SUMMARY
Non-typhoidal Salmonella are an important but poorly characterized cause of paediatric diarrhoea in developing countries. We conducted a hospital-based case-control study in children aged <5 years in Ho Chi Minh City to define the epidemiology and examine risk factors associated with Salmonella diarrhoeal infections. From 1419 diarrhoea cases and 571 controls enrolled between 2009 and 2010, 77 (5·4%) diarrhoea cases were stool culture-positive for non-typhoidal Salmonella. Salmonella patients were more likely to be younger than controls (median age 10 and 12 months, respectively) [odds ratio (OR) 0·97; 95% confidence interval (CI) 0·94–0·99], to report a recent diarrhoeal contact (8·1% cases, 1·8% controls; OR 5·98, 95% CI 1·8–20·4) and to live in a household with >2 children (cases 20·8%, controls 10·2%; OR 2·32, 95% CI 1·2–4·7). Our findings indicate that Salmonella are an important cause of paediatric gastroenteritis in this setting and we suggest that transmission may occur through direct human contact in the home.
Key words: Bacterial infections, epidemiology, paediatrics, Salmonella
INTRODUCTION
Diarrhoea is the second leading cause of death in children aged <5 years globally with an estimated 2·5 billion cases and 1·5 million deaths in this key age group annually [1]. Developing countries bear the brunt of diarrhoeal disease burden in both mortality and morbidity [2, 3]. The non-typhoidal members of the bacterial genus Salmonella (i.e. excluding Salmonella Typhi and Salmonella Paratyphi pathovars) represent a significant public health problem in industrializing and industrialized countries and have been estimated to account for 1–5% of all cases of gastroenteritis in the former group [3, 4]. However the source and transmission of gastrointestinal non-typhoidal Salmonella infections in developing countries are not well documented [5]. Infants and young children suffer substantially more from the effects of Salmonella infections compared to other age groups and are therefore also at higher risk of secondary complications [6]. Obtaining local, specific epidemiological data on enteric pathogens such as Salmonella is crucial to understand and combat paediatric diarrhoea [7].
Vietnam is a rapidly developing country with a population of more than 88 million people (over seven million are aged <5 years), a child mortality rate of 24/1000 live births and an average per capita income of about US$1100 per year [1, 8]. Diarrhoea is the seventh most common cause of death in children aged <5 years in Vietnam and accounts for 4% of the deaths in this age group per year [9].
To assess important epidemiological aspects of diarrhoeal disease in children aged <5 years resident in Ho Chi Minh City, Vietnam, we conducted a large hospital-based case-control study. Here, we present results regarding the prevalence, clinical presentation, treatment and risk factors associated with symptomatic Salmonella diarrhoeal infections in hospitalized children in this large, urban, industrializing setting.
METHODS
Study sites
This study was conducted at three hospitals in central Ho Chi Minh City. The Hospital for Tropical Diseases (HTD) is the tertiary referral hospital for infectious diseases in Ho Chi Minh City and surrounding provinces in southern Vietnam. Children's Hospital 1 (CH1) and Children's Hospital 2 (CH2) are the two main paediatric hospitals in Ho Chi Minh City.
Cases
All children aged <5 years with acute diarrhoeal disease admitted to the Gastrointestinal Department of CH1, CH2 and HTD from May 2009 to April 2010 in Ho Chi Minh City were eligible for participation in the study. We defined a case of diarrhoea as ⩾3 loose stools or at least one bloody loose stool within a 24-h period. We defined a case of Salmonella infection as a diarrhoeal infection stool culture-positive for Salmonella spp. Due to resource and personnel constraints, the first five patients meeting the inclusion criteria from each of the three study sites on weekdays were included in the study. Children were excluded if they did not live within Ho Chi Minh City, had been pre-treated with antimicrobials, had multiple complications unrelated to diarrhoeal disease or did not have informed consent of the parent or guardian.
Controls
Controls were children aged <5 years from Ho Chi Minh City attending the nutrition wards of CH1 and CH2 from March 2010 to December 2010. Children who had symptoms of diarrhoea or respiratory illness, were currently on an antimicrobial regimen, were aged >60 months, did not live in a district of Ho Chi Minh City or did not have informed consent of the parent or guardian were excluded. There was no limit on the number of controls enrolled daily.
Sample collection and questionnaire
Stool specimens for both cases and controls were collected on the day of admission to hospital and prior to any prescribed antimicrobial therapy. All samples were stored in a refrigerator (4 °C) and were transported on the same day to the laboratory for analysis. Treating clinicians were asked to complete a simple case report form for information on symptoms and duration of disease. In addition, a short questionnaire was administered to the child's parent or guardian by study nurses to gather information on basic demographics, socioeconomic indicators and potential risk factors for infection. We additionally recorded the location of the residences of the children with Salmonella infections using a GPS receiver. Addresses were anonymized to ensure patient confidentiality. Climate data was obtained from the Vietnam Southern Regional Meteorological Station.
