Significance
DNA damage challenges genome integrity. Bulky DNA lesions, which are subject to nucleotide-excision repair, induce homologous recombination (HR). However, because there is no direct generation of double-strand breaks (DSBs), the underlying mechanism has been obscure. By investigating UV-induced lesions in nonreplicating G2 cells of budding yeast, we found that translesion DNA synthesis (TLS) and HR are redundant in repair. Using a physical assay that detects recombination between circular sister chromatids, we establish that UV-induced recombination is not attributable to DSBs but instead is associated directly with expanded ssDNA gaps and is increased in cells defective in TLS.
Abstract
Repair of DNA bulky lesions often involves multiple repair pathways such as nucleotide-excision repair, translesion DNA synthesis (TLS), and homologous recombination (HR). Although there is considerable information about individual pathways, little is known about the complex interactions or extent to which damage in single strands, such as the damage generated by UV, can result in double-strand breaks (DSBs) and/or generate HR. We investigated the consequences of UV-induced lesions in nonreplicating G2 cells of budding yeast. In contrast to WT cells, there was a dramatic increase in ssDNA gaps for cells deficient in the TLS polymerases η (Rad30) and ζ (Rev3). Surprisingly, repair in TLS-deficient G2 cells required HR repair genes RAD51 and RAD52, directly revealing a redundancy of TLS and HR functions in repair of ssDNAs. Using a physical assay that detects recombination between circular sister chromatids within a few hours after UV, we show an approximate three-fold increase in recombinants in the TLS mutants over that in WT cells. The recombination, which required RAD51 and RAD52, does not appear to be caused by DSBs, because a dose of ionizing radiation producing 20 times more DSBs was much less efficient than UV in producing recombinants. Thus, in addition to revealing TLS and HR functional redundancy, we establish that UV-induced recombination in TLS mutants is not attributable to DSBs. These findings suggest that ssDNA that might originate during the repair of closely opposed lesions or of ssDNA-containing lesions or from uncoupled replication may drive recombination directly in various species, including humans.
DNA bulky lesions, such as UV-induced pyrimidine dimers and interstrand crosslinks caused by some cancer drugs, can be recognized and processed by nucleotide excision repair (NER). Because of their important links with human pathologies, including cancer, the underlying repair mechanisms and their impact on genome instability have been studied extensively (1, 2). The repair of bulky lesions is initiated by NER, a versatile repair system comprising up to ∼30 proteins/enzymes that are well conserved from yeast to mammals (3). The major steps in NER include damage recognition, followed by endonucleolytic incisions at the 5′ and 3′ sides of the lesion to remove a 25- to 30-nt oligonucleotide containing the damage, and DNA synthesis and ligation to fill the gap, thereby returning the DNA region to its original state (3, 4).
Repair of bulky lesions often is associated with homologous recombination (HR), as suggested for the removal of DNA interstrand crosslinks (5), likely accounting for subsequent genome instability. Because there is no direct generation of double-strand breaks (DSBs), the underlying mechanism of recombination, including initiation, is generally not well understood. Single-strand nicks and ssDNA regions arising during NER near replication forks could result in replication fork collapse in S-phase and lead to recombination (6, 7). However, there is relatively little information about the direct induction of recombination by bulky lesions during nonreplicating G1 or G2 stages of the cell cycle.
As we and others have reported, DSBs can be formed as secondary products during processing of ssDNA lesions arising from agents such as methyl methanesulfonate (MMS) (8-10) at doses that result in closely opposed lesions. Because NER can produce ssDNA gaps of ∼30 nt for a variety of bulky lesions, there is a greater likelihood of secondary generation of DSBs than with base-excision repair, which generates short resection regions. However, gap formation and subsequent refilling during NER are tightly coordinated, with repair synthesis starting after incision on the 5′ side of the lesion (which precedes the 3′ incision), resulting in rapid completion of repair (11, 12). This coordination might prevent the conversion of ssDNA gaps into more deleterious DSBs, thereby preserving genome stability. In the event that long gaps are produced, surveillance mechanisms including checkpoint activation (13, 14) may prevent cell-cycle progression until repair has been completed.
The repair of bulky lesions by NER poses another risk when lesions are closely opposed and the repair polymerase encounters a lesion on the template strand preventing further synthesis. In the case of DNA replication, lesions on template strands are tolerated by translesion DNA synthesis (TLS) polymerases that provide error-prone or error-free bypass of the blocking lesions (15, 16). TLS enables NER to process a variety of bulky lesions, including drug-induced DNA crosslinks (5, 17). With increases in dose, TLS could take on an even more important role in repair, because there would be increased likelihood of lesions in the template strand. In addition, the need for TLS increases with gap size, because there is increased likelihood of encountering a lesion during repair synthesis.
We have addressed the role that recombination might play in the repair of lesions produced by UV (UV-C) in nongrowing G2 cells, especially under conditions of reduced TLS, when large gaps might be expected. Using physical assays that detect gaps as well as recombinational linkage between sister chromatids that are circular (18), we establish that HR and TLS have redundant roles in maintaining chromosome stability after UV irradiation, likely because of the ability to repair ssDNA gaps containing a lesion. Unexpectedly, DSBs appear to have a minor role, at most, in the molecular changes.
Results
Lack of TLS and Recombinational Repair Greatly Increase UV Sensitivity of G2 Cells.
Two types of DNA lesions are generated by UV irradiation, cyclobutane pyrimidine dimers (CPDs) and 6-4 pyrimidine-pyrimidone products (6-4PPs) (19, 20). They are subject to NER and block replication if unrepaired. In yeast, as well as in most eukaryotes, the TLS polymerases η (Pol η) and ζ (Pol ζ) provide replication bypass, allowing duplication of the genome. The Pol η of the budding yeast Saccharomyces cerevisiae, which is encoded by the RAD30 gene, can bypass CPDs accurately (21). The TLS error-prone Pol ζ, a two-subunit enzyme encoded by REV3 (catalytic) and REV7 (accessory), can bypass a broad range of lesions including 6-4PPs (22).
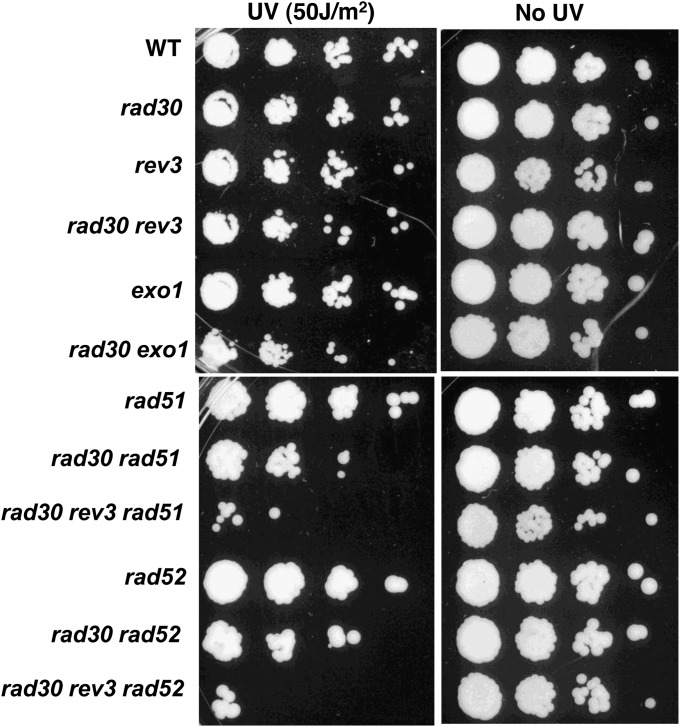
Here, we examine the response to UV irradiation of haploid budding yeast mutants deficient in TLS and recombination repair. All yeast mutants used were derived from haploid strains containing a circular form of chromosome III (Chr III) (23). We first determined survival after UV-C irradiation (50 J/m2) of cells that were nocodazole-arrested in the G2 stage of the cell cycle (>90% arrest; see Table S1). Previously, McDonald et al. (24) showed that the combination of TLS/recombination mutations greatly increased the UV sensitivity of stationary-phase cells (which were likely to be mostly in G1). As shown in Fig. 1, the absence of either or both TLS polymerases in NER-proficient cells has little effect on the sensitivity of G2 cells, indicating that a large amount of damage (∼15,000 CPDs) (25) is well tolerated in the absence of TLS. Also, there is high UV tolerance in G2 cells that are HR deficient because of a lack of either Rad52 or Rad51. However, the double TLS/recombination mutants that lack the TLS polymerase Rad30 and either Rad51 or Rad52 are ∼10-fold more UV sensitive. Loss of an additional TLS polymerase Rev3 (i.e., rev3Δ rad30Δ rad52Δ) increases the sensitivity by another ∼100-fold, demonstrating that the TLS and HR systems have an important role in preventing UV-induced killing of G2 cells even though NER is functional. These results suggest that TLS and HR are redundant in protecting G2 cells from UV irradiation. As described in the following sections, we investigated the repair processes in the G2-arrested cells and found that the repair occurred within a few hours after UV irradiation. Because UV-irradiated cells that are resuspended in medium without nocodazole remain in G2 for at least 2 h (see legend of Table S1), the resistance of cells that are at least TLS+ or HR+ in Fig. 1 is attributed to events in G2 (i.e., before cell-cycle progression).
