Abstract
Hepatic insulin resistance is a principal component of type 2 diabetes, but the cellular and molecular mechanisms responsible for its pathogenesis remain unknown. Recent studies have suggested that saturated fatty acids induce hepatic insulin resistance through activation of the toll-like receptor 4 (TLR-4) receptor in the liver, which in turn transcriptionally activates hepatic ceramide synthesis leading to inhibition of insulin signaling. In this study, we demonstrate that TLR-4 receptor signaling is not directly required for saturated or unsaturated fat-induced hepatic insulin resistance in both TLR-4 antisense oligonucleotide treated and TLR-4 knockout mice, and that ceramide accumulation is not dependent on TLR-4 signaling or a primary event in hepatic steatosis and impairment of insulin signaling. Further, we show that both saturated and unsaturated fats lead to hepatic accumulation of diacylglycerols, activation of PKCε, and impairment of insulin-stimulated IRS-2 signaling. These data demonstrate that saturated fat-induced insulin resistance is independent of TLR-4 activation and ceramides.
The development of hepatic insulin resistance is closely linked to ectopic lipid deposition, obesity and nonalcoholic fatty liver disease (NAFLD) and is a major factor in the pathogenesis of type 2 diabetes, leading to increased risk of dyslipidemia, hypertension, and cardiovascular disease (1, 2). However, the cellular mechanism responsible for this phenomenon is unknown. Recently, two main schools of thought have gained support. In one, an imbalance between lipid supply/synthesis relative to rates of fatty acid oxidation or conversion of diacylglycerols (DAGs) to triacylglycerols (TAGs) in the liver results in net accumulation of DAGs. This then leads to activation and membrane translocation of PKCε and consequently inhibition of insulin-stimulated insulin receptor kinase phosphorylation of IRS proteins and an impaired activation of the downstream insulin-signaling cascade (3–10). Dietary fat sources containing a relatively high proportion of saturated fat include animal products such as lard (∼35–40% of total fat from saturated fat) and heavy cream (∼65% of total fat from saturated fat), although unsaturated fats are prevalent in vegetable products such as safflower oil (∼90–100% of total fat from unsaturated fat). Accordingly, studies using lard oil infusions have suggested that specifically saturated fatty acids activate TLR-4 signaling through the adaptor protein MyD88 leading to activation of IκB kinase, up-regulation of de novo ceramide synthesis enzymes, synthesis of ceramides, and ceramide-induced activation of protein phosphatase 2A, which directly inhibits insulin signaling at the level of protein kinase B (Akt) phosphorylation (11, 12). In this model, TLR-4 receptor signaling (12) and ceramide synthesis (13) are both critical for saturated fat-induced insulin hepatic resistance. However, unsaturated fat-induced insulin resistance is not dependent on the TLR-4 receptor (12) or ceramide synthesis (13, 14). The aim of our study was to test the hypothesis that overconsumption of saturated fats leads to hepatic insulin resistance through a specific mechanism involving activation of the TLR-4/MyD88 pathway and ceramide accumulation and not through the previously established DAG-PKCε–dependent mechanism that is general to all fatty acids.
Results
Saturated and Unsaturated Fat Feeding Results in Hepatic DAG Accumulation, PKCε Activation, and Impairment of Insulin Signaling, but Not Increased Hepatic Ceramides.