Microbiology
Stool specimens were cultured on MacConkey agar (MC, Oxoid), xylose-lysine-deoxycholate agar (XLD, Oxoid), and selenite broth (Oxoid) media and incubated at 37 °C overnight. The overnight selenite broth, which nourished possible Salmonella, was subcultured on MC and XLD agars for identification of Salmonella organisms. Identification of potential Salmonella was performed using conventional biochemical (API 20E biochemical strip tests) according to the standard operating procedure. All isolated pathogens were recorded and kept for future testing by freeze drying and storage at −20 °C.
Data analysis
Data were entered into a database using Excel 2007 (Microsoft, USA) and analysed using Stata/IC version 9.2 (StataCorp., USA). χ2 and Fisher's exact tests were used to compare proportions between groups and Mann–Whitney U tests were used for non-parametric data. Univariate analyses were performed to assess factors associated with symptomatic non-typhoidal Salmonella infection. Factors found to be significantly associated with infection in the univariate analysis were then included in a multivariate logistic regression model to simultaneously control for any effects of confounding. Evidence of effect modification was also investigated to determine if the effect of one exposure on the outcome of Salmonella infection was influenced by other exposures of interest. Two-sided P values ⩽0·05 were considered statistically significant throughout. ArcMap software version 9.2 (ESRI, USA) was used for geographical mapping of patient addresses.
Ethics
Informed consent was required from all parents or guardians for the child to participate in the study. Ethical approval was granted by the Oxford Tropical Research Ethics Committee (OxTREC no. 0109) and the local scientific and ethical committees of the three participating hospitals.
RESULTS
Descriptive characteristics
A total of 1419 children with acute diarrhoea and 571 asymptomatic individuals (without diarrhoea and with stool samples microbiologically culture negative for Salmonella) were enrolled between May 2009 and December 2010 from the three defined healthcare facilities in central Ho Chi Minh City, Vietnam. For 77 (5·4%) diarrhoea cases, patient stool culture yielded Salmonella on the day of hospital admission and therefore met the criteria for a Salmonella case. Of these, 45 (58%) were serogroup B, ten (13%) were serogroup C, five (6%) were serogroup D, two (3%) were S. Arizonae and 15 (20%) were ungroupable. Sixty-four per cent of the Salmonella diarrhoeal cases and 53% of asymptomatic individuals (referred hereon as ‘controls’) were male (χ2 test, P = 0·086), as shown in Table 1. Patients with Salmonella infection were, on average, marginally younger (median 10 months) than controls (median 12 months) (Mann–Whitney U test, P = 0·015), although an equal proportion (78%) of Salmonella patients (60/77) and controls (443/571) were being actively breastfed, or were breastfed as infants. Information on the specific duration of breastfeeding was not collected. Conversely, a greater proportion of controls (70/571, 12%) were malnourished (weight for age Z score ⩽–2) [10], compared to Salmonella patients (5/77, 7%) (P < 0·001).
Table 1.
Baseline, socioeconomic and behavioural characteristics of Salmonella diarrhoea cases and asymptomatic controls aged <5 years from three hospitals in Ho Chi Minh City, Vietnam
| Exposure | Salmonella patients | Controls | P † |
|---|---|---|---|
| n = 77 (%) | n = 571 (%) | ||
| Male sex | 63·6 | 53·2 | 0·087 |
| Breastfed | 77·9 | 77·6 | 0·947 |
| Age (months) | 0·379 | ||
| 0–6 | 24·7 | 16·8 | |
| 7–12 | 37·7 | 34·5 | |
| 13–18 | 19·5 | 19·3 | |
| 19–24 | 5·2 | 9·8 | |
| 25–36 | 7·8 | 8·6 | |
| 37–48 | 2·6 | 5·8 | |
| 49–60 | 2·6 | 5·3 | |
| Weight for age Z score <–2‡ | 6·5 | 12·3 | <0·001* |
| Contact with symptomatic§ | 8·1 | 1·8 | <0·001* |
| Previous illness | 14·3 | 7·0 | 0·026* |
| Residence | 0·852 | ||
| Rural | 11·7 | 12·4 | |
| Urban | 88·3 | 87·6 | |
| Household monthly income | 0·690 | ||
| < US$145 | 16·9 | 22·9 | |
| US$146–242 | 37·7 | 34·7 | |
| US$243–483 | 31·2 | 27·3 | |
| US$484–725 | 7·8 | 10·0 | |
| > US$725 | 6·5 | 5·1 | |
| Water source | 0·251 | ||
| Government pipe | 63·6 | 58·7 | |
| Well | 28·6 | 36·6 | |
| Other|| | 7·8 | 4·7 | |
| Bottled drinking water | 20·8 | 20·1 | 0·953 |
| Boil water | 89·6 | 93·5 | 0·205 |
| Toilet§ | 0·018* | ||
| Inside | 81·8 | 74·4 | |
| Outside | 11·7 | 24·9 | |
| Hand washing after using toilet§ | 0·015* | ||
| Always | 33·8 | 39·8 | |
| Occasionally | 14·9 | 9·5 | |
| No/don't know | 14·9 | 6·2 | |
| Not applicable¶ | 36·5 | 44·7 | |
| >4 adults in household | 29·9 | 26·4 | 0·524 |
| >2 children in household | 20·8 | 10·2 | 0·006* |
| Daycare/nursery§ | 14·3 | 15·4 | 0·773 |
| History of probiotic usage | 13·7 | 72·7 | <0·001* |
| Market food§ | 75·3 | 57·9 | 0·004* |
| Pet in household | 24·7 | 27·2 | 0·646 |
χ2 or Fisher's exact test as appropriate.
