Abstract
The monoamine oxidase isoenzymes (MAOs) A and B play important roles in the homeostasis of monoaminergic neurotransmitters. The combined deficiency of MAO A and B results in significantly elevated levels of serotonin (5-hydroxytryptamine), norepinephrine, dopamine, and β-phenylethylamine; in humans and mice, these neurochemical changes are accompanied by neurodevelopmental perturbations as well as autistic-like responses. Ample evidence indicates that normal levels of monoamines in the hippocampus, amygdala, frontal cortex, and cerebellum are required for the integrity of learning and memory. Thus, in the present study, the cognitive status of MAO A/B knockout (KO) mice was examined with a wide array of behavioral tests. In comparison with male wild-type littermates, MAO A/B KO mice exhibited abnormally high and overgeneralized fear conditioning and enhanced eye-blink conditioning. These alterations were accompanied by significant increases in hippocampal long-term potentiation and alterations in the relative expression of NMDA glutamate receptor subunits. Our data suggest that chronic elevations of monoamines, because of the absence of MAO A and MAO B, cause functional alterations that are accompanied with changes in the cellular mechanisms underlying learning and memory. The characteristics exhibited by MAO A/B KO mice highlight the potential of these animals as a useful tool to provide further insight into the molecular bases of disorders associated with abnormal monoaminergic profiles.
Keywords: adult neurogenesis, autism-spectrum disorder, cognition
Monoaminergic neurotransmitters, such as serotonin (5-HT), norepinephrine (NE), and dopamine (DA), are known to play a critical role in the modulation of mood and emotion, as well as the control of motor, perceptual, and other cognitive functions (1). Abnormal levels of these monoamines have been associated with several neuropsychiatric disorders (2–5). The enzymatic metabolism of these neurotransmitters is mainly catalyzed by monoamine oxidase (MAO), a mitochondrial-bound flavoprotein (6). The two MAO isoenzymes, A and B, are encoded by different genes next to each other on the X chromosome (7), with 70% amino acid identity (8) and identical intron-exon organization (9). MAO A has a higher affinity for 5-HT, NE, and DA (6, 10). In contrast, MAO B prefers phenylethylamine (PEA) as substrate (11), although it can degrade 5-HT, NE, and DA in the absence of MAO A (12). As a consequence, the lack of both isoenzymes in mice results in significantly higher monoamine levels than those observed in single knockout (KO) counterparts (12). The deficiency of both MAO isoenzymes in humans has been found to be associated with severe intellectual and developmental disabilities, hypotonia, failure to thrive, and autistic-like symptoms (including sociocommunicative deficits and perseverative manifestations) (13–15). Although similar alterations were also featured by MAO A KO mice, the severity of the changes was typically more pervasive in MAO A/B KO mice, supporting the idea that the severity of autistic-like features may be correlated to the amounts of monoamine levels, particularly at early developmental stages (16). In previous work, we found that MAO A KO mice exhibited significant increases in fear conditioning but intact motor learning (17). Here we report abnormalities in higher cognitive functions in MAO A/B KO mice and a possible cellular mechanism underlying these defects.
Results
MAO A/B KO Mice Exhibited Similar Spatial Learning in a Morris Water Maze.
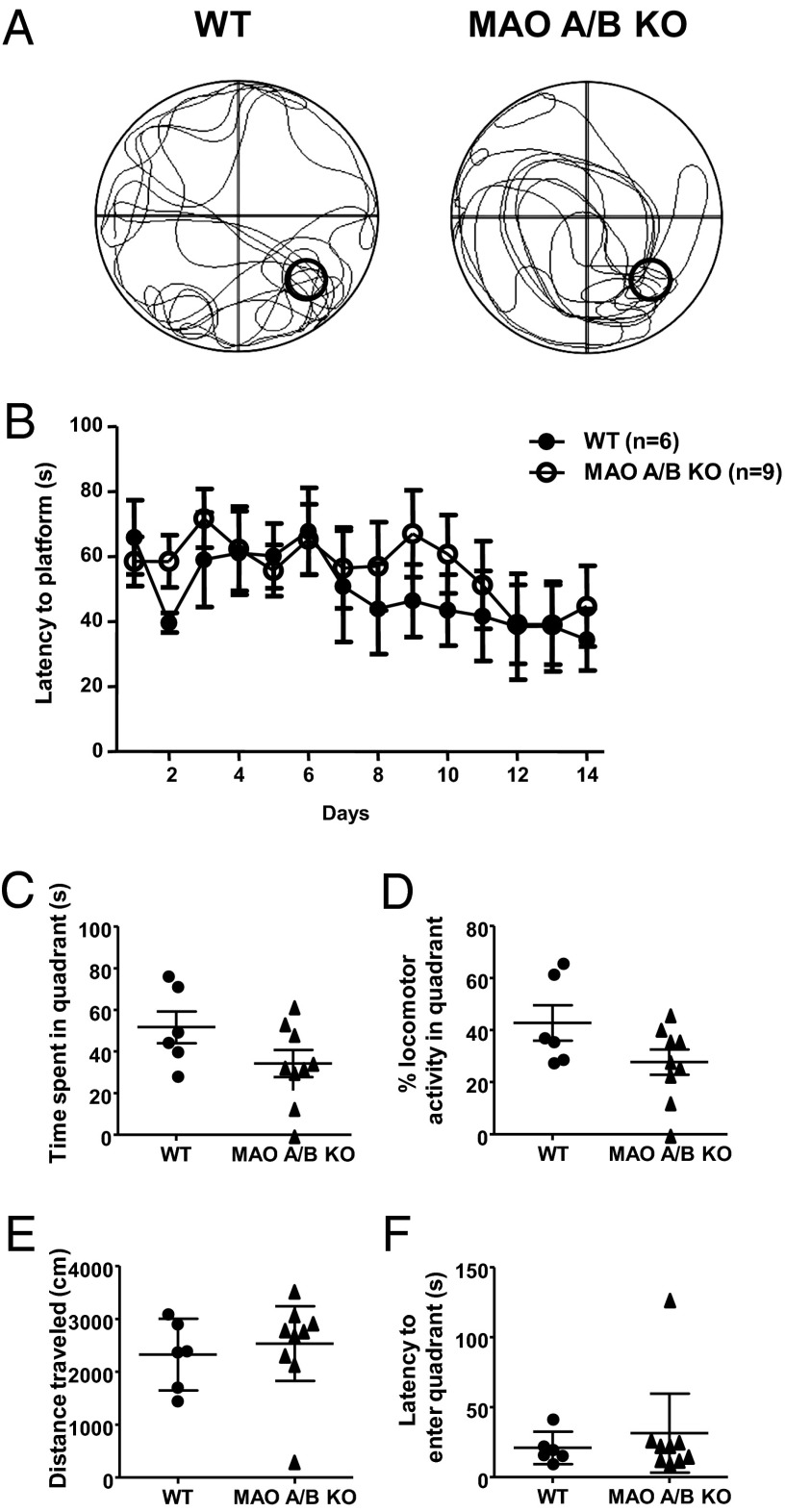