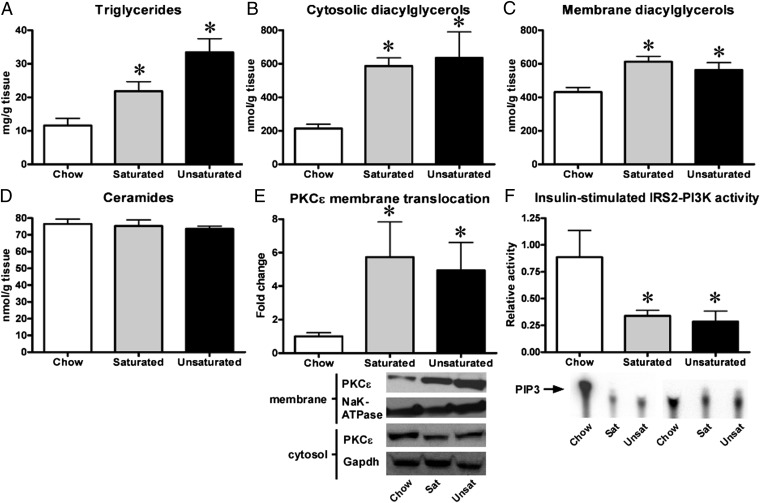
We studied male Sprague-Dawley rats fed a high-fat diet for 3 d, a well-established model of primary lipid-induced hepatic insulin resistance (15). To assess the response to a diet rich in either saturated or unsaturated fatty acids, we fed these rats either a lard- or a safflower-based diet. We investigated the accumulation of relevant lipid metabolites and assessed hepatic insulin signaling in these rats. Neither diet affected body weight. However, both diets resulted in an increase in plasma fatty acid concentrations (100–200 μM) and a mild increase in fasting plasma glucose concentrations (20–30 mg/dL). Fat feeding led to development of hepatic steatosis with a two- to threefold increase in liver triglyceride content (Fig. 1A), a threefold increase in cytosolic liver diacylglycerols (Fig. 1B and Fig. S1), and a 40–50% increase in membrane diacylglycerols (Fig. 1C, Fig. S1), but surprisingly, neither saturated nor unsaturated fat feeding resulted in increased liver ceramides (Fig. 1D). We did not observe an increase in mRNA expression of any enzymes involved in de novo ceramide synthesis with fat feeding (Table S1). We found that the increased hepatic diacylglycerol levels were associated with an approximately fivefold increase in PKCε translocation to the plasma membrane (Fig. 1E). In accordance with this, insulin-stimulated IRS2-associated PI3-kinase activity (Fig. 1F) was decreased by 60–75% with both types of fat diet. In response to insulin-stimulated PI3-kinase activity, Akt translocates to the plasma membrane, which is an essential step in the activation of Akt (16). Upon activation, Akt then translocates to the nucleus and cytosol to phosphorylate various substrates (16) such as FoxO1 (17) and GSK3 (18), which are important hepatic regulators of gluconeogenesis and glycogen metabolism, respectively. Akt2 is considered to be the principal isoform in hepatic insulin action in vivo (19). Consistent with impaired PI3-kinase activity, we found that fat feeding inhibited insulin-stimulated Akt2 translocation to the plasma membrane by 30–40% (Fig. S2A). Although insulin-stimulation led to a marked increase (∼75-fold) in phosphorylated, activated nuclear Akt2 in chow-fed rats, this effect was inhibited 50–60% by fat feeding (Fig. S2B), whereas phosphorylation of the key nuclear Akt2 substrate FoxO1 was reduced by 40–50% (Fig. S2C).
Fig. 1.
Fat feeding leads to hepatic steatosis and impairment of insulin signaling in rats. Three-day high-fat feeding based on either saturated (sat.) or unsaturated (unsat.) fat resulted in a marked increase in hepatic triglycerides in (A), cytosolic (B) and membrane DAGs (C) in rats. However, neither type of fat led to an increase in hepatic ceramide content (D). The increased DAG content was associated with increased membrane translocation of PKCε (E) and an impairment of insulin-stimulated IRS2-associated PI3-K activity (F). n = 5–10 per group. *P < 0.05.
TLR-4/MyD88 Knockdown Prevents Development of Fatty Liver Through Appetite Reduction in Mice Fed Saturated Fat, but Has No Direct Effects on Hepatic Insulin Signaling.
