red). Data in C and D are representative of two independent experiments with four or five mice per group.
Unless cultured in the presence of IL-23, isolated colonic neutrophils down-regulated IL-22 mRNA, suggesting that IL-23 signals sustain IL-22 expression (Fig. 2B; also see Table S1). In addition, IL-23 signals may act to promote the maintenance of an IL-22–producing neutrophil phenotype (Fig. S1C). In this regard, addition of IL-23 resulted in up-regulation of IL-23R and retinoic acid-related orphan receptor gT (RORγt) mRNA levels. Colon neutrophils also express aryl hydrocarbon receptor (Ahr) mRNA; however, IL-23 stimulation did not affect Ahr expression. Although IL-22–producing colonic neutrophils are a subset of those isolated during DSS-induced colitis (Fig. 2C), they are recruited to the colon continuously. Interestingly, we found that IL-22 was present in the granules of colonic neutrophils (Fig. 2D). Together, these data define an innate cell population of mucosal neutrophils that rapidly produce IL-22 via granule exocytosis in response to IL-23 signals.
Neutrophils Contribute to IL-22 Levels During Colitis and Enhance Antimicrobial Peptide Production.
To address whether neutrophils are the source of heightened IL-22 during DSS-induced colitis, we depleted colonic neutrophils using the RB6-8C5 (anti-Gr1) antibody. Compared with controls, RB6-8C5–treated mice showed profound depletion of colonic neutrophils with negligible effect on the presence of other innate populations (Fig. 3A). To minimize the influx of immature neutrophils to the colon in response to systemic neutrophil depletion, antibodies were given for 6 h in vivo, and then colons were cultured ex vivo with antibody and complement. The depletion of neutrophils on day 7 of DSS treatment resulted in significant reduction in IL-22 production from the middle and distal colon compared with the control-treated group (Fig. 3B). This result demonstrates that colonic neutrophils augment local IL-22 levels during acute colitis.
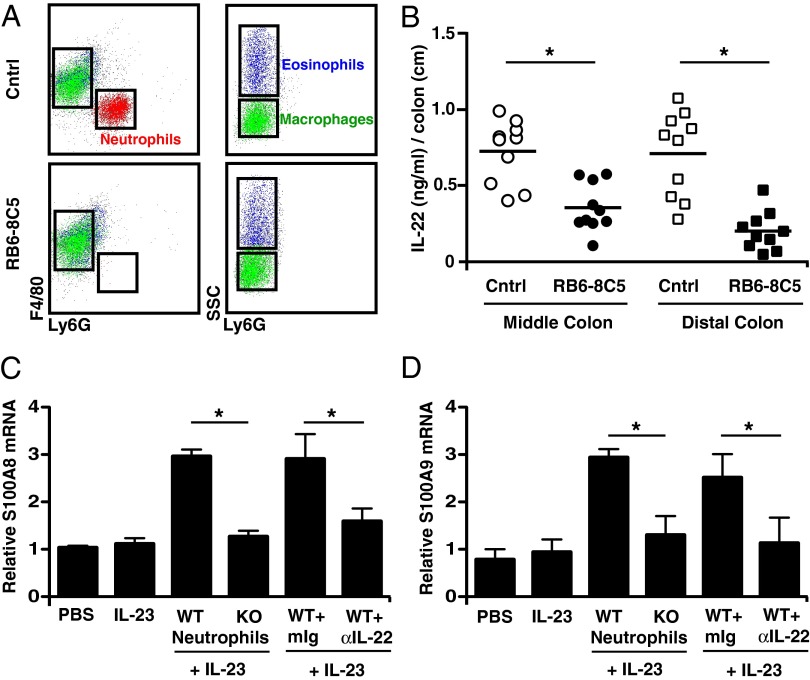
Fig. 3.
