Abstract
For decades, complement has been recognized as an effector arm of the immune system that contributes to the destruction of tumor cells. In fact, many therapeutic strategies have been proposed that are based on the intensification of complement-mediated responses against tumors. However, recent studies have challenged this paradigm by demonstrating a tumor-promoting role for complement. Cancer cells seem to be able to establish a convenient balance between complement activation and inhibition, taking advantage of complement initiation without suffering its deleterious effects. Complement activation may support chronic inflammation, promote an immunosuppressive microenvironment, induce angiogenesis, and activate cancer-related signaling pathways. In this context, inhibition of complement activation would be a therapeutic option for treating cancer. This concept is relatively novel and deserves closer attention. In this paper, we will summarize the mechanisms of complement activation on cancer cells, the cancer-promoting effect of complement initiation, and the rationale behind the use of complement inhibition as a therapeutic strategy against cancer.
Keywords: Complement system, cancer therapy, tumor microenvironment, inflammation, immunosuppression, angiogenesis
1. Introduction
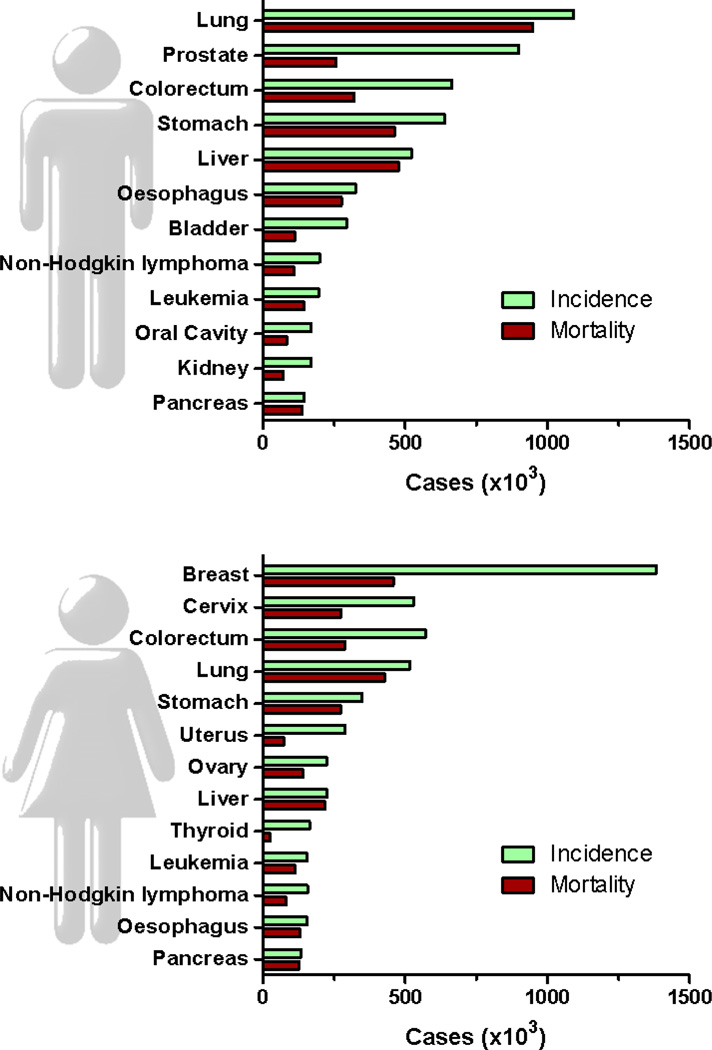
Cancer is a major public health problem causing millions of deaths worldwide [1]. In the United States, one in four deaths is due to cancer [2]. Breast cancer in females and lung cancer in males are the most frequently diagnosed cancers and the leading causes of cancer death for each sex (Fig. 1). Despite substantial advances in the chemotherapeutic management of cancer, more than half of all cancer patients do not respond to therapy or relapse, dying from metastatic disease. In addition, cancer chemotherapy is usually accompanied by severe side effects. These facts have led to the belief that in some tumor types, traditional therapies have reached a “therapeutic plateau” [3]. More personalized therapies with higher tumor specificity and less toxicity are a clinical need.