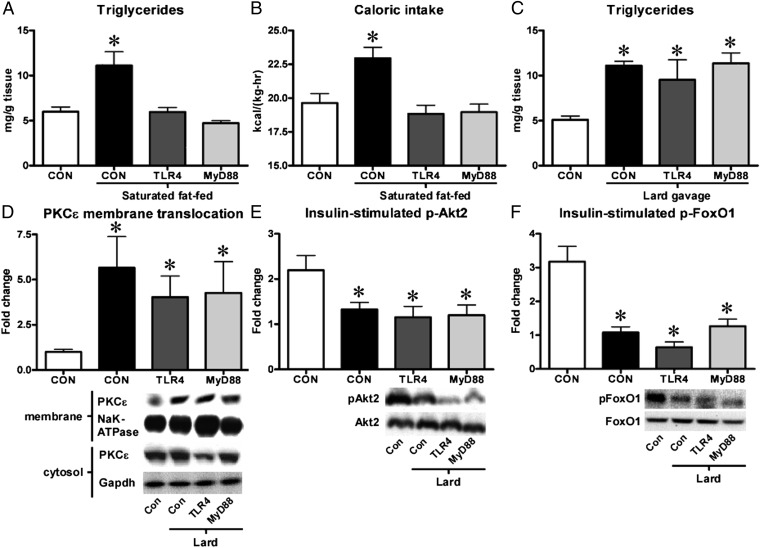
To examine the postulated direct role of TLR-4 receptor signaling specific to saturated fat-induced hepatic insulin resistance, we treated mice with antisense oligonucleotides (ASOs) targeting either TLR-4, its adaptor protein MyD88 or a control and fed them a diet rich in saturated fat for 10 d. Although fat-fed mice treated with a control ASO developed fatty liver (Fig. 2A), knockdown of either TLR-4 or MyD88 prevented hepatic steatosis from occurring (Fig. 2A). To better understand this phenotype, we performed metabolic cage studies on these mice. We found that although knockdown of TLR-4 or MyD88 did not affect energy expenditure (Fig. S3A) or the respiratory exchange ratio (Fig. S3B), it significantly lowered the caloric intake of mice fed a high-fat diet (Fig. 2B) and was associated with increased plasma levels of the anorexic cytokine TNF-α (Fig. S3C). To circumvent the effects of TLR-4 or MyD88 on appetite and examine the direct effects on insulin-stimulated Akt2 phosphorylation and activity, we decided to expose chow-fed mice to lipid gavage with saturated fat-rich lard. After 6 h, the lard gavage resulted in a threefold increase in plasma triglycerides in all mice, compared with ungavaged control mice (Fig. S4A). Lipid gavage led to a doubling of liver triglycerides (Fig. 2C) and cytosolic diacylglycerols (Fig. S4B). Following the gavage, we observed a three- to sixfold increase in membrane translocation of PKCε (Fig. 2D) as well as a 35–45% and ∼60% reduction in insulin-stimulated phosphorylation of Akt2 (Fig. 2E) and FoxO1 (Fig. 2F), respectively. These findings thus indicate that the TLR-4/MyD88 pathway is not directly eliciting the inhibitory effects of saturated fat on insulin signaling.
Fig. 2.
TLR-4/MyD88 knockdown in mice reduces caloric intake, but does not directly protect mice from saturated fat-induced defects in hepatic insulin signaling. Mice treated with ASOs against either TLR-4 or MyD88 were protected against hepatic triglyceride deposition (A) when fed a diet rich in saturated fat because of a decreased caloric intake (B). However, TLR-4/MyD88 knockdown did not protect mice from hepatic triglyceride accumulation (C), membrane translocation of PKCε (D), and impairment of insulin-stimulated Akt2 (E) and FoxO1 (F) phosphorylation following lipid gavage with lard. n = 5–10 per group. *P < 0.05. Con, gavaged control.
TLR-4-Deficient Mice Are Not Protected from Development of Fatty Liver, Ceramide and DAG Accumulation, PKCε Activation, and Hepatic Insulin Resistance When Fed a Diet Rich in Saturated Fat.