Fig. 1.
Cell survival after UV irradiation in G2. Logarithmically growing cells were arrested in G2 with nocodazole and were treated with UV-C (50 J/m2) in ice-cold water. UV-irradiated and mock-treated controls then were plated to YPDA medium in serial 10-fold dilutions. Survival was determined after 2 d of growth.
TLS Deficiency Leads to Accumulation of ssDNA Gaps and Reduced Chromosome Repair After UV Irradiation.
To address the roles that TLS and HR play in the toleration of UV-induced damage in nocodazole-arrested G2 cells, we followed whole-chromosome changes using pulsed-field gel electrophoresis (PFGE), which can display the yeast karyotype that extends from ∼200 kb to ∼2,200 kb. Beginning shortly after exposure to 50 J/m2, there were reductions in the intensity of chromosome bands that were size dependent—the bigger the chromosome, the greater the reduction (Fig. 2A). During the subsequent incubation of cells in rich medium (YPDA) containing nocodazole, the intensities decreased even further over the first hour. This trend reversed over the next hours with nearly complete restitution by 4 h after irradiation. Interestingly, although there was a decrease in chromosome bands, there was no apparent fragmentation based on a lack of “smearing” below each band, which would be expected with random DSB breaks resulting from ionizing radiation (Fig. 2C). Instead, slow-moving DNA (SMD) appeared just below the well but disappeared almost completely by 4 h after UV irradiation. The SMD had been observed earlier by Giannattasio et al. (14) in yeast cells that were UV-irradiated in G1, but in their study there was no reduction in the SMD over the course of the study and nor was there any restitution of chromosomes (comparable results were obtained with G1 cells of the strains used in the present study). We also observed SMD during the repair of MMS lesions in apurinic/apyrimidinic AP endonuclease-deficient yeast G2 cells (9). SMD was proposed to result from the formation of repair intermediates containing long ssDNA regions as previously was established for G1 cells (14).
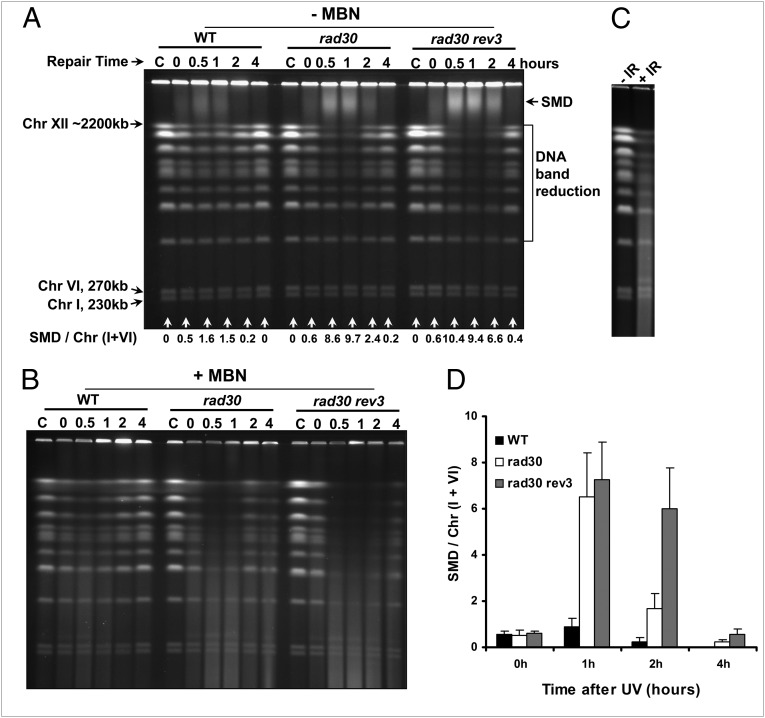
Fig. 2.
TLS deficiency leads to the accumulation of ssDNA gaps and reduced repair efficiency. G2-arrested WT cells and TLS haploid mutants were treated with 50 J/m2 UV and were incubated in YPDA with nocodazole for up to 4 h (30 °C). Cells were collected to make DNA plugs and then were subjected to no MBN treatment (−MBN) (A) or to MBN treatment (+MBN) (B). (C) G2 cells were irradiated with 800 Gy ionizing radiation (+IR) or were not irradiated (−IR). (D) Quantitation of SMD. After PFGE, chromosomes were visualized by ethidium bromide staining. In all figures, the letter “C” above the first lane stands for mock-treated control samples. The SMD is detected as a wide band of DNA between the largest chromosome (Chr XII) and the wells. The relative amounts of SMD were quantified using the ratio of the amount of material in the SMD region to the DNA in the small chromosomes that experienced little, if any, loss and are shown as “SMD/Chr I+VI”. Values shown in D are mean ± SD calculated from four independent experiments. Results shown in A and B are representative of multiple experiments.
The amount of SMD was increased in cells lacking RAD30 (Fig. 2A) as well as in cells lacking the TLS Pol ζ (Rev3; Fig. S1). We note that SMD can vary depending on the amount that is retained in the well. However, the differences between WT cells and TLS mutants become clearer when examining the relative loss of chromosomes, and the difference is especially evident for the larger chromosomes (Fig. 2A and Fig. S1). Because there was little, if any, loss of the smallest chromosomes, Chr I and Chr VI, it was possible to quantitate the relative amount of SMD across experiments by determining the ratio of SMD/(Chr I+VI). There was a consistent and statistical difference between WT cells and TLS mutants (Fig. 2D). The chromosome bands were restituted gradually with time but with differing efficiency, more slowly in rad30Δ and rev3Δ mutants than in WT cells. In rad30Δ rev3Δ mutants, the chromosome restitution was clearly delayed further (∼2 h; Fig. 2 A and D), demonstrating a quantitative impact of the TLS polymerases. Although rad30Δ rev3Δ mutants had additional delay, there was restitution of the chromosome bands by 4 h.
Previous studies suggested that SMD appearing after UV irradiation or MMS treatment likely results from long ssDNA formed during NER and possibly from recombination intermediates (9, 14). To establish the presence of ssDNA, we treated the agarose plugs containing chromosomal DNA with mung bean nuclease (MBN), which can degrade ssDNA tails and gaps and cleave hairpin loops (described in ref. 18). Treatment with MBN eliminated SMD and resulted in the appearance of fragmented chromosomes (Fig. 2B) as found after exposure to ionizing radiation (Fig. 2C). These findings were similar to those in the study of Giannattasio et al. (14), who used S1 nuclease in G1 cells. Unlike S1 nuclease, which also acts on ssDNA nicks (26, 27), MBN is specific to ssDNA tails and gaps. Previously, we had addressed resection at random DSBs and found that duplex DNA with resected ssDNA tails of a few hundred to thousands of bases also exhibited retarded mobility on PFGE (referred to as “PFGE-shift”) with a maximum apparent increase in molecular weight of 150 kb (18, 28). However, there was no evidence of SMD or trapping of DNA in the well. Therefore, we concluded that the SMD is the result of DNA repair intermediates containing ssDNA gaps.