Considered malnourished [10].
Responses for symptomatic contact from 74 cases, 565 controls; for toilet use from 72 cases, 567 controls; for hand washing from 74 cases, 571 controls; for daycare/nursery from 77 cases, 566 controls; for probiotics from 31 cases, 394 controls; and for market food from 73 cases, 570 controls.
Rain water, from a government truck during road construction or other source.
Child in diapers and therefore would not require hand washing.
P value ⩽0·05.
The clinical and treatment characteristics of Salmonella gastrointestinal infections
Profuse watery diarrhoea was most the commonly reported form of diarrhoea, found in 45/77 (59%) patients with Salmonella infection followed by 26 (34%) with diarrhoea containing mucus, and six (8%) with bloody diarrhoea (Table 2). Mild (37·2–39 °C) and severe (>39 °C) fever were common, and the majority of children experienced vomiting. Illness prior to hospitalization occurred for a median of 2 days [interquartile range (IQR) 2–3] and children remained in hospital for a median of 5 days (IQR 3–8), with discharge permitted only by a treating clinician on the observation of the cessation of symptoms. Attending clinicians prescribed treatment to Salmonella patients prior to a microbiological stool investigation or an available antimicrobial susceptibility profile. In total, 67·5% (52/77) of Salmonella infections were prescribed an antimicrobial by the attending physician during their hospital stay. Over half (38/52, 73%) of the prescribed antimicrobials were fluoroquinolones, of which the most commonly used were ciprofloxacin (23/52, 44%) and norfloxacin (10/52, 19%). Additionally, rehydration therapy and probioitic supplements were administered to the majority of patients (lyophilized probiotics reconstituted in a beverage). Sixty-four per cent (33/52) of those prescribed antimicrobials were prescribed probiotic therapy concurrently with the antimicrobials.
Table 2.
Clinical features of Salmonella infections in Vietnamese children hospitalized with diarrhoea (n = 77)
| Diarrhoea type | n | % |
| Bloody | 6 | 7·8 |
| Mucoid | 26 | 33·8 |
| Watery | 45 | 58·5 |
| Patient characteristics | Median | IQR |
| Average no. episodes/day | 5 | 3–8 |
| Length of illness*, days | 2 | 2–3 |
| Length of stay in hospital, days | 5 | 3–8 |
| Symptoms | n | % |
| Fever | 58 | 75·4 |
| Abdominal pain | 13 | 16·9 |
| Anorexia | 35 | 45·5 |
| Cough | 22 | 28·6 |
| Dehydration | 5 | 6·5 |
| Headache | 1 | 1·3 |
| Vomiting | 50 | 64·9 |
IQR, Interquartile range.
Prior to hospitalization.
