Abstract
Numerous cell types have shown a remarkable ability to detect and move along gradients in stiffness of an underlying substrate—a process known as durotaxis. The mechanisms underlying durotaxis are still unresolved, but generally believed to involve active sensing and locomotion. Here, we show that simple liquid droplets also undergo durotaxis. By modulating substrate stiffness, we obtain fine control of droplet position on soft, flat substrates. Unlike other control mechanisms, droplet durotaxis works without imposing chemical, thermal, electrical, or topographical gradients. We show that droplet durotaxis can be used to create large-scale droplet patterns and is potentially useful for many applications, such as microfluidics, thermal control, and microfabrication.
Keywords: droplet control, elasticity, soft matter, wetting, mechanosensing
The control of liquids on surfaces is essential for microfluidics (1), microfabrication (2), and coatings (3–5), to name but a few applications. Wetting is typically manipulated by controlling interfacial energies (6). Heterogeneous surface chemistries have been exploited to pattern (7, 8) and transport droplets (3, 9). Gradients in temperature or electric potential can drive droplet motion (3, 9). Alternatively, surface topography can control the spreading of fluids. For example, isotropically rough surfaces can exhibit superhydrophobicity (10, 11), whereas anisotropic surfaces exhibit anisotropic spreading (12) and even directed droplet transport (13–15). Here, we introduce a method to control droplets on surfaces inspired by the biological phenomenon of durotaxis—the ability of many eukaryotic cell types to move along gradients in the stiffness of their extracellular matrix (16–19). Although the current explanation of durotaxis involves active sensing of matrix stiffness and actomyosin-based motility (18), we show here that even simple liquid droplets display durotaxis. Furthermore, we show that durotaxis can be exploited to achieve large-scale droplet patterning. A simple theory explains how drops move toward softer parts of a substrate, and quantitatively captures the droplet distribution on patterned surfaces. Droplet durotaxis is prominent on soft substrates, which are significantly deformed by liquid surface tension (20, 21).
The spreading of liquid droplets on stiff, flat surfaces is primarily described by the contact angle. In equilibrium, a small droplet takes the shape of a spherical cap with uniform contact angle θ determined by Young’s law: 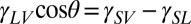 . Here, indices L, S, and V of interfacial energies, γ, represent liquid, solid, and vapor, respectively (6). Spontaneous droplet motion typically occurs in two main cases. First, if the actual contact angle of a droplet differs from its equilibrium contact angle, the droplet will be driven to spread/contract until it reaches its equilibrium shape (6). Second, if there is a difference between the equilibrium contact angle on either side of a droplet, the droplet will be driven toward the more wetting side. This is typically achieved by modifying the interfacial energies across the droplet (9). Here, we show that gradients in substrate stiffness can also drive droplet motion on soft substrates.
. Here, indices L, S, and V of interfacial energies, γ, represent liquid, solid, and vapor, respectively (6). Spontaneous droplet motion typically occurs in two main cases. First, if the actual contact angle of a droplet differs from its equilibrium contact angle, the droplet will be driven to spread/contract until it reaches its equilibrium shape (6). Second, if there is a difference between the equilibrium contact angle on either side of a droplet, the droplet will be driven toward the more wetting side. This is typically achieved by modifying the interfacial energies across the droplet (9). Here, we show that gradients in substrate stiffness can also drive droplet motion on soft substrates.