As shown in Fig. 2B, the MBN cutting had the greatest effect on samples that had large amounts of SMD and had no effect on chromosomal DNA from unirradiated cells. Here, we also show that the restituted chromosomes from WT cells at 4 h were not sensitive to MBN, suggesting complete repair of ssDNA gaps, unlike G1 cells (14). Consistent with a greater amount of SMD, the MBN revealed much more gapped DNA in the rad30Δ and rad30Δ rev3Δ mutants during post-UV incubation. However, the restituted chromosomes at 4 h were less subject to MBN degradation, demonstrating that the gapped regions also could be repaired in the TLS mutants. The repair appeared to be slower than in WT cells, as reflected by the somewhat greater MBN degradation of the DNA from the TLS mutants at 2 and 4 h. This difference is consistent with the comparable survivals of WT cells and the double-TLS mutants shown in Fig. 1.
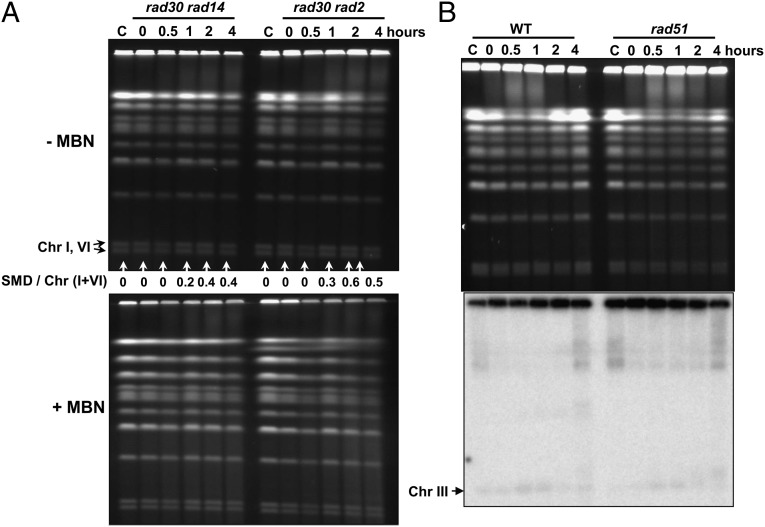
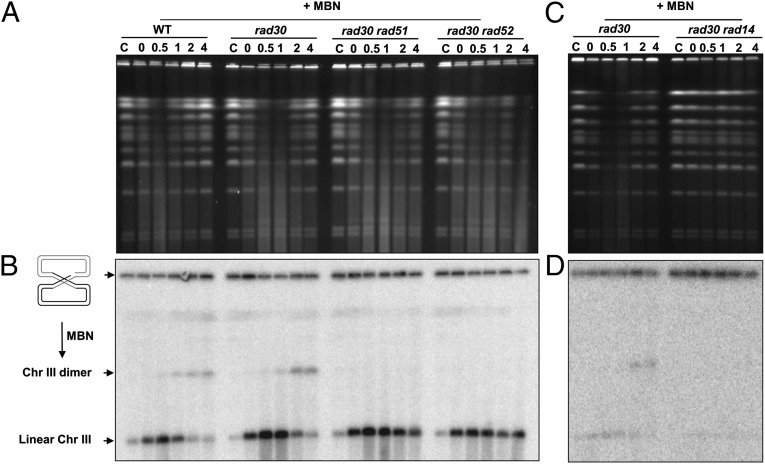
Because NER can repair most UV-induced damage, we asked what role it might play in SMD formation in the irradiated G2 cells. We created rad14Δ rad30Δ or rad2Δ rad30Δ double mutants that were deficient in NER. Rad14 is a homolog of human XPA protein, which forms a complex with ssDNA endonucleases Rad1 and Rad10 and nicks the damaged DNA strand on the 5′ side of a lesion (29, 30), whereas Rad2, a homolog of human XPG, leads to incision at the 3′ side (31). Comparing the results in Fig. 2 and Fig. 3A, we conclude that SMD formation is downstream of NER. Furthermore, the chromosomal DNA from the double mutants, unlike that from the rad30 single mutant, was not sensitive to MBN (Fig. 3A). These findings also suggest that there are no other mechanisms for the generation of gaps after UV treatment of the G2 cells in the absence of NER.
Fig. 3.
SMD was prevented by blocking NER but was not affected by HR deficiency. G2/M-arrested WT cells and rad30Δ rad14Δ, rad30Δ rad2Δ, and rad51Δ mutants were treated with 50 J/m2 UV and were incubated in YPDA with nocodazole for up to 4 h (30 °C). Cells were collected to make DNA plugs. (A) After PFGE of DNA with or without MBN treatment, chromosomes were visualized by ethidium bromide staining. (B) After PFGE, chromosomes were visualized by ethidium bromide staining and Southern blot with a probe that identifies Chr III. Results shown in A and B are representative of multiple experiments.
HR Is Required to Repair UV Lesions in G2 Cells Lacking TLS.
The finding that chromosomes still can be restituted after the appearance of SMD in the absence of TLS suggests an alternative efficient repair system(s) for dealing with the ssDNA, and such repair systems might explain the UV resistance of the TLS mutants (Fig. 1). Because deletion of either of the TLS genes REV3 or RAD30 or the HR genes RAD52 or RAD51 did not markedly increase the sensitivity of G2 cells, but simultaneous inactivation of both pathways did, we investigated the contribution of HR to the appearance of SMD and the restitution of chromosomes.
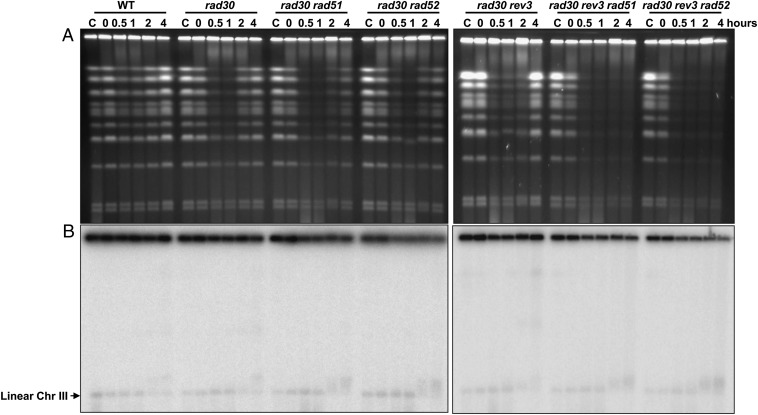
As shown in Fig. 4A, UV irradiation of double mutants defective for a TLS gene and an HR gene (i.e., rad30Δ rad51Δ or rad30Δ rad52Δ) results in the accumulation of SMD and eventual restitution of the chromosomes as seen in single mutants, although restitution may be somewhat delayed. (Deletion of RAD51 alone had no apparent effect on chromosome disappearance and restitution when compared with WT cells, as shown in Fig. 3B.) A complete blockage of TLS and HR in the rad30Δ rev3Δ rad51Δ triple mutant also did not prevent the accumulation of SMD (Fig. S2). However, the restitution of full-size chromosomes was completely blocked (Fig. 4 and Fig. S2). The disappearance of the chromosomes in the triple mutants may be caused by chromosomes being trapped in the well, because MBN treatment generated fragmented chromosomes and a linear Chr III band (see ethidium bromide-stained and probed gels in Fig. S2). Also, if DNA is retained in the well, the amount of SMD appearing in the gel (ethidium bromide staining) would not reflect all the SMD, thereby possibly explaining the apparently similar amounts of SMD in the rad30Δ rev3Δ rad51Δ and rad30Δ rev3Δ rad52Δ mutants and the TLS single mutants. Overall, these data suggest that HR is not involved in the generation of SMD but plays a critical role in the downstream repair. HR is indispensable only when both TLS polymerases are absent. The findings with the UV-irradiated G2 cells are consistent with the greatly increased sensitivity of the triple mutants described in Fig. 1.