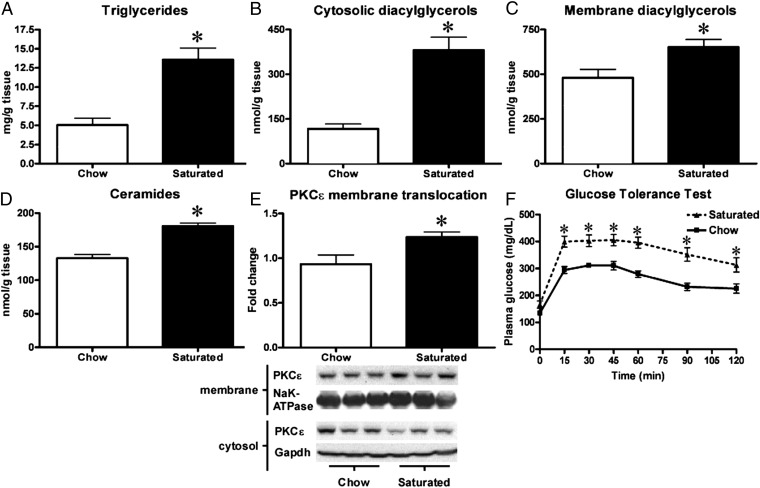
To expand on the results we had obtained through our TLR-4/MyD88-ASO studies, we decided to examine if 10ScNJ mice carrying a spontaneous deletion in the TLR-4 gene were immune to saturated fat-induced insulin resistance. Because the TLR-4 pathway had apparent effects on appetite and other groups had reported that 10ScNJ mice were less inclined to develop obesity (20), we decided to supplement the solid saturated fat diet with liquid heavy cream in the drink. Heavy cream derives ∼95% of its total calories from fat, of which ∼65% are from saturated fat. After 15 d, the saturated fat-fed mice had a significantly higher weight gain than the chow-fed mice (2.7 g ± 0.2 vs.1.3 g ± 0.2) and an increased body fat mass (4.1 g ± 0.3 vs. 1.6 g ± 0.3). Saturated fat-fed mice developed hepatic steatosis with an increase of two- to threefold in liver triglycerides (Fig. 3A), threefold in cytosolic DAGs (Fig. 3B), and ∼30% in membrane DAGs (Fig. 3C). Interestingly, we observed a ∼20% rise in hepatic ceramides in the TLR-4–deficient mice when fed a saturated fat diet (Fig. 3D). Consistent with the accumulation of DAGs, there was a ∼30% increase in activation and membrane translocation of PKCε (Fig. 3E). To assess the impact of saturated fat feeding on insulin sensitivity in TLR-4–deficient mice, we performed i.p. glucose tolerance tests (IPGTTs). The mice fed saturated fat were clearly glucose intolerant and insulin resistant, as reflected by higher plasma glucose concentrations at all time points (Fig. 3F) and higher plasma insulin concentrations in the fasted state and at 90 min (Fig. S5).
Fig. 3.
TLR-4–deficient mice are not protected from saturated fat-induced hepatic steatosis and hepatic insulin resistance. Saturated fat-feeding of TLR-4–deficient mice resulted in hepatic steatosis and an increase in hepatic triglycerides (A), cytosolic- (B), and membrane DAGs (C) as well as ceramides (D). Fatty liver development was associated with membrane translocation of PKCε (E) and insulin resistance as assessed by IPGTT (F). n = 7–10 per group. *P < 0.05.
TLR-4 Deficient Mice Develop Hepatic Insulin Resistance When Fed a Diet Rich in Saturated Fat.
