Abstract
Manduca sexta larvae are a model for growth control in insects, particularly for the demonstration of critical weight, a threshold weight that the larva must surpass before it can enter metamorphosis on a normal schedule, and the inhibitory action of juvenile hormone on this checkpoint. We examined the effects of nutrition on allatectomized (CAX) larvae that lack juvenile hormone to impose the critical weight checkpoint. Normal larvae respond to prolonged starvation at the start of the last larval stage, by extending their subsequent feeding period to ensure that they begin metamorphosis above critical weight. CAX larvae, by contrast, show no homeostatic adjustment to starvation but start metamorphosis 4 d after feeding onset, regardless of larval size or the state of development of their imaginal discs. By feeding starved CAX larvae for various durations, we found that feeding for only 12–24 h was sufficient to result in metamorphosis on day 4, regardless of further feeding or body size. Manipulation of diet composition showed that protein was the critical macronutrient to initiate this timing. This constant period between the start of feeding and the onset of metamorphosis suggests that larvae possess a molt timer that establishes a minimal time to metamorphosis. Ligation experiments indicate that a portion of the timing may occur in the prothoracic glands. This positive system that promotes molting and the negative control via the critical weight checkpoint provide antagonistic pathways that evolution can modify to adapt growth to the ecological needs of different insects.
Keywords: size regulation, ecdysone, biochronometry
Time keeping is an essential feature of biological systems. The best understood timing systems are the circadian clocks that coordinate biological processes with the day–night cycle (1). It is becoming clear, however, that the coordination of growth and development also involves other types of timers that provide avenues by which environmental conditions, notably nutrition, can impact these processes. Such timers may be involved in once in a lifetime events like puberty in humans (2) or metamorphosis in insects, or in recurring events such as the molting cycles of nematodes (3) and arthropods. Work on the nematode, Caenorhabditis elegans, has led the way in understanding the molecular basis of some of these other types of timers, such as the heterochronic genes that lead the animal through successive larval stages (4) and, more recently, a molt timer that times the progression through the recurring molts independent from the developmental processes that it regulates (5, 6). This timer acts in the hypodermal cells through positive- and negative-feedback loops involving lineage-abnormal (lin)-42A, some nuclear receptors, and the let-7 family of microRNAs.
In insects, as in other arthropods, nematodes, and other Ecdysozoa, larval growth is punctuated by periodic molts, as successive larger cuticles are formed to accommodate ongoing growth. The growth and molting processes are influenced by a number of environmental factors, the chief of which is food availability and quality. A major focus has been on control of body size, and larvae of the tobacco hornworm moth, Manduca sexta, have been a major model system in this area for nearly 40 y since the first demonstration by Nijhout and Williams (7) of the phenomenon of a “critical weight” during the last larval stage. Critical weight represents a body size “check-point” that, once achieved, leads to the endocrine cascade resulting in the termination of feeding and initiation of metamorphosis. It is operationally defined as the time at which starvation no longer delays the timing of metamorphosis. This metamorphic checkpoint is intimately associated with the circulating titers of juvenile hormone (JH), which can suppress the entry into metamorphosis in the last larval stage (8–10). At the metamorphic critical weight, circulating JH is removed, both by the inhibition of JH biosynthesis by the corpora allata (CA) and by the appearance of a JH-specific esterase in the blood (11–13), thereby allowing the larva to embark on its metamorphic program.
Although the progression through the larval instars can be quite stereotyped under optimal conditions, under conditions of poor nutrition, larvae can undertake “intercalary” molts (7), suggesting that there are positive factors that drive molting even if nutritional conditions are suboptimal. Callier and Nijhout (14) described that, under poor food conditions, larvae could nevertheless undergo molting using a mechanism that is independent of size, and, indeed, appears to be independent of the brain, the source of the prothoracicotropic hormone (PTTH) that normally drives the ecdysteroid surges needed for molting (15). We examine the effects of challenges in nutrition on allatectomized (CAX) larvae that no longer have JH to impose a size-dependent suppression of metamorphosis. The removal of JH revealed that larvae also possess a molt timer that establishes a minimal time to metamorphosis during the final instar. The timer depends on feeding, primarily on a protein source, to initiate timing, but once initiated it then keeps time in a manner that is independent of further feeding or of body size.
Results
Allatectomy and the Response to Starvation.
The transition from feeding to the wandering stage is associated with a suite of behavioral, physiological, and morphological changes. The larvae cease feeding and initiate sustained locomotion and digging behavior (16). They begin to void their gut contents, which are no longer formed into discrete pellets, and their dorsal body coloration changes as insecticyanin is removed from the dorsal epidermis (17) and ommochrome pigments are deposited into the epidermis beside the heart (18, 19). These changes are linked together as a common response to a small surge of ecdysteroid in the absence of JH that irreversibly commits the larva to a metamorphic pathway (20, 21). In our CAX larvae, we often could not monitor changes in epidermal coloration because the overlying cuticle had melanized during the molt to the fifth instar because of the absence of JH (22–24). Therefore, we identified this transition based on the other physiological and behavioral changes that accompany wandering, as described above.
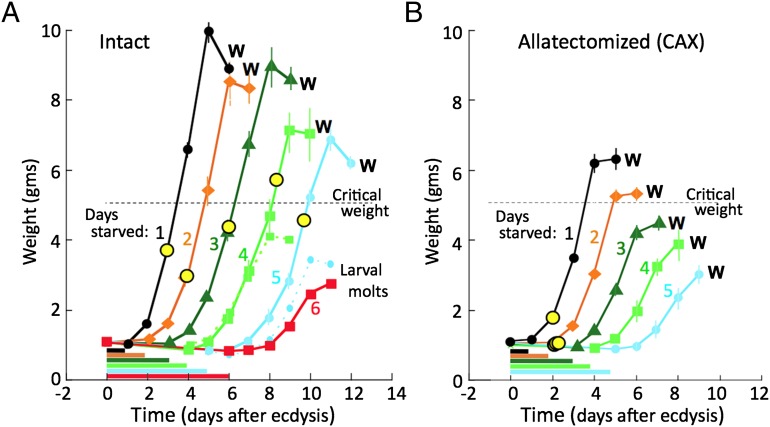
Newly ecdysed, intact, fifth instar larvae were held without food for periods ranging up to 6 d and then given normal diet and weighed daily until they started wandering (Fig.1A). These control larvae gradually lost weight during the starvation period, but they remained hydrated because they were given wet tissues as a supply of water. The weight gain on the first day after return to food was progressively less as the starvation period was increased, presumably due to the deteriorating condition of the larvae. Larvae survived starvation up through 5 d but longer periods resulted in substantial mortality. Larvae starved only 1 or 2 d always eventually wandered and pupated after they were returned to their normal food. With starvation of 3 d or longer, however, an increasing percent of larvae opted out of the metamorphic pattern and molted into a supernumerary sixth larval stage. The growth curves of the latter larvae showed that they were always the slower growing larvae in their cohort (Fig. 1A), and they are not considered in our subsequent analysis. As seen in Fig. 1A, for larvae that pupated at the end of the fifth instar, the period that they fed before wandering increased with the duration of the starvation period. As expected from the work of Nijhout and Williams (7), larvae from all of the starvation groups exceeded the critical weight of 5 g before wandering, but the longer that the larvae were starved, the less was their weight at the onset of metamorphosis. Therefore, in normal larvae, prolonged starvation is followed by an extension of the feeding period so that larvae can at least approach a normal weight before the cessation of feeding at wandering.
