Abstract
Cognitive effort leads to a seeming cacophony of brain oscillations. For example, during tasks engaging working memory (WM), specific oscillatory frequency bands modulate in space and time. Despite ample data correlating such modulation to task performance, a mechanistic explanation remains elusive. We propose that flexible control of neural oscillations provides a unified mechanism for the rapid and controlled transitions between the computational operations required by WM. We show in a spiking network model that modulating the input oscillation frequency sets the network in different operating modes: rapid memory access and load is enabled by the beta–gamma oscillations, maintaining a memory while ignoring distractors by the theta, rapid memory clearance by the alpha. The various frequency bands determine the dynamic gating regimes enabling the necessary operations for WM, whose succession explains the need for the complex oscillatory brain dynamics during effortful cognition.
Keywords: theta band, alpha band, beta band, gamma band, selective gating
As effortful cognition unfolds multiple frequency bands of oscillations play out in the brain. However, no coherent theory exists to explain the progression of frequencies and how they relate to implementing the tasks at hand. Recently it has been speculated that individual bands may implement elementary computations constituent in cognitive tasks (1, 2) as is seen during working memory (WM) performance. Human electrophysiology studies show clearly that memory maintenance correlates positively with oscillations in the theta band (4–8 Hz, refs. 3–8), in the beta band (13–30 Hz, ref. 9), and in the gamma band (30–120 Hz, refs. 10–12). On the other hand, suppression of irrelevant information is associated with oscillations in the alpha band (8–13 Hz, refs. 13–15). In lateralized WM tasks where the subject should ignore cues in one hemifield, alpha power increases in the hemisphere encoding such irrelevant information (8, 16, 17). However, a mechanistic theory explaining why oscillations of various frequency bands wax and wane in time and space during effortful tasks and in particular WM remains outstanding.
WM actively maintains and processes information necessary to carry out actions and decisions. WM is critically capacity limited to a handful of items “held on-line” (18, 19), and operated upon in real time (20). Thus, WM requires obsolete memories to be rapidly cleared and to selectively prevent irrelevant information from being loaded. One of the central unresolved issues is how these WM operations, and in particular selective gating, are carried out by the brain circuits. Ample data (21–24) and classical theoretical arguments (25, 26) point to the selectively tuned persistent neuronal activity as the prevalent mechanism for WM storage (27–29). However, what causal role oscillatory frequency dynamics play in sustained activity and in task performance has remained unclear.
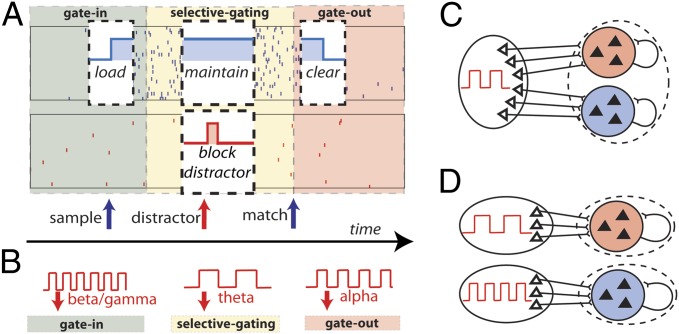
We propose a resolution of these issues by constructing a computational framework that mechanistically links the individual oscillatory bands with implementing canonical WM computations. The oscillatory frequency bands define distinct dynamical regimes (or gating modes) for the WM network: the gamma band allows coexistence of persistent activity and a ground state (a transient input can initiate a persistent state), the alpha-band oscillations allow only the ground state (a transient input can evoke only a transient response), and theta-band oscillations set the network into a regime where an a priori active persistent state remains stable, yet a de novo transient stimulus evokes only a transient response. By modulating the frequency of externally driven oscillations, the WM network shifts between dynamical regimes enabling the necessary canonical computations. We show how in a local version of the network (Fig. 1C), where oscillations are common to all of the neural populations, temporal modulation of the frequencies allows all WM operations to be executed. Consistent with data, the theta band is for memory maintenance and the alpha band ensures functional inhibition. In a model where information is segregated in two hemispheres (Fig. 1D), a lateralized increase in alpha power blocks erroneous activations by distractors.
Fig. 1.
Outline of the working memory task, gating modes, and network models. (A) Delay match-to-sample task with distractors and the various operations to be executed by the network with the underlying gating modes. The two rastergrams represent the two populations B (in blue) and R (in red). The phases of operations are outlined in a white box showing the succession of the gating modes and operations: (I) Gate-in mode: the sample stimulus (blue arrow) activates population B (load). (II) Selective-gating: the distractor stimulus (red arrow) is not able to activate population R persistently (block distractor) and the memory in population B is held (maintain). (III) Gate-out: upon match-stimulus presentation, persistent activity is deactivated in the blue population (clear). (B) Input oscillations enabling the gating-modes: beta–gamma band ensures the gate-in mode at the beginning of the task, theta band ensures the selective-gating mode during the delay period (memory maintenance together with protections from the distractors), and alpha band ensures the gate-out mode at the task completion (memory is rapidly cleared). (C) Local two-population unit network: populations B and R receive input by sources modulated by the shared background oscillation. (D) Bihemispheric two-population unit network: populations B and R receive input by sources modulated by independent background oscillations.