Fig. 1.
Estimated new cancer cases (incidence) and deaths (mortality) for the leading cancer types worldwide in 2008. Source: Globocan 2008 (http://globocan.iarc.fr/)
A variety of strategies based on the understanding of the molecular events associated with cancer initiation and progression are now under intensive investigation. In fact, some rationally targeted therapies have already shown a remarkable effectiveness in selected populations [4]. Targeted therapy refers to a new generation of cancer drugs designed to interfere with a specific target that is believed to have a critical role in tumor cell proliferation and survival. Most of these strategies are based on the identification of targetable signaling proteins critical for tumor growth or progression. Alternatively, cancer therapies directed at immune modulation have also been pursued, but with only modest advances to date [5, 6]. A better understanding of the molecular interactions between tumors and the immune system should lead to better anticancer therapies. In this review we will present emerging data on the relationship between complement, an essential part of the innate immunity, and cancer progression. We will focus on the therapeutic potential of targeting complement activation in cancer, examining the rationale behind this strategy and the aspects that must be investigated before it can be considered an anticancer strategy that is ready for clinical testing.
2. The complement system
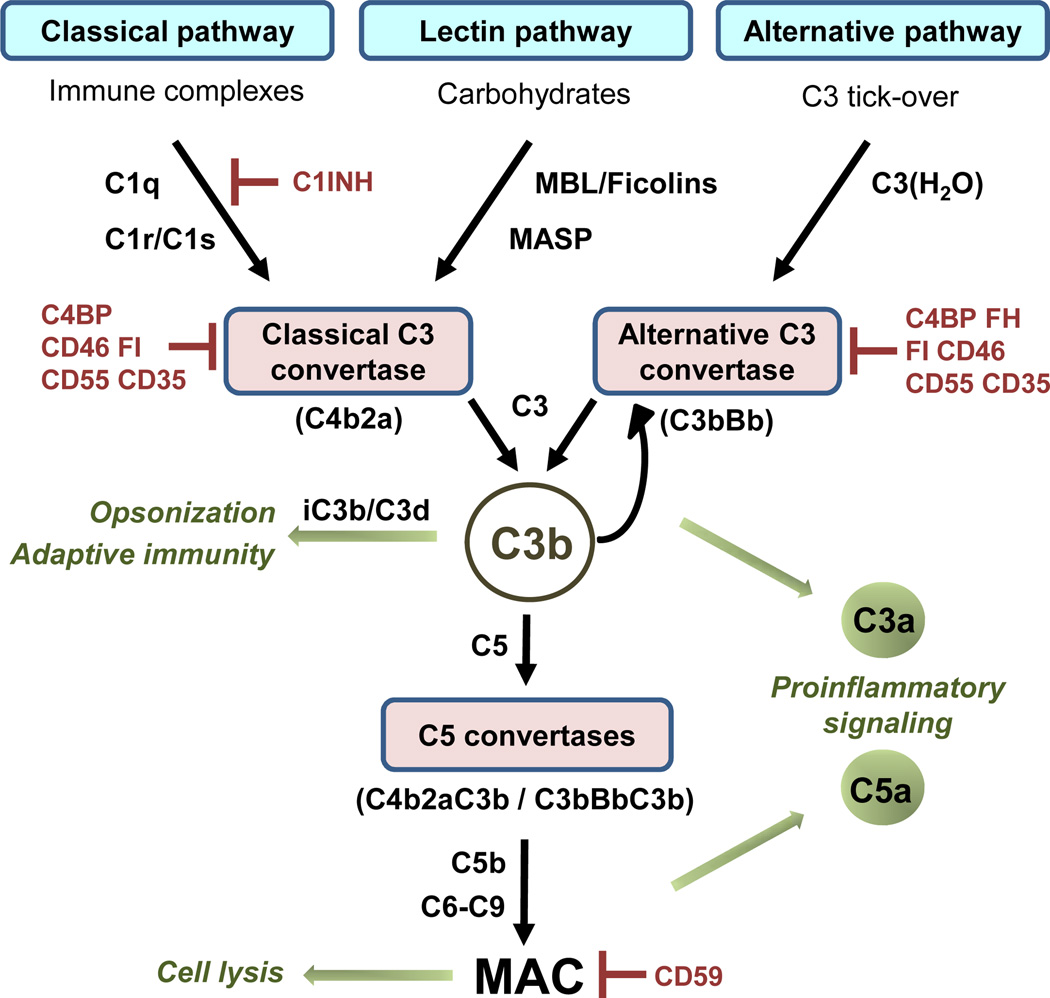
Complement has evolved as a first defense against non-self cells or unwanted host elements. The spectrum of complement-mediated functions ranges from direct cell lysis to the control of humoral and adaptive immunity. This system also regulates a number of immunological and inflammatory processes that contribute to body homeostasis [7]. Complement activities are mediated by more than 50 circulating or cell surface-bound proteins. There are three pathways of complement activation: the classical, the alternative, and the lectin pathways (Fig. 2). The three complement pathways differ in their mechanisms of target recognition but converge in the activation of the central component C3. After this activation, C5 is cleaved, and the assembly of the pore-like membrane attack complex (MAC) is initiated. The enzymatic cleavage of C3 and C5 leads to the production and release of anaphylotoxins C3a and C5a, two important inflammatory mediators and chemoattractants [8]. Complement is tightly controlled by several proteins whose main function is to prevent activation. Complement inhibitors are grouped into two categories, soluble regulators and membrane-bound regulators. These regulators naturally protect self cells and tissues from unwanted complement activation [9].
