Abstract
A growing number of agents targeting ligand-induced Wnt/β-catenin signaling are being developed for cancer therapy. However, clinical development of these molecules is challenging because of the lack of a genetic strategy to identify human tumors dependent on ligand-induced Wnt/β-catenin signaling. Ubiquitin E3 ligase ring finger 43 (RNF43) has been suggested as a negative regulator of Wnt signaling, and mutations of RNF43 have been identified in various tumors, including cystic pancreatic tumors. However, loss of function study of RNF43 in cell culture has not been conducted, and the functional significance of RNF43 mutations in cancer is unknown. Here, we show that RNF43 inhibits Wnt/β-catenin signaling by reducing the membrane level of Frizzled in pancreatic cancer cells, serving as a negative feedback mechanism. Inhibition of endogenous Wnt/β-catenin signaling increased the cell surface level of Frizzled. A panel of 39 pancreatic cancer cell lines was tested for Wnt dependency using LGK974, a selective Porcupine inhibitor being examined in a phase 1 clinical trial. Strikingly, all LGK974-sensitive lines carried inactivating mutations of RNF43. Inhibition of Wnt secretion, depletion of β-catenin, or expression of wild-type RNF43 blocked proliferation of RNF43 mutant but not RNF43–wild-type pancreatic cancer cells. LGK974 inhibited proliferation and induced differentiation of RNF43-mutant pancreatic adenocarcinoma xenograft models. Our data suggest that mutational inactivation of RNF43 in pancreatic adenocarcinoma confers Wnt dependency, and the presence of RNF43 mutations could be used as a predictive biomarker for patient selection supporting the clinical development of Wnt inhibitors in subtypes of cancer.
The evolutionarily conserved Wnt/β-catenin signaling pathway plays critical roles in embryonic development and adult tissue homeostasis (1, 2). Wnt signaling regulates the turnover of the transcription cofactor β-catenin and controls key developmental gene expression programs (3). In the absence of Wnt pathway activation, cytosolic β-catenin is degraded by the β-catenin destruction complex, consisting of adeomatous polyposis coli (APC), AXIN1/2, and glycogen synthase kinase 3α/β (GSK3α/β). Wnt ligand activates its two receptors, Frizzled and LRP5/6, and inactivates the β-catenin destruction complex. Stabilized β-catenin enters the nucleus, binds to the TCF family of transcription factors, and activates transcription. Secretion of Wnt proteins requires Porcupine (PORCN), a membrane bound O-acyltransferase dedicated to Wnt posttranslational acylation (4, 5). Precise regulation of Wnt signaling is critical and various feedback control mechanisms exist to ensure proper signaling output.
Aberrant activation of Wnt/β-catenin signaling has been implicated in tumorigenesis, and many downstream components of the Wnt pathway are mutated in cancers (6). Truncation mutations of APC are found in 80% of colorectal cancer. Stabilization mutations of CTNNB1 (β-catenin) and loss of function mutations of AXIN1/2 are also found in cancers. Despite intense research, targeting Wnt/β-catenin signaling in cancers harboring downstream pathway mutations remains challenging because of the lack of tractable targets (7, 8). However, there are several potential targets upstream in the Wnt signaling pathway, and various agents, including LRP6 antibody (9, 10), Frizzled antibody (11), and Porcupine inhibitor (12), are being developed. However, it is challenging to develop therapeutic agents without a defined patient population, and we do not have enough knowledge about human tumors dependent on ligand-induced Wnt/β-catenin signaling.
We have shown that transmembrane E3 ubiqutin ligase ZNRF3 negatively regulates Wnt/β-catenin signaling through promoting the degradation of Frizzled, and the activity of ZNRF3 is inhibited by R-spondin proteins (13). Ring finger 43 (RNF43) is structurally related to ZNRF3. Intestinal-specific deletion of both Znrf3 and Rnf43 induces hyperproliferation of intestinal crypts and formation of intestinal adenoma in mice (14). These studies suggest that RNF43 serves as a negative regulator of Wnt/β-catenin signaling similar to ZNRF3. However, a cellular system in which RNF43 plays a critical role has not been identified, and, therefore, in vitro loss-of-function studies of RNF43 have not been possible. RNF43 is frequently mutated in intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) of the pancreas (15,16). IPMN and MCN are potential precursors to pancreatic ductal adenocarcinoma (PDAC), which is extremely aggressive and associated with a dismal prognosis and few proven therapeutic options (17–20).
Here, we studied the function of RNF43 in human pancreatic adenocarcinoma cells. We found that RNF43 suppressed Wnt signaling by decreasing membrane levels of Frizzled in pancreatic cancer, functioning as a negative feedback mechanism. By testing Wnt dependency in a large panel of pancreatic cancer cell lines, we discovered that all cell lines whose proliferation was strongly inhibited by a Porcupine inhibitor had homozygous RNF43 loss-of-function mutations. Growth of RNF43-mutant lines was blocked upon depletion of β-catenin or RNF43 reexpression. Treatment of RNF43-mutant tumors in vivo with a Porcupine inhibitor resulted in growth inhibition and differentiation. Therefore, we propose that RNF43 mutation can serve as a predictive biomarker for identifying Wnt ligand-dependent pancreatic cancers that may be responsive to upstream Wnt pathway inhibitors.
Results
Negative Regulation of Wnt Signaling by RNF43 in Pancreatic Cancer Cells.
Because RNF43 is frequently mutated in cystic pancreatic tumors (15, 16), we hypothesized that RNF43 is an essential regulator of Wnt/β-catenin signaling in pancreatic cancer. RNF43 loss-of-function experiments were performed in YAPC, a pancreatic adenocarcinoma cell line. Depletion of RNF43 using two independent siRNAs (Fig. S1A) significantly increased SuperTopflash (STF) Wnt reporter activity, either in the absence or presence of Wnt3a conditioned medium (Fig. 1A). LGK974 is a Porcupine inhibitor that specifically inhibits Wnt secretion and is under clinical evaluation (www.clinicaltrials.gov/ct2/show/NCT01351103?term=lgk974&rank=1). RNF43 siRNA-induced STF activity in the absence of exogenous Wnt3a is inhibited by Porcupine inhibitor LGK974 and IWP-2 (12) (Fig. 1B), indicating that this activity depends on the expression of endogenous Wnt proteins. These results suggest that RNF43 actively suppresses autocrine Wnt/β-catenin signaling in YAPC cells.