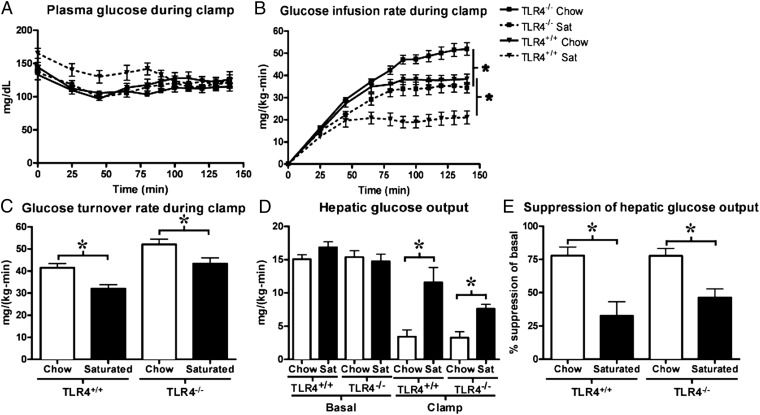
To further investigate the impact of saturated fat feeding on insulin sensitivity in the setting of TLR-4 deficiency, we performed hyperinsulinemic-euglycemic clamp experiments comparing TLR-4–deficient 10ScNJ mice fed either regular chow or saturated fat for 10 d and compared them with age- and weight-matched WT mice (10ScSnJ). To account for the documented alterations in appetite that accompany TLR-4 deficiency, we matched the weight gain in TLR-4–deficient and control mice fed saturated fat over their respective chow groups (saturated fat-fed TLR-4–deficient mice gained 1.9 g ± 0.5 and control gained 1.5 g ± 0.6, more than their respective chow groups). Although plasma glucose levels were not different during the clamp (Fig. 4A), the glucose infusion rates required to maintain euglycemia were ∼40% lower in both TLR-4–deficient and control mice when fed saturated fat compared with chow (Fig. 4B) reasserting that they were indeed insulin-resistant. Whole-body glucose turnover (Fig. 4C) was decreased by 20–30% in both TLR-4–deficient and control mice when fed saturated fat. Basal hepatic glucose production was not different; however (Fig. 4D), both the high fat fed TLR-4–deficient and control mice manifested pronounced hepatic insulin resistance (Fig. 4 D and E). Although mice fed a chow diet displayed effective suppression of glucose production during the hyperinsulinemic-euglycemic clamp (77.8 ± 6.5% for control and 77.1 ± 5.6% for TLR-4 deficient, respectively), this suppression was reduced in mice fed the saturated fat diet (to 32.5 ± 10.7% for control and 46.4 ± 6.5% for TLR-4 deficient, respectively) (Fig. 4E).
Fig. 4.
Saturated fat-fed TLR-4–deficient mice develop hepatic insulin resistance. Although plasma glucose levels were similar (A), the glucose infusion rates required to maintain euglycemia during the hyperinsulinemic-euglycemic clamp were significantly lower in both control and TLR-4–deficient mice fed saturated (sat) fat (B) compared with chow. Whole body glucose turnover was reduced 20–30% by saturated fat feeding (C). Basal hepatic glucose production was not different, but insulin’s ability to suppress hepatic glucose production was impaired in both control and TLR-4–deficient mice fed saturated fat compared with chow (D and E). n = 7–12 per group. *P < 0.05.