Contact Angle Dependence on Substrate Stiffness
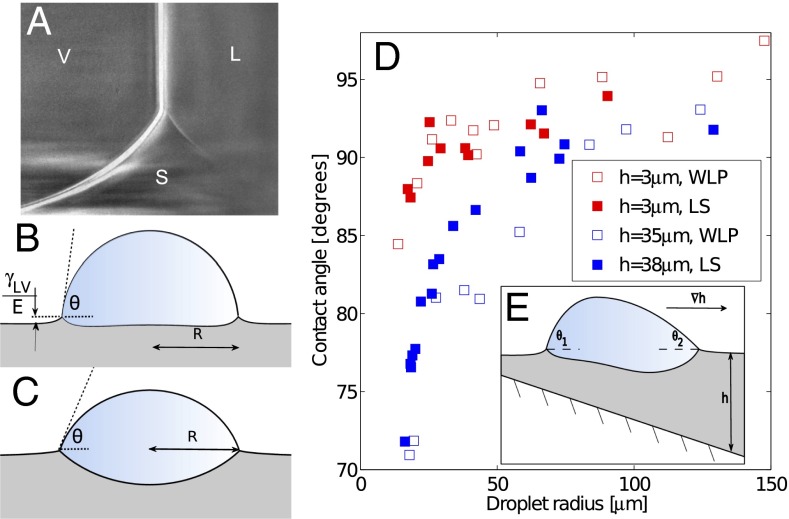
On soft substrates, the apparent contact angle varies with droplet size and substrate stiffness (22, 23). This breakdown of Young’s law occurs because droplet surface tension can significantly deform soft substrates, as shown by the X-ray micrograph in Fig. 1A (22–33). Droplet surface tension pulls up at the contact line creating a ridge, while the droplet’s internal (Laplace) pressure pushes down into the substrate over the contact area, creating a dimple. Dimple formation leads to a contact angle change: when the Laplace pressure is sufficiently large, the droplet bulges down into the substrate, taking a lenticular shape and causing it to appear more wetting, as illustrated in Fig. 1 B and C. The apparent contact angle, θ, is defined here as the angle of the liquid–vapor interface at the contact line relative to the undeformed solid surface far from the droplet. For large droplets, we expect the contact angle to be consistent with Young’s law. For very small drops, the shape mimics that of drops floating on a liquid with the same interfacial tensions as the soft substrate—the contact angle is determined from a Neumann triangle construction (22, 23, 34). The critical droplet radius below which the apparent contact angle starts to deviate from Young’s law is 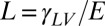 , where L is an elastocapillary length and E is the Young’s modulus of the substrate (23). For hard solids,
, where L is an elastocapillary length and E is the Young’s modulus of the substrate (23). For hard solids, 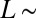 molecular scales, so contact angle changes are insignificant. For soft solids, L can be macroscopic; gels with
molecular scales, so contact angle changes are insignificant. For soft solids, L can be macroscopic; gels with 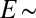 kPa have L ∼ 10 μm.
kPa have L ∼ 10 μm.
Fig. 1.
Droplets deform soft substrates, causing Young’s law to fail. (A) X-ray image of the contact line of a water droplet on a soft, silicone gel substrate. The ridge is pulled up by the droplet surface tension. 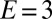 kPa, and the substrate is 22 μm thick. The droplet radius is ∼1 mm. (B) The equilibrium of a sessile droplet on a soft surface with
kPa, and the substrate is 22 μm thick. The droplet radius is ∼1 mm. (B) The equilibrium of a sessile droplet on a soft surface with 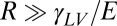 and (C) a soft surface with
and (C) a soft surface with 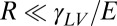 . (D) Symbols show measured contact angles of glycerol droplets on a silicone gel as a function of droplet radius, R. Data are shown for thin silicone gel layers of h = 3 μm (red) and thicker layers of h = 35, 38 μm (blue). Filled/open points were measured by laser scanning (LS)/white-light optical profilometry (WLP). The large-drop contact angle was measured as
. (D) Symbols show measured contact angles of glycerol droplets on a silicone gel as a function of droplet radius, R. Data are shown for thin silicone gel layers of h = 3 μm (red) and thicker layers of h = 35, 38 μm (blue). Filled/open points were measured by laser scanning (LS)/white-light optical profilometry (WLP). The large-drop contact angle was measured as 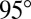 (23). (E) Schematic profile of a droplet on a soft surface of varying thickness, h.
(23). (E) Schematic profile of a droplet on a soft surface of varying thickness, h.
Two examples of the dependence of the macroscopic contact angle on droplet size are shown in Fig. 1D. Here, glycerol droplets rest on thin, flat silicone gel layers (CY52-276A/B; Dow Corning; 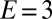 kPa) spin coated on a stiff glass coverslip. In the samples shown, the film thicknesses are
kPa) spin coated on a stiff glass coverslip. In the samples shown, the film thicknesses are 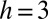 , 35, and 38 μm. We measured θ using a laser scan (laser profilometer with white-light probe sensor; Solarius) and an optical profilometer (NewView 7300; Zygo Corporation). Further details are given in Materials and Methods. For droplets larger than 50 μm, θ approaches the macroscopic value of
, 35, and 38 μm. We measured θ using a laser scan (laser profilometer with white-light probe sensor; Solarius) and an optical profilometer (NewView 7300; Zygo Corporation). Further details are given in Materials and Methods. For droplets larger than 50 μm, θ approaches the macroscopic value of 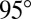 , measured for millimetric droplets. For droplets smaller than 50 μm, θ is significantly reduced. For a given droplet size, the contact angle is smaller on the thicker substrate. In other words, small droplets appear to wet thick substrates more strongly than thin substrates.
, measured for millimetric droplets. For droplets smaller than 50 μm, θ is significantly reduced. For a given droplet size, the contact angle is smaller on the thicker substrate. In other words, small droplets appear to wet thick substrates more strongly than thin substrates.