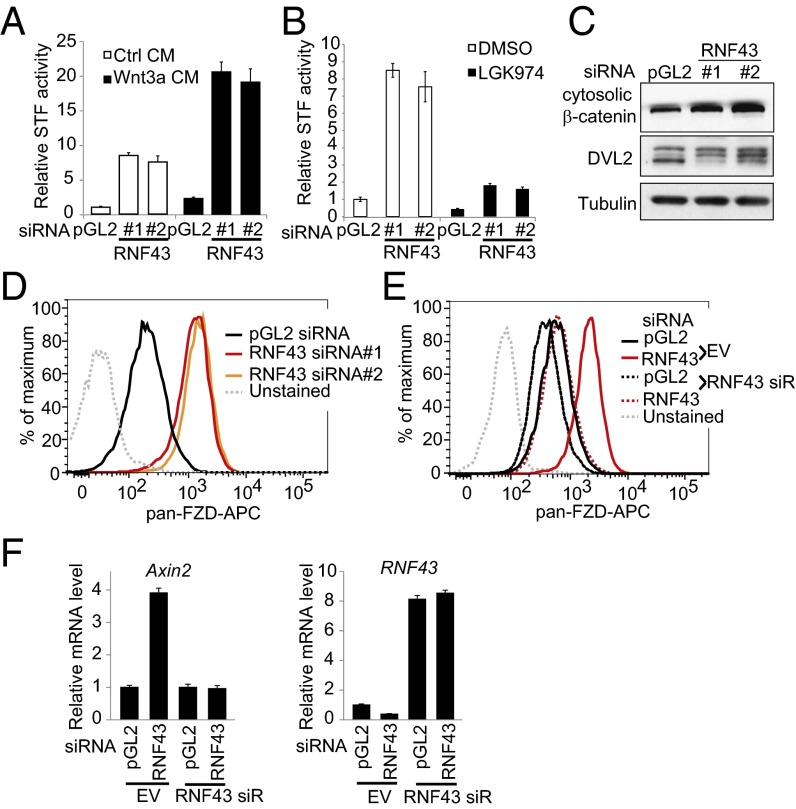
Fig. 1.
Negative regulation of Wnt signaling by RNF43 in pancreatic cancer cells. (A) Depletion of RNF43 increases STF activity in YAPC pancreatic cancer cell line. YAPC-STF cells were transfected with indicated siRNA in the absence or presence of Wnt3a conditioned medium (CM), and STF luciferase reporter activity was measured. pGL2 siRNA is a negative control. (B) RNF43 siRNA-induced activation of STF depends on endogenous Wnt. YAPC-STF cells were transfected with indicated siRNA and then treated with DMSO or 1 μM porcupine inhibitor LGK974. STF reporter activity was then measured. (C) Depletion of RNF43 increases cytosolic β-catenin and phosphorylation of DVL2. (D) Depletion of RNF43 increases the cell surface level of Frizzled (FZD). YAPC cells were transfected with indicated siRNA, and membrane levels of Frizzled were analyzed by flow cytometry using pan-Frizzled antibody 18R5. (E) Flow cytometric analysis of membrane Frizzled in YAPC cells stably expressing empty vector (EV), siRNA-resistant RNF43, and transfected with indicated siRNA. (F) Depletion of RNF43 increases expression of AXIN2.
We next performed biochemical assays to characterize the function of RNF43 in YAPC cells. Depletion of RNF43 increased the level of cytosolic β-catenin (Fig. 1C), in agreement with increased STF reporter activity (Fig. 1A). Dishevelled (DVL) is an intracellular signaling protein downstream of Frizzled, and its phosphorylation is stimulated by Frizzled activation. Depletion of RNF43 increased phosphorylation of DVL2 (Fig. 1C), suggesting that the activity of Frizzled is increased. Indeed, depletion of RNF43 markedly increased the cell surface level of Frizzled (Fig. 1D). To rule out the possibility that the effect of RNF43 siRNA is mediated by off-target activity, we performed a cDNA rescue experiment by stably expressing siRNA-resistant RNF43 (RNF43 siR) in YAPC cells. As seen in Fig. 1E, expression of siRNA-resistant RNF43 largely abolished the effect of RNF43 siRNA on Frizzled levels, suggesting the activity of RNF43 siRNA is on target. Depletion of RNF43 also increased the expression of AXIN2, a β-catenin target gene, and this effect is abolished by the expression of RNF43 siR (Fig. 1F). On the basis of the threshold cycle (Ct) values observed in a quantitative RT-PCR assay, RNF43 is dominantly expressed compared with ZNRF3 in YAPC cells (Fig. S1B). Consistent with the idea that RNF43 targets Wnt receptors for degradation, RNF43 can be coimmunoprecipitated with Frizzled and LRP6 (Fig. S2). Together, these results suggest that RNF43 negatively regulates Wnt/β-catenin signaling by decreasing membrane expression of Frizzled.
Wnt/β-Catenin Signaling Suppresses the Membrane Expression of Frizzled.
Wnt/β-catenin signaling is under exquisite control, with multiple negative feedback control mechanisms to ensure the proper signaling output. We and others have shown that ZNRF3 and RNF43 are β-catenin target genes (13, 14). Although such data suggest that ZNRF3 and RNF43 might suppress membrane Frizzled as a negative feedback loop, the effect of endogenous Wnt/β-catenin signaling on Frizzled expression has never been examined. We found that independent β-catenin siRNAs significantly increased the cell surface level of Frizzled in YAPC cells (Fig. 2A). As expected, depletion of β-catenin decreased the mRNA levels of β-catenin target genes AXIN2 and RNF43 (Fig. 2B). Consistent with this observation, treatment of Porcupine inhibitor IWP-2 or LGK974 also increased the cell surface level of Frizzled (Fig. 2 C and D) and decreased the mRNA levels of AXIN2 and RNF43 (Fig. 2E). These results suggest that activation of the Wnt/β-catenin pathway strongly down-regulates the membrane level of Frizzled, likely through increasing the expression of RNF43 and ZNRF3.