Fig. 1.
The effects of the removal of the corpora allata from fifth instar Manduca larvae on their growth rates and the timing of the formation of their leg imaginal primordia (yellow circles) after starvation for various periods. Larvae were starved from the beginning of the fifth larval instar for the durations indicated by the horizontal bars, and then given their standard diet and tracked until they started wandering (W) or initiated a supernumerary larval molt. (A) Control larvae. Groups that underwent a larval molt were those starved for 6 d and the subgroups for the 4- and 5-d treatments are represented by the small symbols and dashed lines. (B) CAX larvae that lacked JH during and after the starvation period. Groups represent five larvae per group. Symbols show the mean ± SEM for the various days. In cases in which no variance is shown, it is less than the height of the symbol. Critical weight estimate comes from ref. 7.
The response of CAX larvae to a similar regimen of starvation and refeeding is shown in Fig. 1B. Because these larvae lacked JH, none of them underwent a supernumerary larval molt. Interestingly, the daily growth increments shown by the intact and CAX groups were identical under all starvation conditions, showing that JH does not influence daily weight gain in the last instar. A striking difference, however, was that, whereas the control larvae showed a homeostatic extension of their feeding period to compensate for prolonged starvation, the CAX larvae did not and wandered in 4 d after the return to food, regardless of the duration of prior starvation. Consequently, these CAX larvae wandered at dramatically smaller sizes than the corresponding control group. Those starved for 2 d just surpassed the critical weight at the time of wandering whereas those starved for longer periods wandered at progressively smaller weights that were well below the critical weight. Therefore, without their corpora allata, the larvae no longer exhibited a critical weight checkpoint that delays metamorphosis until an appropriate body size is attained. The removal of this inhibitory control system revealed that there is a constant time between the start of feeding and onset of wandering, suggesting that feeding initiates a “timer” system that results in the endocrine initiation of metamorphosis 3–4 d later, regardless of body size.
Although the CAX larvae appear not to use larval body size to determine the time of wandering, it was still possible that they were somehow assessing the state of growth of imaginal tissues to gate their entry into metamorphosis. Indeed, imaginal disc injuries that extend the period of rapid divisions of the discs extend the time spent in the last larval instar in a variety of insects (25–30). Consequently, to assess the state of imaginal tissue development, we determined the time when the leg imaginal primordia formed and initiated growth in the two groups of larvae. With the aid of a dissecting microscope, the formation and growth of the leg imaginal disc can be monitored underneath the transparent leg cuticle of the intact larva (31). Therefore, we checked CAX and control larvae on a daily basis to assess the time of formation of their leg imaginal primordia. In the control larvae, the formation of the leg primordia was suppressed by starvation. They began to form only after the larva was given food, but with a delay based on the duration of the prior starvation, in parallel with the delay in the onset of wandering (yellow circles in Fig. 1A). In the CAX larvae, by contrast, the leg imaginal primordia formed by 2 d after ecdysis even though most of the groups were still starving (31) (Fig. 1B). In the series of starved CAX larvae, then, the time between the formation of the leg disc and the start of wandering did not have a fixed duration and varied from 3 to 7 d. Therefore, the precocious formation and growth of imaginal discs and primordia do not speed the entry into metamorphosis. Larvae wandered 4 d after the resumption of feeding, regardless of when the discs were formed. Therefore, the duration of the feeding period in the CAX larvae appears to be uncoupled from the growth of the imaginal structures as well as from that of the larva itself.
Relationship of the Duration of the Feeding Period to the Onset of Metamorphosis.
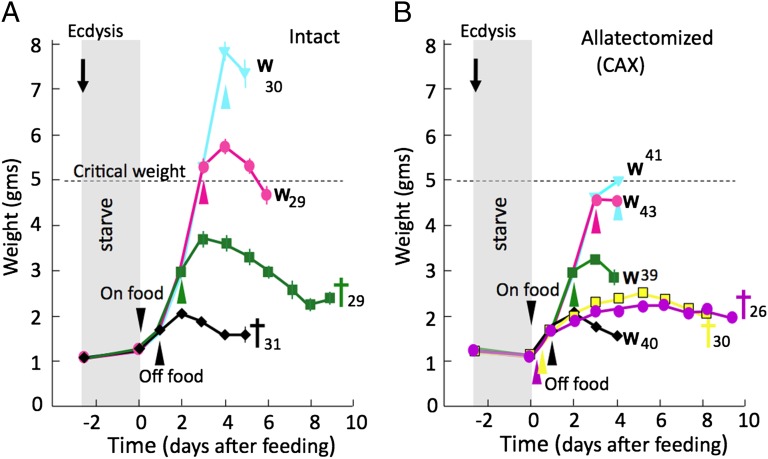
The above section suggests that, in CAX larvae, a cue associated with the initiation of feeding starts a feeding period of fixed duration, the length of which is independent of body size. To understand more about the nature of the feeding cue, we starved newly ecdysed larvae for 2.5 d and then supplied them with normal food for a fixed period, followed again by starvation with a water source alone. For intact larvae, we again saw the impact of the critical weight (Fig. 2A). No larvae survived to wandering if they had food for only 1 d. Of those that fed for 2 d, 75% died before signs of either molting or metamorphosis whereas the remaining 25% began wandering after a considerable delay. Those that were fed 3 or 4 d had attained critical weight by the time that food was withdrawn, and they showed a delayed wandering at 5–6 d after the resumption of feeding.
Fig. 2.
The effects of the duration of the feeding period on the subsequent survival and timing of metamorphosis of fifth instar larval Manduca. Growth curves are for control (A) and for CAX (B) larvae that were starved for 2.5 d from the beginning of the last instar, given food for the indicated period, and then maintained with only a source of water. For the larvae that were fed on standard diet for only 6 or 12 h, they were subsequently given a sucrose-only diet to maintain their energetic needs. W, onset of wandering; crosses, time of death. Each point represents the mean weight, and the error bars represent SE. Numbers are the number of larvae in each group. Cross indicates death without beginning metamorphosis.