Droplet Durotaxis
We hypothesized that the dependence of θ on soft substrate thickness could be exploited to manipulate droplets on chemically homogeneous, flat surfaces. The data in Fig. 1D suggest that a droplet on a soft substrate with nonuniform thickness will have a nonuniform contact angle, as shown in Fig. 1E. By analogy with droplet motion driven by gradients of interfacial energy, we expect droplets to move spontaneously along gradients in substrate thickness. Here, droplet motion is not created by an external force or gradients in interfacial energy, but differences in substrate stiffness. We use “stiffness” in the sense of a spring constant, describing how much a surface is displaced by an external force. Thus, a substrate’s stiffness depends both on its elastic modulus and thickness. Intriguingly, motion along stiffness gradients has been observed in living cells. This phenomenon, called “durotaxis,” can be driven by gradients in the elastic modulus of the substrate (16–18) or by gradients in the substrate thickness (19).
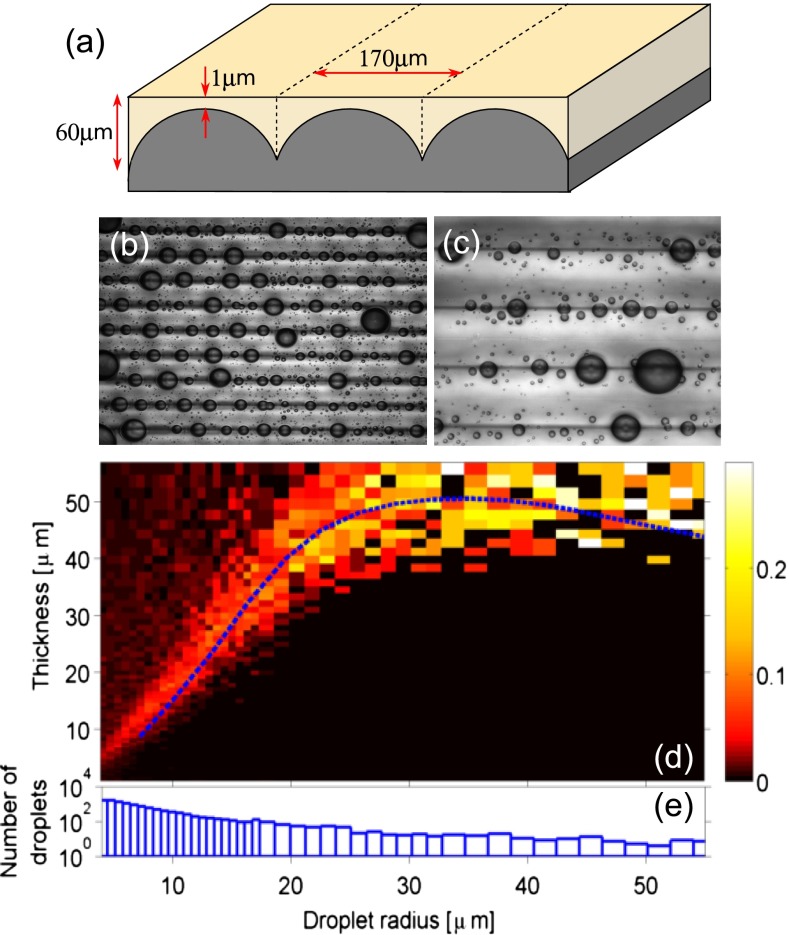
To test this hypothesis, we observed droplets on soft substrates with flat surfaces and gradients in thickness. We coated stiff lenticular sheets with silicone gel (Fig. 2A). The lenticules are cylindrical caps with radii of 91 μm spaced periodically with wavelength of 170 μm. The resulting gel layer had strong thickness gradients but a flat surface (quantified in SI Text).
Fig. 2.
Droplets move on flat surfaces with stiffness gradients. (A) Schematic diagram of flat chemically homogeneous substrates with gradients of stiffness. A flat layer of soft, silicone gel is deposited on a hard, lenticular array creating gradients in the thickness (or stiffness) of the gel. (B and C) Photographs of glycerol droplets after deposition with an atomizer. The dark horizontal bands are located at the thickest regions of the substrate. The spacing between the bands is 170 μm. B and C were taken 5 min and 5 h after application, respectively. (D) Probability density of final droplet locations from 13,300 droplets as a function of substrate thickness and drop radius. The dashed curve is the theoretical prediction for final position of moving droplets. (E) Size distribution of observed droplets.