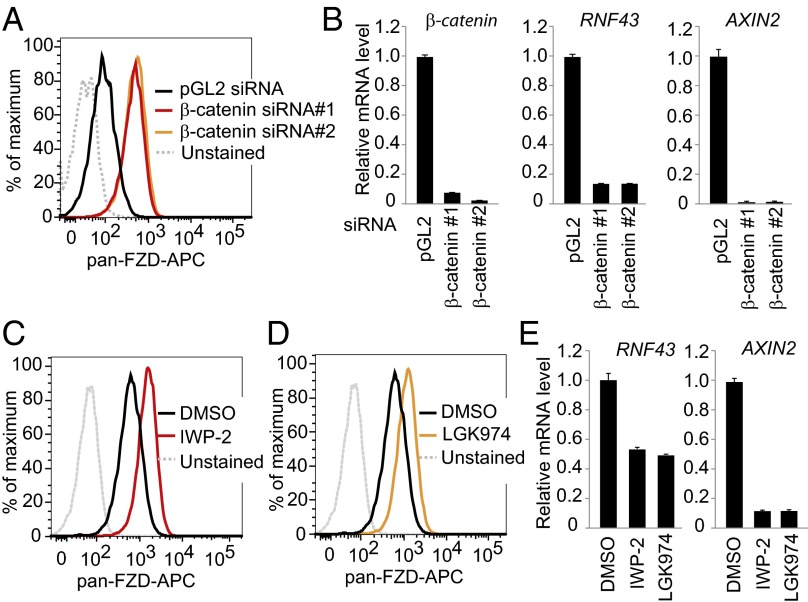
Fig. 2.
Wnt/β-catenin signaling suppresses membrane expression of Frizzled in pancreatic cancer cells. (A) Depletion of β-catenin increases the cell surface level of Frizzled. YAPC cells were transfected by indicated siRNA, and membrane levels of Frizzled were analyzed by flow cytometry. (B) Depletion of β-catenin decreases mRNA level of AXIN2 and RNF43. Cells were treated as in A, and relative mRNA levels of indicated genes were analyzed by quantitative RT-PCR. (C–E) Porcupine inhibitor increases the cell surface level of Frizzled. YAPC cells were treated with 3 μM IWP-2 or 1 μM LGK974 and subjected to flow cytometry analysis for membrane Frizzled (C and D) or gene expression analysis by quantitative RT-PCR (E).
Characterization of RNF43 Mutations in Pancreatic Cancer.
Discovery of LGK974, a potent and selective inhibitor of Porcupine, offered us a unique chemical tool to systematically examine Wnt dependency in a large panel of pancreatic cancer cell lines. Foci formation assay was used because it is more sensitive than routine growth assays such as CellTiter-Glo, and it is less affected by the different growth rates of various cell lines. Of the 39 pancreatic cancer cell lines screened, LGK974 only showed strong growth inhibitory activity in three cell lines, HPAF-II, PaTu 8988S, and Capan-2 (Fig. S3).
We next sought to determine the genetic lesion that could confer Wnt dependency. Because RNF43 is a negative regulator upstream of the Wnt pathway mutated in cancer, we performed RNF43 exon sequencing in all pancreatic cancer cell lines. Strikingly, all three LGK974-sensitive cell lines had mutations of RNF43 and loss of wild-type allele (HPAF-II, E174X; PaTu 8988S, F69C; Capan-2, R330fs) (Fig. 3A). E174X and R330fs mutations truncate the majority of RNF43 protein and most likely inactivate the protein. The functional consequence of F69C mutation is less clear. To define the function of the F69C mutation, we stably expressed C-terminal HA-tagged wild-type RNF43, RNF43 lacking the RING domain (ΔRING), and RNF43 F69C in YAPC cells. Consistent with the function of RNF43 in regulating Frizzled turnover, overexpression of wild-type RNF43 decreased the membrane level of Frizzled, whereas overexpression of RNF43 ΔRING showed dominant-negative activity and increased the membrane level of Frizzled (Fig. 3B). Overexpression of RNF43 F69C modestly increased the membrane level of Frizzled (Fig. 3B), suggesting that the F69C mutant has partial dominant-negative activity upon overexpression. Further, overexpression of RNF43 ΔRING, and to a lesser degree overexpression of RNF43 F69C, increased DVL2 phosphorylation (Fig. 3C) and potentiated Wnt3a-induced STF reporter activity (Fig. 3D). These results suggest that RNF43 negatively regulates Wnt signaling through decreasing membrane expression of Frizzled, and that F69C is an inactivating mutation of RNF43. We sought to understand why F69C mutant is inactive in suppressing Wnt signaling. Although the expression of F69C mutant in total lysates is comparable to that of wild-type RNF43 (Fig. 3C), the level of F69C mutant on the cell surface is much lower than that of wild-type RNF43 in FACS assay (Fig. 3E). These results suggest that F69C mutation significantly inhibits membrane targeting of RNF43. Targeting of RNF43 to the plasma membrane is most likely critical for its Wnt inhibitory activity. Introduction of one extra cysteine residue into the extracellular domain of RNF43 might affect correct pairing of cysteine residues and block proper folding and membrane targeting of RNF43.