Discussion
The specific lipid species and molecular mechanisms by which hepatic steatosis results in hepatic insulin resistance has been a hotly debated topic. We found that overfeeding of both saturated- and unsaturated fat-rich diets activates a DAG-PKCε mechanism resulting in inhibition of insulin-stimulated, IRS-2–associated PI3-kinase activity and an impairment of downstream insulin signaling as previously described (4, 21). Recent studies have proposed that specifically saturated fatty acids cause hepatic insulin resistance through activation of TLR-4 receptor signaling (12) and ceramide synthesis (13). We did not observe an increase in liver ceramides by feeding rats a 3-d high-fat diet enriched with either saturated or unsaturated fat, thus suggesting that ceramide accumulation is not a primary event in the development of lipid-induced hepatic insulin resistance or required for lipid-induced impairment of insulin signaling. Although LPS is known to bind and activate the TLR-4 receptor (22) and induce ceramide synthesis (23), it has been controversial whether saturated fatty acids bind and activate the receptor (24). Fetuin-A has been suggested to act as an adaptor protein mediating the interaction between saturated fatty acids and TLR-4 receptor (25). Although previous studies have clearly established an integral role of the TLR-4 receptor in mediating innate immunity (26, 27), our findings, both in mice treated with antisense oligonucleotides targeting TLR-4 and its adaptor protein MyD88 as well as in TLR-4–deficient mice, clearly demonstrate that TLR-4 does not mediate the direct actions of any lipids in causing hepatic insulin resistance. We did, however, note clear effects of TLR-4 signaling in the regulation of appetite, which is consistent with other recent studies (28). Studies that have implicated TLR-4 and ceramides in mediating saturated fat-induced insulin resistance in vivo have relied heavily on data obtained through systemic lard oil and fatty acid infusions (12, 13, 29), an approach that is likely to provoke an unphysiological inflammatory response—especially given the high degree to which common laboratory reagents, especially those used to complex fatty acids, are contaminated with bacterial lipopeptides and LPS (24). By feeding rats either a lard- or safflower-based diet, we were able to directly, and under physiological conditions, evaluate which specific lipid species accumulate in the liver, and through which mechanisms these cause impairment of hepatic insulin action. Under these conditions, we found that in contrast to hepatic ceramide content and regardless of the nature of the source of fat, lipid-induced hepatic insulin resistance is associated with increased hepatic diacylglycerol accumulation. This was accompanied by increased PKCε signaling and impairment of downstream insulin receptor kinase signaling—a mechanism that has also recently been implicated in hepatic insulin resistance in humans (30, 31). Studies have implicated inflammatory pathways in the etiology of hepatic insulin resistance (32), sepsis is known to be associated with insulin resistance (33, 34), and inflammatory cytokines have been found to be elevated in obesity (35–37) and capable of impairing hepatic insulin sensitivity (38, 39). However, a recent study, using several strains of immune-deficient mice found that these mice were not protected from hepatic insulin resistance induced by short-term high-fat feeding (40). Taken together with our findings, this would suggest that although there may be an associative relationship between obesity and inflammation, the latter is likely not a primary driver of lipid-induced hepatic insulin resistance. In conclusion, our studies identify that DAG-PKCε signaling, not the TLR-4–ceramide pathway, is the key trigger in both saturated fatty acid and unsaturated fatty acid-induced hepatic insulin resistance and support previous studies in both animals and humans that have highlighted the therapeutic potential of targeting the DAG-PKCε signaling mechanism in treating hepatic insulin resistance.
Methods
Animals.
Sprague-Dawley rats (180 g) were purchased from Charles River, C57/BL6, 10ScSnJ (stock 000476); 10ScNJ (stock 003752) mice were purchased from Jackson Laboratories at 10 and 7–8 wk of age, respectively. All animals were males. The animals were housed at Yale University School of Medicine and maintained in accordance with the Institutional Animal Care and Use Committee guidelines.
Antisense oligonucleotides.
Antisense oligonucleotides (ISIS Pharmaceuticals) were injected i.p. every other day for 3 wk before experimentation. ASO sequences were TLR-4: CCACATTGAGTTTCTTTAAG and MyD88: TACACTTGACCCAGGTTGCT. Knockdown was between 65% and 90% as validated by Western blotting and/or quantitative PCR.
Diets.
The unsaturated fat-rich safflower-based diet was 112245 from Dyets (∼0–1% myristate, ∼5% palmitate, ∼2% stearate, ∼12% oleate, ∼80% linoleate). The saturated fat-rich lard-based diet was D12492 from Research Diets (∼1%, myristate, ∼20% palmitate, ∼12% stearate, ∼34% oleate, ∼28% linoleate). Both diets contained 60% kcal from fat. Heavy cream contained ∼12% myristate, ∼31% palmitate, ∼11% stearate, ∼24% oleate, and ∼3% linoleate (molar ratio).
Acute Rat Insulin Infusions.
For acute insulin signaling experiments, catheterized rats were given a primed (200 mU/kg) continuous (4 mU⋅kg−1⋅min−1) infusion of insulin (Novolin, Novo Nordisk) for 20 min.