Colonic neutrophils contribute to IL-22 levels and up-regulate epithelial cell-derived antimicrobial peptides. (A) Colon cells from DSS-treated BL/6 mice that received either anti-rat Ig (Cntrl) or anti-Gr1 (RB6-8C5) for 18 h were stained with anti-Ly6C, anti-Ly6G, anti-CD11b, and anti-F4/80 and were analyzed by flow cytometry. (B) Middle (○) and distal (□) colon tissue from DSS-treated BL/6 mice that received Cntrl (open symbols) or RB6-8C5 (filled symbols) for 6 h was cultured for 2.5–3 d in the presence of respective antibodies and complement. Supernatant was analyzed for IL-22 protein levels by ELISA. (C and D) Neutrophils isolated from colons of either WT or Il22−/− (KO) DSS-treated mice were sorted and then cultured with the colon-derived YAMC epithelial cell line for 48 h. Cells were stimulated with IL-23 in the presence or absence of either a control mouse antibody or blocking anti–IL-22 antibody. Epithelial cell lysates were quantitated for S100A8 (C) and S100A9 mRNA (D) expression by real-time PCR and were normalized to GAPDH mRNA. Data are representative of two independent experiments. *P < 0.05.
Because DSS treatment is thought to damage epithelial cells directly, resulting in abnormal influx of microbes, we investigated whether IL-22–producing neutrophils act to up-regulate antimicrobial peptide production in epithelial cells. For these studies, we used an epithelial cell line, YAMC, derived from colons of young adult mice (30). Colonic neutrophils from DSS-treated or lung neutrophils from asthmatic mice were cocultured with YAMC epithelial cells, and IL-23 was added to the cultures to sustain IL-22 production. IL-23–stimulated neutrophils from DSS-treated WT mice induced a two- to threefold increase in S100A8 and S100A9 antimicrobial peptide mRNA in YAMC cells compared with IL-23–stimulated neutrophils from DSS-treated Il22−/− (KO) mice (Fig. 3 C and D). Similar results were observed using neutrophils sorted from lungs of asthmatic mice (Fig. S4C). Because neutrophils can express S100A mRNA, the contribution of neutrophil-derived IL-22 in the observed induction of epithelial cell-derived S100A mRNA was assessed both by using colonic neutrophils from DSS-treated Il22−/− mice and by including a blocking anti–IL-22 antibody (Fig. 3 C and D). The results establish that IL-22 produced by mucosal neutrophils up-regulates the expression of antimicrobial peptide mRNA by colonic epithelial cells.
IL-22–Producing Neutrophils Contribute to the Resolution of Colitis.
In view of our findings that infiltrating neutrophils produce IL-22 that might contribute to epithelial integrity and antimicrobial peptide production following DSS-mediated injury, we established a model to assess the ability of transferred neutrophils to protect colitic mice. Neutrophils were generated from cultures of bone marrow from WT or Il22−/− mice (CD11b+Ly6B.2 (7/4)+FSCintSSCint) and were transferred into IL-22–deficient recipient mice on day 4 of DSS treatment (when neutrophils relocate to the colon). Recipient mice were killed 2 d later (day 6 of treatment), and the severity of colitis and epithelial damage was scored, and the production of antimicrobial peptides by the colonic epithelium was assessed (Fig. 4).