We sprayed glycerol droplets onto the surface with an atomizer and observed them in reflection on a light microscope. Examples are shown in Fig. 2 B and C. The dark lines indicate the deepest part of the substrate. We found that droplets spontaneously moved from thin regions to thick regions (Movies S1–S3). Depending on their size, the droplets continued to move detectably for up to 1 h, and we left them for 5 h after deposition to reach “steady state.” Fig. 2C shows a typical steady-state image taken 5 h after deposition. We analyzed 92 such images, containing 13,300 droplets to determine their final positions. Droplet radii and center positions were detected automatically using a circular-Hough transform (droplets <20 μm in radius), or by identifying points on the droplet perimeter by hand and fitting a circle through these points (larger droplets). The results are shown in Fig. 2D as the probability density of droplets ending up on a portion of the substrate of a given thickness. Fig. 2E shows the number of droplets recorded in each size range. Large droplets all move to the deepest part of the substrate. Note that the substrate is 60 μm thick at its deepest point. Droplets below 25 μm in radius are most likely to be found where the substrate thickness is roughly 1.5 times their radius; in deeper regions, the probability density is roughly uniform and indistinguishable from the uniform coverage by the atomizer. All droplets are almost perfectly excluded from regions shallower than a drop size-dependent critical thickness.
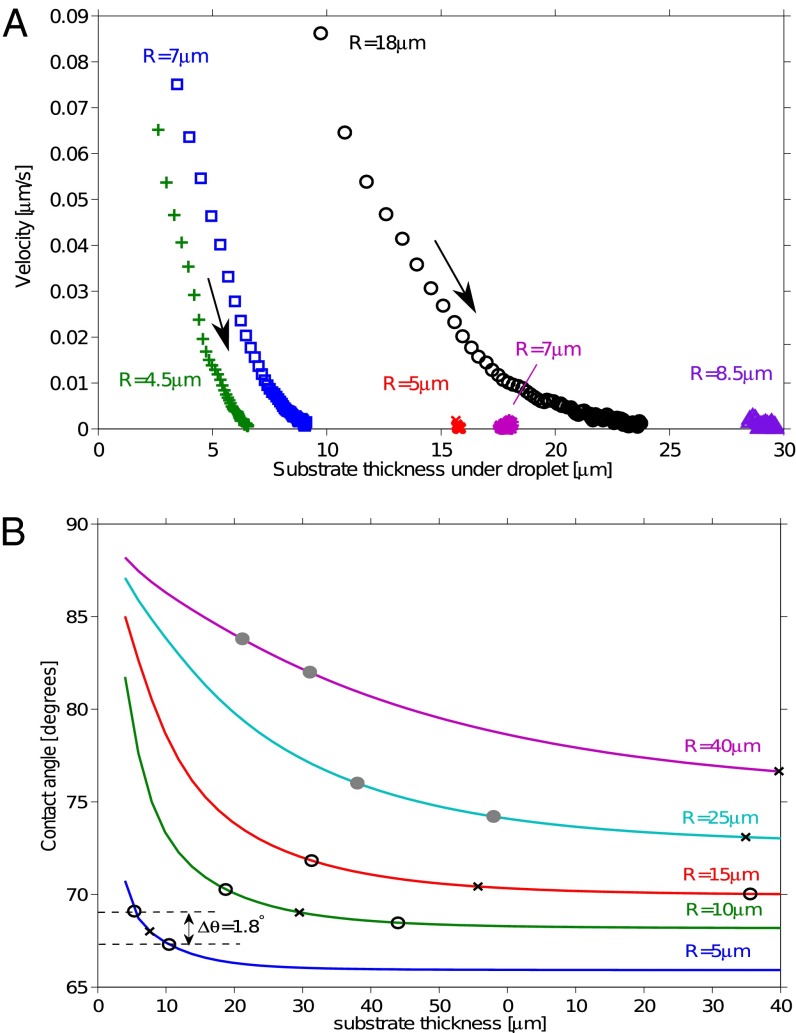
Representative droplet trajectories are shown in Fig. 3A, starting within a minute of initial deposition. This shows droplet velocity as a function of underlying substrate thickness. Droplets are driven toward thicker regions of the substrate, with speed decreasing as they move. Small droplets deposited over thick regions of the substrate do not move.
Fig. 3.
Droplet motion on stiffness gradients. (A) Speed of droplets moving from thin to thick regions of the gel as a function of gel thickness at droplet center, h. Trajectories extracted from image sequences (see Movies S1–S3). Drop speed increased as drop radius increased and substrate thickness decreased. Droplets came to rest when 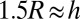 . Droplets starting at locations much thicker than their radii did not move. (B) Solid curves show the theoretical contact angle as a function of substrate thickness for droplets of different sizes. The x symbols show droplet center and o symbols show droplet front and back at thickness where
. Droplets starting at locations much thicker than their radii did not move. (B) Solid curves show the theoretical contact angle as a function of substrate thickness for droplets of different sizes. The x symbols show droplet center and o symbols show droplet front and back at thickness where 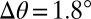 . The o’s are filled when the droplet straddles the trough in the substrate, and otherwise empty.
