Mammals are eukaryotic organisms, yet they can harbor up to hundreds of trillions of bacteria in their intestines through an elegant form of mutualism (1). To extract maximal benefit from these bacteria, the mucosal immune system must respond in a tolerogenic manner while remaining poised to vigorously react to potential pathogens (2). Thus, intestinal immune cells are charged with an exceedingly difficult task: to know when to remain tolerant while always be poised to vigorously respond to a microbial attack (3). The intestinal epithelium greatly facilitates this decision-making process by creating a physical barrier between luminal bacteria and underlying immune cells. The vast majority of intestinal bacteria are unable to penetrate through the mucus-coated epithelial lining, allowing immune cells to focus attention on a limited number of bacteria that manage to circumvent the barrier. However, under conditions of barrier compromise, there is a greatly increased influx of intestinal bacteria into the subepithelial space that results in direct stimulation of proinflammatory immune responses (4). These immune responses must be rapidly and potently regulated to prevent development of chronic inflammatory bowel diseases (IBDs), such as ulcerative colitis and Crohn’s disease (5). Over the last decade, a remarkable number of discoveries have led to an increasingly sophisticated view of the mucosal immune regulation during homeostasis and disease (3). In PNAS, Zindl et al. (6) present another significant advance in the understanding of how immune cells regulate mucosal inflammation and promote healing. Using a mouse model of acute intestinal inflammation (colitis) induced by damage to the epithelial barrier, the authors analyzed accumulation of neutrophils and the cytokine IL-22 to establish a striking relationship between the two that ultimately leads to resolution of intestinal inflammation.
In response to intestinal insult/injury, a combination of innate and adaptive immune cells are sequentially recruited and activated at sites of tissue damage. These immune cells initially protect the host from microbial invasion and further tissue damage; however, intestinal bacteria and injured cells also act as danger signals to alert the immune system to respond in a proinflammatory manner (5). Thus, some of the immune cells that infiltrate the intestine following injury may initially worsen tissue damage. Of the numerous immune cells that are recruited to the intestine during inflammation, neutrophils are among the first responders (7).
While recruitment of neutrophils to sites of intestinal injury or microbial invasion is a crucial component of the inflammatory response, migration of neutrophils across the epithelium is associated with disruption of barrier function that can increase recruitment of more neutrophils due to enhanced leakage of luminal contents into the mucosa (8, 9). Furthermore, neutrophils that are activated by microbial products release proinflammatory mediators such as chemokines/cytokines including IL-8, growth-regulated oncogene (GRO)α, macrophage inflammatory protein (MIP)1α, TNFα, IL-1β and lipid mediators such as leukotriene B4 (LTB4) that have multiple consequences including recruitment and activation of other leukocytes (10). In addition to promoting recruitment of defending leukocytes, neutrophils that phagocytose bacteria and microbial products release copious amounts of reactive oxygen species (ROS) into the immediate tissue environment. Superoxide anion produced by an NADPH oxidase is rapidly converted to hydrogen peroxide, which, in turn, is modified to hypochlorous acid by myeloperoxidase released from exocytosed neutrophils granules. These ROS damage nearby cells/tissue by indiscriminately oxidizing/chlorinating proteins and damaging DNA. Furthermore, the granules released by activated neutrophils also contain potent peptides and enzymes that not only help kill microbes but also degrade proteins and attract additional leukocytes. One abundant enzyme released from degranulating neutrophils is elastase, which has been shown to degrade barrier-forming proteins on epithelial cells.
If recruitment and activation of neutrophils and other immune cells were to continue unabated, then the intestinal mucosa would ensue severe and potentially life-threatening damage, as is seen in fulminant cases of IBD (11). However, under normal circumstances, there is resolution of the acute inflammatory response. It is now well appreciated that, in addition to injuring tissues, neutrophils play an active role in resolution of intestinal inflammation. For this to occur, a complex series of events must ensue that includes subsequent production/release of anti-inflammatory mediators along with removal of infiltrated neutrophils. Neutrophils help to clean out inflamed areas by actively engulfing microbes and inflammatory debris in addition to switching from release of proinflammatory molecules to active participation in the production/release of a variety of lipid mediators that include lipoxins, resolvins, and protectins (12). These agents counteract the recruitment of more neutrophils, thereby promoting resolution in addition to helping in the healing process by promoting epithelial cell migration. Similarly, certain proteases produced by neutrophils serve to inactivate chemokines/cytokines, thereby diminishing further leukocyte recruitment. Dead and dying neutrophils are cleared from sites of inflammation, through efficient phagocytosis by local and recruited macrophages. Lipid mediators such as resolvin E1 play important roles in enhancing macrophage-mediated clearance of neutrophils, thus lessening the risk of release of neutrophil-derived enzymes from dead cells (13).
In addition to neutrophils themselves, cytokines have been shown to function in the resolution of inflammation at mucosal surfaces. IL-17 is primarily derived from Th17 cells and may limit intestinal inflammation via the induction of chemokines, cytokines, and growth factors from epithelial cells. IL-22 is a cytokine of the IL-10 superfamily that plays a crucial role in the resolution of intestinal inflammation (14, 15). IL-22 is produced predominantly by natural killer (NK) cells, Th17/Th22 CD4+ T cells, and innate lymphoid cells (ILCs), and the primary cellular targets of this cytokine in the intestine are epithelial cells (16, 17). On binding to the IL-22 receptor (IL-22R1, also known as IL22RA1) and activating STAT-3 in intestinal epithelial cells, IL-22 mediates two major functions (18). First, it induces the production of antimicrobial peptides including β-defensins, S100 calcium-binding proteins, and regenerating gene family (Reg) family proteins. These factors are critical for host protection against bacterial pathogens (19). Second, IL-22 stimulates proliferation, differentiation, and migration of intestinal epithelial cells, which aids in wound repair and restitution of the barrier following insult. These combined functions of IL-22 likely contribute to the beneficial roles it plays in experimental and human IBD (14).