The CAX larvae were similarly starved for 2.5 d and then supplied with food for various periods. For these larvae, we tested feeding periods as short as 6 h, but the larvae fed for only 6 or 12 h were subsequently given a diet block that contained only sucrose, but no lipid or protein, so they would have some source of energy but could not grow. Consequently, they could live long enough for us to determine their developmental response to the treatment (Fig. 2B). CAX larvae of the 6-h group survived for up to 9 d, but none showed any sign of wandering. A similar response was seen for most of the larvae given a 12-h pulse of food, but the remaining 25% initiated wandering 4 d after the start of the food pulse. For feeding durations of a day or longer, the larvae were supplied with just water after the test feeding period, and they consistently initiated wandering 4 d after the start of the food pulse (Fig. 2B). Interestingly, for the CAX larvae that had food for only 24 h, their weight at wandering was scarcely greater than their weight at the start of the instar, yet they wandered at the same time as starved CAX larvae that fed continuously after the return to food. This result reinforces the conclusion that, in the absence of the CA and JH, the endocrine system is oblivious to the body size of the larva. These data also show that the requirement for feeding is a phasic one, with a feeding period between 12 and 24 h being sufficient to initiate a timer that will result in the pulse of ecdysone that will cause wandering on day 4. Once initiated, the events of the timer apparently run to completion without further need for food intake or increase in body size.
Although CAX larvae that were given food for as short a period as 12–24 h subsequently wandered on time and initiated metamorphosis, they were not able to pupate successfully. Indeed, for most of the treatment groups, the extended period of feeding shown by intact larvae, compared with their CAX counterparts, resulted in a higher success rate at pupation and the formation of larger pupae (Table 1). Thus, JH, through its involvement in the critical weight checkpoint, can override the timer to ensure that sufficient growth occurs to allow a successful metamorphosis.
Table 1.
The ability of normal and allatectomized larvae to wander and form normal pupae after feeding for various periods of time
| Feeding duration* | N | % Wander | % Pupa | Wander weight† |
| Normal larvae | ||||
| 1 d | 31 | 0 | 0 | — |
| 2 d | 29 | 38 | 10 | 4.3 |
| 3 d | 29 | 100 | 88 | 6.0 |
| 4 d | 30 | 97 | 94 | 7.8 |
| Allatectomized (CAX) larvae | ||||
| 6 h‡ | 28 | 67 | 0 | 2.1 |
| 12 h‡ | 30 | 90 | 0 | 2.3 |
| 1 d | 61 | 67 | 0 | 1.8 |
| 2 d | 39 | 100 | 28 | 4.0 |
| 3 d | 43 | 95 | 58 | 4.4 |
| 4 d | 40 | 98 | 68 | 4.6 |
Newly ecdysed fifth instar larvae were starved for 2.5 d, and then fed the standard diet for various durations followed by maintenance with just a water source.
Average weight (g) of wandering larvae.
Maintained on sucrose diet after feeding on standard diet.
Effect of Nutrition on CAX and Intact Larvae: Feeding on Incomplete Diets.
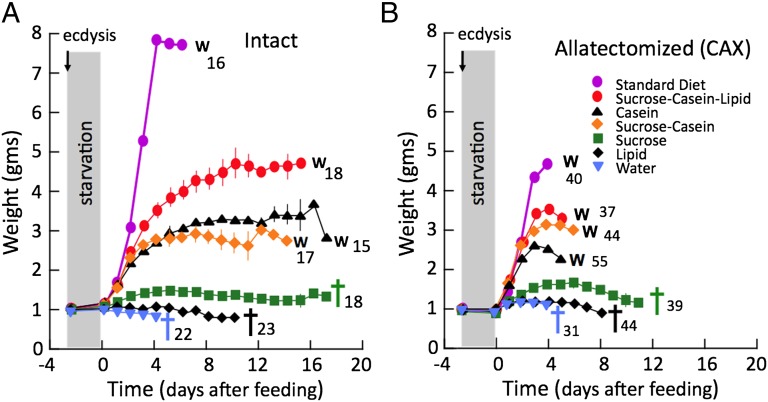
In Manduca, dietary sugars have an important impact on the JH levels (32). To determine whether the timer was sensitive to particular dietary components, we starved last instar larvae for 2.5 d and then provided them with test diets that lacked one or more of the normal macronutrient classes. As seen in Fig. 3A, larvae that were supplied with lipid or carbohydrate (sucrose) as their only source of macronutrients lived for 1–2 wk but showed little weight gain and eventually died without either molting or starting metamorphosis. By contrast, diets with protein (casein) supported weight gain, and most larvae eventually either attempted another larval molt or started wandering. With casein alone, only 27% (n = 15) of the larvae showed any type of molting response, and these underwent a supernumerary larval molt about 9 d after the start of feeding. When the diet contained both casein and sucrose, 82% (n = 17) of the larvae undertook a molt, with 71% opting for metamorphosis and starting wandering whereas 12% showed a supernumerary molt. The larvae undergoing metamorphosis were typically the faster growing larvae in the treatment group. For those that wandered, the time to wandering was markedly delayed over that seen for larvae that were placed on standard diet (15 d versus 6 d, respectively). A few larvae fed with casein alone (1/11) or sucrose plus casein (3/13) underwent a mixed molt that included a clearing over the heart, characteristic of wandering, and head capsule slippage, a feature of a larval molt.
Fig. 3.
The effects of the dietary composition on survival and timing of metamorphosis in control and CAX fifth instar larvae of Manduca. Growth curves are for control (A) and for CAX (B) larvae that were starved for 2.5 d from the beginning of the last instar and then given diets of the indicated composition until they either wandered (W) or died (cross). Each symbol is the mean (± SEM) of the group on the given day. In cases in which no variance is shown, it is less than the height of the symbol. Numbers are the number of larvae in each group.
For CAX larvae given the same suite of test diets, none showed a supernumerary larval molt, as expected because they lacked JH. As with intact larvae, a protein source was most important for survival and the entry into metamorphosis. CAX larvae fed on casein alone started wandering after 5 d at an average weight of 2.8 g. Addition of sucrose delayed wandering for a day with larval size increasing to 3.3 g. Further addition of a lipid source reduced the delay to 4.3 d, with a larval weight of 3.9 g. CAX larvae fed on only lipid or sucrose showed extended survival, but little weight gain, and only 10% and 40%, respectively, survived to show any signs of wandering. Those that wandered did so at 7.5 and 8.5 d after the start of feeding, respectively (Fig. 3B). Therefore, of the three major classes of macronutrients, protein was much more effective than either carbohydrate or lipid in initiating the timer.