Fig. 4.
The redundancy of TLS and HR in repairing UV damage in G2. G2-arrested WT cells and HR and TLS haploid mutants were treated with 50 J/m2 UV and were incubated in YPDA with nocodazole for up to 4 h (30 °C). Cells were collected at the indicated times and processed for PFGE analysis. Chromosomes were visualized by ethidium bromide staining (A) and by Southern blotting (B) with a probe that identifies Chr III. Results shown are representative of multiple experiments.
Compromised TLS Activity Leads to Increased Recombinant Molecules After UV Irradiation.
The requirement for HR in the absence of TLS could be caused by the generation of DSBs, possibly through overlapping repair regions as previously suggested (32, 33) or somehow during the generation of the ssDNAs. Using our previously described circular chromosome assay that can detect random DSBs efficiently at frequencies of only a few DSBs per yeast genome (18, 23), we investigated whether TLS deficiency leads to increased DSBs during the processing of UV damage. In this assay, a circular Chr III, which is ∼300 kb, is retained in the well during PFGE. A random DSB will generate a linear chromosome that enters the gel and forms a distinct band during PFGE.
As shown in Fig. 4, there was at most only a small induction of DSBs following UV exposure of WT cells, based on the limited increase in linear Chr III molecules. The amounts of linear Chr III bands were similar to those in the rad30Δ, rad30Δ rad51Δ and rad30Δ rad52Δ mutants. Because DSBs are expected to accumulate in the HR mutants, these results suggest that there is little generation of DSBs. Previously, we had estimated that the background spontaneous level of DSBs in nongrowing cells is around one or two DSBs per yeast genome (23). Based on a two- to three-fold increase, at most, in band density after UV irradiation (Fig. 4, the 0.5- and 1-h lanes) over the background density (Fig. 4, lane “C”), the transient formation of DSBs resulting from exposure to 50 J/m2 UV is at most two to six DSBs per yeast genome. Given the experimental variation, there appears to be little development of DSBs from UV-induced lesions.
When the DNA plugs from UV-irradiated cells were treated with MBN, however, a large increase in the linear Chr III band was detected, especially at the 0.5- and 1-h time points (Fig. 5), corresponding to the time of the maximum appearance of SMD molecules (Fig. 4A). This increase provides further evidence that many of the circular molecules contained ssDNA gaps. After incubation, the ChrIII band densities of the WT and rad30Δ cells returned to the levels seen in unirradiated cells. However, some DSBs remained in the rad30Δ rad51Δ and rad30Δ rad52Δ strains (Fig. 4), as is consistent with the reduced levels of chromosome restitution at 2 and 4 h seen on ethidium bromide-stained gels (Fig. 4 and Fig. S2).
Fig. 5.
Compromised TLS function leads to the generation of recombinants. G2-arrested WT cells and HR, TLS, and NER haploid mutants were treated with 50 J/m2 UV and were incubated in YPDA with nocodazole for up to 4 h (30 °C). Cells then were collected to make DNA plugs and were treated with MBN. Chromosomes were visualized by ethidium bromide staining (A and C) and by Southern blotting (B and D) with a CHA1 probe that identifies Chr III. Linear Chr III and Chr III dimers (as described in ref. 18) are indicated. Results shown are representative of multiple experiments.
ssDNA has long been suspected to be a component in recombination (models are summarized in ref. 34). The Chr III and PFGE approach we used here also provides an opportunity to detect recombination between sister chromatids. In our previous study we had detected the appearance of a double-sized chromosome III (Chr III dimer) band on PFGE in G2 cells exposed to ionizing radiation (18). This Chr III dimer can be formed only when two sister chromatids combine with each other and thus provides an unambiguous way to detect interchromosome recombination. As shown in Fig. 4, a similar dimer band was not apparent after UV irradiation. However, treatment of the DNA sample with MBN revealed the presence of dimer molecules (Fig. 5 and Fig. S2). Because MBN cuts at ssDNA gaps, these molecules must have come from circular Chr III dimers or the intermediates of recombination between the Chr III sister chromatids The recombinant product was clearly detectable by 1 h after irradiation of G2 WT cells, reaching a maximum 2–4 h postirradiation. Although the absolute number of Chr III dimers could not be determined, the relative difference in dimer bands across the same gels could be compared based on band density. (Sample loading was comparable, as determined by the chromosome bands in the ethidium bromide-stained gels of the smallest chromosomes, Chr I and VI, which experienced little change during incubation, as shown in Fig. 5 and Fig. S2.) We found that by 2 h after irradiation the rad30Δ cells had approximately three times more Chr III dimers than the WT cells (Fig. S2). The amount of dimer molecules appears to be related to the amount of ssDNA. The Chr III linear band generated after MBN treatment was approximately two- to three-fold greater for the rad30Δ cells than for the WT cells. Formation of the Chr III dimer was detected after SMD began to appear. It is possible that completed recombinants lacking ssDNA gaps are generated also, but these molecules would remain in the well after MBN treatment. Because there was no dimer band in the rad30Δ rad51Δ and rad30Δ rad52Δ double mutants, the generation of recombinants requires RAD52 and RAD51.
Overall, these results establish that recombinant molecules are generated during incubation after UV treatment of G2 cells and that reduced TLS leads to more recombinants. We note that for the rad30 rev3 double TLS mutant there was no apparent increase of Chr III dimer over that in rad30 (Fig. S2). Possibly there is an adverse effect of rev3 deletion or the loss of both TLS polymerases on the recombination process (35–37).
Recombinant Molecules Do Not Correlate with DSBs.
The increase in recombinant molecules following MBN treatment (Fig. 5B) did not appear to correlate with the few DSBs (Fig. 4B) observed with the WT cells or the TLS mutants, which had more ssDNA gaps. However, the recombination required NER, as shown by the absence of Chr III dimers after MBN treatment of the UV-irradiated rad14Δ rad30Δ and rad2Δ rad30Δ double mutants (Fig. 5D). These results could be explained by a recombination mechanism initiated by ssDNA gaps or even by nicks rather than DSBs.
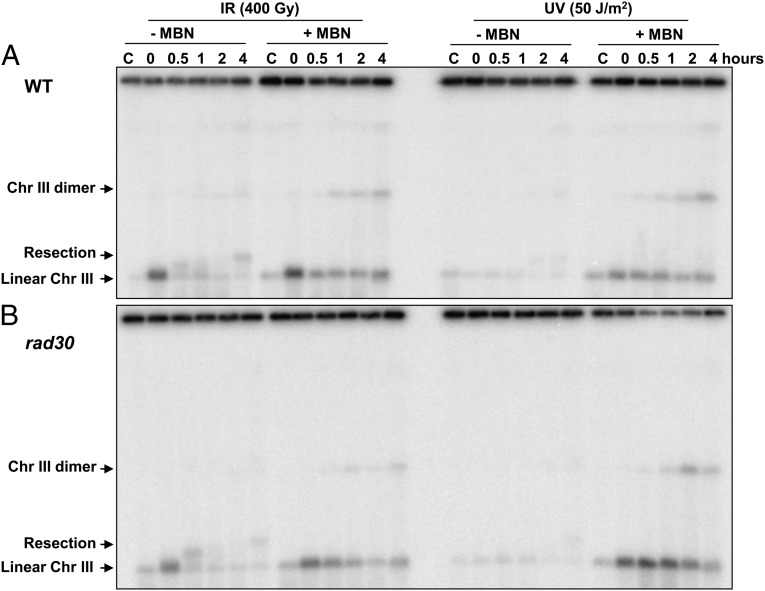
To address the relationship between DSB induction and recombination, we compared the efficiency of DSB induction with the formation of recombinants after exposure of the G2 cells to ionizing radiation and UV. The WT and rad30Δ G2 cells were irradiated with 400 Gy or 50 J/m2. After irradiation and incubation, samples were split and treated with MBN to determine the gapped molecules generated in vivo and the generation of Chr III dimer molecules. As shown in Fig. 6, a large number of DSBs generated by ionizing radiation subsequently were lost through repair. In these experiments there was only a small increase in broken molecules during post-UV incubation. However, despite a greater than 20-fold difference in DSBs between cells exposed to ionizing radiation and UV-irradiated cells, there were comparable amounts of recombinant molecules in the WT cells. For the rad30Δ cells, there were nearly three-fold more recombinants after UV irradiation than after exposure to ionizing radiation. The time frame for the generation of recombinants by exposure to ionizing radiation and UV irradiation was comparable despite the immediate formation of DSBs in the cells exposed to ionizing radiation. If DSBs were the source of recombinant molecules, there should be far fewer recombinants after UV irradiation, contrary to our observations. Thus, DSBs do not seem to be the source of recombinant molecules.