Typical locomotor tracks of wild-type (WT) and MAO A/B KO mice are shown (Fig. 1A) for mice in the spatial learning probe trials. Although the MAO A/B KO mice showed less-focused locomotion (Fig. 1A), both genotypes displayed an equivalent latency to reach the platform [Fig. 1B; genotype: F(1, 14) = 0.23; nonsignificant (NS)] during the spatial learning training phase. Moreover, two-way ANOVA analysis for repeated measures revealed that both genotypes showed a decrease in latency over time [time: F(13, 182) = 3.02; P < 0.001], indicating that they both learned to locate the platform. In the probe trial, although MAO A/B KO mice showed numerically lower values, the two genotypes did not differ significantly in amount of time in the platform-associated quadrant [Fig. 1C; F(1, 13) = 2.99; NS] after platform spatial training. Similarly, MAO A/B KO mice exhibited a reduction in the percentage locomotor activity in the platform-associated quadrant [Fig. 1D; F(1, 13) = 3.43; NS]; however, this effect did not reach statistical significance (P < 0.09). WT and MAO A/B KO mice did not exhibit significant differences in either overall distance [Fig. 1E; F(1, 13) = 0.23; NS] or latency to reach the quadrant in which the platform was located [Fig. 1F; U(6,9) = 23; NS]. In addition, no significant difference was detected in long-term object recognition memory between genotypes (Fig. S1; SI Materials and Methods).
Fig. 1.
MAO A/B KO mice exhibited similar spatial learning and memory. (A) Typical locomotor tracks of WT and MAO A/B-deficient mice in the probe trial after spatial learning acquisition. (B) Latencies to reach the platform throughout the spatial learning phase. (C and D) Time spent in the platform or the percentage locomotor activity in the goal quadrant after spatial learning training. (E and F) Distances traveled by mice and latencies to reach the platform-associated quadrant in the probe trial. Values are represented as mean ± SEM.
MAO A/B KO Mice Showed Enhanced Contextual and Cue Memory and Generalization of Fear to Novel Contexts.
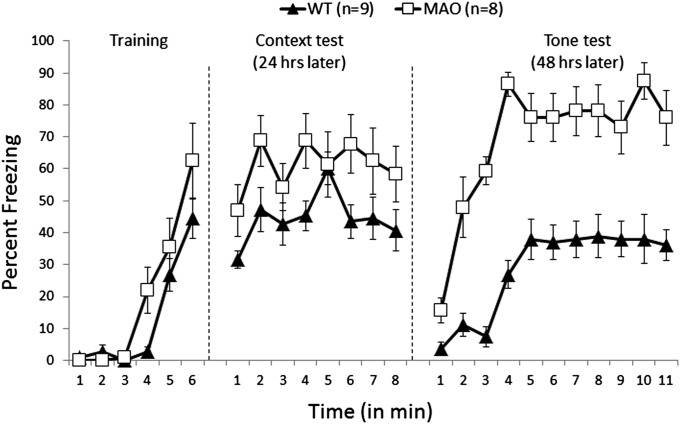
MAO A/B KO and their WT littermates were subjected to fear conditioning. No significant difference was observed between MAO A/B KO and WT mice in the number of crossings (Fig. S2; SI Materials and Methods) and baseline freezing during the first 3 min (Fig. 2). The overall percentage freezing was not different between the two genotypes during training [immediate postshock freezing during minutes 4, 5, and 6; F(1, 13) = 2.26; NS; Fig. 2]. Mice were tested for their contextual memory after 1 d. MAO A/B KO mice demonstrated significantly enhanced contextual learning (overall freezing during 8 min) compared with WT mice [genotype: F(1, 14) = 21.84; P < 0.001]. On day 3, mice were tested for cue learning in a novel context. MAO A/B KO mice showed significantly elevated levels of freezing compared with the WT mice during the first 3 min (without tone) [F(1, 13) = 26.43; P < 0.001] and the next 8 min (with tone) [F(1, 14) = 241.51; P < 0.0001] in the new context (Fig. 2). Because MAO A/B KO mice showed significantly higher freezing in the new context, we compared the freezing during the third minute with that in the fourth minute (first minute of tone exposure). MAO A/B KO mice showed a more robust response to the tone [F(1, 14) = 22.12 ; P < 0.001] compared with a similar analysis for WT mice [F(1, 12) = 8.76; P < 0.01].
Fig. 2.
MAO A/B KO mice showed enhanced and overgeneralized fear. In the training, 1, 2, and 3 denote 3 min of baseline (pretone foot shock) and 4, 5, and 6 represent three posttone foot-shock intervals. The context test was for 8 min. In the tone test, 1, 2, and 3 represent 3 min of baseline (in a novel context) before 8 min of tone. Freezing scores are expressed as the percentage of total observations. Values are represented as mean ± SEM.
MAO A/B KO Mice Displayed Enhanced Eye-Blink Conditioning.