Results
To home in on a specific example, we require our models to correctly perform the delayed match to sample (DMS) with distractors task (30). In this task, a visual sample cue is presented to the subject followed by a delay period; during this period a random number of test cues are presented, yet the subject should respond only when the test cue matches the sample cue. To model this task we considered two stimulus-selective neural population units (R representing red or B for blue) that can be independently activated from a resting state to a persistent state by a sensory stimulus (Fig. 1A). For the task to be executed, the neural circuitry needs to perform four computational operations: load the sample, maintain the sample trace during the delay period, block the irrelevant distractor activation, and clear the sample trace upon match (Note that we do not focus on the “read-out” response–decision mechanism).
We suggest that the correct execution of the four operations is ensured by setting the short-term memory network in appropriate gating modes: gate-in, selective-gating, and gate-out (Fig. 1B). When in the gate-in mode, the system allows a sensory stimulus to be stored as a memory into the network. The selective-gating mode corresponds to maintaining previously encoded memories, while blocking the loading of new sensory stimuli into the memory store. In the gate-out mode, the system impedes any memory load or maintenance. We propose that the WM system selects the gating modes by controlling input oscillations projected to the network (Fig. 1B).
Oscillations Influence Persistent Activity in a Frequency-Dependent Manner.
We determine the dynamic gating modes (gate-in, selective-gating, and gate-out) using a single population model to characterize how oscillations impact reverberant sustained spiking activity. The individual neurons are in an excitable regime such that the network is bistable and can be activated in a persistent state or deactivated to the resting state (Materials and Methods). In addition to the recurrent excitation and constant inhibition, the neurons receive excitatory random external spiking inputs from independent sources, the firing rate of which is modulated by a global input oscillation of frequency  .
.
We find that when the input oscillations are within the beta–gamma band (e.g., 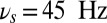 ), the persistent state can be both activated by the sensory stimulus and maintained in the face of the input oscillation (Fig. 2A). In dynamical terms, the network is in a regime with two stable states: the ground state (with a low random activity) and a persistent state. The sensory stimulus effectively transitions the network from the ground to the persistent state. The other two bands put the network into more nuanced dynamical states. When the oscillations start after the activation of the persistent state by the transient sensory stimulus, the theta-band oscillations (e.g.,
), the persistent state can be both activated by the sensory stimulus and maintained in the face of the input oscillation (Fig. 2A). In dynamical terms, the network is in a regime with two stable states: the ground state (with a low random activity) and a persistent state. The sensory stimulus effectively transitions the network from the ground to the persistent state. The other two bands put the network into more nuanced dynamical states. When the oscillations start after the activation of the persistent state by the transient sensory stimulus, the theta-band oscillations (e.g., 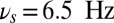 ) do not perturb the persistent memory trace (no erasing). Alpha-band oscillations (e.g.,
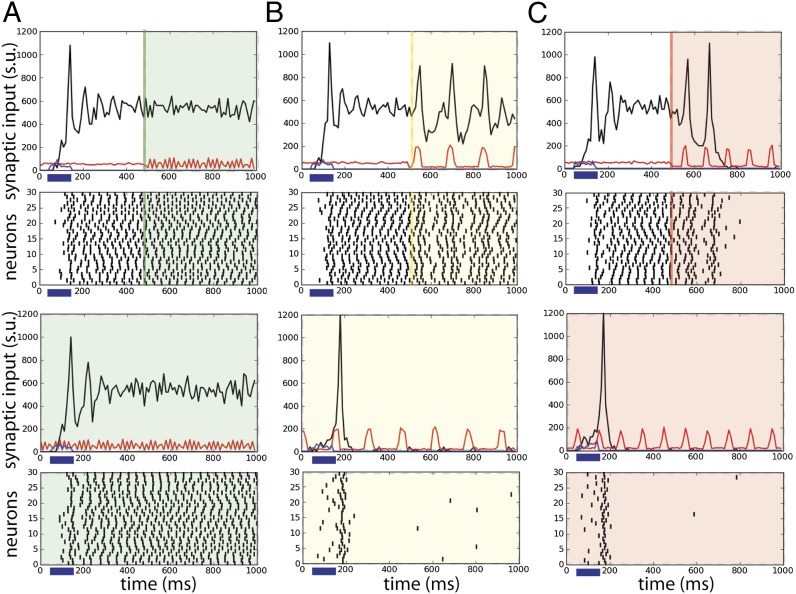
) do not perturb the persistent memory trace (no erasing). Alpha-band oscillations (e.g., 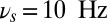 ), on the other hand, efficiently deactivate the persistent activity (erasing the trace). When the oscillations are present before and during the stimulus presentation, both the theta and alpha bands prevent the activation of the persistent activity. Thus, the theta oscillations put the network into a dynamical regime where ongoing persistent activity that has been activated before the onset of theta remains stable, yet new transitions from the ground state to the persistent state are effectively forbidden or selectively gated. Alpha oscillations, on the other hand, define a dynamical regime for the network where no persistence is stable, and only the ground state is possible. Hence theta-band selectively prevents activation yet ensures persistence (example shown in Fig. 2B), but the alpha band efficiently assures clearance (Fig. 2C). It is interesting to find that the oscillation-induced erasing is not due to a decrease excitation during the oscillatory input (Fig. S1).