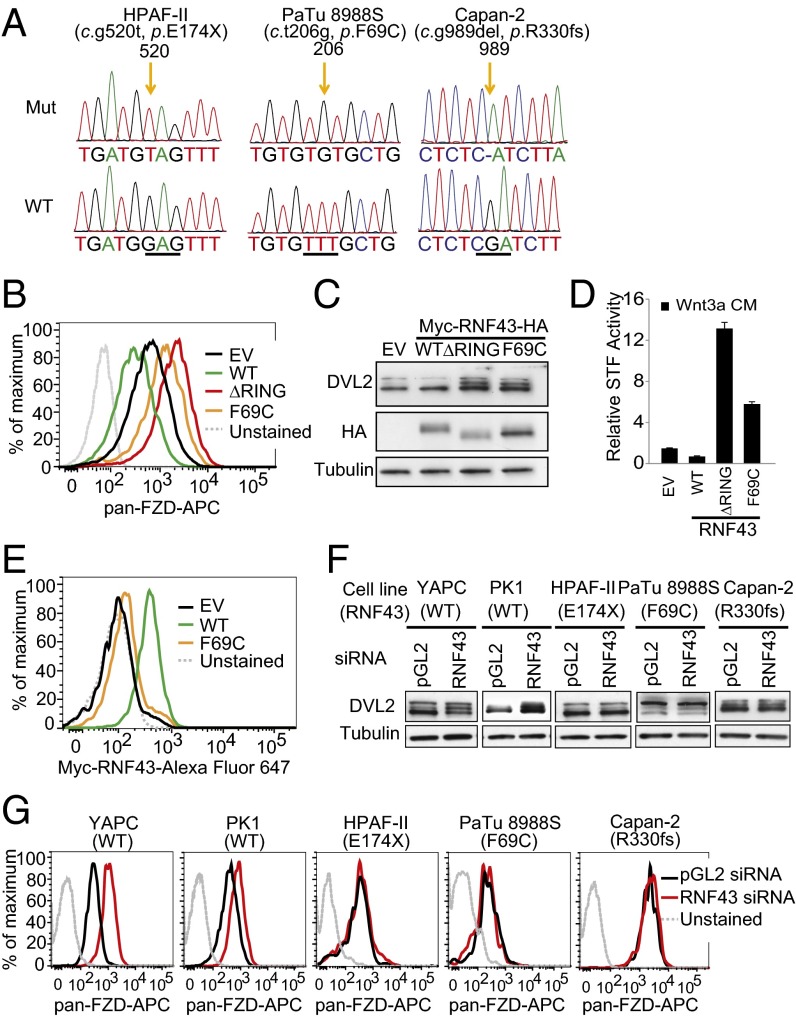
Fig. 3.
Characterization of RNF43 mutations in pancreatic cancer. (A) Mutations of RNF43 in HPAF-II, PaTu 8988S, and Capan-2. Corresponding sequence of wild-type RNF43 is shown at the bottom. (B) Flow cytometric analysis of membrane Frizzled in YAPC cells stably expressing empty vector (EV), wild-type (WT) RNF43, or mutant (ΔRING or F69C) RNF43. (C) DVL2 phosphorylation was analyzed by immunoblotting in the same cells described in B. (D) Wnt3a-induced STF reporter activity in YAPC-STF cells expressing empty vector (EV), wild-type (WT) RNF43, or mutant (ΔRING, or F69C) RNF43. (E) Flow cytometric analysis of membrane Myc-RNF43 in YAPC cells stably expressing empty vector (EV), wild-type (WT) RNF43, or mutant (F69C) RNF43. (F) Depletion of RNF43 increases DVL2 phosphorylation in pancreatic cell lines with wild-type RNF43 (YAPC and PK1), but not those with mutant RNF43 (HPAF-II, PaTu 8988S, and Capan-2). (G) Flow cytometric analysis of membrane Frizzled in pancreatic cancer cell lines treated with RNF43 siRNA.
We next examined the function of RNF43 in RNF43-mutant cell lines. Although depletion of RNF43 increased DVL2 phosphorylation in YAPC and PK1, two pancreatic cancer cell lines with wild-type RNF43, depletion of RNF43 in HPAF-II, PaTu 8988S, and Capan-2 cells did not increase DVL2 phosphorylation (Fig. 3F). Consistently, depletion of RNF43 increased the cell surface level of Frizzled in RNF43–wild-type, but not in RNF43-mutant, pancreatic cancer cell lines (Fig. 3G). Taken together, these results suggest that Frizzled levels are no longer inhibited by RNF43 in pancreatic cancer cell lines with RNF43 mutations.
RNF43 Mutation Predicts Sensitivity to Wnt Inhibition in Pancreatic Cancer Cell Lines.
We next characterized Wnt dependency in pancreatic adenocarcinoma cell lines. In foci formation assay, LGK974 strongly inhibited the growth of RNF43-mutant cells (PaTu8988S, HPAF-II, and Capan-2), without significant effect on RNF43–wild-type cells (PK1 and YAPC) (Fig. 4A). Importantly, the growth inhibitory effect of LGK974 in RNF43-mutant cells was rescued by exogenous Wnt3a (Fig. 4A), suggesting that the effect of LGK974 was mediated by blocking Wnt secretion. Immunoblot and quantitative PCR assays indicated that LGK974 decreased the expression of MYC, a β-catenin target gene (21) and increased the expression of cyclin-dependent kinase inhibitor p21 in RNF43-mutant, but not RNF43–wild-type, cell lines (Fig. 4 B and D). LGK974 decreased cytosolic β-catenin (Fig. 4C) and inhibited the expression of the β-catenin target gene AXIN2 (Fig. 4D) in both RNF43-mutant and RNF43–wild-type cell lines, demonstrating that all these cell lines have active autocrine Wnt signaling. LGK974 blocked EDU incorporation in RNF43-mutant cell lines, suggesting that Wnt inhibition in these cells leads to cell cycle arrest (Fig. 4E). Further, LGK974 induced the expression of differentiation markers MUC2 and MUC5A/C (Fig. 4D) and increased mucin production as visualized by Alcian Blue staining (Fig. 4F) in RNF43-mutant cells, consistent with cellular differentiation. LGK974 also strongly inhibited the proliferation of PaTu 8988S and HPAF-II in soft agar assay (Fig. 4G). Capan-2 cannot be tested because it does not grow in soft agar assay. Note that although many cell lines had autocrine Wnt signaling, only RNF43-mutant lines clearly depend on autocrine Wnt signaling for growth (Fig. S3). Therefore, RNF43 mutation status can potentially be used to enrich for patients who are likely to respond to upstream Wnt inhibitors.