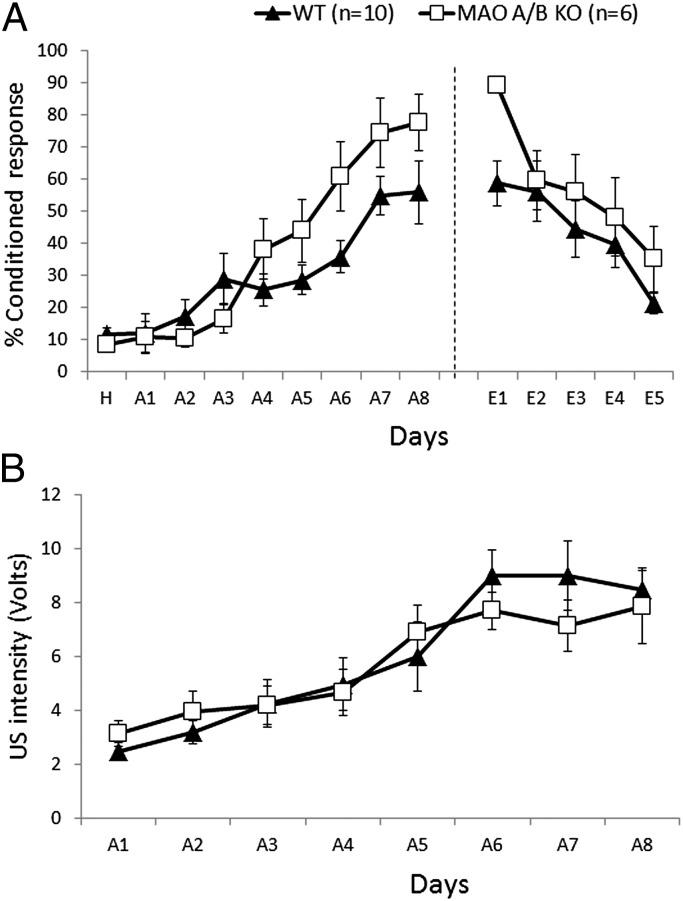
MAO A/B KO and WT mice were tested for motor learning, using the delay eye-blink conditioning paradigm. No difference was observed in the performance of the two genotypes during habituation and the first 3 d of acquisition. MAO A/B KO mice performed significantly better [overall percentage conditioned response (CR)] than WT mice from day 4 through the end of the acquisition phase [genotype: F(1, 14) = 6.96; P < 0.02; Fig. 3A]. After the acquisition phase, mice underwent 5 d of extinction using tone-alone trials. MAO A/B KO mice showed significantly higher conditioned responses than WT mice on day 1 of extinction [F(1, 14) = 10.43; P < 0.01; Fig. 3A]. However, this difference does not indicate that MAO A/B KO mice had a deficit in extinction, as their learning was significantly higher during acquisition. As opposed to these differences in the acquisition and extinction phases, no difference was observed in the unconditioned stimulus (US) intensities between the two genotypes during acquisition [F(1, 14) = 0.06; NS; Fig. 3B].
Fig. 3.
MAO A/B KO mice showed enhanced delay eye-blink conditioning. (A) Percentage of conditioned response exhibited by WT and MAO A/B KO mice during 8 d of acquisition and 5 d of extinction. (B) Periorbital shock (unconditioned stimulus) intensity used during 8 d of acquisition. The unconditioned stimulus intensity (the minimal voltage required to elicit as eye-blink/head turn response) was adjusted daily for each animal. Values are represented as mean ± SEM.
MAO A/B KO Mice Exhibited Significantly Enhanced hippocampal LTP Compared with both WT and MAO A KO Littermates.
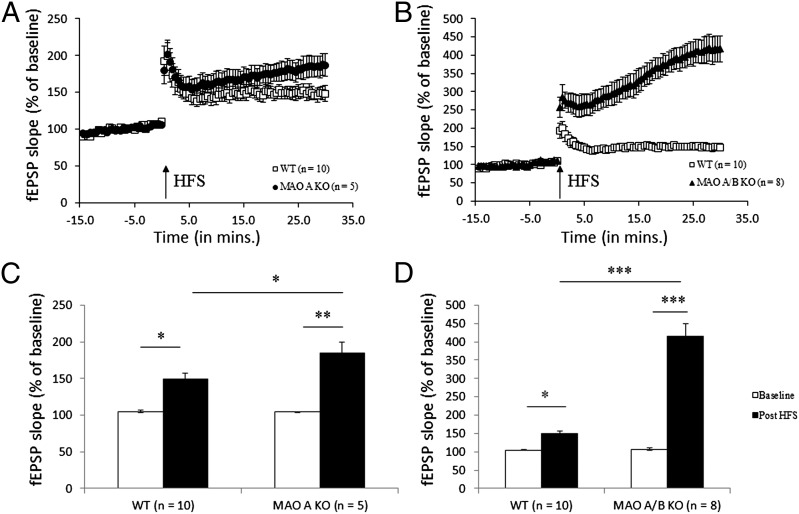
Abnormal fear processing in MAO A (17) and MAO A/B KO mice led us to further investigate whether these learning abnormalities were correlated with alterations in electrophysiological events underlying cognition such as long-term potentiation (LTP). To address this question, we performed extracellular recordings in hippocampal CA1. Hippocampal slices were used from MAO A/B KO, MAO A KO, and WT mice. No difference was observed between the three genotypes during 15 min of stable baseline. LTP was induced using two theta burst stimulations separated by a 10-s interval. MAO A KO mice displayed significantly enhanced LTP compared with WT mice [F(1, 14) = 5.13; P < 0.05; Fig. 4 A and B], and MAO A/B KO mice showed significantly elevated LTP compared with both MAO A KO [F(1, 12) = 24.56; P < 0.001] and WT mice [F(1, 17) = 66.72; P < 0.0001; Fig. 4 C and D].
Fig. 4.