Seasonality and location
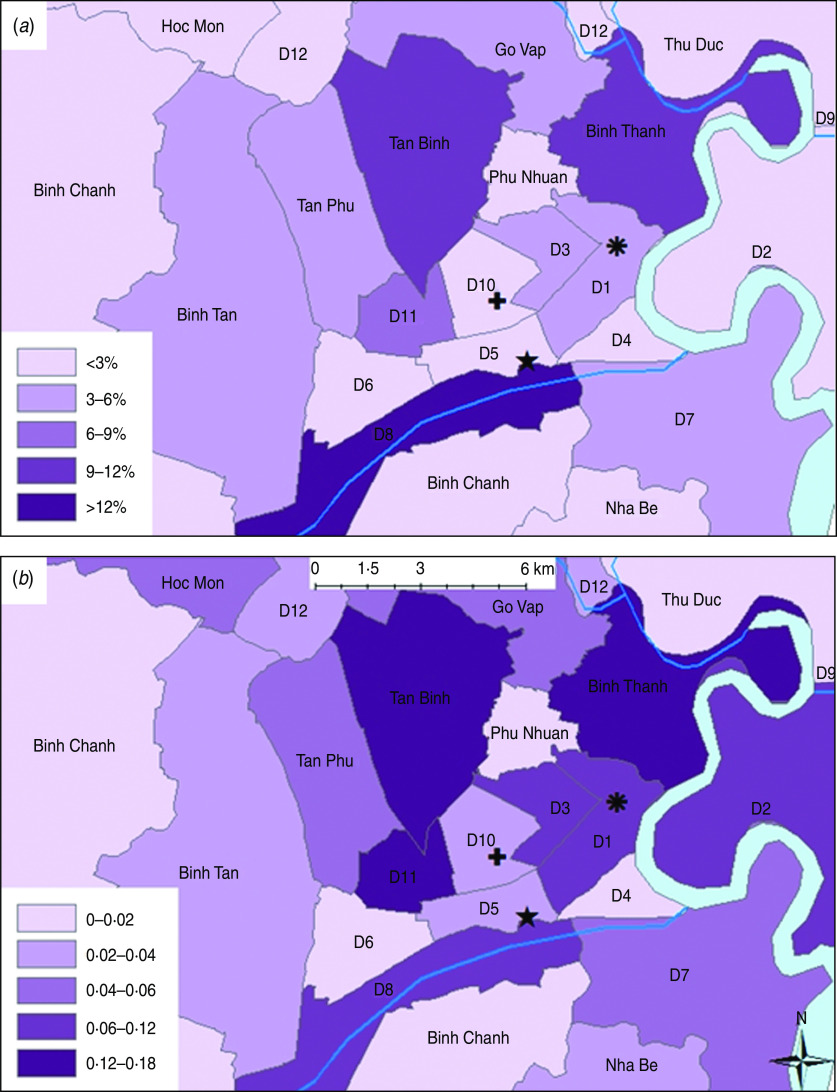
The south of Vietnam has two main seasons: a wet season from May to November and a humid dry season from December to April. The proportion of diarrhoea cases presenting to hospital that had a culture-confirmed diagnosis for Salmonella was the highest in August (12/193, 12·9%) and the lowest in February (2/80, 2·3%). We did not identify a strong association between Salmonella infections and average monthly temperature (Spearman's correlation coefficient r = 0·389, P = 0·080) and there appeared to be no association between Salmonella infection and average monthly rainfall (Spearman's r = 0·14, P = 0·665). The GPS coordinates of each case were plotted and case counts per district revealed the highest proportion of Salmonella patients (12/77, 16%) lived in district 8 in Ho Chi Minh City (Fig. 1a). However, when comparing the ratio of Salmonella cases to cases of diarrhoea caused by other bacterial or viral agents collected for 2009–2010 (Fig. 1b), less centrally located districts such as district 11, Tan Binh and Binh Thanh seemed to have proportionally heavier burdens of Salmonella infection with ratios of Salmonella culture-positive diarrhoeal cases to culture-negative cases of 0·18 (7/39), 0·15 (8/52) and 0·13 (9/69), respectively.
Fig. 1.
[colour online]. The geographical distribution of Salmonella cases in children aged <5 years admitted to the three study hospitals in central Ho Chi Minh City. The hospital locations are as follows: ✚ Children's Hospital 1;  Children's Hospital 2; ★ Hospital for Tropical Diseases. (a) Proportion of total Salmonella cases from each district; (b) Ratio of cases of Salmonella to cases of another bacterial or viral aetiology.
Children's Hospital 2; ★ Hospital for Tropical Diseases. (a) Proportion of total Salmonella cases from each district; (b) Ratio of cases of Salmonella to cases of another bacterial or viral aetiology.