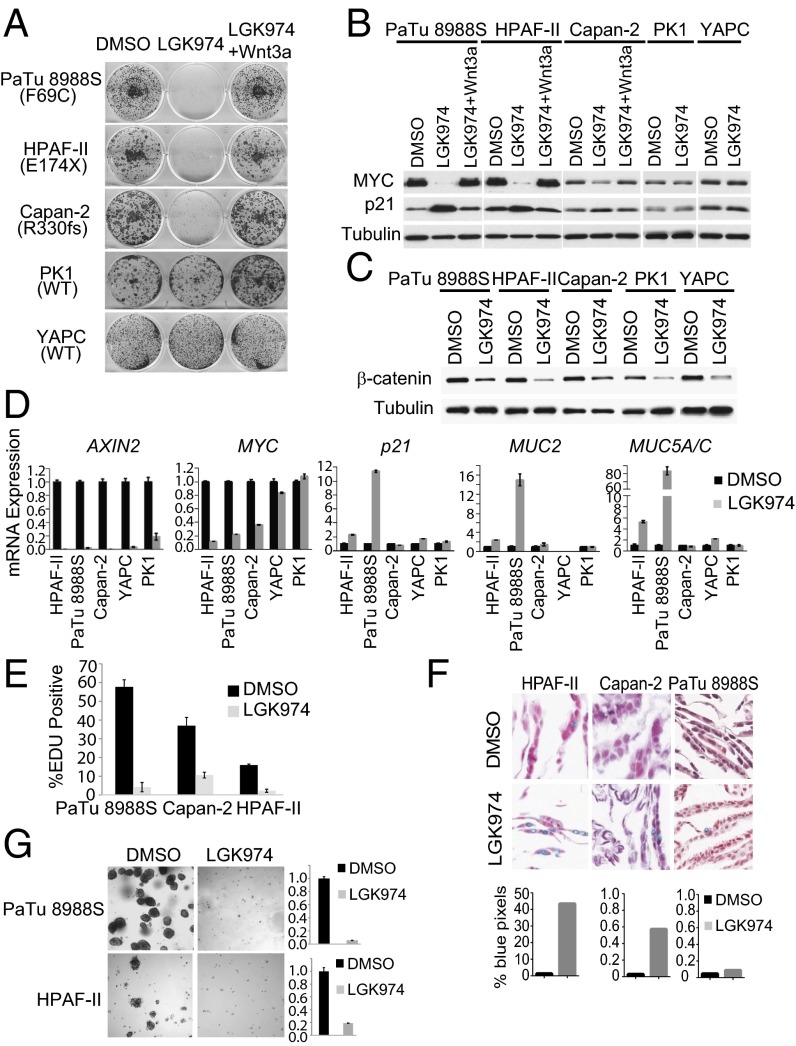
Fig. 4.
LGK974 specifically inhibits the growth of pancreatic cancer cell lines harboring RNF43 mutation. (A) LGK974 specifically inhibits the growth of pancreatic cancer cell lines with RNF43 mutation. Pancreatic cancer cell lines were treated with DMSO, 1 μM LGK974, or LGK974 together with recombinant Wnt3a in foci formation assay. (B) LGK974 decreases the protein expression of MYC and increases the protein expression of p21 in RNF43 mutant pancreatic cancer lines. (C) LGK974 decreases cytosolic β-catenin in all pancreatic cancer cell lines. (D) LGK974 decreases mRNA expression of MYC and induces mRNA expression of p21, MUC2, and MUC5A/C in RNF43-mutant pancreatic cancer lines. (E) LGK974 inhibits EDU incorporation in pancreatic cancer lines with RNF43 mutation. (F) Representative images of Alcian blue staining for mucin of cells treated with DMSO or LGK974 (Upper). (F, Lower) Percentage of blue positive pixels. (G) LGK974 inhibits the growth of pancreatic cancer cell lines with RNF43 mutation in soft agar assay. Representative images of cells treated with DMSO or LGK974 were shown (Left). The number of surviving cells was quantified by Alama blue staining (Right).
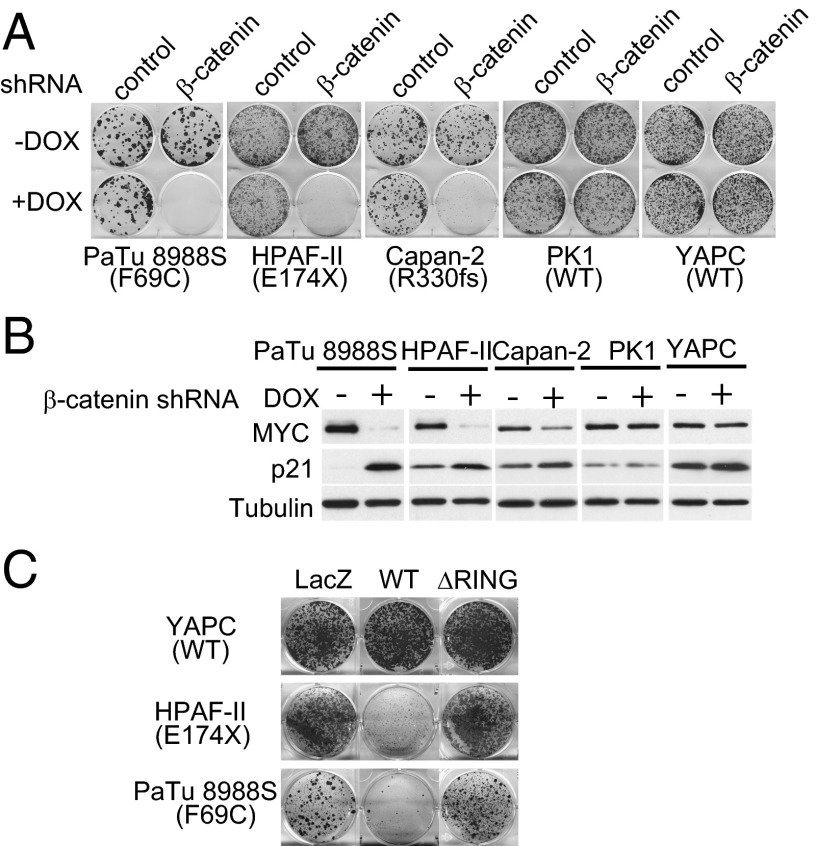
Frizzled proteins potentiate both canonical Wnt/β-catenin signaling and noncanonical Wnt signaling. Therefore, mutational inactivation of RNF43 would conceivably increase both canonical and noncanonical Wnt signaling (13). We used β-catenin shRNA to determine the contribution of Wnt/β-catenin signaling to the growth of the pancreatic cancer lines. Inducible expression of a previously validated β-catenin shRNA (22) strongly inhibited the proliferation of RNF43-mutant (PaTu8988S, HPAF-II, and Capan-2), but not RNF43–wild-type (PK1 and YAPC) pancreatic cancer lines (Fig. 5A). Depletion of β-catenin also decreased the expression of β-catenin target gene AXIN2 and MYC and increased the expression of p21, MUC2, and MUC5A/C in RNF43-mutant cell lines (Fig. 5B and Fig. S4). These results suggest that the effect of LGK974 on cell growth is likely mediated by canonical Wnt/β-catenin signaling. We next examined whether RNF43 mutation is required for the growth of RNF43 mutant cells. As seen in Fig. 5C, expression of wild-type RNF43, but not LacZ or RNF43 ΔRING, significantly inhibited the proliferation of RNF43-mutant PaTu 