Fig. 2.
Simplified scheme of the pathways of complement activation. Patternrecognition molecules such as Clq, MBL (mannose-binding lectin), or ficolins bind to surface structures and initiate the formation of the classical C3 convertase. The alternative C3 convertase results from spontaneous hydrolysis of C3 (tick-over). C3 convertases cleave C3 into C3b and C3a. Deposition of C3b leads to the generation of additional C3 convertases (self-amplification) and the C5 convertase, which cleaves C5 into C5a and C5b. In the terminal pathway, interactions between components C5b, C6, C7, C8, and C9 lead to the formation of the lytic membrane attack complex (MAC). The C3b degradation fragments iC3b and C3d participate in phagocytosis and are linked to adaptive immune responses. While the C3b and its degradation fragment iC3b participate in both phagocytosis and in adaptive immune response, the C3dg fragment is only involved in adaptive immune responses. The anaphylatoxins C3a and C5a trigger immune reactions upon binding to their receptors (C3aR, C5aR, C5L2). Complement regulators (indicated in red) prevent unwanted complement activation (C1INH: CI inhibitor; C4BP: C4 binding protein; FI: Factor I; FH: Factor H).
3. Complement activation in cancer cells
Malignant transformation is accompanied by the acquisition of genetic and epigenetic alterations that distinguish the transformed cells from their normal counterparts. This process dramatically changes cell-surface proteins, glycosylation, and phospholipids patterns [10–15]. Cancer-related membrane modifications can be recognized by innate and adaptive immune mechanisms that protect the host against the development of cancer [16]. This is the basis of the immune surveillance hypothesis, which proposes that the immune system surveys the body for tumor-associated molecules, eliminating many, if not most, emerging tumors [17]. The immune surveillance activity is one of the three phases proposed in the inmmunoediting theory: elimination (immune surveillance), equilibrium, and escape [18]. If tumor cells are able to get pass the elimination phase, they enter an equilibrium period during which surviving tumor cells keep dividing and acquiring genetic and epigenetic abnormalities under the immunological pressure. This pressure contributes to the selection of tumor cell variants that are resistant to immune effectors. During the escape phase, tumor cells effectively evade the immune system.