Enhanced hippocampal LTP in MAO A/B KO mice compared with WT and MAO A KO mice. (A and B) Slopes of fEPSPs expressed as percentage of the average values during the course of recording, including 15 min of stable baseline and 30 min after theta burst stimulations. (C and D) Comparison of fEPSPs slope values during the 5 min before HFS with the last 5 min after HFS. Values are represented as mean ± SEM *P < 0.05; **P < 0.001; ***P < 0.0001. HFS, high-frequency stimulation.
MAO A/B KO Mice Have Altered Expressions of NMDA Receptor Subunits and Enhanced Adult Hippocampal Neurogenesis.
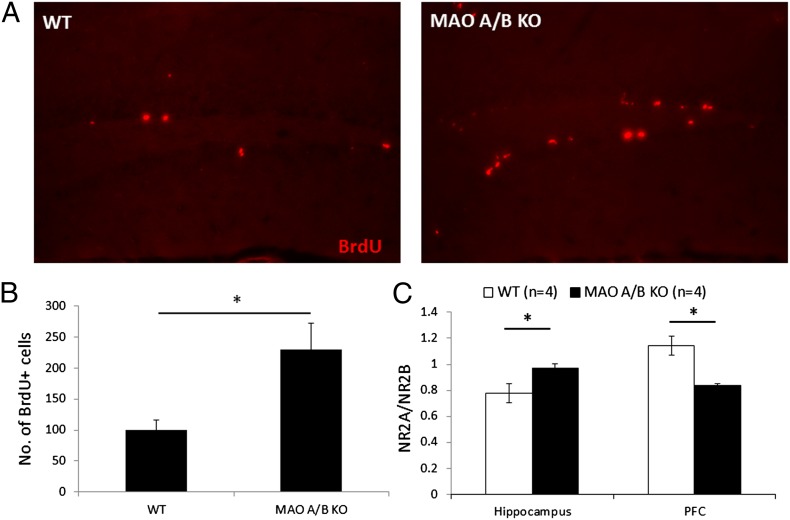
NMDA receptors are critically involved in plasticity and cognition. Thus, we examined whether the enhanced learning and hippocampal LTP were correlated with any alterations in the levels of these receptors. The expression of NMDA receptor subunits (NR2A and NR2B) was investigated in the prefrontal cortex, hippocampus, amygdala, and cerebellum. Relative levels of NR2A:NR2B were increased by ∼25% in the hippocampus [WT = 0.78 ± 0.07; MAO A/B KO = 0.97 ± 0.03; F(1, 6) = 6.128; P < 0.05; Fig. 5]. However, in the prefrontal cortex, relative levels of NR2A:NR2B were decreased by ∼27% [WT = 1.14 ± 0.07; MAO A/B KO = 0.84 ± 0.02; F(1, 6) = 16.544; P < 0.01; Fig. 5]. There was no significant difference in the relative expression of NR2A and NR2B in the amygdala and cerebellum (Fig. S3; SI Materials and Methods). MAO A/B KO mice also showed enhanced adult hippocampal neurogenesis, as assessed by number of bromodeoxyuridine+ (BrdU+) cells. Neurogenesis was increased by ∼130% in MAO A/B KO mice compared with WT mice [WT = 22.25 ± 3.50; MAO A/B KO = 51.25 ± 9.42; F(1, 6) = 8.33; P < 0.03; Fig. 5].
Fig. 5.
Increased adult hippocampal neurogenesis and altered NMDA receptor levels in MAO A/B KO mice compared with WT. (A) BrdU-labeled cells in the dentate gyrus subgranular zone of WT (Left) and MAO A/B KO (Right) mice. (B) Number of BrdU+ cells in the dentate gyrus subgranular zone of WT and MAO A/B KO mice. (C) Relative expression of NR2A and NR2B levels as assessed by Western blots. Values are represented as mean ± SEM *P < 0.05.
Discussion
The analysis of the cognitive abnormalities in MAO A/B KO mice showed that these animals exhibited significant enhancements of emotional and motor, but not spatial or declarative, learning. These behavioral changes were accompanied by an altered expression of the NMDA receptor subunits in the hippocampus and the prefrontal cortex. In addition, we found an increased adult hippocampal neurogenesis in MAO A/B KO mice. The present results complement and extend previous findings from our group and others on the characterization of the phenotypic consequences of combined MAO A and MAO B deficiency in humans and mice (12, 14–16, 18).
MAO A/B KO mice exhibited a statistical trend (albeit not significant) toward impaired water-maze memory. In contrast, these animals displayed a significant enhancement in fear learning akin to that previously reported for MAO A-deficient mice (17). This apparent divergence may signify that the higher retention of memories in MAO-deficient mice can only be observed during the processing of emotional memories. The enhanced fear in MAO A/B KO mice could result from elevated monoamine levels in the amygdala (12, 19–22). Enhanced amygdaloid functioning in MAO A/B KO mice resulting from the presence of high levels of monoamines could also underlie our previous observation of increased anxiety and decreased social interactions in these animals (23–25). It is very unlikely that the enhanced fear learning in MAO A/B KO mice is because of their higher reactivity to sudden painful stimuli. We have shown in our previous study that MAO A/B KO mice do not display any difference in latency to lick their paws in the hot plate test (16).
The elevated and overgeneralized fear in MAO A/B KO mice is in keeping with our previous results (12) and at variance with our previous findings in MAO A KO mice (17). Given that MAO B KO mice exhibited low anxiety (26), the most likely explanation for the divergent phenotypes between MAO A and MAO A/B KO mice may lie in the greater neurochemical imbalances exhibited by the latter mutants. The overgeneralized fear also raises the possibility of context discrimination in MAO A/B KO mice. Hippocampus plays an essential role in the processing of contextual stimuli. Manipulations that affect hippocampal synaptic plasticity (e.g., LTP) also affect context learning and indicate that hippocampus plays a particularly salient role in the discrimination of meaningful contexts (27, 28). Adult hippocampal neurogenesis is another form of neural circuit plasticity that results in the generation of new neurons in the dentate gyrus throughout life and plays a crucial role in pattern separation (29). Because monoaminergic neurotransmitters modulate adult neurogenesis (30), we examined the number of newborn cells in the dentate gyrus of MAO A/B KO mice. The enhancement in adult neurogenesis challenges the possibility that a deficit in context discrimination contributed to overgeneralized fear. Surprisingly, the increase in adult neurogenesis contrasts with our previous observations (18, 31), where we showed a diminished proliferation of neural stem cells in the developing and adult subventricular zone (18) in MAO A/B KO mice and reduced differentiation of neural cells in MAO A KO embryonic stem cell lines (31). This indicates that the regulation of neurogenesis in these two regions is very different (e.g., multiple receptor subtypes mediate the potent, partly selective of each neurogenic zone, stimulatory action of 5-HT on adult brain cell proliferation) (32).