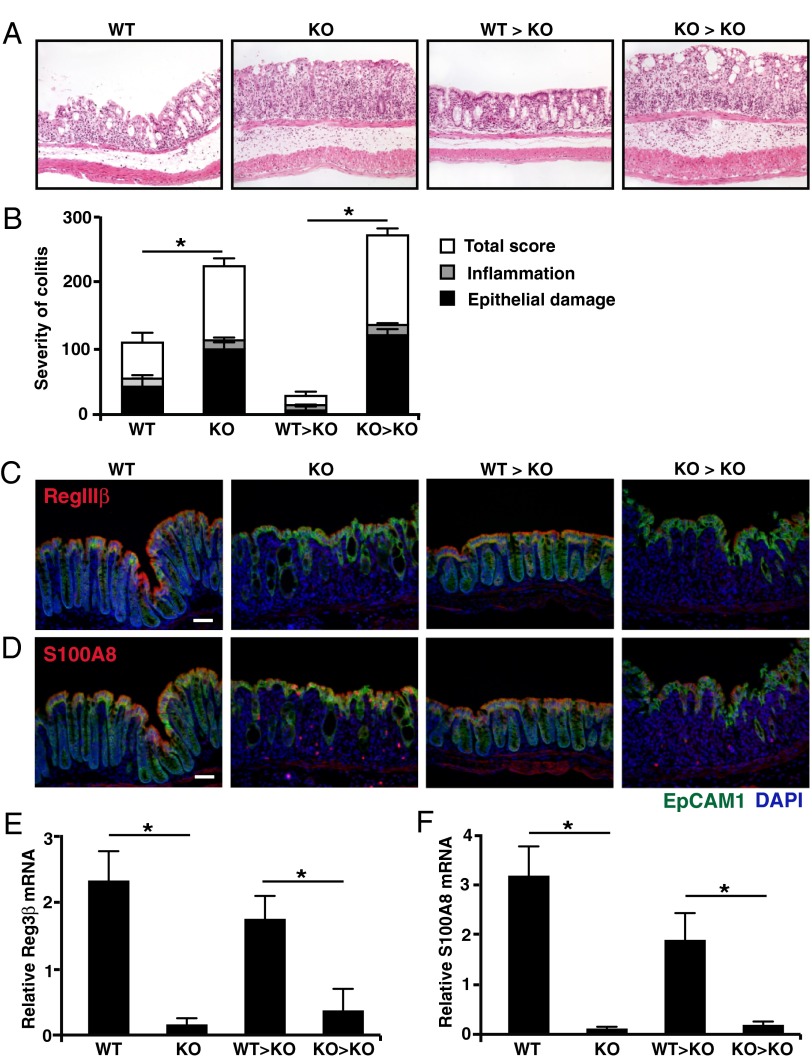
Fig. 4.
IL-22–producing neutrophils play a protective role during DSS-induced colitis. Colon tissue from day 6 DSS-treated BL/6 mice (WT), Il22−/− (KO) mice, and Il22−/− mice that on day 4 received an i.p. injection of sorted 2 × 106 bone marrow neutrophils from either BL/6 (WT → KO) or Il22−/− (KO → KO) mice was stained with H&E (A) for blinded histological scoring (B) or was stained with EpCAM1/CD326 (green) and DAPI (blue) and with anti-RegIIIβ (red) (C) or with anti-S100A8 (red) (D) for protein expression. (Scale bars, 70 μm.) (E) Reg3β and (F) S100A8 mRNA expression from laser-captured colon epithelial cells was analyzed by real-time PCR, and gene-specific mRNA was normalized to GAPDH mRNA. Data are representative of three independent transfer experiments with four or five mice per group. *P < 0.05.
In mice that did not receive transfers of neutrophils, epithelial damage in the middle and distal colons of Il22−/− (KO) mice was increased significantly compared with that of WT mice on day 6 of DSS treatment (Fig. 4 A and B). This increase was associated with a marked decrement in the production of RegIIIβ and S100A8 peptides by the colonic epithelium of IL-22–deficient mice (Fig. 4 C and D). The transfer of neutrophils derived from Il22−/− mice into DSS-treated Il22−/− mice (KO → KO) did not significantly alter colon pathology (Fig. 4 A and B), nor did it rescue production of antimicrobial peptide (Fig. 4 C and D). In contrast, the transfer of bone marrow-derived neutrophils from WT mice to DSS-treated Il22−/− mice (WT → KO) resulted in significant protection of the colon from DSS-induced epithelial cell damage, which correlated with enhanced expression of RegIIIβ and S100A8 peptides by colonic epithelial cells (Fig. 4 A–D).