), on the other hand, efficiently deactivate the persistent activity (erasing the trace). When the oscillations are present before and during the stimulus presentation, both the theta and alpha bands prevent the activation of the persistent activity. Thus, the theta oscillations put the network into a dynamical regime where ongoing persistent activity that has been activated before the onset of theta remains stable, yet new transitions from the ground state to the persistent state are effectively forbidden or selectively gated. Alpha oscillations, on the other hand, define a dynamical regime for the network where no persistence is stable, and only the ground state is possible. Hence theta-band selectively prevents activation yet ensures persistence (example shown in Fig. 2B), but the alpha band efficiently assures clearance (Fig. 2C). It is interesting to find that the oscillation-induced erasing is not due to a decrease excitation during the oscillatory input (Fig. S1).
Fig. 2.
Frequency-dependent influence of oscillations on the reverberant spiking persistent state. The network responses to a transient excitatory external stimulus (t = 50–150 ms) depend on the frequency content of the background oscillatory input. First and third rows show the rastergram of 30 representative neurons; second and fourth rows show average population input from recurrent connections (black), background activity (red), and external stimulus (blue) in arbitrary/normalized units. For each frequency the background oscillation is switched on either after the stimulus presentation (first and second rows) or before (third and fourth rows). (A) Beta–gamma-band oscillations (here 45 Hz) are compatible with persistent state maintenance: neither erasing nor blocking is seen. (B) Theta-band oscillations (here 6.5 Hz) maintain an a priori persistent state while blocking de novo activations. (C) Alpha-band oscillations (here 10 Hz) inhibit reverberant activity: the persistent state is deactivated by oscillations onset and is prevented from being activated by the transient stimulus.
To show that the different oscillatory bands generically set the network into distinct dynamic gating regimes, we analyze two key measures of network behavior: the “erase” probability P(erase) and the “block” probability P(block). P(erase) is the fraction of trials where the oscillations erase a preactivated persistent trace. P(block) gives the fraction of trials where a de novo trace is not allowed to be turned on by a transient stimulus (Materials and Methods).
We find that P(erase) differs from P(block) as a function of 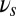 , allowing us to define functional gating modes for the network. The gate-in mode is defined by probability that the persistent activity is both activated by the sensory stimulus and is persistently maintained in the face of oscillations (the memory trace is neither erased nor blocked):
, allowing us to define functional gating modes for the network. The gate-in mode is defined by probability that the persistent activity is both activated by the sensory stimulus and is persistently maintained in the face of oscillations (the memory trace is neither erased nor blocked): 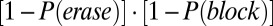 (Fig. 3B, green line). The selective-gating mode reflects the probability that an existing sustained state is maintained, yet no new activation by the sensory stimulus is allowed:
(Fig. 3B, green line). The selective-gating mode reflects the probability that an existing sustained state is maintained, yet no new activation by the sensory stimulus is allowed: 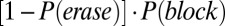 (Fig. 3B, yellow line). The gate-out mode, defining the no-memory state, is tracked by the probability that a de novo persistent state is prevented from being activated and an existing state is also deactivated:
(Fig. 3B, yellow line). The gate-out mode, defining the no-memory state, is tracked by the probability that a de novo persistent state is prevented from being activated and an existing state is also deactivated: 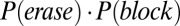 (Fig. 3B, red line).
(Fig. 3B, red line).
Fig. 3.
Gating modes of the networks determined by the oscillations frequency. (A) Erasing probability (dashed line) and blocking probability (continuous lines). Red stars correspond to the values of the oscillation in Fig. 2B and black triangles to the value in Fig. 2C. (B) Probability of the gating modes determined by the joint probability: of erase and block (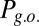 , red line), not-erase and not-block (
, red line), not-erase and not-block (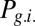 , green curve), and not-erase and block (
, green curve), and not-erase and block (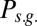 , yellow curve). The gate-out mode corresponds to domination of
, yellow curve). The gate-out mode corresponds to domination of 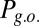 and falls in the range 8.5 Hz
and falls in the range 8.5 Hz 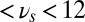 Hz with maximal value at
Hz with maximal value at 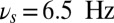 (red star). The gate-in mode corresponds to the dominance of
(red star). The gate-in mode corresponds to the dominance of 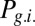 and falls in the range
and falls in the range  . The selective-gating mode corresponds to the dominance of
. The selective-gating mode corresponds to the dominance of 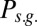 and falls in the range 4.5 Hz
and falls in the range 4.5 Hz 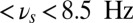 , with maximal value at
, with maximal value at 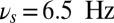 (black triangle). The filled space around the curves represent SEM.
(black triangle). The filled space around the curves represent SEM.
Our studies (Fig. 3B) show that the gate-in mode is ensured by beta–gamma-band oscillations (> 12 Hz). The selective-gating mode, and thus the ability to selectivity maintain in memory a previously loaded item, is ensured by the theta-band oscillations (3–8 Hz), in agreement with experiments showing that theta oscillations are associated with memory maintenance (4–7). Finally, the gate-out mode, where a memory cannot be either loaded nor maintained, is ensured by the alpha-band oscillations (8–12 Hz), thus in agreement with experiments showing that alpha is associated with functional inhibition (14, 15).