There is no direct evidence to support the contention that complement can eliminate nascent tumors. However, considering that complement is designed for the recognition of non-self elements, it is logical to assume that changes in the composition of tumor cell membranes make these cells a target for complement recognition. Consistent with this assumption, a number of clinical studies have reported an activation of complement in cancer patients [19–23]. Indirect evidence for a role of complement in immunosurveillance also comes from the fact that cancer cells develop a variety of strategies to avoid complement-mediated damage [24]. Their best-known escape mechanism is the overexpression of complement regulatory proteins, a subject that has been extensively reviewed [25–29]. According to the immunoediting hypothesis, this overexpression is indicative of a selective pressure created by complement activation in the tumor microenvironment that sculpts cancer cells to evade the harmful effects of complement.
Other indirect evidence of complement activation by cancer cells comes from the increased complement activity found in biological fluids from cancer patients [30–34]. Complement activation has also been observed in in vitro studies of cancer cell lines. Lung cancer cells deposit C5 and generate the active product C5a more efficiently than do non-malignant bronchial epithelial cells [35]. However, the antigens responsible for this activation and the pathway/s involved are not yet known. The classical pathway has been identified as the main contributor to complement activation on subcutaneously inoculated TC-1 cervical cancer cells [36]. In vitro studies have shown spontaneous activation of the classical complement pathway by two neuroblastoma cell lines [22]. In the case of primary tumors, there are few studies pointing to a specific activation pathway. Lucas et al. [21] have suggested that a tumor-specific immune response occurs in papillary thyroid carcinomas, with activation of the classical complement cascade. Follicular and MALT lymphomas also deposit elements of the classical pathway [23], and alterations in this pathway have been described in patients with chronic lymphocytic leukemia [37, 38]. In contrast, the results of other studies have suggested that lymphoma and myeloma cells activate the alternative pathway [19, 39–41]. Moreover, both the alternative and the classical pathway seem to be involved in some cases [42]. The lectin pathway of complement activation has been found to be significantly increased in colorectal cancer patients [33].
In general, the information about the pathways activated by cancer cells is fragmented. Most studies on this subject were published years ago, and results are confusing, most likely because of the high heterogeneity among different tumor types studied. Each tumor has its own unique antigenic identity and a characteristic profile of complement regulators. This variety of complement recognition molecules and regulators should result in a diversity of activation pathways. To make things more complicated, there are extrinsic complement activation pathways mediated by soluble and membrane-bound proteases, such as serine proteases of the coagulation and fibrinolysis systems [43–46]. Lung cancer cells can produce C5a in the absence of serum, likely through the action of an extrinsic pathway mediated by an uncharacterized trypsin-like serine protease [35]. Thus, a more systematic analysis of the pathways and mediators by which cancer cells activate complement is needed. Such studies would greatly improve our understanding of the dynamic interplay between complement and cancer and would offer the opportunity to identify new molecular biomarkers.
Complement components, or their activation products, have been proposed as markers in other pathologies in which this system is involved [47–49]. Lung cancer patients show significantly higher plasma levels of complement proteins and activation fragments than do control donors [32, 35], and elevated complement levels are correlated with lung tumor size [30]. Complement-related proteins are also elevated in biological fluids from patients with other types of tumor [32–34, 50]. More interestingly, complement activity can be associated with clinical outcome. For example, a positive correlation has been observed between survival time and the initial activity of the classical pathway of complement in patients with chronic lymphocytic leukemia [51]. High MASP-2 levels in serum have been found found to be an independent prognostic marker of recurrence and reduced survival in colorectal cancer [52]. High levels of complement regulatory proteins have also been associated with poor prognosis in different malignancies [53–55], and plasma complement components may also be useful as early predictive markers of response to chemotherapy [56].