. The o’s are filled when the droplet straddles the trough in the substrate, and otherwise empty.
Mechanism of Droplet Durotaxis
A simple theory quantitatively captures the final position of moving droplets. Extending our analogy with droplet motion driven by gradients of interfacial energy, we expect droplets to move spontaneously when the contact angle difference across the droplet, 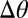 , exceeds a critical value,
, exceeds a critical value, 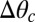 (6). Using our previous theory (22, 23), we calculated the expected contact angle of a droplet as a function of substrate thickness, as shown by the curves in Fig. 3B (SI Text). This was used to estimate
(6). Using our previous theory (22, 23), we calculated the expected contact angle of a droplet as a function of substrate thickness, as shown by the curves in Fig. 3B (SI Text). This was used to estimate 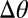 as a function of droplet size and position, as shown by the gray and black symbols in the figure. In the shallower parts of the substrate,
as a function of droplet size and position, as shown by the gray and black symbols in the figure. In the shallower parts of the substrate, 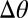 can be as much as
can be as much as 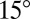 , depending on the droplet size. As the substrate thickness increases,
, depending on the droplet size. As the substrate thickness increases, 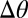 decreases. Thus, the presumed driving force for droplet motion decreases with increasing thickness. Assuming that the droplet stops when
decreases. Thus, the presumed driving force for droplet motion decreases with increasing thickness. Assuming that the droplet stops when 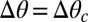 , we can determine the final position of the droplet as a function of its radius. Superimposing the theoretical final droplet positions on top of the experimental data in Fig. 2D, we find good agreement between theory and experiments for a critical value
, we can determine the final position of the droplet as a function of its radius. Superimposing the theoretical final droplet positions on top of the experimental data in Fig. 2D, we find good agreement between theory and experiments for a critical value 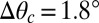 . This is consistent with contact angle hysteresis measurements for macroscopic glycerol droplets on flat, silicone gel-coated surfaces using a contact angle goniometer (VCA Optima; AST Products). Hysteresis was sufficiently small as to be undetectable within the accuracy of the machine
. This is consistent with contact angle hysteresis measurements for macroscopic glycerol droplets on flat, silicone gel-coated surfaces using a contact angle goniometer (VCA Optima; AST Products). Hysteresis was sufficiently small as to be undetectable within the accuracy of the machine 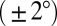 .
.
To confirm this mechanism and rule out other causes of droplet motion, we repeated our experiments replacing the soft silicone gel with a stiff silicone elastomer (Sylgard 184 with 10:1 ratio; Dow Corning; 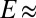 1.8 MPa). For this material, our theory predicts no significant substrate deformation or contact angle differences. Indeed, we found no droplet motion (see Movies S1–S3).
1.8 MPa). For this material, our theory predicts no significant substrate deformation or contact angle differences. Indeed, we found no droplet motion (see Movies S1–S3).
Patterning with Durotaxis
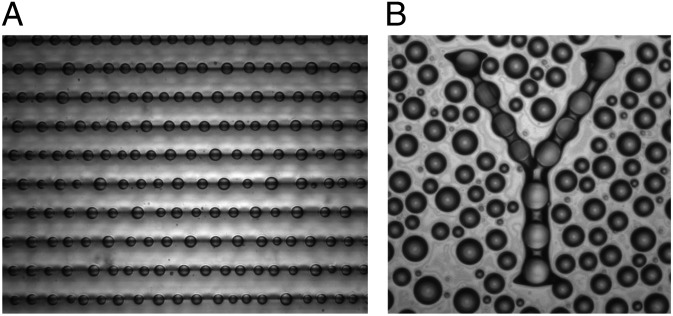
The images in Fig. 2 B and C demonstrate the potential of droplet durotaxis for controlled pattern formation. This is greatly enhanced when droplets are allowed to coarsen, coalesce, and evaporate. Fig. 4A shows water droplets on the substrate studied above, where droplets have been condensed from the ambient atmosphere by cooling of the substrate. While droplets grow by condensation, long-range forces—likely elastic in origin—drive their coalescence (Movies S1–S3). This growth rapidly drives droplets to the thickest region of the soft substrate. With the removal of cooling, the remaining small droplets rapidly evaporate, leaving the largest droplets precisely localized over the deepest regions of the substrate. Fig. 4B shows water droplets condensed onto a flat, silicone layer coated on an arbitrary substrate pattern—in this case, the letter “Y,” created by a flat 35-μm–deep etch into a silicon wafer. The silicone layer is a few micrometers thick on the wafer surrounding the “Y.”
Fig. 4.