The frequency tunings we observed were largely invariant to modulating a number of network properties. Our extensive searches indicate that the tuning for the gating modes is stable to changes in the duty cycle of the oscillations (Fig. S2 A and E), the network size (Fig. S2C), and the synaptic strength (Fig. S2D). Of all of the parameters we explored, the membrane time constant τ had the strongest influence on the optimal frequency values for the gating modes (Fig. S2 B and F). It is interesting to note that the value of the membrane time constant that has been experimentally determined for pyramidal neurons (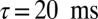 , refs. 31, 32), and that we used, also yields the frequency tuning most consistent with the experimental data on memory retention (4–7) and distractor suppression (14, 15).
, refs. 31, 32), and that we used, also yields the frequency tuning most consistent with the experimental data on memory retention (4–7) and distractor suppression (14, 15).
Execution of the Working Memory Task by Oscillation Modulation.
To assess the prototypical DMS with distractor task, we first consider a local network, presumably localized in the prefrontal cortical circuit where the persistent activity representing the two items is robust to distractors (23). Data also show that cells responding to different items, presented in the same visual hemifield, are intermixed (23, 33) so the two item-specific neuronal populations are likely modulated by the same oscillation source (Fig. 1C).
As the DMS task unfolds, the sample is presented transiently, a distractor arrives during the delay period, and the response is elicited at the end of the trial. Depending on the phase of the task, the frequency of the background oscillation shifts to optimal values (as determined by the curves in Fig. 3B) to select among the different gating modes: in the selective-gating mode 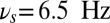 (black triangle in the figure), in the gate-out mode
(black triangle in the figure), in the gate-out mode 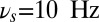 (black star in the figure), and in the gate-in mode
(black star in the figure), and in the gate-in mode 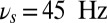 (every value > 20 Hz is acceptable in this case).
(every value > 20 Hz is acceptable in this case).
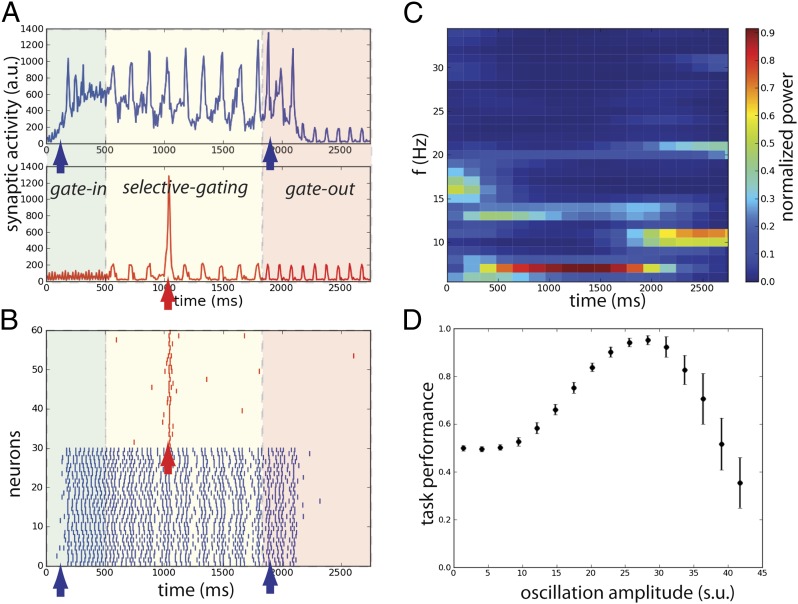
We can see that the appropriate frequency-control sequence can implement all of the operations for the DMS task (Fig. 4). The input oscillation is initially in the gamma frequency range enabling the gate-in mode and the memory in population B is loaded by the sample stimulus. After few hundred milliseconds delay, the oscillation frequency shifts to theta as in (7), switching the network into the selective-gating mode. The distractor is presented when the system is in this mode, which causes the block of activation of population R and the maintenance of memory in population B. At match presentation, the system recognizes the read-out (we stress again that we do not focus on the read-out mechanism) and the oscillation frequency is switched to alpha enabling the gate-out mode. At the match-stimulus offset, the memory in population B is cleared.
Fig. 4.
Flexible frequency control of shared oscillations implements the sequential requirements of working memory within a local network. (A) Average synaptic input simulating a local-field potential of a network performing correctly the DMS task of the population B (Upper) and population R (Lower). Colored areas represent different values of oscillations frequency: gamma band 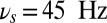 (green), theta band
(green), theta band 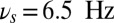 (yellow), and alpha band
(yellow), and alpha band 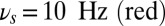 . (B) Rastergram of 30 representative neurons for each population. (C) Time-frequency power spectrum of the average synaptic input summed for populations B and R. (D) Task performance of the local network described by a psychometric curve with respect to the amplitude of the oscillations in the three gating modes. The amplitude of the oscillations is varied equally in the theta, alpha, and gamma bands.
. (B) Rastergram of 30 representative neurons for each population. (C) Time-frequency power spectrum of the average synaptic input summed for populations B and R. (D) Task performance of the local network described by a psychometric curve with respect to the amplitude of the oscillations in the three gating modes. The amplitude of the oscillations is varied equally in the theta, alpha, and gamma bands.
It is noteworthy that the network oscillations in the theta band track the input frequency during the task (see the time-frequency plot, Fig. 4C). Such theta oscillations have been observed in the WM system during the delay period and correlate positively with delay-response task performance in humans (6). Note that in Fig. 4C also the higher harmonics of the theta band (harmonic at 13 Hz) and alpha band (harmonic at 20 Hz) appear. Also it is striking that the response to the distractor is a rapid transient increase of the firing rate whose amplitude, consistent with the results of ref. 23, is qualitatively equal to the response to the item presentation at the beginning of the task (Fig. 4B): the transient sensory response to the distractor is not suppressed, yet the distractor is unable to activate the persistent memory trace.