To quantitate alterations in antimicrobial peptide production that resulted from neutrophil-specific deficiency of IL-22, laser-capture microdissection (LCM) was used (Fig. 4 E and F). Epithelial cells isolated from DSS-treated WT mice (WT) expressed >15–fold higher RegIIIβ and S100A8 mRNA as compared with DSS-treated Il22−/− mice (KO) mice. The transfer of bone marrow-derived neutrophils from Il22−/− mice into DSS-treated Il22−/− mice (KO → KO) had no detectable effect on antimicrobial peptide levels, whereas transfers of WT neutrophils (WT → KO) rescued RegIIIβ or S100A8 mRNA expression by colonic epithelial cells. Thus, neutrophil-derived IL-22 significantly ameliorates the severity of DSS-induced epithelial damage and restores antimicrobial peptide protection.
Discussion
In this study, we find that colon-infiltrating neutrophils produce IL-22 in response to coordinated signaling by IL-23 and TNF-α, establishing a role for granulocytes in supporting enhanced epithelial barrier function. Consistent with our findings, mice with defective colonic recruitment of neutrophils caused by CXCR2 deficiency had impaired resistance to the rodent intestinal pathogen, C. rodentium (24), protection against which is highly dependent on IL-23–induced IL-22 (9, 31). Therefore, neutrophils recruited to the colon during the acute phase of colitis have a dual role in host protection: directly killing microbes and indirectly signaling epithelial cells via IL-22/IL-22R to amplify antimicrobial peptide production and enhance barrier function.
MyD88/TNF-α signals that are triggered in response to transcytosed microbes or their proinflammatory products contribute to increased IL-22 production in the colons of DSS-treated mice (Fig. S2B). In this regard, several published reports indicate that colonic macrophages serve a protective role in DSS-induced colitis (32, 33). Because, in addition to IL-23, colonic macrophages produce abundant TNF-α during DSS-induced colitis (34), colonic macrophages and IL-22–producing colonic neutrophils likely cooperate to protect the epithelial barrier during acute colitis, where there is coincident recruitment of both cell populations (Fig. 1). Notably, it has been reported that patients with psoriasis treated with the TNF-α inhibitor, etanercept (TNFRII-Fc), had decreased levels of IL-17 and IL-22 in their serum (27). Although this finding could reflect a global reduction in inflammation, it also is conceivable that TNF-α blockade might result in dampening of IL-22 production, an effect that could be detrimental to the restoration of epithelial integrity in colitis. This possibility will require further study.
The finding that colon-recruited neutrophils represent a critical source of IL-22 for restoration of epithelial integrity suggests that sustained production of IL-22 requires coordinate interplay with other innate immune cells previously identified as sources of IL-22 in the response to mucosal injury or inflammation (e.g., ILC subtypes, NK cells, dendritic cells, and γδ T cells). However, during acute colitis, rapid release of granule-packaged IL-22 from colonic neutrophils may be more beneficial than IL-22 production from other innate and lymphocyte populations, which can take hours to days. The production of IL-22 by such a variety of cell types no doubt reflects the indispensible role of this cytokine in maintaining and repairing the intestinal barrier. In this regard, NK1.1+ cells have been shown to contribute to IL-22 levels in the colon around day 4 after initiation of DSS treatment (4), preceding the recruitment of peak numbers of IL-22–producing neutrophils around days 6–8 after DSS treatment (Fig. 1A), which, in turn, precedes the development and recruitment of Th17 or Th22 cells around days 10–12 after DSS treatment (35). Therefore, various IL-22–producing cells contribute to the maintenance or restitution of epithelial integrity at different stages of disease, supporting a sequential recruitment model in which different components of the innate and adaptive immune system are orchestrated for sustained production of IL-22. Alternatively, or perhaps in addition, different populations of IL-22–producing cells might contribute to regional resolution of colitis. In future studies it will be important to consider several factors in understanding the interplay of innate and adaptive immune cells that produce IL-22: (i) the schedule of recruitment to the colon, (ii) regional positioning of cells, and (iii) potential targeting of different IL-22R–expressing cells.