Patterning droplets with durotaxis. (A) Photograph of water droplets deposited by condensation onto a flat chemically homogeneous surface with varying stiffness. The dark horizontal bands are located at the thickest regions of the substrate. The spacing between these lines is 170 μm. The patterning process is shown in SI Text. (B) Droplets deposited by condensation onto soft, flat surface coated a “Y” shape etched into a silicon wafer. Field of view is 620 μm wide.
Conclusions
In conclusion, simple liquid droplets undergo durotaxis on stiffness gradients on soft substrates. This motion can be used to move droplets without chemical, thermal, or topographical gradients. Durotaxis can be used to pattern droplets over large scales, and may also be useful for microfluidics (9), microfabrication and self-assembly (2, 35), and condensers (5). We note that the behavior of droplets on stiffness gradients is analogous to the collective behavior of adatoms adsorbed onto the surface of a crystal (36). Thus, droplets may be a convenient macroscopic analog for atomic adsorption and redistribution. Our results also have implications for the biological mechanisms involved in cellular durotaxis. Even though drops move to softer substrates and cells typically migrate to stiffer substrates, the phenomenon of durotaxis by simple liquid droplets indicates that active stiffness sensing may not be required.
Materials and Methods
Substrate Fabrication.
We created our substrates by coating a flat layer of silicone on a lenticular, polyester-resin substrate (Lenstar Plus–Thin; Pacur). For the soft substrates, we used a silicone gel (CY52-276A/B; Dow Corning), whereas for the harder substrates we used a silicone elastomer (Sylgard 184). The fabrication process is shown in SI Text. We spin-coated a 2% (wt/vol) solution of polystyrene in toluene on a glass slide to form a flat coating of polystyrene. The silicone was premixed and degassed in a vacuum chamber, and then a small drop was sandwiched between the polystyrene film and the lenticular substrate. A flat weight ( 200 g) was placed on the sample to compress the gel layer. After curing overnight, the weight was removed and the coated substrate was gently peeled off the polystyrene.
200 g) was placed on the sample to compress the gel layer. After curing overnight, the weight was removed and the coated substrate was gently peeled off the polystyrene.
X-ray Imaging of the Contact Line.
A flat, soft substrate was made by spin coating a 22-μm–thick layer of the silicone gel on a glass slide. A droplet of pure, deionized, water (Millipore; 18 M ⋅cm at 25° C) was placed on the substrate and allowed to equilibrate for several minutes. The contact line was then visualized using X-ray imaging performed using the transmission X-ray microscopy at the 32-ID-C beamline in the Advanced Photon Source (APS) of the Argonne National Laboratory. High-resolution, 50 nm/pixel micrographs were recorded using bright light at a photon energy of 8 keV generated by the APS (37). As we used a short exposure time of less than 0.2 s per snapshot, X-ray effects—such as polymer degradation—should be negligible (38). A Zernike phase-ring provided sufficient phase contrast to clearly visualize the droplet interfaces, so no contrast agent was required (39, 40).
⋅cm at 25° C) was placed on the substrate and allowed to equilibrate for several minutes. The contact line was then visualized using X-ray imaging performed using the transmission X-ray microscopy at the 32-ID-C beamline in the Advanced Photon Source (APS) of the Argonne National Laboratory. High-resolution, 50 nm/pixel micrographs were recorded using bright light at a photon energy of 8 keV generated by the APS (37). As we used a short exposure time of less than 0.2 s per snapshot, X-ray effects—such as polymer degradation—should be negligible (38). A Zernike phase-ring provided sufficient phase contrast to clearly visualize the droplet interfaces, so no contrast agent was required (39, 40).
Contact Angles.
We measured droplet contact angles using two separate techniques: first, with an optical profilometer (NewView 7300; Zygo Corporation), and second, with a laser-scan device (laser profilometer with white-light probe sensor; Solarius). First, we measured the height from the undeformed surface 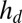 of droplets on a range of substrate thicknesses. Second, we used an image of the droplet to measure its apparent radius, R. When
of droplets on a range of substrate thicknesses. Second, we used an image of the droplet to measure its apparent radius, R. When 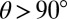 , R represents the actual radius of the droplet. When
, R represents the actual radius of the droplet. When 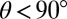 , R represents the footprint radius of the droplet. We calculated the contact angle θ by assuming that the droplet surface is a spherical cap of height
, R represents the footprint radius of the droplet. We calculated the contact angle θ by assuming that the droplet surface is a spherical cap of height 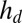 and radius R: if
and radius R: if 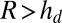 ,
, 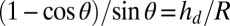 , whereas if
, whereas if 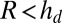 ,
, 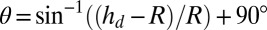 . On a rigid substrate with no wetting ridge, this technique will give the correct contact angle. However, on soft substrates, this value systematically overestimates θ by an amount on the order the ratio of the ridge height divided by the droplet radius. This systematic error is
. On a rigid substrate with no wetting ridge, this technique will give the correct contact angle. However, on soft substrates, this value systematically overestimates θ by an amount on the order the ratio of the ridge height divided by the droplet radius. This systematic error is 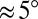 for the smallest droplets and decreases with increasing radius (23).
for the smallest droplets and decreases with increasing radius (23).