We statistically assessed the performance of the operations of load, maintain, block distractor, and clear (Materials and Methods) finding that all of them are performed with high probability (Fig. S3A). Furthermore there is an optimal amplitude for the oscillations to maximize the overall task performance (Fig. 4D): for small amplitudes, the system is not able to block the distractor activation, so the performance is at chance, and for large values the task performance approaches zero because the oscillations perturb the item loading and maintenance of the memory trace. Finally, we found that the theta phase significantly modulates the ability of the network to block distractors, but clearing is only weakly modulated by the phase of the oscillation in the alpha range (Fig. S3 B and C).
WM Task Implementation in a Bihemispheric Network: Role of Hemisphere Lateralized Alpha-Band Oscillations in Distractor Inhibition.
In WM tasks where the stimuli are presented in separate visual hemifields, alpha-band oscillation shows lateralized increase in the distractor hemisphere contralateral to the relevant stimuli (8). This is interpreted as alpha-inhibiting areas encoding irrelevant information (note that WM is processed contralaterally, ref. 34, similarly to the visual system). Our model, properly modified to reflect such lateralization, gives a mechanistic explanation for this alpha-band modulation. We consider a bihemispheric WM system consisting of the short-memory storage compartments located in the opposite cortical hemispheres (Fig. 1D). This bihemispheric network differs from the local network above in the sense that oscillations can be modulated differently between the two cortical hemispheres.
To execute the lateralized DMS task with distractors, the oscillation frequency in the network is modulated as follows (Fig. 5). The population selective to the distractor (ipsilateral to the relevant stimulus) receives alpha-band oscillations 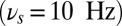 that are present from the task start, throughout the delay period until the task completion. This alpha oscillation sets the ipsilateral subnetwork into the gate-out mode. The oscillation in the relevant population B (contralateral to the relevant stimulus) starts in gamma-band frequency
that are present from the task start, throughout the delay period until the task completion. This alpha oscillation sets the ipsilateral subnetwork into the gate-out mode. The oscillation in the relevant population B (contralateral to the relevant stimulus) starts in gamma-band frequency  ensuring the gate-in mode. Then this is shifted to the theta band
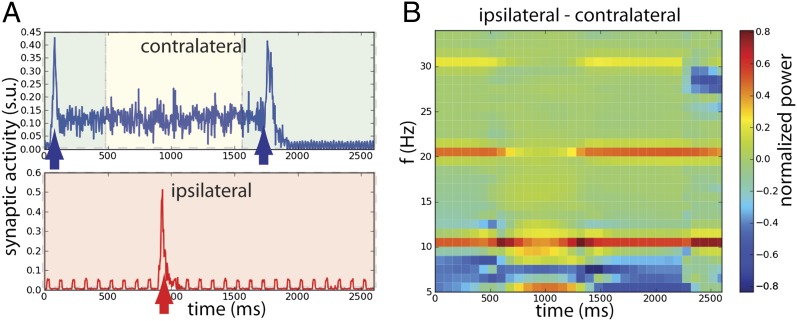
ensuring the gate-in mode. Then this is shifted to the theta band 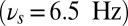 during maintenance phase of the task setting the system in a selective-gating mode. Before the end of the task at 1,550 ms, the oscillation in the contralateral population can relax back to the gamma band. The simulated local field potentials (LFPs) and the gating modes are represented together (Fig. 5A). The plot of the difference between time-frequency characteristics of the two populations (Fig. 5B) shows that there is an increase in the alpha-band power for the ipsilateral (irrelevant, Fig. S4D) vs. the contralateral (relevant, Fig. S4C) population during the task execution. Note that the small dip in the alpha-power difference at the distractor presentation is due to the transient activation of the network by this input. The gate-out mode in the ipsilateral population blocks the distractor to activate the persistent state. In contrast, the selective-gating and the gate-in modes in the contralateral population allow the loading and maintenance of the sample stimulus. The strong match stimulus directly suppresses the memory trace at the offset of match presentation, at 1,800 ms (see LFP and rastergram in Fig. 5A and Fig. S4A).
during maintenance phase of the task setting the system in a selective-gating mode. Before the end of the task at 1,550 ms, the oscillation in the contralateral population can relax back to the gamma band. The simulated local field potentials (LFPs) and the gating modes are represented together (Fig. 5A). The plot of the difference between time-frequency characteristics of the two populations (Fig. 5B) shows that there is an increase in the alpha-band power for the ipsilateral (irrelevant, Fig. S4D) vs. the contralateral (relevant, Fig. S4C) population during the task execution. Note that the small dip in the alpha-power difference at the distractor presentation is due to the transient activation of the network by this input. The gate-out mode in the ipsilateral population blocks the distractor to activate the persistent state. In contrast, the selective-gating and the gate-in modes in the contralateral population allow the loading and maintenance of the sample stimulus. The strong match stimulus directly suppresses the memory trace at the offset of match presentation, at 1,800 ms (see LFP and rastergram in Fig. 5A and Fig. S4A).
Fig. 5.