4. Promotion of cancer growth by complement
Recognition of cancer cells by the complement system has been traditionally associated with an effector activity that contributes to the destruction of the tumor cells. Accordingly, researchers have designed a wide variety of strategies to increase complement activation in the context of immunotherapy against tumors [29]. However, as early as in 1975, Shearer et al. reported that complement has the capacity to stimulate growth when cells are treated with low concentrations of antitumor antibodies [57]. More recent studies have demonstrated a tumor-promoting role of complement in mouse models [8, 58].
Although the finding that complement elements can act as tumor promoters may be considered unexpected, the idea is entirely consistent with the cancer immunoediting theory. Based on this theory, recognition of cancer cells by complement elements creates a selective pressure that leads to the expansion of new tumor populations that are able to control complement activation. In this context, cancer cells could take advantage of the convenient balance created between complement activation and inhibition (Fig. 3)
Fig. 3.
The complement system has a dual action in cancer. Experimental data support the idea that complement is activated by tumors. However, some studies also suggest that malignant cells evade the harmful effects of complement and make use of some complement effector molecules to promote cancer growth. Unfortunately, the exact mechanisms and consequences of this duality are not very well known.
This new perspective on the role of complement in cancer was proposed in a syngeneic mouse model of cervical cancer [36]. Complement deficiencies in this model and pharmacological blockade of complement-related mediators have been associated with impaired tumor growth [36]. In particular, C5a, a potent chemoattractant and proinflammatory mediator generated locally in the tumor microenvironment, seemed to be an essential contributor to tumor growth. Later studies with mouse models have supported this seminal observation. C5a contributes to lung cancer progression [35], and genetic complement deficiencies, including C5aR knockdown, impair ovarian tumor growth in mice [59]. Contrasting results have been obtained in immunodeficient mice injected with human SKOV-3 ovarian adenocarcinoma cells transfected with mouse C5a. Tumor cells that overexpress C5a have been found to show a significant reduction in tumor progression [60]. More intriguingly, results obtained in a syngeneic lymphoma model suggest that the impact of C5a on tumor growth is concentration-dependent [60]. Taken together, all these animal models support the idea that complement activation is able to regulate tumor growth. A variety of cancer-related biological processes may be involved in this regulation. In the next sections, data on the potential role of complement in the modulation of chronic inflammation, immunosuppression, angiogenesis, and cancer cell signaling will be reviewed.
4.1. Complement in tumor-associated inflammation
Inflammation is a protective biological process that removes harmful stimuli from the organism. Experimental data support the idea that chronic inflammation and adaptive immune responses are critically involved in tumor immunosurveillance [61]. Appropriately polarized inflammation triggered by the use of Mycobacterium bovis impairs bladder cancer progression [62]. Furthermore, infiltration of certain types of leukocytes within the tumor microenvironment is associated with anti-tumor T-cell responses and a good prognosis [63–65].
Nevertheless, the relationship between cancer and inflammation is complex and subject to opposing forces. In particular, tumor-associated inflammation can contribute to multiple hallmark capabilities by supplying bioactive molecules to the tumor microenvironment [66]. Thus, while acute responses are considered part of the defense against neoplastic cells, sustained inflammation in the tumor microenvironment increases the risk of neoplastic transformation and has many tumor-promoting effects [67]. Chronic exposure to irritants, including tobacco smoke, radon, and asbestos, considerably increases the risk of developing lung cancer [68]. Persistent inflammation is characterized by stromal accumulation of certain types of cytokines, chemokines, grow factors, matrix remodeling proteases, and reactive oxygen species [69]. These events critically compromise tissue homeostasis and create a tumor-supportive microenvironment.
The role of complement in inflammation is well known [70]. Complement breakdown reactions lead to the generation of powerful immune effectors, such as the anaphylatoxins C5a and C3a. Anaphylatoxins are potent chemoattractants for eosinophils, monocytes, and T lymphocytes [71, 72]. In addition, the interaction of these molecules with their receptors stimulates the release of granule-based enzymes, cytokines, eicosanoids, and reactive oxygen species, all of which contribute to innate immune functions and/or tissue damage [71–73]. Deregulated complement activity is involved in the pathogenesis of some chronic inflammatory diseases [74, 75].