8988S and HPAF-II cells but had no effect on RNF43-wild-type YAPC cells. Reintroduction of wild-type RNF43 presumably overcomes the minor dominant negative activity of endogenously expressed RNF43 F69C in PaTu 8988S cells. Capan-2 cannot be tested in this assay because viral infection efficiency of this cell line is too low, and we could not obtain stably infected cells by using retrovirus expressing LacZ or RNF43 ΔRING. Together, these results suggest that Wnt/β-catenin signaling enhanced by RNF43 mutation is driving the proliferation of RNF43-mutant pancreatic cancer cells and that suppression of Wnt/β-catenin signaling in these cells induces cell cycle arrest and cellular differentiation.
Fig. 5.
Depletion of β-catenin or reexpression of RNF43 inhibits the proliferation of pancreatic cancer cells with RNF43 mutation. (A) Depletion of β-catenin specifically inhibits the growth of pancreatic cancer cells with RNF43 mutation in foci formation assay. The expression of β-catenin shRNA was induced by doxycycline (DOX). (B) Depletion of β-catenin decreases the protein expression of MYC and increases the protein expression of p21 in pancreatic cancer cells with RNF43 mutation. (C) Reexpression of RNF43 inhibits the proliferation of pancreatic cancer cells with RNF43 mutation. Cells were infected with retrovirus encoding LacZ, wild-type (WT), or mutant (ΔRING) RNF43 and subjected to foci formation assay.
Blocking Wnt Secretion Inhibits the Growth of RNF43-Mutant Pancreatic Tumors in Vivo.
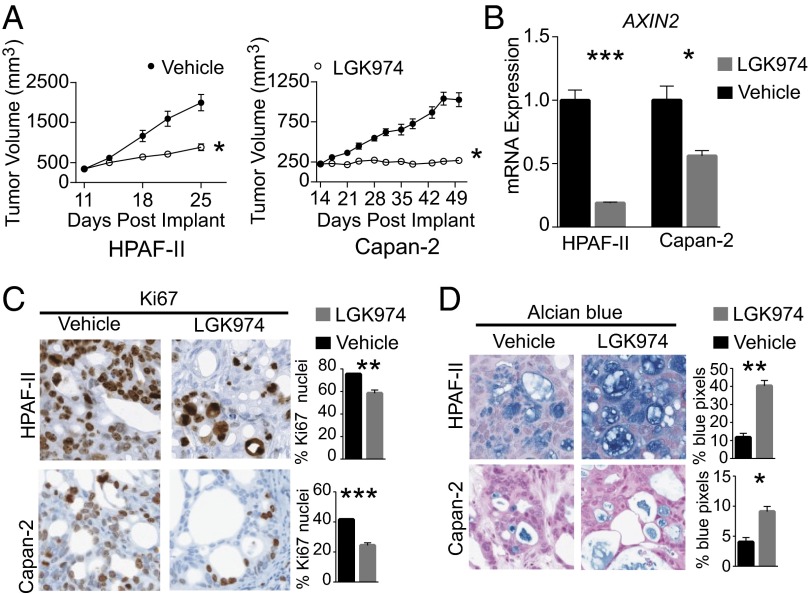
To analyze the role of Wnt pathway activation in the maintenance of RNF43-mutant pancreatic tumors in vivo, we used two PDAC xenograft models (HPAF-II and Capan-2) (Fig. 6A). Treatment of mice bearing HPAF-II xenografts with 5 mg/kg LGK974, oral gavage (p.o.) twice daily (BID) for 14 d resulted in significant inhibition of tumor growth (T/C = 33%) relative to vehicle treatment. Furthermore, treatment of mice bearing Capan-2 xenografts with 5 mg/kg LGK974, p.o. BID for 35 d achieved tumor stasis (T/C = 5%). Consistent with the mechanism of action of LGK974, expression of the β-catenin target gene AXIN2 was decreased in treated HPAF-II and Capan-2 xenografts (Fig. 6B). Similar to in vitro findings in RNF43-mutant PDAC cells, treatment with LGK974-induced cell cycle arrest and differentiation within the xenograft tumors (Fig. 6 C and D).
Fig. 6.