Fig. 6.
Comparison between ionizing radiation and UV for the induction of DSB and generation of recombinants. G2-arrested WT cells (A) and rad30 (B) haploid mutants were treated either with 400 Gy ionizing radiation (IR, Left) or 50 J/m2UV (Right) and then were incubated in YPDA with nocodazole for up to 4 h (30 °C). Cells were collected to make DNA plugs and were processed further with or without MBN treatment, followed by PFGE and Southern blotting to detect Chr III linear, resected linear, and recombinant dimer molecules, as indicated.
UV-Induced Recombination Requires Gap Expansion.
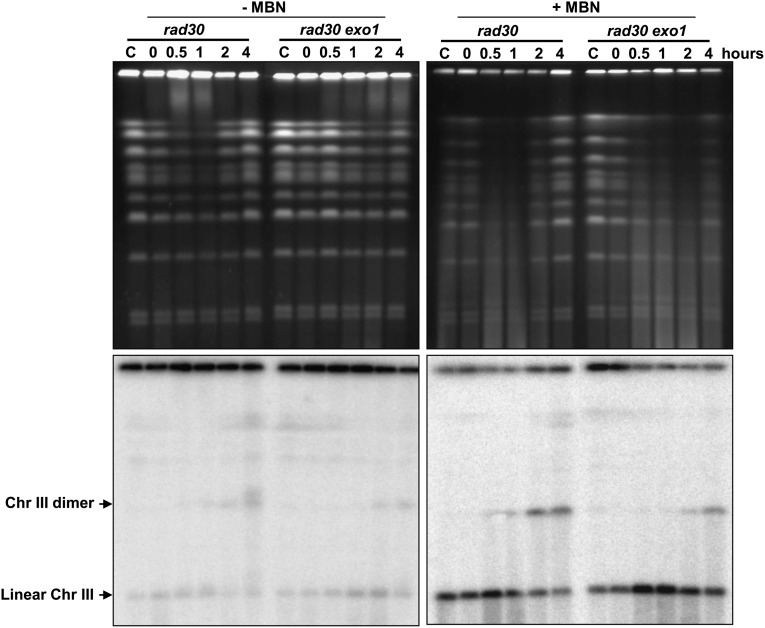
Recently, Giannattasio et al. (14) showed that the ssDNA gaps formed after UV irradiation in G1 yeast cells could be expanded by Exo1 to up to 500 nt, and the resulting ssDNA led to checkpoint activation. We deleted EXO1 in a rad30Δ mutant to examine whether the generation of Chr III dimers after UV was dependent on large stretches of ssDNA. As shown in Fig. 7, there was a nearly 2-h delay in the appearance of ssDNA based on the formation of SMD in cells not treated with MBN. Similarly, the appearance of Chr III recombinants was delayed in cells treated with MBN and there were fewer recombinant molecules in the rad30Δ exo1Δ strain than in the rad30Δ strain. These results indicate that recombination after UV exposure requires gap expansion to long ssDNA from the initial NER excision tract of ∼30 nt and is provided largely by Exo1.
Fig. 7.
exo1 delays the appearance of both SMD and Chr III dimer. G2-arrested rad30 and rad30 exo1 haploid mutants were treated with UV (50 J/m2) and were incubated in YPDA with nocodazole for up to 4 h (30 °C). Cells then were collected to make DNA plugs and were processed with or without MBN treatment. Chromosomes were visualized by ethidium bromide staining and by Southern blotting with a CHA1 probe that identifies Chr III linear, resected, and recombinant dimer molecules, as indicated.
Discussion
ssDNA gaps are potentially genome destabilizing. If unrepaired, they can lead to DSBs during subsequent replication. In addition, ssDNA is more susceptible to mutagens because there is no complementary strand to template the correction of lesions (38). Here, we show that the combined three systems available in most eukaryotes to provide toleration of bulky lesions—NER, TLS, and HR—prevent and repair ssDNA that can arise in G2 cells. The HR-mediated repair appears to act directly on the ssDNA and, unexpectedly, not through DSB intermediates. The repair of the ssDNAs would allow cells to proceed into the next cell cycle. In the absence of ssDNA repair, DSBs can appear in the subsequent round of replication, which may explain the UV sensitivity of TLS/HR double mutants irradiated in G2.
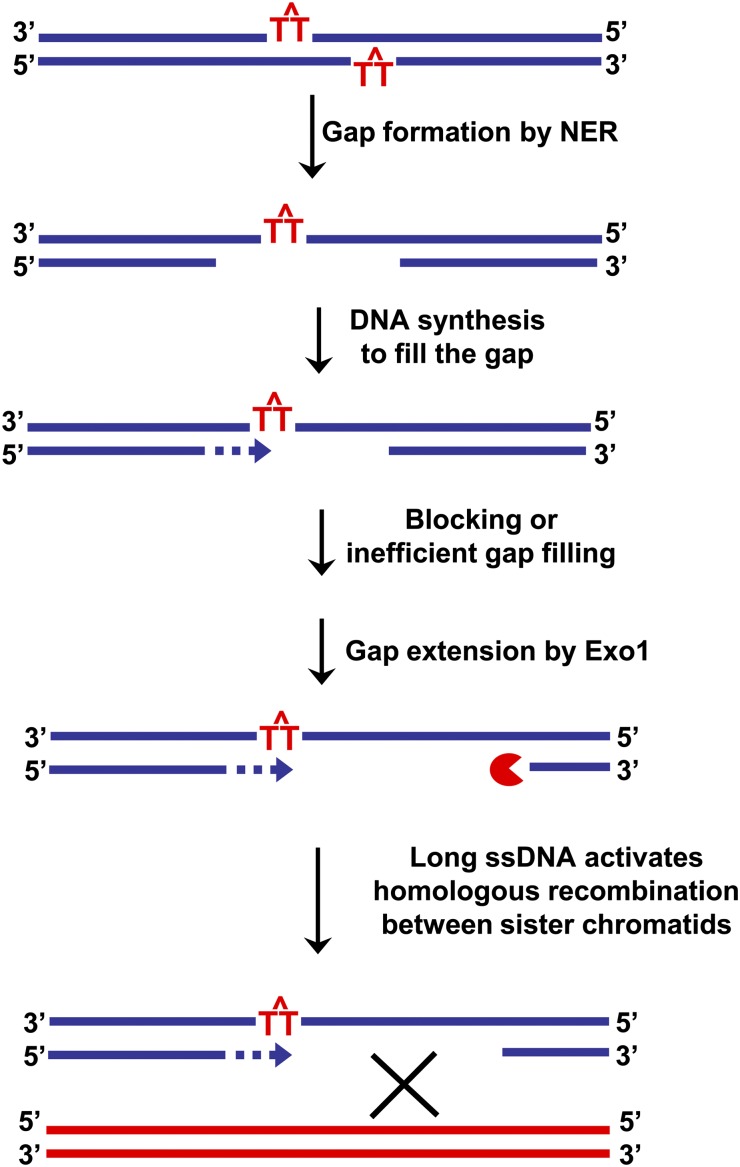
The filling of the excised region during NER is highly coordinated with the excision steps so that normally there are only short-lived small ssDNA intermediates (11). In bacteria, the damaged oligonucleotide is removed by the UvrD helicase just before or during the repair synthesis step by DNA polymerase I (39). The accumulation of ssDNA in TLS mutants after UV irradiation of G2 cells could result from closely opposed lesions. After NER of one of the lesions, the subsequent repair synthesis would encounter the second lesion, which could be bypassed by TLS polymerases (described in Fig. 8). Consistent with this result, the error-prone Y family DNA polymerase Pol κ has been shown to have an important role in NER for repair synthesis in mammalian cells (40, 41). Apart from its other functions, this polymerase might be used to deal with closely opposed DNA lesions. The absence of TLS may result in small gaps being enlarged by Exo1, leading to SMD, as shown previously for cells arrested in G1 (14) and also found for G2 cells in this study. In support of this view, we have observed a dramatic difference between WT cells and TLS mutants including (i) elevated formation of SMD in rad30 or rev3 mutants along with more SMD in the double mutant and (ii) a greater amount of linear Chr III band in TLS mutants after in vitro MBN treatment that converts gaps into DSBs. Even when TLS is functioning in WT cells, there is a small amount of SMD. This SMD may be caused by insufficient TLS proteins, by some types of lesions not being efficiently bypassed, or, occasionally, by efficient Exo1-mediated gap expansion before TLS recruitment. In any case, we have shown that SMD results from long ssDNA gaps (or from some sort of downstream repair intermediates) in UV-irradiated G2 cells and that the formation of SMD is not Rad51 or Rad52 dependent. A related gap-expansion phenomenon also was observed in bacteria. Earlier studies showed that, although the majority of UV lesions in Escherichia coli result in short repair patches of ∼20–30 nt, a small proportion leads to long patches of up to several hundred nucleotides (42). In addition, mutants deficient in polymerase I or its 5′ exonuclease activity led to more long patches that were proposed to be due to gap expansion by a 3′ exonuclease and resynthesis by DNA polymerases II or III (42, 43). The polymerases II and III had been shown to generate longer patches than polymerase I (44).