Socioeconomic and behavioural characteristics
Responses from the questionnaire administered to the parents/guardians of both cases and controls were evaluated to determine trends in socioeconomic or behavioural factors in the overall population as well as any important differences between the two groups. From the resulting questionnaire data, a low proportion of children reported having been in recent contact with an individual symptomatic for diarrhoea or a recent previous diarrhoeal episode, although children with Salmonella infection were more likely to have reported both (Table 1). In regard to sanitation and water supply, over half of Salmonella patients (49/77, 64%) and controls (335/571, 59%) used a municipal government pipeline, which was followed less commonly by wells, 29% (22/77) and 37% (209/571), respectively. Roughly 20% of children with Salmonella infection (16/77) and controls (117/571) reported drinking only bottled water, while almost all Salmonella patients (69/77, 90%) and controls (534/571, 94%) reported boiling their water prior to drinking. Hand washing behaviour differed between the groups, with those positive for Salmonella more commonly reporting not washing hands or not knowing if hands were washed (36/71, 15%) than controls (35/571, 6%). Yet surprisingly, Salmonella patients were more likely to have an indoor toilet (63/72, 82%) compared to controls (142/567, 74%).
In order to assess general living conditions, we evaluated the level of household crowding by measuring the proportion of Salmonella patients and controls who reported having more than the median number of adults (n = 4) and children (n = 2) in the household, as estimated from our larger population of 1419 diarrhoeal cases and 571 controls. The households of ~30% of Salmonella patients (23/77) and controls (151/571) had more than four adults yet the patients' households had greater than the median of two children more frequently (16/77, 21%) than controls' households (57/571, 10%). Additionally, monthly income distributions were comparable between Salmonella patients and controls, with the majority of households (cases and controls) having an income of between US$145 and US$480 per month.
More children with Salmonella reported living in households regularly purchasing meat and vegetables from outdoor markets (55/73, 75%) compared to only 58% (330/570) of controls. Pet ownership (mainly cats and dogs), was ~25% in children with Salmonella infection (19/77) and controls (155/571). Finally, Salmonella patients reported regularly consuming probiotics prior to diarrhoea much less frequently (7/51, 14%) than controls (280/440, 64%).
Exposure analysis
Symptomatic Salmonella gastrointestinal infection was associated with having contact with a recently symptomatic individual [odds ratio (OR) 4·90, 95% confidence interval (CI) 1·7–13·9], living in a household where meat and vegetables were primarily purchased at an outdoor market (OR 2·22, 95% CI 1·3–3·9), recent previous diarrhoeal illness (OR 2·21 95% CI 1·1–4·5), not washing hands or not knowing if hands were washed after using the toilet (OR 2·67, 95% CI 1·3–5·5), age (OR 0·98, 95% CI 0·96–1·00) and having >2 children living in the household (OR 2·32, 95% CI 1·2–4·8). The type of toilet (indoor/outdoor) present in the household was also found to be important. Living in an urban or rural district modified the effect of toilet type on the risk of contracting a Salmonella infection (test for homogeneity of ORs, P = 0·038) such that living in a household with an outdoor toilet was not a risk factor in rural districts (OR 2·41, 95% CI 0·6–9·1), whereas it appeared to be strongly protective for those living in urban districts (OR 0·24, 95% CI 0·1–0·6). Probiotics also demonstrated a protective effect (OR 0·09, 95% CI 0·04–0·22).
After controlling for the factors that were found to be statistically significant in the univariate analysis as listed above, several risk factors remained independently associated with Salmonella infections in the multivariate model (Table 3). These risk factors included having had a symptomatic contact [adjusted OR (aOR) 5·98, 95% CI 1·8–20·4], age (aOR 0·97, 95% CI 0·94–0·99), living in a household where meat and vegetables were primarily purchased at an outdoor market (aOR 2·27, 95% CI 1·2–4·2), having >2 children in the household (aOR 2·32, 95% CI 1·2–4·4·7) individuals with an outdoor toilet living in predominantly urban districts (aOR 0·25, 95% CI 0·09–0·72).
Table 3.
Selected univariate and multivariate analysis of risk factors for symptomatic Salmonella gastroenteritis
| Risk factor | OR | 95% CI | aOR | 95% CI |
|---|---|---|---|---|
| Previous illness | 2·21 | 1·08–4·52 | 1·77 | 0·73–4·26 |
| Symptomatic contact | 3·14 | 1·12–8·83 | 5·98 | 1·76–20·36 |
| Age (months) | 0·98 | 0·96–1·00 | 0·97 | 0·94–0·99 |
| Absence of hand washing | 2·67 | 1·29–5·53 | 1·99 | 0·85–4·63 |
| Purchasing market food | 2·22 | 1·27–3·88 | 2·27 | 1·22–4·24 |
| >2 children in household | 2·32 | 1·26–4·29 | 2·32 | 1·15–4·67 |
| Outside toilet | ||||
| Urban | 0·27 | 0·11–0·70 | 0·25 | 0·09–0·72 |
| Rural | 1·67 | 0·41–6·81 | 1·53 | 0·30–7·86 |
OR, Odds ratio; C,: Confidence interval; aOR, adjusted odds ratio.