Contextual fear conditioning is also dependent on the hippocampus (33), suggesting that the enhancement of fear learning could be a result of a hyperfunctioning hippocampus. Higher contextual learning in MAO A/B KO mice was accompanied by direct physiological alterations in the hippocampus. Synaptic plasticity was enhanced in MAO A KO mice and greatly enhanced in MAO A/B KO mice. Enhanced LTP in MAO A KO mice is in accord with our previous observation of their higher contextual fear learning (17). Interestingly, we did not find a correlation between increased LTP and water maze performance in MAO A/B KO mice. Enhanced LTP, contextual learning and low sensitivity to ketamine suggest a possible alteration in NMDA receptors, which are key receptors underlying synaptic plasticity in several areas of the brain (34, 35). We found an increase in the expression of NR2A subunits in the hippocampus compared with the WT controls. LTP in the CA1 region of the hippocampus has been shown to be dependent on NR2A subunit-containing NMDA receptors, which is mediated by Ras-GRF2/Erk Map Kinase signaling (36). Targeted gene KO of NR2A or its C-terminal domain is sufficient to impair hippocampal LTP and cause learning deficits including Pavlovian fear conditioning (37). Increased NR2A subunit-expressing NMDA receptors in the hippocampus thus could underlie enhanced LTP and contextual fear learning in MAO A/B KO mice. In addition, NR2B-containing NMDA receptors negatively regulate neurogenesis in the adult hippocampus by activating nNOS activity. Thus, a shift toward NR2A-containing NMDA receptors in the hippocampus of MAO A/B KO mice could contribute to the enhanced neurogenesis (38).
In contrast to the hippocampus, prefrontal cortex (PFC) in MAO A/B KO mice was found to have a higher expression of NR2B compared with WT mice. PFC plays an important role in working memory (39–41), emotional memory (42, 43), attention regulation (44), and behavioral inhibition (45, 46). NR2B-containing NMDA receptors in the PFC play a crucial role in its normal functioning (47). Higher levels of NR2B could mediate enhanced fear conditioning in MAO A/B KO mice, as NMDA receptors containing NR2B subunits play a critical role in contextual fear learning (48). A similar enhancement of NR2B has been seen in a previous model of autism in the neocortex (48). Alterations in the expression levels of these subunits may be linked to other alterations documented in the cortex of MAO A/B KO mice, such as the increased dendritic arborization in the pyramidal cells of the orbitofrontal cortex and the absence of barrel fields (16).
We found enhanced magnitude of conditioned eye-blink responses in MAO A/B KO mice, whereas no such differences were found in MAO A KO mice (17). Abnormalities in eye-blink conditioning have been associated with cerebellar alterations (49). Accordingly, we previously showed that the cerebella of MAO A/B KO, but not MAO A KO, mice exhibit significant reductions of Purkinje cells number and enhancements of the molecular and granule cells layers (16). This further supports our hypothesis that the severity of phenotype in MAO A/B KO mice results from much higher levels of monoamines than that of MAO A KO mice. Similar to fear conditioning, the mice were equated for the effect of the shock on behavior by adjusting the shock intensity to be just above the threshold for eliciting a head turn. Hence, the enhanced eye-blink conditioning in MAO A/B KO mice could not be interpreted as an enhanced effect of shock. Furthermore, impairment was observed in reversal learning in MAO A/B KO mice, but that could be a “carry over” of the enhanced learning in the acquisition phase. Abnormal eye-blink conditioning in MAO A/B could be accounted for by aberrant cerebellar–forebrain interactions (50). Monoaminergic inputs in the cerebellum include NEergic and 5-HTergic afferents from the locus coeruleus and raphe nucleus, respectively (51), and have been shown to modulate synaptic transmission in Purkinje cells and other cerebellar neurons. Significantly elevated levels of these neurotransmitters in the cerebellum could cause enhanced eye-blink learning in MAO A/B KO mice. Finally, we note a study of eye-blink conditioning in autistic children (52) in which it was reported that the autistic children learned this task faster than age-matched normal control children, just as is the case for our MAO A/B KO mice. Future studies are warranted to find out the neuroanatomical abnormalities in the cerebellum in MAO A/B KO mice.
In summary, we report that the combined absence of MAO A and B causes cognitive abnormalities accompanied by alterations in NMDA receptor subunits NR2A and NR2B and enhanced synaptic plasticity in the hippocampus. These behavioral phenotypes are much more pronounced in MAO A/B KO mice compared with MAO A KO mice (17). These mice may serve as a useful model to study the relationship between MAOs, abnormal monoamine processing, and neuropsychiatric disorders, including autistic-like features. From a translational perspective, these mice may serve as an interesting model to develop interventions to these neuropsychiatric disorders. Furthermore, they may serve as an effective experimental platform to aid in the screening of drug candidates for efficacy in treatment of these disorders and to study the role of monoamine neurotransmitters in cognitive regulation. Our study was limited by the use of only male adult mice, and future studies are required to include the analysis of sex differences and to address the developmental aspect of these phenotypes. Moreover, different monoamines including 5-HT, NE, and DA have been implicated in the pathogenesis of neurodevelopmental disorders, and future studies are required to dissect out their individual contribution to the observed phenotype in our study.
Materials and Methods
Animals.