Fig. 8.
Model for ssDNA gap induction of recombination. After UV irradiation, the processing of closely opposed lesions by NER could generate a gap opposite a UV lesion, which is normally tolerated by TLS. In the absence of TLS or when the gap is not efficiently refilled, it is subject to gap expansion by Exo1. The resulting gap or the stalled nascent strand can lead to recombinational interactions, including strand invasion to generate recombinant intermediates. The homologous sequence in the sister chromatid serves as a template allowing the gap opposite the lesion to be filled.
Based on an earlier study, irradiation with 50 J/m2 UV-C (254 nm) would yield ∼0.5 pyrimidine dimers/kb (25). If NER generates ∼30-nt excised regions (3) that are distributed randomly, approximately 0.004 closely opposed lesions (within 30 nt of each other) would be expected per kilobase. The frequency actually may be larger, because the appearance of closely opposed lesions does not follow dose-squared kinetics, at least at lower doses (32). Thus, yeast chromosomes with a length of around 300 kb would contain an average of approximately one pair of closely opposed lesions that might be expected to require damage bypass during repair. This estimate is reflected in our PFGE results, in which the disappearance of chromosomes was size dependent; irradiation with 50 J/m2 UV led to no apparent loss of the smallest chromosomes, Chr I and VI (∼200 kb each). Our findings with Chr III suggest that there is little conversion of closely opposed lesions into DSBs, possibly indicating repair interference, unlike the situation in closely opposed MMS-induced lesions (8).
In this study, we establish that HR can repair the ssDNA gaps that arise in G2 cells when TLS is defective, thereby acting as a back-up in gap repair. Contrary to findings in G1 cells, which lack the opportunity for recombinational repair (14), in the G2 cells we found efficient loss of SMD at later times, restitution of chromosomes, the disappearance of MBN-sensitive sites, and high survival rates, all of which required Rad52 and Rad51. Although recombination repair can restore collapsed replication forks (45, 46), there is little direct evidence that HR directly processes and repairs replication-blocking lesions located on ssDNA. Using a plasmid-based assay, Adar et al. (47) showed that gaps opposite an abasic site or a benzo[a]pyrene-guanine adduct could be repaired by HR in mammalian cells. However, it was not clear whether the lesions on the plasmid were direct substrates for recombination repair or were converted into other types of HR-responsive lesions in vivo. In addition, it is not known whether such lesion-containing gaps were reparable only during S phase or could be processed in nonreplicating stages.
The repair of ssDNA gaps in G2 cells appeared to occur through an HR mechanism that is independent of DSB formation. Lesions other than DSBs, including ssDNA breaks (SSBs), have been proposed as initiators of recombination in many early models of recombination (34). More recently, an SSB was shown to initiate gene conversion to generate mating-type switching in fission yeast (48). Also, recombination-activating gene (RAG)–mediated nicks efficiently stimulate HR during V(D)J recombination (49). In other studies using enzymes that generate site-specific nicks, SSBs could promote recombination (50, 51). However, in many of these studies it was not clear whether HR was initiated by SSBs per se or by subsequent formation of DSBs.
Using an assay that directly measures recombination by the appearance of Chr III dimer molecules from sister chromatids, we have concluded that ssDNA can initiate HR in nonreplicating G2 cells. First, the formation of Chr III dimers required Rad51/52-mediated HR. Second, there was no correlation between the number of DSBs and the appearance of Chr III dimers. Many more chromosome dimers were produced with exposure to UV than by exposure to ionizing radiation, although ionizing radiation produced vastly more DSBs. In addition, there is no correlation between the timing of DSB induction and Chr III dimer formation. Although ionizing radiation directly induces DSBs, followed within several minutes by repair-related resection, Chr III recombinant molecules appeared at about the same time after gamma and UV irradiation (∼1 h; Fig. 6). That ssDNA is required for at least the initiation of recombination comes from the observations that both NER and ExoI are needed for the generation of SMD as well as recombinants and that deficiencies in TLS, which greatly increase SMD, lead to corresponding increases in recombinant molecules.
Although the mechanism by which an ssDNA gap could trigger sister chromatid recombination remains to be determined, the ssDNA gaps directly formed by the NER excision step are insufficient to activate HR. Deletion of EXO1 greatly reduced the amount of ssDNA as well as recombinant molecules (Fig. 7). Exo1 is a structure-specific nuclease possessing both 5′–3′ exonuclease and 5′-flap endonuclease activity and is involved in a variety of DNA metabolic processes (52). Previous results with G1 cells demonstrated that Exo1-extended gaps can lead to the activation of a cellular checkpoint (14). Possibly, additional events following Exo1-dependent checkpoint activation also can trigger HR repair. Also, the expanded gaps might participate directly in a pathway that initiates recombination, similar to the recombinational role that resection plays at DSBs.
Fig. 8 presents a model that summarizes our findings and describes how ssDNA gaps could be channeled to HR for repair when TLS is defective. Although we have established several of the parameters required for the generation of recombination, the mechanisms of interactions between sister chromatids and the actual exchange events remain to be determined. After ssDNA formation, subsequent step(s) in recombination require homology search and might include pairing of the ssDNA with the homologous double-strand region of the sister chromatid or localized unwinding of the blocked nascent strand and homology search as has been suggested for template switching at uncoupled replication forks (53). Regardless of the initiating scenario, the subsequent events are expected to include strand invasion and synthesis from the sister chromatid DNA. Given our recent findings that sister chromatid cohesion has a role in preventing DSB-induced recombination between homologous chromosomes (54), it would be interesting to determine whether this cohesion effect extends to ssDNA.
Although the current study used recovery after UV irradiation as a model for repair of bulky lesions and recombination, the mechanism for the generation of ssDNA and the efficient recombinational repair of gaps likely is the same for a variety of bulky lesions. These findings, which are expected to expand our understanding of mechanisms of HR and the complementary relationship between TLS and HR, have direct implications for genome stability in other eukaryotes, including humans.
Materials and Methods
Yeast Strains.
Yeast strains used in this study are derivatives of two isogenic haploid strains, MWJ49 and MWJ50 (MATα leu2-3,112 ade5-1 his7-2 ura3Δ trp1-289), which contain a circular form of Chr III (23). The deletion mutants rad30, rev3, rad50, rad51, rad52, exo1, and their derived double or triple mutants were created by replacing the relevant ORF in these strains with selectable markers as previously described (55).