LGK974 inhibits tumor growth of RNF43-mutant pancreatic tumors in vivo. (A) HPAF-II (Left) or Capan-2 (Right) xenograft-tumor-bearing mice were treated with 10 mL/kg vehicle, p.o., BID (filled circles) or 5 mg/kg LGK974, p.o., BID (open circles) for 14 d (HPAF-II) or 35 d (Capan-2). The tumor volume of vehicle and LGK974-treated mice is plotted as the mean ± SEM (n = 8 per treatment group). (B) Quantitative RT-PCR of tumor AXIN2 levels after 3 d of treatment, 7 h after final dose. Graphs represent mean ± SEM (n = 3 per treatment group). (C) Representative images of Ki67 staining by immunohistochemistry (Left) after 14 d of treatment. (Right) Percentage of nuclei positive for Ki67 after 14 d of treatment. Graphs represent mean ± SEM (n = 3 per treatment group). (D) Representative images of Alcian blue staining for mucin (Left) after 14 d of treatment. (Right) Percentage of blue pixels after 14 d of treatment. Graphs represent mean ± SEM (n = 3 per treatment group). *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In this study, we have demonstrated that RNF43 serves as a negative feedback regulator of Wnt pathway in pancreatic cells by suppressing membrane expression of Frizzled. We have shown that Wnt/β-catenin signaling potently inhibits Frizzled membrane expression, likely through induction of RNF43. These findings provide an explanation for why pancreatic cancer cells acquire mutations in RNF43 to escape from this powerful negative feedback regulation and achieve increased Wnt/β-catenin signaling. Three Wnt-dependent pancreatic adenocarcinoma cell lines have been identified by using the Porcupine inhibitor LGK974 and, strikingly, all of these cell lines harbor RNF43 loss-of-function mutations. The proliferation of these RNF43-mutant cell lines in vitro is inhibited upon β-catenin depletion or RNF43 reexpression, and their growth in vivo is also inhibited by LGK974. Our data establish RNF43 as a tumor suppressor in pancreatic adenocarcinoma and suggest that the presence of RNF43 mutations may serve as a useful biomarker for patient selection during the clinical development of Wnt inhibitors.
ZNRF3 and RNF43 are transmembrane E3 ubiquitin ligases that potently inhibit Wnt signaling through targeting Wnt receptor for degradation (13, 14). These E3 ligases have a relatively small extracellular domain similar to protease-associated domains, which are found in proteases and membrane receptors and have unknown function (23). We discovered that R-spondin interacts with the extracellular domain of these E3 ligases and inhibits their function (13). ZNRF3 and RNF43 have a large intracellular domain, which also likely plays a regulatory role. How ZNRF3 and RNF43 specifically recognize Frizzled/LRP6 complex is still not clear. It is possible that both the extracellular domain and intracellular domain of ZNRF3 and RNF43 are involved in Wnt receptor targeting.
The Wnt/β-catenin signaling pathway is critical for the development of the exocrine pancreas (24–27). Inhibition of Wnt/β-catenin signaling disrupts acinar but not islet cell development (24, 25, 27). Conversely, inducible expression of stabilized β-catenin in the pancreas of adult mice increases the proliferation of acinar cells with minimal effects on islet cells in adult mice (28). Recent evidence suggests there is an ongoing and selective requirement for Wnt/β-catenin signaling in the adult exocrine pancreas, promoting acinar cell self-renewal and regeneration (29). Future studies will be needed to elucidate the role of RNF43 in the regulation of Wnt/β-catenin signaling during pancreatic development and homeostasis. Like KRAS mutations, RNF43 mutations are found at a high frequency in pancreatic cancer precursor lesions IPMNs and MCNs (16), suggesting that RNF43 mutations may contribute to the initiation of PDAC. There is mounting evidence that acinar cells, through a process of ductal reprogramming, may serve as the cell of origin of PDAC (30–34). In mice, activation of oncogenic KRAS in adult acinar cells is insufficient to induce PDAC (31). Aberrant Wnt/β-catenin signaling in acinar cells due to inactivation of RNF43 may provide an additional stimulus needed to promote proliferation and tumorigenesis. Importantly, increased Wnt/β-catenin signaling resulting from RNF43 mutation is also required for maintaining the transforming phenotype of pancreatic cancer lines. Wnt dependency of RNF43 mutant cell lines is remarkable considering all these cell lines also have KRAS mutation.
There is growing evidence that Wnt pathway activation may contribute to the maintenance and/or progression of PDAC (35). Expression profiling of Wnt pathway components and immunohistochemistry for nuclear β-catenin indicate that the Wnt/β-catenin pathway is commonly activated in PDAC (36-39). The level of cytosolic and nuclear β-catenin is positively correlated with pancreatic intraepithelial neoplasia grade and development of PDAC (36, 39), suggesting that β-catenin signaling promotes the progression of PDAC. Wnt ligands, such as WNT2b, WNT7b, and WNT11, are strongly expressed in PDAC cell lines (38), raising the possibility of autocrine Wnt pathway activation. Consistent with autocrine Wnt pathway activation, we have found that LGK974 decreases the expression of AXIN2 in 22 of 34 (65%) pancreatic cancer cell lines in vitro (Fig. S3). The presence of Wnt/β-catenin pathway activation is necessary but not sufficient to confer Wnt dependency (Fig. S3). Only PDAC cell lines with autocrine Wnt signaling and RNF43 loss-of-function mutations showed clear sensitivity to LGK974 in a foci formation assay. These results suggest that RNF43-mutant PDACs may have a higher probability of being Wnt ligand-dependent compared with RNF43–wild-type tumors.
Not all PDAC cell lines with RNF43 mutations are sensitive to LGK974. We have found three pancreatic cancer lines carrying homozygous mutations of RNF43, PaTu8988T (F69C), Panc10.05 (M18fs), and PL45 (M18fs), that are not sensitive to LGK974 (Fig. S3). Note that PaTu 8988S and PaTu8988T were derived from the same patient, and Panc10.05 and PL45 were also derived from the same patient. These results suggest that additional molecular mechanisms can render RNF43-mutant tumors independent of Wnt signaling.