Four- to 6-mo-old MAO A/B KO (12), MAO A KO, and their WT littermate mice were pathogen-free housed in ventilated cages on a 12-h light/dark cycle at 22 °C and 35% humidity with ad libitum access to food and water. All testing was performed during the light phase of the cycle. Separate mice were used for each behavioral experiment, electrophysiology, immunohistochemistry, and Western blots. All experiments conformed to the Animal Welfare Act, Guide to Use and Care of Laboratory Animals, the US Government Principles of the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training guidelines on the ethical use of animals, and the Institutional Animal Care and Use Committee (IACUC), University of Southern California.
Spatial Memory.
Spatial memory was measured using the Morris water maze, as previously described (53). The water maze consisted of a large circular metal tub (83 cm × 150 cm in diameter) filled with opaque water maintained between 29 °C and 32 °C. Six unique visual cues were placed on the walls of the maze at equal distances for spatial reference. The protocol consisted of two phases: platform training and spatial memory learning. In phase 1, for platform training, mice were individually exposed to the water for a 2-min probe trial. A 32-cm platform (14 cm in diameter) was placed 1 cm above the water in the middle of a quadrant to facilitate platform learning. Mice [MAO A/B KO (n = 11) and WT (n = 7)] were individually placed in the water and allowed to freely explore until they reached the platform. A 2-min cutoff time was used to minimize fatigue. Mice that failed to reach the platform were gently guided. On the five subsequent trials, the position of the platform was randomly rotated between quadrants.
In phase 2, at the start of the spatial memory phase, mice were exposed to a 2-min probe trial without the presence of any platform. The platform was added and placed 1 cm below the water in the middle of a quadrant. Mice were individually placed in the water and allowed to freely explore until they reached the platform. A 2-min cutoff time was used to minimize fatigue. Mice that failed to reach the platform were gently guided. A total of five training trials were conducted every day until the WT mice were able to consistently find the platform within 20 s. Mice that were unable to locate the platform on the final day were removed from the study (1 WT and 2 MAO A/B KO mice in total were removed from the study). Mice were then subjected to a 2-min probe trial without the presence of a platform. Spatial learning was assessed by using the total time spent in the platform-associated quadrant after phase 2.
Fear Conditioning.
Mice were trained in a fear conditioning chamber using a protocol described before (54). Behavior was recorded with a video camera. On day one, mice were individually placed in a 10.5 × 12 × 12-inch conditioning chamber with electrifiable flooring (Coulbourne Instruments). To enhance the salience of the chamber, three walls had broad, 1 inch red and white diagonal stripes at 45° and one wall of the chamber was Plexiglas to allow video recording. One milliliter vanilla extract was placed in a small Petri dish below the cage flooring and out of the animal’s reach to enhance the salience of the context. After a 3-min baseline period, three tone-foot-shock pairings (tone, 20 s, 80–85 dB, 2 kHz; foot shock, 1 s, 0.7 mA) separated by 1-min intervals were delivered. The shock was delivered during the last second of the tone. Tone and foot-shock delivery were controlled by software (Lab Linc Operant Control Software; Coulbourne Instruments). The chamber was cleaned with 70% (vol/vol) ethanol between individual sessions. Contextual fear conditioning was assessed 24 h after training by placing the mouse back in the same test chamber for 8 min without tone or foot shock. Freezing to altered context was tested in a novel chamber (12 × 8 × 5 inches) 48 h after training for 3 min. Afterward, in this second chamber, the training tone (cue) was delivered for 8 min to evaluate tone conditioning. The chamber was cleaned between sessions with 70% (vol/vol) isopropyl alcohol. Freezing behavior, defined as the absence of all visible movement of the body except from movement necessitated by respiration was scored every 5 s and expressed as percentage of the observation period. All freezing analysis was done by an observer blind to the genotypes.
Delay Eye-Blink Conditioning.
Mice were implanted with a 4-pin head stage (Digi-Key) and had four Teflon-coated stainless steel wires and one bare stainless steel wire (0.003 inch bare and 0.0055 inch coated; A-M Systems, Inc.). The bare wire was connected to a gold pin (Arrow Electronics, Inc.), and dental cement was used to hold it to the 4-pin connector. This bare wire was used as a grounding electrode and was implanted in the neck muscle. The coated wires were implanted s.c. in the orbicularis oculi of the left upper eyelid (55): two for delivering periorbital shocks and two for recording differential electromyograms. After the surgery, mice were individually housed and were given 1 wk recovery before use in the behavioral experiments. During the training, mice [MAO A/B KO (n = 6) and WT (n = 10)] were placed individually within Plexiglas cylinders in a sound-attenuated chamber and received a single habituation session for 1 d without tone and shock, and spontaneous eye-blink activity was recorded. After habituation, mice were trained with delay eye-blink conditioning for a period of 7 d (A1–A7, the acquisition period) with a 350-ms tone (2kHz, 80–85 dB; 4040A function generator; BK Precision) as the conditioned stimulus, coterminating with a 100-ms shock (100 Hz, biphasic; SD9 stimulator, Grass Instruments) as the unconditioned stimulus. The unconditioned stimulus level was adjusted daily for each mouse to give a small head turn before each day of training. The interstimulus interval was kept at 250 ms, and the intertrial interval was randomized between 20 and 40 s (average, 30 s). Every training session consisted of 100 trials in 10 blocks. Each block consisted of one conditioned stimulus-alone (first) trial and 9 paired trials. After 7 d of acquisition, mice were exposed to 5 d of extinction. In this phase, mice were given conditioned stimulus-alone trials (100 each) for 5 d and their rate of extinction was determined. Electromyographic activity was processed as before (55). The percentage of conditioned response was the ratio of the number of conditioned responses to the number of valid trials. For details on software and data analysis, please see SI Materials and Methods and ref. 54.
Preparation of the Hippocampal Slices.
After 30–40 s deep isoflurane anesthesia (Isocare), each mouse was decapitated, and the brain was removed and kept for 2–3 min in icy artificial cerebrospinal fluid (ACSF). The brain was then transferred to a stage of Vibratome (RBC) and was cut into 450-µm thick coronal hippocampal slices. Slices were stabilized at least for 1 h in a holding chamber, where they were submerged in oxygenated ACSF (room temperature) consisting of 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.3 mM MgSO4, 26 mM NaHCO3, 2.4 mM CaCl2, and 10 mM glucose.