UV and Ionizing Irradiation.
Haploid yeast cells were synchronized in G2 with nocodazole (15 μg/mL) as described in ref. 9; the extent of arrest is described in Table S1. For UV irradiation, cells in ice-cold water were exposed to 50 J/m2 UV-C [254 nm, 0.85 J/(m2⋅s)] using cold cathode model 782L10 lamps from American Ultraviolet C). Post-UV incubation in nocodazole is described in Table S1. For ionizing irradiation, cells kept on ice were irradiated in a 137Cs irradiator (J. L. Shepherd Model 431) at a dose rate of 2.3 krad/min to a total dose of 400 Gy. After irradiation, cells were returned to YPDA medium [1% yeast extract, 2% (wt/vol) Bacto-Peptone, 2% (wt/vol) dextrose, 60 mg/mL adenine sulfate] containing nocodazole (G2) and were incubated for up to 4 h (30°C). Cells were collected at various times before and after irradiation for analysis by PFGE and Southern blot probing.
PFGE Analysis.
Detection of UV-induced damage and repair were based on PFGE analysis as previously described (23). Briefly, control and UV-treated cells were mixed with low-melting agarose [2% (wt/vol) stock in Tris-EDTA buffer, final concentration 0.6%] containing 1 mg/mL Zymolyase (100 U/mg) (MP Biochemicals) to prepare agarose-embedded DNA plugs. The cells in the plugs then were incubated with a “spheroplasting” solution [1 M sorbitol, 20 mM EDTA, 10 mM Tris (pH 7.5)] for 2 h followed by digestion with proteinase K [10 mM Tris (pH 8.0), 100 mM EDTA, 1.0% N-lauroylsarcosine, 0.2% sodium deoxycholate, 1 mg/mL proteinase K] for 24 h at 30 °C. Yeast chromosome DNA was separated by PFGE using a Bio-Rad CHEF-Mapper XA system (Bio-Rad) on 1% agarose gel (6 V/cm for 24 h at 14 °C, 10–90 s ramp and 120° switching angle). Gels were stained with ethidium bromide, and Southern blot analysis was carried out with a probe specific for Chr III. Autoradiographs were digitized and densitometric analysis was performed using Kodak MI software (version 5.0). Comparison of the relative chromosome band densities of different mutants was done within the same Southern blot. The comparable amounts of DNA loading were judged based on ethidium bromide staining. Note: Comparisons between different blots are not accurate because of variations in Southern transfer and radioactive signals. The PFGE results that are presented in the figures are representative of at least two gels.
MBN Digestion.
MBN (Promega) digestion of DNA in agarose plugs was used to cut/degrade ssDNA regions formed during the repair of UV-induced damage. A 50-μL plug slice was equilibrated at room temperature in 150 μL of TE [10 mM Tris (pH 7.4), 1 mM EDTA], followed by 30-min incubation with 80 units/mL MBN (controls were incubated in reaction buffer without enzyme) and then equilibration with ice-cold Tris-EDTA [10 mM Tris, 50 mM EDTA (pH 8.0)] to stop the reaction. PFGE Southern blot analysis was performed as described above.
Supplementary Material
Acknowledgments
We thank Drs. Julie Horton, Dmitry Gordenin, Shay Covo, and Kin Chan for critical reading of the manuscript and suggestions. This work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences (National Institutes of Health, Department of Health and Human Services) under Project 1 Z01 ES065073 (to M.A.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301676110/-/DCSupplemental.
References
- 1.Kuper J, Kisker C. Damage recognition in nucleotide excision DNA repair. Curr Opin Struct Biol. 2012;22(1):88–93. doi: 10.1016/j.sbi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Nouspikel T. DNA repair in mammalian cells: Nucleotide excision repair: Variations on versatility. Cell Mol Life Sci. 2009;66(6):994–1009. doi: 10.1007/s00018-009-8737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanawalt PC. Subpathways of nucleotide excision repair and their regulation. Oncogene. 2002;21(58):8949–8956. doi: 10.1038/sj.onc.1206096. [DOI] [PubMed] [Google Scholar]
- 4.Mocquet V, et al. Sequential recruitment of the repair factors during NER: The role of XPG in initiating the resynthesis step. EMBO J. 2008;27(1):155–167. doi: 10.1038/sj.emboj.7601948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muniandy PA, Liu J, Majumdar A, Liu ST, Seidman MM. DNA interstrand crosslink repair in mammalian cells: Step by step. Crit Rev Biochem Mol Biol. 2010;45(1):23–49. doi: 10.3109/10409230903501819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriel-Carretero M, Aguilera A. Replication fork breakage and re-start: New insights into Rad3/XPD-associated deficiencies. Cell Cycle. 2010;9(15):2958–2962. [PubMed] [Google Scholar]
- 7.Eppink B, et al. The response of mammalian cells to UV-light reveals Rad54-dependent and independent pathways of homologous recombination. DNA Repair (Amst) 2011;10(11):1095–1105. doi: 10.1016/j.dnarep.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Ma W, et al. The transition of closely opposed lesions to double-strand breaks during long-patch base excision repair is prevented by the coordinated action of DNA polymerase delta and Rad27/Fen1. Mol Cell Biol. 2009;29(5):1212–1221. doi: 10.1128/MCB.01499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma W, Westmoreland JW, Gordenin DA, Resnick MA. Alkylation base damage is converted into repairable double-strand breaks and complex intermediates in G2 cells lacking AP endonuclease. PLoS Genet. 2011;7(4):e1002059. doi: 10.1371/journal.pgen.1002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malyarchuk S, Castore R, Harrison L. DNA repair of clustered lesions in mammalian cells: Involvement of non-homologous end-joining. Nucleic Acids Res. 2008;36(15):4872–4882. doi: 10.1093/nar/gkn450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staresincic L, et al. Coordination of dual incision and repair synthesis in human nucleotide excision repair. EMBO J. 2009;28(8):1111–1120. doi: 10.1038/emboj.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luijsterburg MS, et al. Stochastic and reversible assembly of a multiprotein DNA repair complex ensures accurate target site recognition and efficient repair. J Cell Biol. 2010;189(3):445–463. doi: 10.1083/jcb.200909175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sertic S, Pizzi S, Lazzaro F, Plevani P, Muzi-Falconi M. NER and DDR: Classical music with new instruments. Cell Cycle. 2012;11(4):668–674. doi: 10.4161/cc.11.4.19117. [DOI] [PubMed] [Google Scholar]
- 14.Giannattasio M, et al. Exo1 competes with repair synthesis, converts NER intermediates to long ssDNA gaps, and promotes checkpoint activation. Mol Cell. 2010;40(1):50–62. doi: 10.1016/j.molcel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Woodgate R. What a difference a decade makes: Insights into translesion DNA synthesis. Proc Natl Acad Sci USA. 2007;104(40):15591–15598. doi: 10.1073/pnas.0704219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 17.Beljanski V, Marzilli LG, Doetsch PW. DNA damage-processing pathways involved in the eukaryotic cellular response to anticancer DNA cross-linking drugs. Mol Pharmacol. 2004;65(6):1496–1506. doi: 10.1124/mol.65.6.1496. [DOI] [PubMed] [Google Scholar]
- 18.Westmoreland J, et al. RAD50 is required for efficient initiation of resection and recombinational repair at random, gamma-induced double-strand break ends. PLoS Genet. 2009;5(9):e1000656. doi: 10.1371/journal.pgen.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douki T, Cadet J. Far-UV photochemistry and photosensitization of 2′-deoxycytidylyl-(3′-5′)-thymidine: Isolation and characterization of the main photoproducts. J Photochem Photobiol B. 1992;15(3):199–213. doi: 10.1016/1011-1344(92)85124-d. [DOI] [PubMed] [Google Scholar]
- 20.Tornaletti S, Pfeifer GP. Slow repair of pyrimidine dimers at p53 mutation hotspots in skin cancer. Science. 1994;263(5152):1436–1438. doi: 10.1126/science.8128225. [DOI] [PubMed] [Google Scholar]