DISCUSSION
The epidemiology of gastrointestinal infections caused by Salmonella has been extensively studied in developed countries but there is a paucity of data regarding the prevalence and potential transmission routes of Salmonella in developing countries. This is the first study to exclusively evaluate the epidemiology and risk factors of non-typhoidal Salmonella gastrointestinal infections in children in Vietnam.
We found that fever, anorexia, vomiting and either watery or mucoid diarrhoea were all common features of patients with Salmonella infections, which are typical globally of Salmonella infection presentation [5]. Cases of Salmonella were not found to be associated with average monthly temperature in Ho Chi Minh City, although there was a proportional increase during the warmer months of the year. Previous studies conducted in the UK and Australia have shown that a higher mean ambient temperature leads to an increase in the number of salmonellosis notifications, possibly through an increase in bacterial reproduction at various points along the food chain [11, 12]. Although, whether their findings are an important transmission factor in a developing-country setting remains unclear currently.
Additionally, although it is important to consider that our geographical data is biased as a consequence of hospital referral patterns, the highest proportion of Salmonella patients came from district 8 of Ho Chi Minh City. This district is the area of the city with the greatest density of canals, waterways and is one of the districts with the largest number of temporary urban settlements [13]. Proximity to potentially contaminated water and related poor sanitary conditions may explain the high proportion of Salmonella infections from persons living in this area.
With respect to our controls, young children with Salmonella infections were more likely to have had a symptomatic contact, to be younger, to live in a household where food is purchased primarily at an outdoor market, to live with at least three children in the household and were more likely to use an outdoor rather than an indoor toilet in urban areas. Our findings regarding the young age of infection are consistent with other studies, which have shown that younger children are at greater risk, and have more frequent Salmonella infections than children in older age groups [14]. The majority of Salmonella infections in this study were from infants aged <1 year, with the peak of infections in those aged between 7 and 12 months. Potential reasons for increased susceptibility to Salmonella infections include numerous host factors, such as decreased gastric acidity, immaturity of the gut-associated lymphoid tissue or a lack of serogroup-specific maternal antibodies [14]. Additionally, research conducted in Malawi has demonstrated that children aged between 4 months and 2 years are at increased risk of invasive non-typhoidal Salmonella infection due in part to a lack of Salmonella-specific antibody, impaired bactericidal activity and waning maternal IgG [15, 16], although mechanisms may differ for disease limited to the gastrointestinal tract.
Previous studies have identified Salmonella in the environment of patients with recently identified infections and that multiple cases of the same Salmonella strain may occur in the same household, suggesting the potential for intra-household transmission [17–19]. In a large case-control study using hospital laboratory reports in the USA from 2002 to 2004, Jones et al. found that 20% of households with a primary Salmonella spp. infection reported a subsequent case [6]. However, having had contact with a symptomatic individual is difficult to interpret as it is often unclear if the contact had the same infection, if the period of infectiousness of the contact coincides with the timing of the patient's illness and whether a shared exposure is responsible for the association. We observed that cases were more likely to have several children living in the same household, which has also been shown to be a risk factor in studies of other enteric pathogens in the Congo and Bangladesh [20, 21]. It is reasonable to suggest that multiple children living in a single household may lead to an increase in the frequency of Salmonella transmission, as children aged <5 years are more likely to be infected and also tend to shed asymptomatically for protracted time periods which would broaden the potential transmission window [22]. The possibility of different primary patterns of Salmonella transmission by age warrants more attention.
Although multiple investigations have demonstrated that a considerable proportion of Salmonella spp. transmission occurs through the food chain [3], direct person-to-person Salmonella transmission has been implicated as a more important transmission route in some non-industrialized countries [23]. We found that living in a household where food was purchased at outdoor markets was a risk factor, a consequence of substandard food handling, storage and preparation practices at such settings. Very young children are less likely to eat solid food, so it is likely that a primary risk factor for symptomatic Salmonella is by a direct exposure from either the contaminated environment or family members, not specific food products [6]. Therefore, ensuring a clean local environment and adequate personal hygiene of caregivers are critical prevention measures to limit symptomatic Salmonella infections in very young children in this population.