Unlike other IL-22–producing innate immune cell populations, neutrophils are nonresidents of the colon and are recruited in large numbers in response to mucosal injury. In addition to IL-22, neutrophils also express IL-17 in response to IL-23 (ref. 36 and Fig. 2A); both are cytokines classically produced by Th17 cells. Because neutrophils are short-lived cells and must be recruited to the colon continuously, it is of interest that Th17 cells promote the enhanced recruitment of neutrophils to inflammatory sites, both through their production of factors that increase neutrophil production by the bone marrow, whether directly (e.g., GM-CSF) or indirectly (e.g., IL-17–induced granulocyte colony-stimulating factor, G-CSF), and their production or induction of chemokines that recruit neutrophils (e.g., IL-8). The findings herein therefore identify a previously unappreciated connection in the Th17–neutrophil axis, wherein sustained recruitment of neutrophils that results from Th17-driven chronic inflammation could serve as an important mechanism for both amplifying IL-22–dependent protective effects on the epithelium and eliminating microbes that breach the injured epithelial barrier.
Materials and Methods
Mice.
C57BL/6 mice were purchased from Jackson Laboratories. Il22−/− mice were a gift from W. Ouyang (Genetech, South San Francisco, CA) (37). Il23a−/− mice were provided by Schering-Plough/Merck. All animals were bred and maintained at the University of Alabama at Birmingham (UAB) in accordance with the UAB Institutional Animal Care and Use Committee regulations.
DSS Model of Colitis.
Mice were treated with 2.5–3% (wt/vol) DSS (MP Biomedicals; molecular weight: 36,000–50,000) in their drinking water for 7 d and then were switched to sterile tap water. DSS-treated mice were monitored regularly for signs of disease.
Ex Vivo Colon Cultures.
Colon tissue was flushed, cut into three equal sections (proximal, middle, distal), and then was opened longitudinally. Individual tissue segments were placed in 0.4 mL of R10 medium [Roswell Park Memorial Institute (RPMI) medium 1640 supplemented with 10% (vol/vol) FCS, penicillin (100 IU/mL), streptomycin (100 μg/mL), 1× nonessential amino acids (NEAA), sodium pyruvate (1 μM), β-mercaptoethanol (2.5 μM), and l-glutamine (2 μM)] in a 24-well culture plate and were incubated for 2–3 d at 37 °C, 5% CO2.
Depletion of Neutrophils.
DSS-treated mice were administered 100 μg of either anti-rat IgG (Control; Sigma) or anti-Gr1 (RB6-8C5) for 6–18 h. Colon tissue was cultured as described above. Media were supplemented with 10 μg of either anti-rat Ig or anti-Gr1, respectively, and Low-Tox rabbit complement (Colorado Serum Co.).
LCM.
Unfixed colon tissue frozen in O.C.T. compound (Tissue-Tek) in 6-μm sections on PEN-membrane glass slides (Applied Biosystems) was stained with Cresyl Violet dye (Ambion). Stained epithelial cells (1,000 per cap) were captured on Arcturus HS LCM caps (Applied Biosystems) using a Veritas LCM instrument. RNA was extracted using the Arcturus PicoPure RNA isolation kit (Applied Biosystems). DNA was degraded using an RNase-free DNase set (Qiagen).
Epithelial Cell Cultures.
Conditionally immortalized YAMC cells were kindly provided by R. H. Whitehead (Vanderbilt University, Nashville, TN) (30). Cells were maintained at 33 °C in RPMI 1640 containing 5% FBS (vol/vol), 1 μg/mL insulin, 10 μM α-thioglycerol, 1 μM hydrocortisone, and 10 U/mL IFN-γ. Cells (3.5 × 105) were seeded into 12-well culture plates and incubated overnight at 37 °C without IFN-γ. The next day, the medium was changed to R10 before the addition of 10 ng/mL IL-23 and 3 × 105 neutrophils. Two days later, YAMC cells were washed and processed for RNA isolation using an RNeasy Mini kit (Qiagen).