To maximize the benefits of targeted cancer therapeutics, it is critical to identify those patients most likely to respond to a therapy. Several agents, including Porcupine inhibitor, Frizzled antibody, and LRP6 antibody, are being developed to inhibit ligand-induced Wnt/β-catenin signaling. However, the clinical development of these agents is challenging because it has been difficult to identify Wnt ligand-dependent human tumors. A patient selection strategy based on expression of Wnt ligands or Wnt inhibitors may not be sufficient. Indeed, many pancreatic adenocarcinoma cell lines with autocrine Wnt/β-catenin signaling do not depend on Wnt for in vitro growth (Fig. S3). Furthermore, β-catenin signaling can be activated in a ligand-independent manner (39), so a strategy based on nuclear accumulation of β-catenin would not be reliable either. Our study has established RNF43 as a tumor suppressor that inhibits upstream Wnt signaling in pancreatic adenocarcinoma models. Our finding that all pancreatic cancer cell lines showing strong Wnt dependency carry RNF43 mutations suggests that the presence of RNF43 mutations may serve as a predictive biomarker for selecting patients likely to respond to upstream Wnt pathway inhibitors. In addition to pancreatic tumors, RNF43 is also mutated in endometrioid carcinoma of the uterus (40), mucinous ovarian tumors (41), and liver fluke-associated cholangiocarcinoma (42), supporting the evaluation of upstream Wnt inhibitors in these clinical indications.
Materials and Methods
Pancreatic cancer cells and HEK293 cells were grown in medium recommended by ATCC supplemented with 10% FBS. Retrovirus or lentivirus was produced from HEK293 cells by standard virus packaging procedure using FuGENE 6 (Roche) transfection reagent. Pancreatic cell lines expressing STF reporter, RNF43 constructs, or doxycycline (DOX)-inducible β-catenin shRNA were generated by viral infection and drug selection.
siRNA transfection was performed by using Dharmafect 1 transfection reagent (Dharmacon). Detailed methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Alwin Schuller, Jane Cheng, Colleen Kowal, Hui Gao, Lang Huynh, Raffaella Zamponi, Adriana Donavan, Reginald Valdez for technical assistance and Frank Stegmeier, Gary Vanasse, Jennifer Harris, Andy Boral, John Monahan, Rob McDonald, Michael Schlabach, Rebecca Mosher, Alexander Cao, Robert Schlegel, Alan Huang, Megan Yao, Shaowen Wang, Neil Kubica, Alan Buckler, Leon Murphy, and Vic Myer for advice and helpful discussions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307218110/-/DCSupplemental.
References
- 1. Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Ann Rev Cell Dev Biol 20:781–810. [DOI] [PubMed]
- 2.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herr P, Hausmann G, Basler K. WNT secretion and signalling in human disease. Trends Mol Med. 2012;18(8):483–493. doi: 10.1016/j.molmed.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11(6):791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 7.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 8. Takada K, et al. (2012) Targeted disruption of the BCL9/beta-catenin complex inhibits oncogenic Wnt signaling. Sci Transl Med 4(148):148ra117. [DOI] [PMC free article] [PubMed]
- 9.Ettenberg SA, et al. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci USA. 2010;107(35):15473–15478. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y, et al. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS ONE. 2010;5(9):e12682. doi: 10.1371/journal.pone.0012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurney A, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109(29):11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5(2):100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao HX, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 14.Koo BK, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 15. Furukawa T, et al. (2011) Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 1:161. [DOI] [PMC free article] [PubMed]
- 16.Wu J, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108(52):21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brugge WR. Management and outcomes of pancreatic cystic lesions. Dig Liver Dis. 2008;40(11):854–859. doi: 10.1016/j.dld.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Matthaei H, Schulick RD, Hruban RH, Maitra A. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2011;8(3):141–150. doi: 10.1038/nrgastro.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36(4):831–849, vi. doi: 10.1016/j.gtc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 21.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 22.Scholer-Dahirel A, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci USA. 2011;108(41):17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo X, Hofmann K. The protease-associated domain: A homology domain associated with multiple classes of proteases. Trends Biochem Sci. 2001;26(3):147–148. doi: 10.1016/s0968-0004(00)01768-0. [DOI] [PubMed] [Google Scholar]
- 24.Murtaugh LC, Law AC, Dor Y, Melton DA. Beta-catenin is essential for pancreatic acinar but not islet development. Development. 2005;132(21):4663–4674. doi: 10.1242/dev.02063. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulou S, Edlund H. Attenuated Wnt signaling perturbs pancreatic growth but not pancreatic function. Diabetes. 2005;54(10):2844–2851. doi: 10.2337/diabetes.54.10.2844. [DOI] [PubMed] [Google Scholar]
- 26.Dessimoz J, Bonnard C, Huelsken J, Grapin-Botton A. Pancreas-specific deletion of beta-catenin reveals Wnt-dependent and Wnt-independent functions during development. Curr Biol. 2005;15(18):1677–1683. doi: 10.1016/j.cub.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 27. Wells JM, et al. (2007) Wnt/beta-catenin signaling is required for development of the exocrine pancreas. BMC Dev Biol 7:4. [DOI] [PMC free article] [PubMed]
- 28.Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M. Stabilization of beta-catenin impacts pancreas growth. Development. 2006;133(10):2023–2032. doi: 10.1242/dev.02366. [DOI] [PubMed] [Google Scholar]
- 29.Keefe MD, et al. β-catenin is selectively required for the expansion and regeneration of mature pancreatic acinar cells in mice. Dis Model Mech. 2012;5(4):503–514. doi: 10.1242/dmm.007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp JL, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22(6):737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11(3):291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Habbe N, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci USA. 2008;105(48):18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De La O JP, et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci USA. 2008;105(48):18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171(1):263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JP, 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10(10):683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Aynati MM, Radulovich N, Riddell RH, Tsao MS. Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia. Clin Cancer Res. 2004;10(4):1235–1240. doi: 10.1158/1078-0432.ccr-03-0087. [DOI] [PubMed] [Google Scholar]
- 37.Zeng G, et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia. 2006;8(4):279–289. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasca di Magliano M, et al. Pasca di MM et al Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS ONE. 2007;2(11):e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, et al. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009;15(3):207–219. doi: 10.1016/j.ccr.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kinde I, et al. (2013) Evaluation of DNA from the papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med 5(167):167ra4. [DOI] [PMC free article] [PubMed]
- 41.Ryland GL, et al. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. J Pathol. 2013;229(3):469–476. doi: 10.1002/path.4134. [DOI] [PubMed] [Google Scholar]
- 42.Ong CK, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44(6):690–693. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.