Electrophysiological Recordings.
Hippocampal slices were moved to an interface recording chamber and perfused with ACSF at a rate of 1.5–2 mL/min at 30–32 °C while provided with 95% O2 and 5% CO2. Extracellular field excitatory postsynaptic potentials (fEPSPs) were recorded from stratum radiatum of CA1 using a glass pipette filled with ACSF (2–3 MΩ resistance) in response to current stimulation (twisted nichrome wires, 50 µm) ranging between 20–60 µA at the Schaffer collateral-commissural projection in CA1 stratum radiatum. Recording and stimulation electrodes were positioned in CA1. The glass pipettes were made using standard wall borosilicate tubing, using a pipette puller (Sutter Instruments). Pulses 0.1 ms in duration were given to the stimulating electrode every 30 s. Evoked responses were amplified by an Axoclamp 2A DC amplifier (Axon Instruments), filtered at 6 kHz and digitized at 20 kHz. Data acquisition was controlled by Clampex 7.0 software (Axon Instruments). After a 15-min stable baseline period, 100-Hz high-frequency stimulation (HFS) was delivered twice, separated by 10 s. Recording continued for another 30 min after HFS. To measure LTP values, fEPSP slopes were expressed as the percentage of average fEPSP slope values during the 5 min before HFS and were compared with the last 5 min after HFS. Statistical significance between groups was evaluated by unpaired two-tailed t tests.
Animal Dissection and Tissue Processing.
For adult neural stem cell determination and NMDA receptor subunits analyses, 3-mo-old WT and MAO A/B KO mice were given three i.p. injections of BrdU (50 mg/kg) 24, 16, and 2 h before sacrifice. Mice were transcardially perfused with PBS, and the brain was dissected into two hemispheres. One hemisphere was fixed immediately in cold 4% (wt/vol) paraformaldehyde for immunohistology and stereological analyses. From the remaining hemisphere, the hippocampus, prefrontal cortex, amygdala, and cerebellum were extracted and quickly frozen on dry ice and stored at −80 °C and were processed for protein analyses. For immunohistology, brains were postfixed overnight, cryoprotected, frozen, and cryostat-sectioned in the sagittal plane at 18 μm thickness. Every fifth section in the hippocampus was processed for BrdU immunohistochemistry, using rat monoclonal antibody for BrdU (rat anti-BrdU, 1:100, MCA2060; AbDSerotec) Fluorescent signals were detected using a Zeiss LSM 510 Meta NLO imaging system equipped with Argon, red HeNe, and green HeNe lasers. Panels were compiled in Adobe Photoshop 7.0 (Adobe Systems Inc) and were analyzed using image J.
Western Blot.
Protein lysates from different brain regions (10 µg) were run on 4%–12% (wt/vol) SDS gel (Invitrogen), followed by transfer onto PVDF membrane. Membranes were blocked in 5% (wt/vol) nonfat dry milk (Bio-Rad) for 1 h, followed by incubation in primary antibodies overnight at 4 °C. Primary antibodies used were rabbit anti-NR2A (1:1,000; Millipore), rabbit anti-NR2B (1:1,000; Millipore), and mouse anti-GAPDH (1:8,000; Ambion). Membranes were washed in washing buffer [1× PBS/0.5% (vol/vol) Tween-20] 3 times for 10 min each and incubated in secondary antibodies for 1 h at room temperature. Secondary antibodies used were horseradish peroxidase conjugated goat anti-rabbit and goat anti-mouse (1:5,000; Jackson Immunoresearch). Membranes were washed in washing buffer, followed by incubation in SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific) for 5 min at room temperature. Membranes were exposed to X-ray film for optimal development time (30 s–2 min). X-ray films were scanned with an Epson Expression 800 scanner, and individual bands were quantified using IPLab Gel software (BioVision Technologies). NR2A and NR2B levels from individual brain samples were normalized to respective GAPDH levels. Data represents ratio of normalized NR2A to NR2B.
Statistical Analysis.
Data were analyzed using a one-way ANOVA followed by Neuman–Keuls post hoc analysis unless otherwise mentioned. Data displayed in graphs were reported as mean ± SEM or fold change ± SEM, and P < 0.05 was considered to be significant regardless of the statistical test used.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) Grant R01 MH39085 (to J.C.S.), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant R21HD070611 (to M.B.), and the Boyd and Elsie Welin Professorship (to J.C.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308037110/-/DCSupplemental.