Supplementary Material
Acknowledgments
We thank Andrei Kruglov (The Trudeau Institute) for critical technical advice; T. Schoeb and the Comparative Pathology Laboratory for histology scoring; B. Clodfelder-Miller and the Cellular and Molecular Neuropathology Core at the University of Alabama at Birmingham (UAB) for laser-capture microdissection; M. Spell at the Center for Aids Research Flow Cytometry Core at UAB for sorting; M. Chimento at the High Resolution Imaging Facility at UAB for transmission electron microscopy; D. O’Quinn, J. Oliver, B. J. Parsons, and M. Zeng for expert technical assistance; and G. Gaskins for editorial assistance. This work was supported by grants from the National Institutes of Health (NIH) and the Crohn’s and Colitis Foundation (to C.T.W.) and by T32 Training Grant funds from NIH/National Institute of Allergy and Infectious Diseases and the UAB Training Program in Immunologic Diseases and Basic Immunology (to C.L.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 12509.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300318110/-/DCSupplemental.
References
- 1.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmechel S, et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14(2):204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto-Furusho JK, et al. Colonic epithelial upregulation of interleukin 22 (IL-22) in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16(11):1823. doi: 10.1002/ibd.21235. [DOI] [PubMed] [Google Scholar]
- 4.Zenewicz LA, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, Yang J, Gao Y-D, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151(4):297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 6.Wolk K, Sabat R. Interleukin-22: A novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17(5):367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Brand S, et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118(2):534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 10.Newman WG, Zhang Q, Liu X, Amos CI, Siminovitch KA. Genetic variants in IL-23R and ATG16L1 independently predispose to increased susceptibility to Crohn’s disease in a Canadian population. J Clin Gastroenterol. 2009;43(5):444–447. doi: 10.1097/MCG.0b013e318168bdf0. [DOI] [PubMed] [Google Scholar]
- 11.Chung Y, et al. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16(11):902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenberg GF, Fouser LA, Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 13.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: Regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 14.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31(1):15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awasthi A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182(10):5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams IR, Parkos CA. Colonic neutrophils in inflammatory bowel disease: Double-edged swords of the innate immune system with protective and destructive capacity. Gastroenterology. 2007;133(6):2049–2052. doi: 10.1053/j.gastro.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Parkos CA, Colgan SP, Madara JL. Interactions of neutrophils with epithelial cells: Lessons from the intestine. J Am Soc Nephrol. 1994;5(2):138–152. doi: 10.1681/ASN.V52138. [DOI] [PubMed] [Google Scholar]
- 22.Friedman GB, Taylor CT, Parkos CA, Colgan SP. Epithelial permeability induced by neutrophil transmigration is potentiated by hypoxia: Role of intracellular cAMP. J Cell Physiol. 1998;176(1):76–84. doi: 10.1002/(SICI)1097-4652(199807)176:1<76::AID-JCP9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol. 2008;181(8):5702–5710. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spehlmann ME, et al. CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J Immunol. 2009;183(5):3332–3343. doi: 10.4049/jimmunol.0900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol. 2007;179(1):566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- 26.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caproni M, et al. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: A randomized-controlled trial. J Clin Immunol. 2009;29(2):210–214. doi: 10.1007/s10875-008-9233-0. [DOI] [PubMed] [Google Scholar]
- 28.Naito Y, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18(5):560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 29.Mizoguchi E, et al. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134(2):470–480. doi: 10.1053/j.gastro.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 30.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA. 1993;90(2):587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 32.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102(1):99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80(4):802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 34.Ghia JE, et al. Role of M-CSF-dependent macrophages in colitis is driven by the nature of the inflammatory stimulus. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G770–G777. doi: 10.1152/ajpgi.00453.2007. [DOI] [PubMed] [Google Scholar]
- 35.Monk JM, et al. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J Nutr. 2012;142(1):117–124. doi: 10.3945/jn.111.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werner JL, et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun. 2011;79(10):3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.