Hyperinsulinemic-Euglycemic Clamp.
Were performed as previously described (41). Briefly, following an overnight fast, catheterized mice were infused with 3-[3H]glucose at a rate of 0.05 μCi/min for 120 min to determine basal glucose turnover. Next, a primed infusion of insulin and 3-[3H]glucose was administered at 7.14 mU⋅kg−1⋅min−1 and 0.24 μCi/min, respectively, for 4 min, after which the rates were reduced to 3 mU⋅kg−1⋅min−1 insulin and 0.1 μCi/min 3-[3H]glucose for the remainder of the experiment. Mean plateau insulin levels in mice were between 40.7 and 42.5 μU/mL for all groups. Blood was collected via tail massage for plasma glucose, insulin, and tracer levels at set time points during the 140-min infusion, and a variable infusion of 20% dextrose was given to maintain euglycemia. A 10-μCi bolus injection of [14C]2-deoxyglucose was given at 90 min to determine tissue-specific glucose uptake.
IPGGT.
Overnight fasted mice were injected intraperitoneally with 1 mg/g glucose, and blood was collected by tail bleed at set times for plasma insulin and glucose measurements.
Lard Gavage.
Following an overnight fast, catheterized mice were given an oral gavage of lard (400 μL/25 g body weight) and allowed to rest for 6 h. The mice were then given a primed infusion of insulin (7.14 mU⋅kg−1⋅min−1) for 4 min, after which the rate was reduced (3 mU⋅kg−1⋅min−1 insulin).
Body Composition and Metabolic Cage Studies.
Body composition was determined by 1H magnetic resonance spectroscopy (Bruker Minispec). The comprehensive laboratory animal monitoring system (Columbus Instruments) was used to evaluate activity, energy expenditure, feeding, drinking, and respiratory quotient over the course of 48 h. The data are the 24-h averages normalized to body weight.
Quantitative PCR.
RNA was isolated using an RNeasy kit (Qiagen) according to the manufacturer's instructions and then used for cDNA synthesis using a RT-PCR kit (Qiagen). Quantitative PCR was performed with the Applied Biosystems 7500 Fast Real Time-PCR system and SYBR Green detection reagent.
Western Blotting.
Cytoplasmic and nuclear fractionation was performed with the NE-PER kit (Pierce). PKCε activity was determined as previously described (4). Secondary antibodies were HRP-coupled and ECL reagent (BioVision) was used for detection. Quantification was performed using ImageGauge 4.0 (Fujifilm). For all signaling data, insulin-stimulated values were normalized to the basal values of the same experimental group.
PI3-kinase Activity Assay.
Performed using the method previously described (42). Briefly, liver protein lysates were prepared (in 50 mM Tris⋅HCl, pH 7.4; 1% Triton X-100; 150 mM NaCl; 1% glycerol, 10 mM EDTA, 20 mM NaP2O7, 200 mM NaF, proteinase inhibitors, and 4 mM Na3VO4). Four milligrams of protein per sample were immunoprecipitated with anti -IRS-2 antibody and the resulting immunocomplexes were washed thoroughly in PBS (1% Nonidet P-40, 0.1 mM Na3VO4) and subsequently in 100 mM Tris (pH 7.5, 500 mM LiCl, and 0.1 mM Na3VO4), and 10 mM Tris (pH 7.5, 0.1 M NaCl, 1 mM EDTA, 0.1 mM Na3VO4). A total of 10 μL of 100 mM MgCl2 was added and the complex was heated to room temperature. Next, 10 µL of 2 μg/μL phosphatidylinositol (in 10 mM Tris, pH 7.5, 1 mM EGTA and then 30 μCi/10 µL of [32P] was added. The reaction was stopped after 10 min by the addition of 20 µL of 8 M HCl. The mixture was separated by CHCL3:MeOH (1:1) and the bottom fraction was run on TLC plates before being exposed to X-ray film at −80 °C. Quantification was performed using ImageGauge 4.0 (Fujifilm).