Supplementary Material
Acknowledgments
We thank Zygo Corporation for the use of their optical profilometer and help with measuring contact angles. We thank Valerie Horsley, Anand Jagota, and Elizabeth Jerison for helpful conversations. R.W.S. is funded by the Yale University Bateman Interdepartmental Postdoctoral Fellowship. J.S.W. thanks the Swedish Research Council for support. Support for instrumentation was provided by National Science Foundation Grant DBI-0619674. This research was also supported by the Yale Institute for Nanoscience and Quantum Engineering, and by the Creative Research Initiatives (Functional X-ray Imaging) of the Ministry of Education, Science and Technology/National Research Foundation of Korea. Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the DOE under Contract DE-AC02-06CH11357.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 12505.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307122110/-/DCSupplemental.
References
- 1.Squires T, Quake S. Microfluidics: Fluid physics at the nanoliter scale. Rev Mod Phys. 2005;77(3):977–1026. [Google Scholar]
- 2.Srinivasarao M, Collings D, Philips A, Patel S. Three-dimensionally ordered array of air bubbles in a polymer film. Science. 2001;292(5514):79–83. doi: 10.1126/science.1057887. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhury MK, Whitesides GM. How to make water run uphill. Science. 1992;256(5063):1539–1541. doi: 10.1126/science.256.5063.1539. [DOI] [PubMed] [Google Scholar]
- 4.Wong TS, et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature. 2011;477(7365):443–447. doi: 10.1038/nature10447. [DOI] [PubMed] [Google Scholar]
- 5.Sokuler M, et al. The softer the better: Fast condensation on soft surfaces. Langmuir. 2010;26(3):1544–1547. doi: 10.1021/la903996j. [DOI] [PubMed] [Google Scholar]
- 6.de Gennes P-G, Brochard-Wyart F, Quere D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves. New York: Springer Science; 2004. [Google Scholar]
- 7.Gau H, Herminghaus S, Lenz P, Lipowsky R. Liquid morphologies on structured surfaces: From microchannels to microchips. Science. 1999;283(5398):46–49. doi: 10.1126/science.283.5398.46. [DOI] [PubMed] [Google Scholar]
- 8.Sirringhaus H, et al. High-resolution inkjet printing of all-polymer transistor circuits. Science. 2000;290(5499):2123–2126. doi: 10.1126/science.290.5499.2123. [DOI] [PubMed] [Google Scholar]
- 9.Darhuber A, Troian S. Principles of microfluidic actuation by modulation of surface stresses. Annu Rev Fluid Mech. 2005;37:425–455. [Google Scholar]
- 10.Cassie ABD, Baxter S. Wettability of porous surfaces. Trans Faraday Soc. 1944;40:546–551. [Google Scholar]
- 11.Lafuma A, Quéré D. Superhydrophobic states. Nat Mater. 2003;2(7):457–460. doi: 10.1038/nmat924. [DOI] [PubMed] [Google Scholar]
- 12.Courbin L, et al. Imbibition by polygonal spreading on microdecorated surfaces. Nat Mater. 2007;6(9):661–664. doi: 10.1038/nmat1978. [DOI] [PubMed] [Google Scholar]
- 13.Shastry A, Case MJ, Böhringer KF. Directing droplets using microstructured surfaces. Langmuir. 2006;22(14):6161–6167. doi: 10.1021/la0601657. [DOI] [PubMed] [Google Scholar]
- 14.Lagubeau G, Le Merrer M, Clanet C, Quéré D. Leidenfrost on a ratchet. Nat Phys. 2011;7(5):395–398. [Google Scholar]
- 15.Prakash M, Quéré D, Bush JW. Surface tension transport of prey by feeding shorebirds: The capillary ratchet. Science. 2008;320(5878):931–934. doi: 10.1126/science.1156023. [DOI] [PubMed] [Google Scholar]
- 16.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79(1):144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 18.Trichet L, et al. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc Natl Acad Sci USA. 2012;109(18):6933–6938. doi: 10.1073/pnas.1117810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi YS, et al. The alignment and fusion assembly of adipose-derived stem cells on mechanically patterned matrices. Biomaterials. 2012;33(29):6943–6951. doi: 10.1016/j.biomaterials.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman B, Bico J. Elasto-capillarity: Deforming an elastic structure with a liquid droplet. J Phys Condens Matter. 2010;22(49):493101. doi: 10.1088/0953-8984/22/49/493101. [DOI] [PubMed] [Google Scholar]