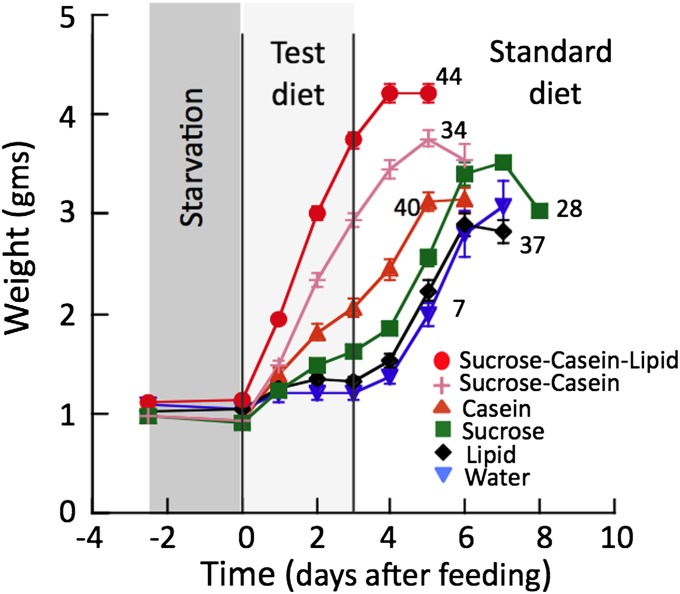
To further explore the impact of different nutrients on the initiation of the timer, we subjected another batch of CAX larvae to 2.5 d of starvation, followed by 3 d on a test diet and then the remainder of their time on their standard food (Fig. 4). Each test group was then compared with CAX larvae that had been given the standard diet after the initial starvation period, or had been starved for the entire 5-d treatment period before being given the standard diet. We reasoned that, if the macronutrients in the test diet could not initiate the timer, then larvae on these diets should wander at the same time as those starved for the entire 5-d period. As assessed by the start of wandering, the timer in CAX larvae given the base diet supplemented with either lipid or sucrose behaved the same as the continuous starvation group, and the larvae started wandering at 4–5 d after being given the standard diet. Larvae given test diets with casein, by contrast, were wandering by 3 d after transfer to normal diet, which was less time than that seen for the continuously starved group but a day longer than those fed the sucrose–casein–lipid diet and 2 d longer than the standard diet group (compare with Fig. 3B). These two experiments support the idea that the quality of the food is important for the initiation of the timer and that the most important component is protein.
Fig. 4.
Growth curves showing the ability of diets of various compositions to initiate the molt timer. Freshly ecdysed CAX larvae were starved for 2.5 d, fed a test diet of the indicated composition for the next 3 d, and then given their standard diet. The last day of the curve for each group is the day of wandering. Groups of larvae given test diets containing just sucrose or just lipid responded to the standard diet like the group that had access to just water during the test period, and they wandered 4 or 5 d after the final transfer. Those given test diets that contained casein wandered after 2–3 d, indicating that their molt timer had started during the test period. Each symbol is the mean (± SEM) of the group on the given day. In cases in which no variance is shown, it is less than the height of the symbol. Numbers are the number of larvae in each group.
Activation of a Timer in Fourth Larval Stage.
To assess whether the timer was present in earlier larval instars, we allatectomized larvae during the molt from the third to the fourth larval instar. These CAX larvae subsequently showed black pigmentation after ecdysis to the fourth instar, and, at the end of the instar, they wandered and started metamorphosis rather than molting to the fifth larval stage. Starvation of these CAX fourth instar larvae for periods beyond 2 d was lethal, so we assessed the effects of refeeding after only 1 or 2 d of starvation. Both groups of starved CAX larvae started wandering 2 d after they were given food. The larvae starved for 1 d wandered at a mean weight of 0.67 g (n = 18; SEM ± 0.05) and those starved for 2 d had a mean wandering weight of 0.36 g (n = 11; SEM ± 0.02). For the latter group, the wandering weight is only 2.7 times their weight when they started to feed and is well under the critical weight expected for a fourth instar of Manduca (14). These results indicate that a timer that is initiated by feeding is a feature of both the penultimate and final larval instars (and likely every instar). Interestingly, the timed interval is significantly shorter for the fourth instar than the fifth, suggesting that the timer has a characteristic period in each instar.
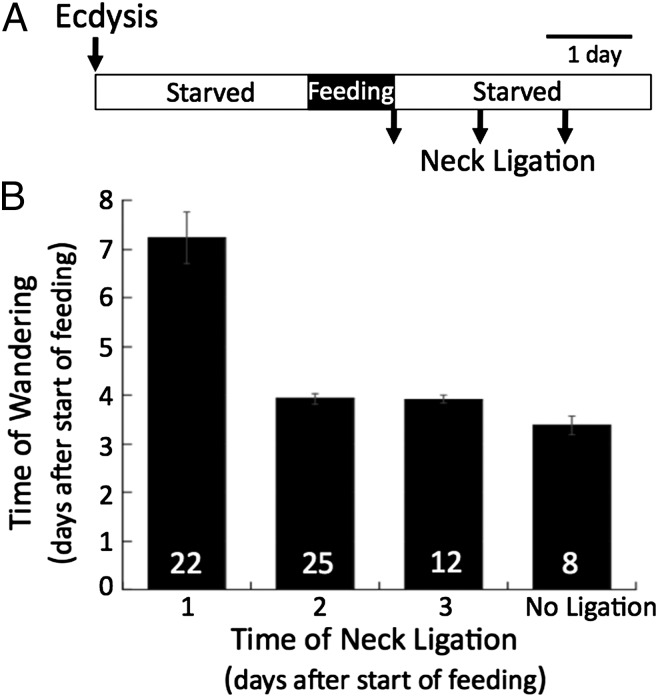
Effect of Neck Ligation on the Timing to Wandering in CAX Larvae.
Feeding of CAX larvae for 24 h is sufficient to initiate a timer that results in wandering on day 4, but what is the nature of the timer and where does it reside? To determine its possible location, we examined the influence of possible factor(s) released from the brain using neck ligation to separate body from the head at various times after the onset of feeding. When CAX larvae were fed 24 h, then either neck-ligated or starved an additional 24 or 48 h before being neck-ligated, all of the bodies subsequently showed the clearing of the epidermis and the voiding of gut contents characteristic of wandering (Fig. 5). Larvae ligated at the end of day 1 delayed showing these signs until about day 7. By contrast, those ligated on day 2 or day 3 (48 or 72 h after the start of feeding) were scored as wandering by day 4, essentially the same time as unligated CAX controls. Therefore, at least the latter half of the timer process appears to occur outside of the head.
Fig. 5.
The effect of decapitation by neck ligation of CAX larvae on the timing of their transition to the wandering stage. (A) The experimental protocol was as follows: newly ecdysed CAX larvae were starved for 2.5 d, fed for 24 h to start their molt timers, and then neck-ligatured at various times after they initiated feeding. (B) Larvae neck-ligatured a day after being given food showed delayed signs of wandering, but those ligated later showed a timing equivalent to the CAX larvae that had just been given the 1-d feeding bout. Bars give the mean and SEM for each group. N is the number of animals in each group.
Discussion
For larvae of M. sexta, the decision to initiate metamorphosis is determined by body size (7, 14, 33). An important concept that has emerged from such studies is the idea of critical weight (7, 34) and that, with the attainment of critical weight, the clearance of JH sets into motion the endocrine events that cause the cessation of feeding and the onset of metamorphosis, locking the animal into its final body size. This study explores the relationship of size and nutrition to the onset of metamorphosis when the JH control system is removed by allatectomy. With the surgical removal of the CA in the last larval stage, the larvae continue to feed and grow and subsequently enter metamorphosis although some tissues may overshoot the pupal stage and show premature adult differentiation (23, 35). We show here that the removal of the CA also removes all vestiges of size control and that the CAX larvae can initiate metamorphosis even if weight gain has been essentially nil. Although CAX larvae that have been partially starved so that they are below the critical weight can readily initiate metamorphosis, they typically do not successfully pupate and eventually die. These observations argue that the critical weight checkpoint, mediated through the CA and JH, ensures that the larva has enough nutrient reserves to deal with the demands of metamorphosis.