Antimicrobials are not universally recommended for the treatment of gastrointestinal non-typhoidal Salmonella infections specifically or for treating non-bloody diarrhoea in children in general [24]. Treatment with antimicrobials is only advised for children with suspected or confirmed septicaemia or additional secondary complications [25]. Almost 70% of the Salmonella-infected individuals in this study received at least one antimicrobial, most commonly a fluoroquinolone, which contradicts internationally recognized guidelines. In fact, some data suggest that antimicrobials may actually prolong shedding of the pathogen in the stool [22], and will presumably increase the potential for the development of antimicrobial resistance. Resistance has implications for treatment failure, increasing treatment costs and protracted therapy for infections that do require an antimicrobial, as second-line drugs are often more expensive and typically require a longer treatment [26]. Furthermore, the treating clinicians prescribed probiotics to over 60% of Salmonella-infected patients, with almost half of the patients concurrently receiving probiotics and antimicrobials. Diagnostics are seldom performed for diarrhoea in settings like Vietnam and patients are prescribed therapy based on clinical presentation and prior to microbiological culture result. More stringent treatment guidelines and the restriction of access to antimicrobials in the community would help to ensure more appropriate antimicrobial practices.
Our analysis suggests that probiotics have a significant prophylactic effect against symptomatic Salmonella in this study. In Vietnam, probiotics vary substantially but generally consist of a lyophilized Lactobacillus spp. in a single-dose sachet, which is normally reconstituted in water or milk prior to consumption. The use of probiotics was not included in the multivariate model due to possible biases introduced by limitations in study design. As controls were collected from the nutritional ward, parents of these children may have been likely to give their child a product supplemented with probiotics (very popular and inexpensive in Vietnam) in a specific effort to care for the nourishment of their child. Parents of diarrhoeal patients may have been less concerned with nutritional issues and therefore may have been less likely to give their child probiotics. However, the strong effect found in the univariate model warrants further scrutiny. A recent large community-based, randomized, double-blind, placebo-controlled trial was conducted in an urban settlement in Kolkata, India, demonstrating that a daily intake of probiotics resulted in significant protection against acute diarrhoea in children aged <5 years with an overall protective efficacy of 14% (95% CI 4–23%, P < 0·01) [27]. These results are positive, as this was the first large, randomized controlled trial for a prophylactic effect of probiotics conducted in a developing country. A recent Cochrane review regarding the use of probiotics for treating acute diarrhoea found probiotics to be associated with a reduction in risk and duration of diarrhoeal diseases in children [28], adding additional plausibility to our observation.
There were several limitations to our study, the most important of which is that this data was entirely from passive detection and was dependent on the healthcare-seeking behaviour of the patients. As such, much of the burden of infection due to Salmonella could remain undocumented, including those less severe cases that did not require medical care. Uncollected differences could have been present between the cases and controls as well, as controls were only collected from two hospitals due to logistical reasons. Selection bias may also have been present as controls were collected from nutrition wards. We found that these individuals were more likely to have a lower weight-for-age Z score than the cases which could potentially introduce biases as nutritional state tends to play a strong role in susceptibility to diarrhoeal infections and could skew noted epidemiological associations through influences of unknown confounders [29]. Or it is possible that controls were likely to have already acquired Salmonella infection which could have contributed to their poor nutritional status.
We surmise that while our findings should be generalized with caution, our study provides a reasonable estimate of the proportion of Salmonella-associated diarrhoea in hospitalized children and highlights some related risk factors for children resident in Ho Chi Minh City. Our findings imply that Salmonella is a common cause of paediatric gastroenteritis in this setting and that transmission may occur through direct human contact within the household, offering some palpable and tractable prospective routes for more focused epidemiological investigations in locations in other rapidly developing cities in Asia.
ACKNOWLEDGEMENTS
We thank the clinical staff of the Hospital for Tropical Diseases, Children's Hospital 1 and Children's Hospital 2 in Ho Chi Minh City for their efforts in conducting this work and the individuals enrolled in the study. We especially acknowledge the efforts of the microbiology laboratories at the Hospital for Tropical Diseases in Ho Chi Minh City, Vietnam. This work was supported through funding from the Wellcome Trust Visions Initiative (UK), the Li Ka Shing foundation (People's Republic of China) and a fellowship (S.B.) from the OAK Foundation (USA) through Oxford University, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.UNICEF/WHO. Diarrhoea: why children are still dying and what can be done. Geneva: The United Nations Children's Fund/World Health Organization, 2009. Report no.: NLM: WS312. [Google Scholar]
- 2.Thapar N, Sanderson PIR. Diarrhoea in children: an interface between developing and developed countries. Lancet 2004; 363: 641–653. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi MB, et al. Nontyphoidal Salmonella from human clinical cases, asymptomatic children, and raw retail meats in Yucatan, Mexico. Clinical Infectious Diseases 2006; 42: 21–28. [DOI] [PubMed] [Google Scholar]
- 4.WHO. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers, 4th revision. Geneva: World Health Organization, 2005. Report No.: WHO/CDD/SER/80.2. [Google Scholar]
- 5.Graham SM. Salmonellosis in children in developing and developed countries and populations. Current Opinion in Infectious Diseases 2002; 15: 507–512. [DOI] [PubMed] [Google Scholar]
- 6.Jones TF, et al. A case-control study of the epidemiology of sporadic Salmonella infection in infants. Pediatrics 2006; 118: 2380–2387. [DOI] [PubMed] [Google Scholar]
- 7.Bodhidatta L, et al. Rotavirus disease in young children from Hanoi, Vietnam. Pediatric Infectious Disease Journal 2007; 26: 325–328. [DOI] [PubMed] [Google Scholar]
- 8.World Bank. The World Bank: gross national income per capita (Vietnam), 2009. (http://data.worldbank.org/country/vietnam). Accessed 11 March 2012.