References
- 1.Feldman RS, Quenzer LF. Fundamentals of neuropsychopharmacology. Sunderland, MA: Sinauer Associates; 1984. p. xvii. [Google Scholar]
- 2.Hornykiewicz O. The mechanisms of action of L-dopa in Parkinson’s disease. Life Sci. 1974;15(7):1249–1259. doi: 10.1016/0024-3205(74)90306-3. [DOI] [PubMed] [Google Scholar]
- 3.Palmer AM, DeKosky ST. Monoamine neurons in aging and Alzheimer’s disease. J Neural Transm. 1993;91(2-3):135–159. doi: 10.1007/BF01245229. [DOI] [PubMed] [Google Scholar]
- 4.Schildkraut JJ. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am J Psychiatry. 1965;122(5):509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 5.Snyder SH, Banerjee SP, Yamamura HI, Greenberg D. Drugs, neurotransmitters, and schizophrenia. Science. 1974;184(4143):1243–1253. doi: 10.1126/science.184.4143.1243. [DOI] [PubMed] [Google Scholar]
- 6.Shih JC. Molecular basis of human MAO A and B. Neuropsychopharmacology. 1991;4(1):1–7. [PubMed] [Google Scholar]
- 7.Lan NC, et al. Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics. 1989;4(4):552–559. doi: 10.1016/0888-7543(89)90279-6. [DOI] [PubMed] [Google Scholar]
- 8.Bach AW, et al. cDNA cloning of human liver monoamine oxidase A and B: Molecular basis of differences in enzymatic properties. Proc Natl Acad Sci USA. 1988;85(13):4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimsby J, Chen K, Wang LJ, Lan NC, Shih JC. Human monoamine oxidase A and B genes exhibit identical exon-intron organization. Proc Natl Acad Sci USA. 1991;88(9):3637–3641. doi: 10.1073/pnas.88.9.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cases O, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268(5218):1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geha RM, Chen K, Wouters J, Ooms F, Shih JC. Analysis of conserved active site residues in monoamine oxidase A and B and their three-dimensional molecular modeling. J Biol Chem. 2002;277(19):17209–17216. doi: 10.1074/jbc.M110920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K, Holschneider DP, Wu W, Rebrin I, Shih JC. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J Biol Chem. 2004;279(38):39645–39652. doi: 10.1074/jbc.M405550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenders JW, et al. Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J Clin Invest. 1996;97(4):1010–1019. doi: 10.1172/JCI118492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito M, et al. MAOA/B deletion syndrome in male siblings with severe developmental delay and sudden loss of muscle tonus. Brain Dev. 2013 doi: 10.1016/j.braindev.2013.01.004. 10.1016/j.braindev.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Whibley A, et al. Deletion of MAOA and MAOB in a male patient causes severe developmental delay, intermittent hypotonia and stereotypical hand movements. Eur J Hum Genet. 2010;18(10):1095–1099. doi: 10.1038/ejhg.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bortolato M, et al. Monoamine oxidase A and A/B knockout mice display autistic-like features. Int J Neuropsychopharmacol. 2013;16(4):869–888. doi: 10.1017/S1461145712000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JJ, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci USA. 1997;94(11):5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng A, et al. Monoamine oxidases regulate telencephalic neural progenitors in late embryonic and early postnatal development. J Neurosci. 2010;30(32):10752–10762. doi: 10.1523/JNEUROSCI.2037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 20.Lavond DG, Kim JJ, Thompson RF. Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- 21.LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270(6):50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- 22.McGaugh JL. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annu Rev Neurosci. 1989;12:255–287. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- 23.Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33(4):901–912. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- 24.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 25.Tillfors M. Why do some individuals develop social phobia? A review with emphasis on the neurobiological influences. Nord J Psychiatry. 2004;58(4):267–276. doi: 10.1080/08039480410005774. [DOI] [PubMed] [Google Scholar]
- 26.Bortolato M, Godar SC, Davarian S, Chen K, Shih JC. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology. 2009;34(13):2746–2757. doi: 10.1038/npp.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shors TJ, Matzel LD (1997) Long-term potentiation: What's learning got to do with it? Behav Brain Sci 20(4):597–614. [DOI] [PubMed]
- 28.Smith DM, Mizumori SJ. Learning-related development of context-specific neuronal responses to places and events: The hippocampal role in context processing. J Neurosci. 2006;26(12):3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djavadian RL. Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol Exp (Warsz) 2004;64(2):189–200. doi: 10.55782/ane-2004-1505. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZQ, Chen K, Ying QL, Li P, Shih JC. Monoamine oxidase A regulates neural differentiation of murine embryonic stem cells. J Neural Transm. 2011;118(7):997–1001. doi: 10.1007/s00702-011-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29(3):450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 33.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends Neurosci. 1993;16(12):521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 35.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87(7):1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 36.Jin SX, Feig LA. Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PLoS ONE. 2010;5(7):e11732. doi: 10.1371/journal.pone.0011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brigman JL, et al. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15(2):50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu M, et al. Negative regulation of neurogenesis and spatial memory by NR2B-containing NMDA receptors. J Neurochem. 2008;106(4):1900–1913. doi: 10.1111/j.1471-4159.2008.05554.x. [DOI] [PubMed] [Google Scholar]
- 39.Baeg EH, et al. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40(1):177–188. doi: 10.1016/s0896-6273(03)00597-x. [DOI] [PubMed] [Google Scholar]
- 40.Granon S, Vidal C, Thinus-Blanc C, Changeux JP, Poucet B. Working memory, response selection, and effortful processing in rats with medial prefrontal lesions. Behav Neurosci. 1994;108(5):883–891. doi: 10.1037//0735-7044.108.5.883. [DOI] [PubMed] [Google Scholar]
- 41.Procyk E, Goldman-Rakic PS. Modulation of dorsolateral prefrontal delay activity during self-organized behavior. J Neurosci. 2006;26(44):11313–11323. doi: 10.1523/JNEUROSCI.2157-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manes F, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125(Pt 3):624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- 43.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163(1):109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 44.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 45.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 46.Morita M, Nakahara K, Hayashi T. A rapid presentation event-related functional magnetic resonance imaging study of response inhibition in macaque monkeys. Neurosci Lett. 2004;356(3):203–206. doi: 10.1016/j.neulet.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 47.Zhao MG, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47(6):859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci USA. 2007;104(33):13501–13506. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Bao S, Lockard JM, Kim JK, Thompson RF. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. J Neurosci. 1996;16(8):2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ito M (1984) The cerebellum and neural control (Raven Press, New York), pp xvii.
- 51.Freedman R, Hoffer BJ, Woodward DJ, Puro D. Interaction of norepinephrine with cerebellar activity evoked by mossy and climbing fibers. Exp Neurol. 1977;55(1):269–288. doi: 10.1016/0014-4886(77)90175-3. [DOI] [PubMed] [Google Scholar]
- 52.Sears LL, Finn PR, Steinmetz JE. Abnormal classical eye-blink conditioning in autism. J Autism Dev Disord. 1994;24(6):737–751. doi: 10.1007/BF02172283. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29(12):3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grammer M, Kuchay S, Chishti A, Baudry M. Lack of phenotype for LTP and fear conditioning learning in calpain 1 knock-out mice. Neurobiol Learn Mem. 2005;84(3):222–227. doi: 10.1016/j.nlm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Lee T, Kim JJ. Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. J Neurosci. 2004;24(13):3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.