In the last instar, then, the continuing presence of JH can delay the entry into metamorphosis, but the removal of JH alone is not sufficient to initiate metamorphosis. This conclusion derives from the fact that larvae that were allatectomized early in the fifth instar molt have no circulating JH by the time of head capsule slippage (as shown by their black pigmentation) (23, 36), but they do not immediately initiate metamorphosis after ecdysis. Rather, they feed for about 3–4 d before they finally start wandering (23). This result argues that the decision to start metamorphosis involves both positive factors associated with feeding as well as the negative influences of JH suppression.
We examined the relationship of nutrition and size to molting in the absence of JH by subjecting CAX larvae to a series of starvation treatments. Intact larvae respond homeostatically to prolonged starvation by extending their feeding period so that critical weight is achieved before starting metamorphosis (Fig. 1A). CAX larvae showed identical growth curves to intact larvae after all starvation regimens, with the striking exception that they stopped feeding and started wandering 4 d after starting to feed regardless of the duration of the prior period of starvation (Fig. 1B) or their weight at wandering. Indeed, in the absence of JH-mediated suppression, the initiation of metamorphosis occurred after a fixed time period that was referenced to feeding, rather than the start of the instar. This size-independent pathway we refer to as the “molt timer.”
We did not explore the temperature sensitivity of this timer, but we assume that its interval is not temperature compensated as is that of a circadian clock (e.g., ref. 37) but rather varies with temperature in parallel with the overall growth rate of the larva. We did, however, examine how the timing interval relates to the duration of feeding, by feeding CAX larvae for various periods and then withdrawing food. After only 12 h on food, a quarter of the CAX larvae had apparently started their molt timer because they wandered 4 d later. After 24 h with food, all of the CAX larvae subsequently wandered by day 4 despite the fact that they had only achieved about 5% of their potential weight gain. These data argue that the molt timer is started by feeding, but, once started, it continues timekeeping without nutrient input. This unique requirement of food at the beginning of an instar corresponds to the “period of indispensable nutrition” defined by Bounhiol (38) for Bombyx mori. In intact larvae, imaginal primordia, such as those associated with the eyes, antennae, and legs, also require this initial feeding period to commit themselves to metamorphic development (39). However, it is not the commitment of these primordia that starts the timer because, in the CAX larvae, their commitment and morphogenetic growth are initiated while the larvae are starving (31) (Fig. 1B), but the onset of timing still requires that these larvae actually feed (Fig. 1B).
JH titers in Manduca larvae are markedly affected by nutrition, with early starvation causing a marked elevation in the JH titer (40). This elevation seems especially sensitive to trehalose levels (32). The molt timer, however, seems to rely on a different set of macronutrient cues. Both carbohydrate and lipid were ineffective in starting the molt timer, but feeding on a protein source, such as casein, resulted in the initiation of time keeping (Figs. 3 and 4). The protein-supplemented basal diet, however, was not as effective as the normal diet that contains all of the macronutrients.
What is the nature of the signal that feeding on protein sends to initiate the molt timer? It is most likely the protein kinase target of rapamycin (TOR), which is the main mediator of cellular nutrient sensing of amino acid levels and regulates their utilization and thereby cellular growth rate (41). Coordination of growth within and among tissues and organs then is mediated by the insulin/insulinlike growth factor (IGF) pathway. In Manduca the prothoracic glands grow during the final instar, and this growth is dependent on amino acids and TOR signaling (42, 43). Accompanying the growth is an increased capacity to secrete ecdysone in response to PTTH, and starvation for the first 2 d of the fifth instar strongly suppresses this response and increases transcript levels of the insulin receptor and 4E-binding protein (4EBP) (a translation inhibitor) (42). Injection of insulin, however, was unable to rescue ecdysone production by the glands although it rescued the starvation-induced deficits in protein synthesis. This lack of effect of insulin may be due to the high JH levels found in starving larvae (40) because, in early final instar Bombyx larvae, JH inhibits ecdysone secretion by the prothoracic glands and also their acquisition of competence to respond to PTTH (44). Alternatively or in addition, it could be due to the lack of sufficient nutrient precursors for the synthesis of ecdysone because amino acids and associated TOR signaling are necessary for both gland growth and molting (43). Whether insulin in the absence of an amino acid source is sufficient to initiate the molt timer in CAX larvae needs to be determined.
Only a quarter of the CAX larvae that fed on the standard diet for 12 h subsequently started wandering in 4 d; the remaining larvae also started metamorphosis, but after a much longer delay (Fig. 2B). This result suggests that, whereas the molt timer typically sets the minimal duration of the feeding period, its activation may not be all or none. Further work needs to be done to define how this timing system responds to suboptimal regimens of nutrition.
The allatectomy experiments revealed the presence of a timer underlying the molt from the last larval stage, but is such a timer a feature of every instar? The concept of critical weight was first developed in M. sexta for the metamorphic molt (7), but subsequent experiments show that a critical weight can be defined for every instar, and occurs when the larva surpasses 4.8 times its initial size at ecdysis (14). Metamorphic changes in the neuroendocrine system allow JH to mediate the effects of the critical weight checkpoint in the last instar, but we do not know the mechanisms that enforce the checkpoint in earlier instars because JH is continually present during the intermolt periods (45). Without an ability to inactivate the critical weight checkpoint in subterminal instars, we cannot directly test for the presence of a timer for a larval–larval molt. Allatectomy of fourth instars clearly shows that, when these animals are forced to precociously enter metamorphosis, a timer is evident and that its timing function scales with either instar or body size. It may be important, however, that the 2-d interval that it times from the onset of feeding to the start of the metamorphic transition is very similar to the duration of the normal fourth instar intermolt, from the start of feeding until PTTH release (46). Also, the period of indispensable nutrition, which corresponds to the amount of feeding needed to initiate the timer, is a feature of every larval molt (38). With the latter data in mind, we speculate that, similar to the inhibitory influences imposed by the critical weight checkpoint, the positive influences of a feeding-activated molt timer is a feature of every larval instar. These two factors then work together to coordinate growth and molting of the larva.