Working memory task execution in a bihemispheric network. (A) Simulated LFP (in synaptic units) of a network performing the DMS task correctly: Upper gives the LFP of the population B (contralateral) that shows persistent activity turned on by the stimulus and turned off by a second presentation of the same stimulus (match) and Lower is the LFP of the population R (ipsilateral) showing that the lateralized distractor causes only a transient network response and no persistent activity. Colored area represents different values of oscillation frequency; gamma: 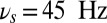 (green), theta:
(green), theta: 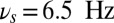 (yellow), and alpha:
(yellow), and alpha: 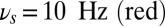 . (B) Difference in the time-frequency power spectrum of the average synaptic activity between the ipsilateral (population R) and contralateral (population B) populations showing clearly preponderance of alpha band in the R population that is irrelevant for the task. Note how this compares with data of figure 2a in ref. 8.
. (B) Difference in the time-frequency power spectrum of the average synaptic activity between the ipsilateral (population R) and contralateral (population B) populations showing clearly preponderance of alpha band in the R population that is irrelevant for the task. Note how this compares with data of figure 2a in ref. 8.
Compiling statistics for numerous sample runs for this network, we see that all of the operations are performed above chance level (Fig. S5A). In fact, the overall task performance depends critically on the alpha amplitude (Fig. S4B). For sufficiently large values of the amplitude the task is performed correctly, because the alpha-oscillation blocks the distractor loading in the ipsilateral population and drops to chance as the alpha amplitude decreases. We also found that the phase of alpha oscillations modulates the amplitude of the transient response (Fig. S5B) consistently with ref. 35. We note that for the lateralized task to be performed correctly only the alpha-band oscillations to the ipsilateral population are strictly necessary (Fig. S6).
Discussion
We propose a paradigm for online cortical information gating by flexible task contingency-dependent control of oscillations. For WM, such flexible gating allows information to be selectively loaded, maintained, blocked, or cleared. In our paradigm, externally driven oscillations set the WM store into distinct gating modes, each defined by the frequency. By shifting the gating modes as a function of the task phase, the WM system performs successfully all of the operations of a DMS task with distractors. Furthermore, we show that each of the frequency range-gating mode associations is consistent with many experimental studies on oscillations in WM.
The mechanism for gating is based on external oscillations determining the transitions between the resting state and active state, the latter corresponding to a memory stored in the attractor framework (25). The frequency of the oscillation modulates the probabilities of these transitions thereby determining three gating modes: gate-in, ensuring that a memory item can be loaded and maintained; selective-gating, ensuring that a given preloaded memory item can be maintained but no de novo items can be loaded; and gate-out, where memory can neither be loaded nor maintained.
We applied the oscillation-induced gating in a local multiitem spiking network, designed to model WM tasks where all items are represented in the same area of the prefrontal cortex. In this version of the model, the populations receive a common background input oscillation. Flexibly varying the frequency of the oscillation in time as the demands of the WM unfold enables the WM system to perform successfully all of the operations. In particular, increasing theta oscillations during the delay period allows selective memory maintenance, which is consistent with experimental observations (6, 7).
We designed a bihemispheric spiking network to explain the lateralization of alpha frequency in delayed-response tasks where relevant and irrelevant information are segregated in different hemifields (8, 16, 17). These experimental results show that alpha activity increases in the hemispheres encoding irrelevant information. In our bihemispheric model, each population receives independent external oscillations. The WM system drives an external alpha oscillation in the population encoding the irrelevant information. Because the alpha oscillations set this subnetwork in a gate-out mode the activation is blocked. In further support for external oscillations controlling the gating modes, it has been shown that repetitive transcranial magnetic stimulation (rTMS)-induced alpha directed to the parietal area, encoding relevant (irrelevant) information, decreases (increases) the task performance (8).
In the bihemispheric network, we show that the operation clear could be executed directly by the match stimulus (Fig. 5 and Fig. S6). This is likely due to transient spike-time synchronization elicited by the excitatory stimulus, as was found in spatial WM networks (36, 37). Note that although we did not describe the read-out process explicitly, previous computational work shows that this can be executed directly by the match stimulus (28). Thus, the match stimulus may result in both the read-out and the clear operations. Such unification of apparently distinguished operations is an important issue that has been addressed in the context of parametric WM (29), yet requiring ad hoc switches between excitation and inhibition. Because the match stimulus is locally directed to one population, these mechanisms would allow the selective removal of the memory of one target item in a network that is encoding multiple items simultaneously.
Pioneering models have proposed that the presence of multiple bands in WM, notably theta and gamma oscillations (38) or beta and gamma oscillations (39), subserves to maintain multiitem memory via a multiplexing mechanism that nests low-frequency with fast-frequency oscillations. However, to our knowledge, none has explained the contraposition between the theta, beta, and gamma bands associated with memory maintenance (6, 9, 10) and the alpha band associated with functional inhibition (15). In our model, the frequency ranges of the gating modes are compatible with this functional segregation of the frequency bands. Indeed, the frequency ranges of the selective-gating and gate-in modes (memory maintained) span in the theta, beta, and gamma bands. In contrast, the frequency range of the gate-out mode (memory not maintained) spans the alpha band. It is notable that the oscillation-induced gating is robust to parameter variations. The specific frequency tuning for the different gating modes is most sensitive to the intrinsic membrane time constant of the neurons. Throughout the paper we used values reported for cortical pyramidal neurons in the experimental literature (31) and notably from primate prefrontal cortex (PFC) (32).