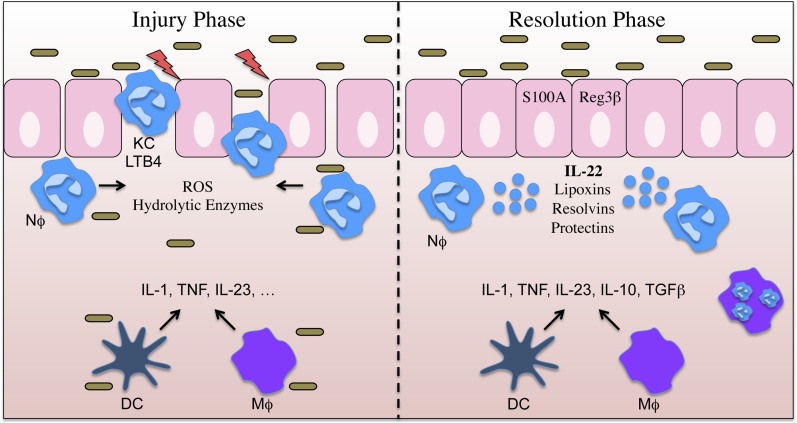
Zindl et al. observe that following the induction of acute dextran sodium sulfate (DSS)-induced intestinal injury, the recruitment of several innate immune cells, namely macrophages and neutrophils, paralleled an increase in IL-22 production (Fig. 1). The authors make the striking observation that neutrophils themselves were an important source of IL-22. Upon stimulation with IL-23 and TNFα, colonic neutrophils produced IL-22. Interestingly, IL-22 was found to localize within neutrophil granules, suggesting that these cells may rapidly release IL-22 via exocytosis when primed by key inflammatory signals. The in vivo relevance of these observations was established by antibody-mediated depletion of neutrophils, resulting in a significant reduction in IL-22 expression. Additionally, elegant adoptive transfer studies using WT or IL-22-deficient neutrophils into DSS-treated IL-22–deficient mice firmly established that IL-22 production by neutrophils is sufficient to induce antimicrobial peptide expression by epithelial cells and to promote the resolution of colitis.
Fig. 1.
Following insult/injury to the intestinal epithelium, microbial-induced local secretion of chemokines (KC, MIP-2, etc.) and other factors promote the recruitment of neutrophils (Nϕ), macrophages (Mϕ), and dendritic cells (DCs). Infiltrating neutrophils become activated and produce ROS and bioactive lipid mediators (LTB4, etc.) that aid in microbial killing, but have the bystander effect of damaging intestinal epithelial cells and compromising barrier function. This injury phase is followed by a resolution phase where IL-23, TNF, and possibly other cytokines induce IL-22 production by neutrophils. IL-22 leads to the production of antimicrobial peptides (S100A8, Reg3β) and healing of the intestinal epithelial barrier.
Until recently, many immune cells present in the inflamed intestine were considered “guilty by association.” There is now beginning to be a deeper appreciation for the complexity of mucosal immune responses during inflammation. For example, proinflammatory Th1 CD4+ T cells are well appreciated to contribute to the pathology of IBD; however, Foxp3+CD4+ regulatory T cells can protect from and even treat intestinal inflammation (20). Thus, as a whole, CD4+ T cells can be considered both bad and good guys when it comes to their roles in regulating pathology. The current discovery by Zindl et al. demonstrates that neutrophils likewise exhibit complex immunological behaviors. They potently generate ROS and tissue-degrading enzymes that ultimately damage neighboring cells, including intestinal epithelial cells, and increase permeability (11). However, once neutrophils are done wreaking havoc, they play an active role in restoring epithelial integrity and inducing antimicrobial defenses. Of course many intriguing questions about how neutrophils and IL-22 regulate intestinal homeostasis remain. Although neutrophil-derived IL-22 is sufficient for antimicrobial defense and restitution of epithelial integrity, is it necessary? Are there different subsets of neutrophils that mediate unique biological functions such as IL-22 production? Do neutrophils release IL-22 from granules? Are activated monocytes/macrophages/dendritic cells critical for neutrophil-mediated IL-22 production? Do neutrophils secrete other cytokines/factors that promote epithelial integrity and/or antimicrobial responses? What are other epithelial cell responses to IL-22 and are they direct or indirect? The answers to these exciting questions that have been inspired by Zindl et al. will hopefully lead to a deeper understanding of how mucosal immune cells and cytokines may be manipulated to treat acute and chronic intestinal inflammation.
Footnotes
The authors declare no conflict of interest.
See companion article on page 12768.
References
- 1.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14(7):676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 4.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 5.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140(6):1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zindl CL,et al. IL-22–producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci USA. 2013;110:12768–12773. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5(4):354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 8.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 9.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: Implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- 10.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazil JC, Louis NA, Parkos CA. The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(7):1556–1565. doi: 10.1097/MIB.0b013e318281f54e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colgan SP, Ehrentraut SF, Glover LE, Kominsky DJ, Campbell EL. Contributions of neutrophils to resolution of mucosal inflammation. Immunol Res. 2013;55(1-3):75–82. doi: 10.1007/s12026-012-8350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis. 2012;18(9):1777–1784. doi: 10.1002/ibd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonnenberg GF, Fouser LA, Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 16.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23(3):159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- 19.Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8(11):829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]