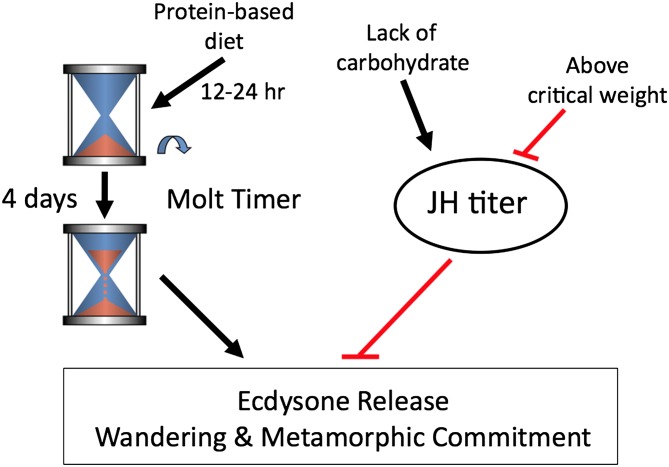
A key question is how does the molt timer fit into the physiological control for growth and molting in the intact larva? Comparison of the molting responses of intact versus CAX larvae points to the relationships seen in Fig. 6. The molt timer sets the minimum duration of the feeding period during an instar. Its timing function is initiated by feeding, but, once set in motion, it appears to be “blind” to the quantity or quality of food ingested. Consequently, in the CAX larvae, which are working with the molt timer alone, suboptimal diets that do not provide enough premetamorphic growth will nevertheless lead to the initiation of a metamorphic molt, which proves to be fatal. In intact larvae that are nutritionally challenged in a similar way, however, their JH system with its critical weight checkpoint monitors nutrient input and body size and delays molting despite the timer having finished its course. Recently, Callier and Nijhout (14) showed that larval size within an instar is likely assessed by the fixed tracheal size and oxygen delivery to the tissues, and critical weight thereby corresponds to the body size at which the tracheal system can no longer deliver oxygen at its maximal rate. Thus, oxygen levels must influence a mechanism upstream of the JH signaling system, not the metamorphic timer.
Fig. 6.
Schematic representation of how nutritional components and size interact to regulate the timing of entry into metamorphosis.
Insects show a diversity of responses to size and nutrition (34). Some insects, like Trogoderma (47), show the phenomenon of retrograde molting when starved, undergoing periodic molts even though reducing in size. In dung beetles, for example, the reduction in food during the last larval stage results in a precocious start of metamorphosis (34) whereas, in Manduca, similar manipulations can result in a delay in this process. The existence of both positive and negative mechanisms that impinge on metamorphosis provide pathways that evolution can modify to adapt growth to the ecological needs of different insects.
Where does the molt timer reside? At this time, we do not know whether the molt timer has a discrete location or whether it is the product of a physiological cascade that is distributed among various cells or tissues. One support of the latter idea comes from the ligation experiments (Fig. 5). CAX larvae that were neck-ligatured a day after refeeding were delayed in showing the symptoms of wandering relative to their CAX controls. By 2 d of feeding, however, the subsequent time courses in the two groups are identical. These results suggest an early phase in the timer that includes a head structure and a second phase that acts outside of the head. Interestingly, Callier and Nijhout (14) found that subsized Manduca eventually molt even if below the critical weight but that this molting may not involve control by the head. An obvious candidate for this second target is the prothoracic glands because these glands are ultimately involved in secreting the ecdysone that causes wandering. Indeed, one can experimentally shorten the intermolt period in final instar CAX Manduca to 2 d by feeding the ecdysone agonist RH-5849 immediately after ecdysis (48). Also, feeding the commercial silkworm B. mori larvae on a diet that contains ecdysone results in rapid, repeated molting so that the larva goes through 11–12 instars rather than the normal five before it is large enough for metamorphosis (49). In Drosophila, TOR signaling, which is usually involved with amino acid utilization, has an impact on the duration of the last larval stage because it regulates ecdysone synthesis in the prothoracic gland (50). Similarly, the insulin-like signaling pathway controls developmental speed in C. elegans (51).
Our study has uncovered a hitherto unknown developmental timer in insects, which determines the time to metamorphosis. We think that such a molt timer is likely a general feature of insect growth and metamorphosis but that its presence is masked by the strong suppressive effects of the JH system in the last larval stage. Given that the nematode C. elegans also has a molt timer, it is tempting to speculate that a conserved timing mechanism might underlie developmental transitions across all ecdysozoans. The presence of such intrinsic timers ensures that each individual reaches sexual maturity even in the face of adverse environmental conditions.
Materials and Methods
Animals and Surgical Procedures.
Larvae of tobacco hornworm M. sexta were individually reared in plastic cups containing standard diet (52) at 25.5 °C under a 12L:12D photoperiod. Allatectomy, the surgical removal of the corpora allata (the glands that secrete JH), was usually done early during the molt from the fourth to the fifth (last) larval stage according to the method of Hiruma (53). Molting larvae were selected about 5–6 h before head capsule slippage and anesthetized by submersion in water for 30 min. The CA was removed through small ventral incisions in the neck cuticle using sharpened no. 5 forceps, and larvae were then replaced in their cups for recovery. A few hours before ecdysis to the fifth instar, the CAX larvae were transferred to individual cups without food but containing a moist absorbent tissue paper as a source of water. The success of the allatectomy was indicated by the larvae changing color to black, rather than green, at the outset of the fifth instar. Larvae with traces of green coloration represented 1–2% of the operated animals, and they were discarded.
Intact larvae were kept on food until a few hours before ecdysis to the fifth instar and then weighed. Those weighing 1.0 g to 1.1 g were transferred to individual cups containing a moist absorbent tissue paper and served as controls. Experiments with groups of control and CAX larvae were always run simultaneously so that both groups experienced the same microheterogeneities in temperature that might occur during extended experiments.
Temporal Diet Manipulation.
Larvae were fed on the standard diet for various periods and then placed in a clean cup with only a wet tissue paper as a water source for the duration of the starvation period. An exception to this protocol was made for two groups of CAX larvae that were fed for only 0.25 or 0.5 d. They were given a block of agar that contained 7% (wt/vol) sucrose to extend their survival through the observation period.
Nutrient Manipulation.
To assess the effect of different nutrients on the time of wandering, larvae were fed a base diet to which we added various combinations of macronutrients, including sucrose, casein (for amino acids), and/or lipid (linseed oil and cholesterol). The base diet contained salts, vitamins, and mold and bacterial inhibitors [15.65 g/L Wesson’s salt, 2.61 g/L sorbic acid, 1.3 g/L methyl parabenzoate, 6.52 g/L ascorbic acid, 0.26 g/L streptomycin, 0.07 g/L kanamycin, 0.11% formaldehyde, and 1.3% (vol/vol) vitamin mixture (stock solution: 1.0 g/L nicotinic acid, 0.5 g/L riboflavin, 0.23 g/L thiamine, 0.23 g/L pyridoxine, 0.23 g/L folic acid, 0.02 g/L biotin)], and solidified with 15.9 g/L nonnutrient Gelcarin PS 402 (FMC BioPolymer). To make diets with different nutrients, 5.8% (wt/vol) casein (39), 7.0% (wt/vol) sucrose (39), and/or 0.54% (vol/vol) raw linseed oil and 4.54 g/L cholesterol were added to the base diet. See Table S1 for the composition of these diets compared with the standard rearing diet for Manduca (52) used in our laboratory.
Determination of Growth Rate and Timing of Wandering.