This work predicts that the probability to block a distractor or erase a memory during WM would be differentially modulated by induced theta vs. alpha oscillations. Such oscillations could be induced by rTMSs as in ref. 8 or by presenting visual stimuli on low-contrast grating background oscillating in time at the required frequency. Furthermore, our computational analysis predicts that the theta phase strongly modulates the ability to ignore the distractors. Alpha-phase dependence has been noted recently also for sensory performance, providing a phase-dependent sampling or clocking for perception (40). It is interesting to note that we find that alpha phase modulates stimulus-evoked transients by about same amount as reported for sensory responses (SI Text, Fig. S5B). However, the distinction between the theta and alpha effects may in fact reflect the difference between the perception and the memory systems.
A central characteristic of WM is its tight capacity limitation that could stem from items either competing for discrete slots (19) or a common resource (41). What might our oscillation-induced gating imply for such a limitation? By design our model falls into the discrete slot category: we have two populations for two items. However, extended to beyond two items it would show common resource behavior: the probability of task performance decreases with item number (see Fig. S7 for details and ref. 42 for opposite behavior). By controlling the oscillatory frequency content as well as timing, one can control the performance of the constituent operations, and thereby define the performance for multiple items. Precisely how the oscillation-based gating could define the model capacity is a subject for future work.
In our model the frequency shifts are externally controlled. This is compatible with the central executive imposing correct dynamics on the WM store (43) and is supported by the theory that the PFC is a flexible modulator of the WM store (44). We de facto propose that the PFC governs the oscillatory frequency thereby controlling the WM gating modes. The lateral prefrontal cortex and its ventro-lateral portions (vLPFC) have been linked to task contingency monitoring and cognitive control (45, 46) making them a likely cortical substrate. The mechanisms for the cortical oscillatory bands are a subject of intense investigation (47). Quite likely the alpha-band activity is generated by an interplay of the thalamus and the cortex, and potentially regulated by frontocorticothalamic loops, involving the pulvinar nucleus and the reticular formation (48, 49) for flexible task-dependent alpha routing (note that the vLPFC is connected to these structures, ref. 45). Cholinergic modulation of alpha may in turn provide a task-dependent mechanism to adjust the coherence of afferent alpha in WM store (50). The theta oscillations may be generated in the PFC intrinsically and through an interplay with hippocampal oscillations (51). Corticohippocampal theta coupling increases at critical epochs of WM tasks (52) in a dopamine (DA) dependent manner. Also WM performance correlates with PFC theta coupling to the ventral tegmental area, a major source of DA (53). Put together these may suggest that flexible frequency control can be achieved by the PFC modulation of acetylcholine effects for alpha and DA effects for theta, each acting through distinct subcorticocortical loops (PFC-mesolimbic for theta and PFC-thalamic for alpha).
In conclusion, the present model is able to perform successfully a WM task with a unified gating mechanism as opposed to previous models based on unrelated mechanisms regrouped together. In addition, the model assigns a functional role to a large span of frequencies encompassing the theta, alpha, beta, and gamma bands. We finally stress that, in this paradigm, oscillations have a pure dynamical role, as opposed to other paradigms where oscillations are carrier of a population code. Because these two levels of descriptions are not mutually exclusive it would be interesting to combine them to further progress in understanding the role of brain oscillations.
Materials and Methods
We study recurrently coupled networks of spiking neurons in an excitable regime. Neurons receive excitatory inputs from three sources: recurrent inputs from neurons in the network, inputs from external sensory stimuli, and inputs from nonspecific background inputs. The background inputs are modulated by oscillations where we controlled, depending on the phase of the task, the frequency 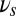 . Full information on neuron and oscillation equations can be found in the SI text.
. Full information on neuron and oscillation equations can be found in the SI text.
We consider several related networks. The first model is a single-population unit network of recurrently coupled excitatory pyramidal neurons. The second model is made up of two independent recurrent excitatory populations that receive background activity modulated by a common oscillation (Fig. 1C). This network represents a “local” two-item discrete WM network. The third model is equal to the second model except in that each population receives a background input that is modulated by an independent oscillation (Fig. 1D). This network represents a “bihemispheric” two-item discrete WM network. Additional information on network parameters, on task performances, and on the numerical analysis can be found in the SI Text.
Supplementary Material
Acknowledgments
The authors thank Romain Brette, Ole Jensen, Christian Machens, and Catherine Tallon-Baudry for constructive discussions. M.D. was partially supported by Ministère de l′Einsegnement Supérieur et de la Recherche (France), Institut National de la Santé et de la Recherche Médicale, and Ecole Normale Superieure. B.S.G. was partially supported by Centre National de la Recherche Scientifique (CNRS), Agence Nationale de la Recherche Grant Dopanic, a CNRS Neuroinformatique et Neurosciences Computationnelles Grant, Neuropole Ile de France, an Ecole de Neuroscience de Paris collaborative grant, and LABEX Institut des Etudes Cognitives.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303270110/-/DCSupplemental.