Hepatic Metabolite Analyses.
Hepatic triglycerides were extracted using a methanol/chloroform-based method (4) and quantified with a colorimetric kit (Genzyme); diacylglycerols and ceramide levels were measured as previously described (4, 43).
Biochemical Analyses.
Plasma glucose concentrations were measured by a glucose oxidase method using a Beckman Glucose Analyzer II. Plasma insulin was measured by RIA kit (Linco Research), and fatty acids were measured by a spectrophotometric technique (Wako NEFA Kit).
Statistical Analysis.
All data are reported as mean ± SEM. Comparisons between groups were made using unpaired two-tailed Student t tests and ANOVA where appropriate. A value of P < 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism 6 software.
Supplementary Material
Acknowledgments
We thank Jianying Dong and Vara Prasad Manchem for their excellent technical support. This project was supported by US Public Health Service Grants R24 DK-085638, R01 DK-40936, P30 DK-45735, and U24 DK-059635 and Veterans Administration Merit Review Award 5I01BX000901.
Footnotes
Conflict of interest statement: S.B. is an employee of ISIS and may own stock in the company.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311176110/-/DCSupplemental.
References
- 1.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 2.Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2010;26(3):202–208. doi: 10.1097/MOG.0b013e328337b0c4. [DOI] [PubMed] [Google Scholar]
- 3.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab. 2012;15(5):574–584. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel VT, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279(31):32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 5.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 8.Boden G, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54(12):3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 9.Neschen S, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2(1):55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Kim JK, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98(13):7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5):585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Holland WL, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121(5):1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Holland WL, et al. Lipid mediators of insulin resistance. Nutr Rev. 2007;65(6 Pt 2):S39–S46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 15.Kraegen EW, et al. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40(11):1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 16.Andjelković M, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272(50):31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003;100(20):11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 19.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 20.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16(6):1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 21.Samuel VT, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117(3):739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poltorak A, et al. Genetic and physical mapping of the Lps locus: Identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis. 1998;24(3):340–355. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 23.MacKichan ML, DeFranco AL. Role of ceramide in lipopolysaccharide (LPS)-induced signaling. LPS increases ceramide rather than acting as a structural homolog. J Biol Chem. 1999;274(3):1767–1775. doi: 10.1074/jbc.274.3.1767. [DOI] [PubMed] [Google Scholar]
- 24.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29(11):1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 25.Pal D, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. July 29, 2012 doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 26.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 28.Orr JS, et al. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61(11):2718–2727. doi: 10.2337/db11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magkos F, et al. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology. 2012;142(7):1444–1446 e2. doi: 10.1053/j.gastro.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumashiro N, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2011;108(39):16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clowes GH, Jr, et al. Blood insulin responses to blood glucose levels in high output sepsis and septic shock. Am J Surg. 1978;135(4):577–583. doi: 10.1016/0002-9610(78)90040-5. [DOI] [PubMed] [Google Scholar]
- 34.Iochida LC, Tominaga M, Matsumoto M, Sekikawa A, Sasaki H. Insulin resistance in septic rats—a study by the euglycemic clamp technique. Life Sci. 1989;45(17):1567–1573. doi: 10.1016/0024-3205(89)90423-2. [DOI] [PubMed] [Google Scholar]
- 35.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 36.Bunout D, et al. Interleukin 1 and tumor necrosis factor in obese alcoholics compared with normal-weight patients. Am J Clin Nutr. 1996;63(3):373–376. doi: 10.1093/ajcn/63.3.373. [DOI] [PubMed] [Google Scholar]
- 37.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 38.Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130(1):43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- 39.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52(11):2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 40.Lee YS, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60(10):2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jurczak MJ, et al. Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012;287(4):2558–2567. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folli F, Saad MJ, Backer JM, Kahn CR. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J Biol Chem. 1992;267(31):22171–22177. [PubMed] [Google Scholar]
- 43.Jurczak MJ, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes. 2011;60(3):890–898. doi: 10.2337/db10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.