Larvae were weighed a few hours before ecdysis to the fifth instar, and then daily after the resumption of feeding and until the time of wandering. The larvae were checked daily for signs that they had entered the wandering stage. For intact larvae, the start of wandering was recognized by a clearing of epidermal pigment over the heart and the appearance of pink pigmentation in the flanking epidermis (54), a sheen appearing on the cuticle, cessation of feeding, enhanced locomotion, retraction of the crochets on the prolegs, and the voiding of gut contents (16). In CAX larvae, the epidermal pigment changes were often masked by the melanization of the cuticle, but the other features of the cessation of feeding (indicated by a lack of weight gain), the voiding of the gut contents, the intense locomotion, the crochet retraction, and the sheen on the cuticle were all clearly evident. For pupation, the wandering larvae were placed in holes bored into wooden blocks, and their pupation success was recorded. Those that formed normal pupae were weighed 8 d after wandering.
Neck Ligation Experiments.
To assess the influence of the head on the timing to wandering, larvae were neck-ligated using waxed dental floss. CAX fifth instar larvae were starved for 2.5 d and then fed for 24 h before they were neck-ligated after various time points. The animals were then placed on dry absorbent tissue so as to monitor the timing of wandering. The latter event was evident by the voiding of liquid feces from the rectum and by the initiation of retraction of the prolegs.
Supplementary Material
Acknowledgments
We thank Binh Nguyen for maintaining the Manduca colony and providing dietary ingredients for the various diets that Y.S. and T.K. prepared. This study was supported by National Science Foundation (NSF) Grant IBN-0344933 and National Institutes of Health Grant GM060122 (to L.M.R.), NSF Grant IBN-0452009 (to J.W.T.), and Japan Society for Promotion of Science (JSPS) Grant 25292195 (to K.H.). T.K. was supported by a Research Fellowship from JSPS.
Footnotes
The authors declare no conflict of interest.
See QnAs on page 12501.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311405110/-/DCSupplemental.
References
- 1.Dunlap JC, Loros JJ, Liu Y, Crosthwaite SK. Eukaryotic circadian systems: Cycles in common. Genes Cells. 1999;4(1):1–10. doi: 10.1046/j.1365-2443.1999.00239.x. [DOI] [PubMed] [Google Scholar]
- 2.Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129(6):675–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- 3.Byerly L, Cassada RC, Russell RL. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev Biol. 1976;51(1):23–33. doi: 10.1016/0012-1606(76)90119-6. [DOI] [PubMed] [Google Scholar]
- 4.Reinhart BJ, Ruvkun G. Isoform-specific mutations in the Caenorhabditis elegans heterochronic gene lin-14 affect stage-specific patterning. Genetics. 2001;157(1):199–209. doi: 10.1093/genetics/157.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruaud AF, Bessereau JL. Activation of nicotinic receptors uncouples a developmental timer from the molting timer in C. elegans. Development. 2006;133(11):2211–2222. doi: 10.1242/dev.02392. [DOI] [PubMed] [Google Scholar]
- 6.Monsalve GC, Frand AR. Toward a unified model of developmental timing: A “molting” approach. Worm. 2012;1(4):221–230. doi: 10.4161/worm.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): Growth of the last-instar larva and the decision to pupate. J Exp Biol. 1974;61(2):481–491. doi: 10.1242/jeb.61.2.481. [DOI] [PubMed] [Google Scholar]
- 8.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): Cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol. 1974;61(2):493–501. doi: 10.1242/jeb.61.2.493. [DOI] [PubMed] [Google Scholar]
- 9.Nijhout HF. Insect Hormones. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 10.Rountree DB, Bollenbacher WE. The release of the prothoracicotropic hormone in the tobacco hornworm, Manduca sexta, is controlled intrinsically by juvenile hormone. J Exp Biol. 1986;120:41–58. doi: 10.1242/jeb.120.1.41. [DOI] [PubMed] [Google Scholar]
- 11.Sparks TC, Hammock BD, Riddiford LM. The haemolymph juvenile hormone esterase of Manduca sexta (L.)-inhibition and regulation. Insect Biochem. 1983;13:529–541. [Google Scholar]
- 12.Browder MH, D’Amico LJ, Nijhout HF. The role of low levels of juvenile hormone esterase in the metamorphosis of Manduca sexta. J Insect Sci. 2001;1:11. [PMC free article] [PubMed] [Google Scholar]
- 13.Hiruma K, Kaneko Y. Hormonal regulation of insect metamorphosis with special reference to juvenile hormone biosynthesis. Curr Top Dev Biol. 2013;103:73–100. doi: 10.1016/B978-0-12-385979-2.00003-4. [DOI] [PubMed] [Google Scholar]
- 14.Callier V, Nijhout HF. Control of body size by oxygen supply reveals size-dependent and size-independent mechanisms of molting and metamorphosis. Proc Natl Acad Sci USA. 2011;108(35):14664–14669. doi: 10.1073/pnas.1106556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith WA, Rybczynski R. Prothoracicotropic hormone. In: Gilbert LI, editor. Insect Endocrinology. London: Academic; 2012. pp. 1–6. [Google Scholar]
- 16.Dominick OS, Truman JW. The physiology of wandering behaviour in Manduca sexta. II. The endocrine control of wandering behaviour. J Exp Biol. 1985;117:45–68. doi: 10.1242/jeb.117.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Riddiford LM, et al. Developmental expression, synthesis, and secretion of insecticyanin by the epidermis of the tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol. 1990;14(3):171–190. doi: 10.1002/arch.940140305. [DOI] [PubMed] [Google Scholar]
- 18.Hori M, Riddiford LM. Isolation of ommochromes and 3-hydroxykynurenine from the tobacco hornworm, Manduca sexta. Insect Biochem. 1981;11:507–513. [Google Scholar]
- 19.Hori M, Riddiford LM. Regulation of ommochrome biosynthesis in the tobacco hornworm, Manduca sexta, by juvenile hormone. J Comp Physiol B. 1982;147:1–9. [Google Scholar]
- 20.Riddiford LM. Hormonal control of insect epidermal cell commitment in vitro. Nature. 1976;259(5539):115–117. doi: 10.1038/259115a0. [DOI] [PubMed] [Google Scholar]
- 21.Riddiford LM. Ecdysone-induced change in cellular commitment of the epidermis of the tobacco hornworm, Manduca sexta, at the initiation of metamorphosis. Gen Comp Endocrinol. 1978;34(4):438–446. doi: 10.1016/0016-6480(78)90284-8. [DOI] [PubMed] [Google Scholar]
- 22.Truman JW, Riddiford LM, Safranek L. Hormonal control of cuticle coloration in the tobacco hornworm: Basis of an ultrasensitive bioassay for juvenile hormone. J Insect Physiol. 1973;19:195–203. [Google Scholar]
- 23.Kiguchi K, Riddiford LM. The role of juvenile hormone in pupal development of the tobacco hornworm, Manduca sexta. J Insect Physiol. 1978;24:673–680. [Google Scholar]