In the case of cancer, transformed cells are recognized by complement but resist complement attack through the shielding role of complement inhibitors. This controlled tumor-mediated complement activation provides a permanent source of complement effectors that could create an inflammatory microenvironment favorable to cancer growth. Complement-bioactive molecules such as C3-, C4-, and C5-derived fragments, Clq, and MAC are prominent elements of the inflammatory tumor microenvironment [76], but, unfortunately, little is known about their function in tumorigenesis. In addition, C3-deficient mice exhibit an impaired production of the pro-inflammatory cytokine IL-6 after partial liver resection [77]. IL-6 is a multifunctional cytokine that displays a wide range of biologic activities in cancer: it inhibits apoptosis, stimulates angiogenesis, and increases drug resistance in patients with advanced tumors [78]. Activation of C3aR and C5aR increases IL-6 mRNA expression [79].
Complement activity also stimulates the expression of transforming growth factor β (TGF-β) [80, 81]. TGF-β displays pro- and anti-tumor effects, depending on the cellular context [82]. In regard to the relationship between complement and other effectors of innate immunity, several studies have demonstrated a synergism between complement and toll-like receptors (TLRs). TLR engagement upregulates nitric oxide synthase-2 and cyclooxygenase-2 and contributes to tumor-associated inflammation [83]. Both C3a and C5a increase the activity of TLR2/6, TLR4, and TLR9 and hence the upregulation of the pro-inflammatory cytokines tumor necrosis factor, IL-6, and IL-1β [84]. Therefore, the role of complement activation in the stromal accumulation of cytokines and growth factors is consistent with its contribution to the inflammatory network in cancer.
Another important feature of tumors is their plasticity in adapting to different physiological conditions. A phenomenon closely related to tumor-associated inflammation is hypoxia [85]. Interestingly, under hypoxic conditions, there is an increase in complement activity because of a downregulation in the expression of factor H and factor I [86], which could lead to the generation of C5a and other proinflammatory molecules. This downregulation is a good example of how tumors engage strategies that not only help them to survive stress but also contribute to cancer progression. We can conclude that tumors are able to trick the anti-tumor effects of inflammation in order to polarize immune responses toward those effectors that facilitate tumor growth. The biologically relevant interplay between complement and inflammation encourages the design of future studies to address the contribution of complement to the modulation of tumor-associated inflammation.
4.2. Complement in immunoregulation
Tumor-mediated immune responses are different from those observed in other pathological conditions [87]. In the past, researchers considered that the immune responses observed within tumors were all the consequence of an immune attack. However, tumors also acquire the ability to circumvent immune recognition and even take advantage of it. Dense infiltrates of both innate and adaptive immune cells are a common feature of solid tumors and have an impact on the clinical outcome of cancer patients. The presence of M2-polarized tumor associated macrophages, myeloid-derived suppressor cells (MDSCs), type II natural killer T cells, regulatory/tolerogenic dendritic cells, and regulatory T cells (Tregs) is associated with cancer progression, whereas Ml-polarized tumor associated macrophages, type I natural killer T cells, and effector T cells contribute to immune-mediated tumor eradication [88–92]. The delicate balance between these opposing cell populations determines the fate of a tumor (i.e., elimination or promotion).
The complement system is critical for the regulation of adaptive T-cell responses [93]. Deficiencies in complement proteins are associated with impaired CD4+ and CD8+ T-cell effector responses, suggesting that complement can sustain adaptive immune responses against tumors [94–98]. On the other hand, complement activation can also promote an immunosuppressive tumor microenvironment. C5a is a potent chemoattractant for MDSCs that is able to increase the number of highly suppressive polymorphonuclear MDSCs in the tumor stroma and to facilitate the tumorimmunosuppressive effects of mononuclear MDSCs. This activation of complement enhances the production of reactive oxygen and nitrogen species within tumor microenvironment, which is accompanied by a subsequent decrease in tumor cytotoxic CD8 T-cell responses [36]. In accordance with these functions, the blockade of C5a in mice bearing lung cancer cells significantly diminishes the number of polymorphonuclear MDSCs in the spleen and the intratumoral expression of immunosuppressive mediators [35].