References
- 1.Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12(2):105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 2.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13(2):121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 3.Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7(4):374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- 4.Tesche CD, Karhu J. Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci USA. 2000;97(2):919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghavachari S, et al. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21(9):3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15(8):1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45(1):147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Sauseng P, et al. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009;19(21):1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 9.Spitzer B, Wacker E, Blankenburg F. Oscillatory correlates of vibrotactile frequency processing in human working memory. J Neurosci. 2010;30(12):4496–4502. doi: 10.1523/JNEUROSCI.6041-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced γ-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18(11):4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard MW, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13(12):1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 12.Pipa G, et al. Performance- and stimulus-dependent oscillations in monkey prefrontal cortex during short-term memory. Front Integr Neurosci. 2009;3:25. doi: 10.3389/neuro.07.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T. ‘Paradoxical’ alpha synchronization in a memory task. Brain Res Cogn Brain Res. 1999;7(4):493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 14.Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9-12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12(8):877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- 15.Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27(12):3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Der Werf J, Jensen O, Fries P, Medendorp WP. Gamma-band activity in human posterior parietal cortex encodes the motor goal during delayed prosaccades and antisaccades. J Neurosci. 2008;28(34):8397–8405. doi: 10.1523/JNEUROSCI.0630-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimault S, et al. Oscillatory activity in parietal and dorsolateral prefrontal cortex during retention in visual short-term memory: Additive effects of spatial attention and memory load. Hum Brain Mapp. 2009;30(10):3378–3392. doi: 10.1002/hbm.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller GA. The magical number seven plus or minus two: Some limits on our capacity for processing information. Psychol Rev. 1956;63(2):81–97. [PubMed] [Google Scholar]
- 19.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390(6657):279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 20.Wang X-J. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24(8):455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- 21.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(3997):652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 22.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 23.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romo R, Brody CD, Hernández A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399(6735):470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 25.Hebb DO. Organization of Behavior: A Neuropsychological Theory. New York: John Wiley and Sons; 1949. [Google Scholar]
- 26.Wilson HR, Cowan JD. A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik. 1973;13(2):55–80. doi: 10.1007/BF00288786. [DOI] [PubMed] [Google Scholar]
- 27.Amit DJ, Brunel N. Model of global spontaneous activity and local structured activity during delay periods in the cerebral cortex. Cereb Cortex. 1997;7(3):237–252. doi: 10.1093/cercor/7.3.237. [DOI] [PubMed] [Google Scholar]
- 28.Brunel N, Wang X-J. Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. J Comput Neurosci. 2001;11(1):63–85. doi: 10.1023/a:1011204814320. [DOI] [PubMed] [Google Scholar]
- 29.Machens CK, Romo R, Brody CD. Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science. 2005;307(5712):1121–1124. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- 30.Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254(5036):1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- 31.McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Burgos G, Rotaru DC, Zaitsev AV, Povysheva NV, Lewis DA. GABA transporter GAT1 prevents spillover at proximal and distal GABA synapses onto primate prefrontal cortex neurons. J Neurophysiol. 2009;101(2):533–547. doi: 10.1152/jn.91161.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuster JM, Jervey JP. Inferotemporal neurons distinguish and retain behaviorally relevant features of visual stimuli. Science. 1981;212(4497):952–955. doi: 10.1126/science.7233192. [DOI] [PubMed] [Google Scholar]
- 34.Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: Evidence for mnemonic “scotomas”. J Neurosci. 1993;13(4):1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dugué L, Marque P, VanRullen R. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J Neurosci. 2011;31(33):11889–11893. doi: 10.1523/JNEUROSCI.1161-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laing CR, Chow CC. Stationary bumps in networks of spiking neurons. Neural Comput. 2001;13(7):1473–1494. doi: 10.1162/089976601750264974. [DOI] [PubMed] [Google Scholar]
- 37.Gutkin BS, Laing CR, Colby CL, Chow CC, Ermentrout GB. Turning on and off with excitation: the role of spike-timing asynchrony and synchrony in sustained neural activity. J Comput Neurosci. 2001;11(2):121–134. doi: 10.1023/a:1012837415096. [DOI] [PubMed] [Google Scholar]
- 38.Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267(5203):1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 39.Kopell N, Whittington MA, Kramer MA. Neuronal assembly dynamics in the beta1 frequency range permits short-term memory. Proc Natl Acad Sci USA. 2011;108(9):3779–3784. doi: 10.1073/pnas.1019676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci USA. 2010;107(37):16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bays PM, Husain M. Dynamic shifts of limited working memory resources in human vision. Science. 2008;321(5890):851–854. doi: 10.1126/science.1158023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Z, Wang X-J, Wang D-H. From distributed resources to limited slots in multiple-item working memory: A spiking network model with normalization. J Neurosci. 2012;32(33):11228–11240. doi: 10.1523/JNEUROSCI.0735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baddeley AD, Hitch G. Recent developments in working memory. Curr Opin Neurobiol. 1998;8(2):234–238. doi: 10.1016/s0959-4388(98)80145-1. [DOI] [PubMed] [Google Scholar]
- 44.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 45.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 47.Wang X-J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90(3):1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saalmann YB, Kastner S. Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol. 2009;19(4):408–414. doi: 10.1016/j.conb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71(2):209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso A, Khateb A, Fort P, Jones BE, Mühlethaler M. Differential oscillatory properties of cholinergic and noncholinergic nucleus basalis neurons in guinea pig brain slice. Eur J Neurosci. 1996;8(1):169–182. doi: 10.1111/j.1460-9568.1996.tb01178.x. [DOI] [PubMed] [Google Scholar]
- 51.Fujisawa S, Buzsáki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72(1):153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benchenane K, et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 2010;66(6):921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20(10):3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.