- 24.Hori M, Hiruma K, Riddiford LM. Cuticular melanization in the tobacco hornworm larva. Insect Biochem. 1984;14:267–274. [Google Scholar]
- 25.Pohley HJ. Experimentelle Untersuchungen uber die Steuerung des Hautungsrhythmus bei der Mehlmotte Ephestia kuhniella Zeller [Experimental investigations of the control of molting rhythms in the meal moth, Ephestia kuhniella Zeller] Wilh Roux's Arch Entwicklungsmech Org. 1960;152:182–203. doi: 10.1007/BF00649885. German. [DOI] [PubMed] [Google Scholar]
- 26.Madhavan K, Schneiderman HA. Hormonal control of imaginal disc regeneration in Galleria mellonella (Lepidoptera) Biol Bull. 1969;137:321–331. [Google Scholar]
- 27.Sehnal F, Bryant PJ. Delayed pupariation in Drosophila imaginal disc overgrowth mutants is associated with reduced ecdysteroid titer. J Insect Physiol. 1993;39(12):1051–1059. [Google Scholar]
- 28.Krishnakumaran A. Injury induced molting in Galleria mellonella larvae. Biol Bull. 1972;142(2):281–292. doi: 10.2307/1540231. [DOI] [PubMed] [Google Scholar]
- 29.Halme A, Cheng M, Hariharan IK. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr Biol. 2010;20(5):458–463. doi: 10.1016/j.cub.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336(6081):579–582. doi: 10.1126/science.1216735. [DOI] [PubMed] [Google Scholar]
- 31.Truman JW, et al. Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science. 2006;312(5778):1385–1388. doi: 10.1126/science.1123652. [DOI] [PubMed] [Google Scholar]
- 32.Jones D, Jones G, Bhaskaran G. Dietary sugars, haemolymph trehalose levels and supernumerary molting of Manduca sexta larvae. Physiol Zool. 1981;54:260–266. [Google Scholar]
- 33.Nijhout HF, Davidowitz G, Roff DA. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J Biol. 2006;5(5):16. doi: 10.1186/jbiol43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callier V, Nijhout HF. Body size determination in insects: A review and synthesis of size- and brain-dependent and independent mechanisms. Biol Rev Camb Philos Soc. 2013 doi: 10.1111/brv.12033. [DOI] [PubMed] [Google Scholar]
- 35.Williams CM. The juvenile hormone. II. Its role in the endocrine control of molting, pupation, and adult development in the Cecropia silkworm. Biol Bull. 1961;116:323–338. [Google Scholar]
- 36.Fain MJ, Riddiford LM. Juvenile hormone titers in the hemolymph during late larval development of the tobacco hornworm, Manduca sexta (L.) Biol Bull. 1975;149(3):506–521. doi: 10.2307/1540383. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman WF, Pittendrigh CS, Pavlidis T. Temperature compensation of the circadian oscillation in Drosophila pseudoobscura and its entrainment by temperature cycles. J Insect Physiol. 1968;14(5):669–684. doi: 10.1016/0022-1910(68)90226-6. [DOI] [PubMed] [Google Scholar]
- 38.Bounhiol JJ. Recherches experimentales sur le determinisme de la metamorphose chez les lepidopteres [Experimental studies on the determination of metamorphosis in Lepidoptera]. Suppl Bull Biol FT. Belg. 1938;34:1–190. French. [Google Scholar]
- 39.MacWhinnie SG, et al. The role of nutrition in creation of the eye imaginal disc and initiation of metamorphosis in Manduca sexta. Dev Biol. 2005;285(2):285–297. doi: 10.1016/j.ydbio.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Cymborowski B, Bogus M, Beckage NE, Williams CM, Riddiford LM. Juvenile hormone titres and metabolism during starvation-induced supernumerary larval moulting of the tobacco hornworm, Manduca sexta (L.) J Insect Physiol. 1982;28:129–135. [Google Scholar]
- 41.Hietakangas V, Cohen SM. Regulation of tissue growth through nutrient sensing. Annu Rev Genet. 2009;43:389–410. doi: 10.1146/annurev-genet-102108-134815. [DOI] [PubMed] [Google Scholar]
- 42.Walsh AL, Smith WA. Nutritional sensitivity of fifth instar prothoracic glands in the tobacco hornworm, Manduca sexta. J Insect Physiol. 2011;57(6):809–818. doi: 10.1016/j.jinsphys.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Kemirembe K, Liebmann K, Bootes A, Smith WA, Suzuki Y. Amino acids and TOR signaling promote prothoracic gland growth and the initiation of larval molts in the tobacco hornworm Manduca sexta. PLoS ONE. 2012;7(9):e44429. doi: 10.1371/journal.pone.0044429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai S, Okuda M, Ohtaki T. Juvenile hormone inhibits ecdysone secretion and responsiveness to prothoracicotropic hormone in prothoracic glands of Bombyx mori. Gen Comp Endocrinol. 1989;75(2):222–230. doi: 10.1016/0016-6480(89)90074-9. [DOI] [PubMed] [Google Scholar]
- 45.Baker FC, Tsai LW, Reuter CC, Schooley DA. In vivo fluctuations of JH, JH acid, and ecdysteroid titer, and JH esterase activity, during development of fifth stadium Manduca sexta. Insect Biochem. 1987;17:989–996. [Google Scholar]
- 46.Truman JW. Physiology of insect rhythms. I. Circadian organization of the endocrine events underlying the moulting cycle of larval tobacco hornworms. J Exp Biol. 1972;57:805–820. doi: 10.1242/jeb.60.2.371. [DOI] [PubMed] [Google Scholar]
- 47.Beck SD, Bharadwaj RK. Reversed development and cellular aging in an insect. Science. 1972;178(4066):1210–1211. doi: 10.1126/science.178.4066.1210. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds SE, Brown AM, Rakesh SK, Riddiford LM, Hiruma K. Induction of supernumerary larval moulting in the tobacco hornworm Manduca sexta: Interaction of bis acylhydrazine ecdysteroid agonists with endogenous Juvenile Hormone. Physiol Entomol. 2009;34:30–38. [Google Scholar]
- 49.Tanaka Y, Takeda S. Ultranumerary larval ecdyses of the silkworm Bombyx mori induced by ecdysone. Naturwissenschaften. 1993;80:131–132. [Google Scholar]
- 50.Mirth CK, Shingleton AW. Integrating body and organ size in Drosophila: Recent advances and outstanding problems. Front Endocrinol (Lausanne) 2012;3:49. doi: 10.3389/fendo.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruaud AF, Katic I, Bessereau JL. Insulin/insulin-like growth factor signaling controls non-Dauer developmental speed in the nematode Caenorhabditis elegans. Genetics. 2011;187(1):337–343. doi: 10.1534/genetics.110.123323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bell RA, Joachim FG. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am. 1976;69:365–373. [Google Scholar]
- 53.Hiruma K. Possible roles of juvenile hormone in the prepupal stage of Mamestra brassicae. Gen Comp Endocrinol. 1980;41(3):392–399. doi: 10.1016/0016-6480(80)90084-2. [DOI] [PubMed] [Google Scholar]
- 54.Truman JW, Riddiford LM. Physiology of insect rhythms. 3. The temporal organization of the endocrine events underlying pupation of the tobacco hornworm. J Exp Biol. 1974;60(2):371–382. doi: 10.1242/jeb.60.2.371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.