- 9.WHO. World Health Organization: Viet Nam health profile, 2011. (http://www.who.int/countries/vnm/en/). Accessed 15 March 2012.
- 10.Albert MJ, et al. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. Journal of Clinical Microbiology 1999; 37: 3458–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Souza RM, et al. Does ambient temperature affect foodborne disease? Epidemiology 2004; 15: 86–92. [DOI] [PubMed] [Google Scholar]
- 12.Lake IR, et al. A re-evaluation of the impact of temperature and climate change on foodborne illness. Epidemiology and Infection 2009; 137: 1538–1547. [DOI] [PubMed] [Google Scholar]
- 13.Habitat for Humanity. Mapping urban poverty in Ho Chi Minh City, Vietnam, 2009. (http://www.habitatvietnam.org/). Accessed 16 February 2012.
- 14.Shimoni Z, et al. Nontyphoid Salmonella bacteremia: age-related differences in clinical presentation, bacteriology, and outcome. Clinical Infectious Diseases 1999; 28: 822–827. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan CA, et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. Journal of Clinical Investigation 2008; 118: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gondwe EN, et al. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proceedings of the National Academy of Sciences USA 2010; 107: 3070–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker J, Bloomfield SF. Survival of Salmonella in bathrooms and toilets in domestic homes following salmonellosis. Journal of Applied Microbiology 2000; 89: 137–144. [DOI] [PubMed] [Google Scholar]
- 18.Schutze GE, et al. The home environment and salmonellosis in children. Pediatrics 1999; 103: e1–e5. [DOI] [PubMed] [Google Scholar]
- 19.Ethelberg S, et al. Household outbreaks among culture-confirmed cases of bacterial gastrointestinal disease. American Journal of Epidemiology 2004; 159: 406–412. [DOI] [PubMed] [Google Scholar]
- 20.Manun'Ebo MN, et al. Influence of demographic, socioeconomic and environmental variables on childhood diarrhoea in a rural area of Zaire. Journal of Tropical Medicine and Hygiene 1994; 97: 31–38. [PubMed] [Google Scholar]
- 21.Pathela P, et al. Diarrheal illness in a cohort of children 0–2 years of age in rural Bangladesh: I. Incidence and risk factors. Acta Pædiatrica 2006; 95: 430–437. [DOI] [PubMed] [Google Scholar]
- 22.Hohmann EL. Nontyphoidal salmonellosis. Clinical Infectious Diseases 2001; 32: 263–269. [DOI] [PubMed] [Google Scholar]
- 23.Feasey NA, et al. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerging Infectious Diseases 2010; 16: 1448–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirinavin S, Garner P. Antibiotics for treating Salmonella gut infections. Cochrane Database of Systematic Reviews 2009, Issue No. 2, Art. No.: CD001167. [DOI] [PubMed] [Google Scholar]
- 25.Khanna R, et al. Diarrhoea and vomiting caused by gastroenteritis in children under 5 years: summary of NICE guidance. British Medical Journal 2009; 338: 1009–1012. [DOI] [PubMed] [Google Scholar]
- 26.Travers K, Michael B. Morbidity of infections caused by antimicrobial-resistant bacteria. Clinical Infectious Diseases 2002; 34: S131–134. [DOI] [PubMed] [Google Scholar]
- 27.Sur D, et al. Role of probiotic in preventing acute diarrhoea in children: a community-based, randomized, double-blind placebo-controlled field trial in an urban slum. Epidemiology and Infection 2010; 1: 1–8. [DOI] [PubMed] [Google Scholar]
- 28.Allen SJ, et al. Probiotics for treating infectious diarrhoea. Cochrane Database of Systematic Reviews 2004, Issue No. 11, Art. No.: CD003048. [DOI] [PubMed] [Google Scholar]
- 29.Schlaudecker EP, Steinhoff MC, Moore SR. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Current Opinion in Infectious Diseases 2011; 24: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]