- 21.McCulloch SD, et al. Preferential cis-syn thymine dimer bypass by DNA polymerase eta occurs with biased fidelity. Nature. 2004;428(6978):97–100. doi: 10.1038/nature02352. [DOI] [PubMed] [Google Scholar]
- 22.Livneh Z, Ziv O, Shachar S. Multiple two-polymerase mechanisms in mammalian translesion DNA synthesis. Cell Cycle. 2010;9(4):729–735. doi: 10.4161/cc.9.4.10727. [DOI] [PubMed] [Google Scholar]
- 23.Ma W, Resnick MA, Gordenin DA. Apn1 and Apn2 endonucleases prevent accumulation of repair-associated DNA breaks in budding yeast as revealed by direct chromosomal analysis. Nucleic Acids Res. 2008;36(6):1836–1846. doi: 10.1093/nar/gkm1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald JP, Levine AS, Woodgate R. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics. 1997;147(4):1557–1568. doi: 10.1093/genetics/147.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resnick MA, Setlow JK. Repair of pyrimidine dimer damage induced in yeast by ultraviolet light. J Bacteriol. 1972;109(3):979–986. doi: 10.1128/jb.109.3.979-986.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhry MA, Weinfeld M. Induction of double-strand breaks by S1 nuclease, mung bean nuclease and nuclease P1 in DNA containing abasic sites and nicks. Nucleic Acids Res. 1995;23(19):3805–3809. doi: 10.1093/nar/23.19.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geigl EM, Eckardt-Schupp F. Repair of gamma ray-induced S1 nuclease hypersensitive sites in yeast depends on homologous mitotic recombination and a RAD18-dependent function. Curr Genet. 1991;20(1-2):33–37. doi: 10.1007/BF00312762. [DOI] [PubMed] [Google Scholar]
- 28.Westmoreland JW, Resnick MA. Coincident resection at both ends of random, γ-induced double-strand breaks requires MRX (MRN), Sae2 (Ctp1), and Mre11-nuclease. PLoS Genet. 2013;9(3):e1003420. doi: 10.1371/journal.pgen.1003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardwell AJ, Bardwell L, Tomkinson AE, Friedberg EC. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science. 1994;265(5181):2082–2085. doi: 10.1126/science.8091230. [DOI] [PubMed] [Google Scholar]
- 30.Guzder SN, Sommers CH, Prakash L, Prakash S. Complex formation with damage recognition protein Rad14 is essential for Saccharomyces cerevisiae Rad1-Rad10 nuclease to perform its function in nucleotide excision repair in vivo. Mol Cell Biol. 2006;26(3):1135–1141. doi: 10.1128/MCB.26.3.1135-1141.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habraken Y, Sung P, Prakash L, Prakash S. Yeast excision repair gene RAD2 encodes a single-stranded DNA endonuclease. Nature. 1993;366(6453):365–368. doi: 10.1038/366365a0. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds RJ. Induction and repair of closely opposed pyrimidine dimers in Saccharomyces cerevisiae. Mutat Res. 1987;184(3):197–207. doi: 10.1016/0167-8817(87)90017-4. [DOI] [PubMed] [Google Scholar]
- 33.St Charles J, et al. High-resolution genome-wide analysis of irradiated (UV and γ-rays) diploid yeast cells reveals a high frequency of genomic loss of heterozygosity (LOH) events. Genetics. 2012;190(4):1267–1284. doi: 10.1534/genetics.111.137927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haber JE (2008) Evolution of models of homologous recombination. Recombination and Meiosis, Genome Dynamics and Stability, ed Egel R, Lankenau D-H (Springer, Berlin), pp 1–64.
- 35.Wittschieben JP, Reshmi SC, Gollin SM, Wood RD. Loss of DNA polymerase zeta causes chromosomal instability in mammalian cells. Cancer Res. 2006;66(1):134–142. doi: 10.1158/0008-5472.CAN-05-2982. [DOI] [PubMed] [Google Scholar]
- 36.Sonoda E, et al. Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J. 2003;22(12):3188–3197. doi: 10.1093/emboj/cdg308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma S, et al. REV1 and polymerase ζ facilitate homologous recombination repair. Nucleic Acids Res. 2012;40(2):682–691. doi: 10.1093/nar/gkr769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Sterling J, Storici F, Resnick MA, Gordenin DA. Hypermutability of damaged single-strand DNA formed at double-strand breaks and uncapped telomeres in yeast Saccharomyces cerevisiae. PLoS Genet. 2008;4(11):e1000264. doi: 10.1371/journal.pgen.1000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Houten B, Eisen JA, Hanawalt PC. A cut above: Discovery of an alternative excision repair pathway in bacteria. Proc Natl Acad Sci USA. 2002;99(5):2581–2583. doi: 10.1073/pnas.062062599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogi T, et al. Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Mol Cell. 2010;37(5):714–727. doi: 10.1016/j.molcel.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Ogi T, Lehmann AR. The Y-family DNA polymerase kappa (pol kappa) functions in mammalian nucleotide-excision repair. Nat Cell Biol. 2006;8(6):640–642. doi: 10.1038/ncb1417. [DOI] [PubMed] [Google Scholar]
- 42.Hanawalt PC, Cooper PK, Ganesan AK, Smith CA. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- 43.Grossman L, Braun A, Feldberg R, Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- 44.Masker W, Hanawalt P, Shizuya H. Role of DNA polymerase II in repair replication in Escherichia coli. Nat New Biol. 1973;244(138):242–243. doi: 10.1038/newbio244242a0. [DOI] [PubMed] [Google Scholar]
- 45.Tourrière H, Pasero P. Maintenance of fork integrity at damaged DNA and natural pause sites. DNA Repair (Amst) 2007;6(7):900–913. doi: 10.1016/j.dnarep.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Ward JD, Barber LJ, Petalcorin MI, Yanowitz J, Boulton SJ. Replication blocking lesions present a unique substrate for homologous recombination. EMBO J. 2007;26(14):3384–3396. doi: 10.1038/sj.emboj.7601766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adar S, Izhar L, Hendel A, Geacintov N, Livneh Z. Repair of gaps opposite lesions by homologous recombination in mammalian cells. Nucleic Acids Res. 2009;37(17):5737–5748. doi: 10.1093/nar/gkp632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaykov A, Arcangioli B. A programmed strand-specific and modified nick in S. pombe constitutes a novel type of chromosomal imprint. Curr Biol. 2004;14(21):1924–1928. doi: 10.1016/j.cub.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Lee GS, Neiditch MB, Salus SS, Roth DB. RAG proteins shepherd double-strand breaks to a specific pathway, suppressing error-prone repair, but RAG nicking initiates homologous recombination. Cell. 2004;117(2):171–184. doi: 10.1016/s0092-8674(04)00301-0. [DOI] [PubMed] [Google Scholar]
- 50.Metzger MJ, McConnell-Smith A, Stoddard BL, Miller AD. Single-strand nicks induce homologous recombination with less toxicity than double-strand breaks using an AAV vector template. Nucleic Acids Res. 2011;39(3):926–935. doi: 10.1093/nar/gkq826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Nierop GP, de Vries AA, Holkers M, Vrijsen KR, Gonçalves MA. Stimulation of homology-directed gene targeting at an endogenous human locus by a nicking endonuclease. Nucleic Acids Res. 2009;37(17):5725–5736. doi: 10.1093/nar/gkp643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran PT, Erdeniz N, Symington LS, Liskay RM. EXO1-A multi-tasking eukaryotic nuclease. DNA Repair (Amst) 2004;3(12):1549–1559. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Karras GI, Jentsch S. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell. 2010;141(2):255–267. doi: 10.1016/j.cell.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 54.Covo S, Westmoreland JW, Gordenin DA, Resnick MA. Cohesin Is limiting for the suppression of DNA damage-induced recombination between homologous chromosomes. PLoS Genet. 2010;6(7):e1001006. doi: 10.1371/journal.pgen.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12(3):259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.