- 21.Mora S, Phou T, Fromental J-M, Pismen LM, Pomeau Y. Capillarity driven instability of a soft solid. Phys Rev Lett. 2010;105(21):214301. doi: 10.1103/PhysRevLett.105.214301. [DOI] [PubMed] [Google Scholar]
- 22.Style RW, Dufresne ER. Static wetting on deformable substrates, from liquids to soft solids. Soft Matter. 2012;8(27):7177–7184. [Google Scholar]
- 23.Style RW, et al. Universal deformation of soft substrates near a contact line and the direct measurement of solid surface stresses. Phys Rev Lett. 2013;110(6):066103. doi: 10.1103/PhysRevLett.110.066103. [DOI] [PubMed] [Google Scholar]
- 24.Shanahan M, de Gennes P-G. L’arête produite par un coin liquide près de la ligne triple de contact solide/liquide/fluide. C R Acad Sci Paris. 1986;302(8):517–521. [Google Scholar]
- 25.Shanahan M. The influence of solid micro-deformation on contact angle equilibrium. J Phys D Appl Phys. 1987;20:945–950. [Google Scholar]
- 26.Carre A, Gastel J-C, Shanahan MER. Viscoelastic effects in the spreading of liquids. Nature. 1996;379:432–434. [Google Scholar]
- 27.Extrand CW, Kumagai Y. Contact angles and hysteresis on soft surfaces. J Colloid Interface Sci. 1996;184(1):191–200. doi: 10.1006/jcis.1996.0611. [DOI] [PubMed] [Google Scholar]
- 28.Pericet-Cámara R, Best A, Butt H-J, Bonaccurso E. Effect of capillary pressure and surface tension on the deformation of elastic surfaces by sessile liquid microdrops: An experimental investigation. Langmuir. 2008;24(19):10565–10568. doi: 10.1021/la801862m. [DOI] [PubMed] [Google Scholar]
- 29.Pericet-Camara R, et al. Solid-supported thin elastomer films deformed by microdrops. Soft Matter. 2009;5(19):3611–3617. [Google Scholar]
- 30.Jerison ER, Xu Y, Wilen LA, Dufresne ER. Deformation of an elastic substrate by a three-phase contact line. Phys Rev Lett. 2011;106(18):186103. doi: 10.1103/PhysRevLett.106.186103. [DOI] [PubMed] [Google Scholar]
- 31.Marchand A, Das S, Snoeijer JH, Andreotti B. Capillary pressure and contact line force on a soft solid. Phys Rev Lett. 2012;108(9):094301. doi: 10.1103/PhysRevLett.108.094301. [DOI] [PubMed] [Google Scholar]
- 32.Limat L. Straight contact lines on a soft, incompressible solid. Eur Phys J E Soft Matter. 2012;35(12):1–13. doi: 10.1140/epje/i2012-12134-6. [DOI] [PubMed] [Google Scholar]
- 33.Lubarda V. Mechanics of a liquid drop deposited on a solid substrate. Soft Matter. 2012;8(40):10288–10297. [Google Scholar]
- 34.Neumann F. Vorlesungen ber die Theorie der Capillaritt. Leipzig, Germany: B. G. Teubner; 1894. [Google Scholar]
- 35.Widawski G, Rawiso M, François B. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature. 1994;369:387–389. [Google Scholar]
- 36. Villain J, Duport C, Nozières P (1995) Elastic instabilities in crystal growth. 25 Years of Non-Equilibrium Statistical Mechanics, Vol 445 of Lecture Notes in Physics, eds Brey JJ, Marro J, Rubí JM, San Miguel M (Springer, Berlin), pp 177–188.
- 37.Chu YS, et al. Hard-x-ray microscopy with Fresnel zone plates reaches 40 nm Rayleigh resolution. Appl Phys Lett. 2008;92:103119. [Google Scholar]
- 38.Weon BM, et al. Ablation and deposition of poly(dimethylsiloxane) with X-rays. ChemPhysChem. 2010;11(1):115–118. doi: 10.1002/cphc.200900841. [DOI] [PubMed] [Google Scholar]
- 39.Weon BM, et al. Colloid coalescence with focused x rays. Phys Rev Lett. 2011;107(1):018301. doi: 10.1103/PhysRevLett.107.018301. [DOI] [PubMed] [Google Scholar]
- 40.Weon BM, Lee JS, Kim JT, Pyo J, Je JH. Colloidal wettability probed with x-ray microscopy. Curr Opin Coll Int Sci. 2012;17(16):388–395. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.