Complement activation also affects the activity of Tregs, an important immunosuppressive population of T cells. Activation of C5a and C3a receptors in Tregs triggers pAKT-dependent phosphorylation of the transcription factor Foxol, downregulating the levels of Foxp3 and, therefore, the suppressive Treg activity [99]. In addition, blockade of C3aR and C5aR in responder CD4+ T cells can promote their differentiation towards a Treg phenotype [100]. However, C5a at high concentrations significantly promotes Treg differentiation, which suggests that this factor may regulate Treg differentiation in a concentration dependent manner [60].
Other complement effectors can also contribute to tumor-mediated immunoregulation. Crosslinking of CD46 with C3b or monoclonal antibodies combined with CD3 activation leads to the development of Tregs [101]. The ligation of the complement C3 activation product iC3b to CR3 on antigen-presenting cells results in antigen-specific systemic tolerance [102]. Furthermore, iC3b/CR3 interaction upregulates the expression of TGF-β2 and IL-10, two pivotal contributors of tumor-mediated immunosuppression [102]. Recently, it has also been demonstrated that iC3b promotes the generation of powerful immunosuppressing MDSCs [103]. In conclusion, there is evidence to support the hypothesis that complement modulates the immune response generated by tumor cells. However, only fragmentary results are available concerning the impact of complement activation on the immune tumor microenvironment (Table 1). Of note, the outcome with respect to tumor growth can depend on the levels of complement effectors in the local tumor microenvironment [60].
Table 1.
Effects of complement on the immune tumor microenvironment of different murine cancer models1.
| Tumor type2 | Cervical cancer (TC-1) |
Lung cancer (3LL) |
Ovarian cancer (transgenic Tg+) |
Ovarian cancer (SKOV-3) |
Lymphoma (RMA) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental model | C3 KO3 |
C5aR KO |
C5aR AP |
C5aR AP |
C5aR AA |
C3 KO |
C3 Het. |
C5aR KO |
C5atransfection (low expression) |
C5a transfection (low expression) |
C5a transfection (high expression) |
||
| Tumor growth | ↓ | ↓ | ↓ | ↓ | ↓ (n.s.) | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ||
| Tumor | Immune cell populations | Total MDSCs | ↑ (n.s.) | = | = | ↑ (n.s.) | = | = | |||||
| MO-MDSC | = | ||||||||||||
| PMN-MDSCs | ↓ | ||||||||||||
| B cells | = | = | |||||||||||
| Macrophages | = | = | t | = | |||||||||
| CD8+ T cells | ↑ (n.s.) | ↑ (n.a.) | ↓ | = | = | ↓ | |||||||
| CD4+ T cells | = | ↓ | |||||||||||
| NK | ↓ | = | |||||||||||
| Tregs | ↓ | = | |||||||||||
| Immunomodulatory molecules | RNS/ROS | ↓ | |||||||||||
| iNOS | = | ||||||||||||
| Arginase-1 | ↑ (n.s.) | ↓ | ↓ | ↓ | |||||||||
| LAG3 | ↓ | ||||||||||||
| IL-6 | ↓ | ↓ (n.s.) | = | ||||||||||
| IL-10 | ↓ | ↓ (n.s.) | = | ||||||||||
| CTLA4 | ↓ | = | |||||||||||
| PDL1 | ↓ | = | |||||||||||
| TGF-β | = | = | = | ||||||||||
| IL-12 | = | ||||||||||||
| IFN-γ | = | ||||||||||||
| TNF-α | = | = | ↓ | ||||||||||
| Grazyme B | = | = | = | ||||||||||
| Perforin | = | = | = | ||||||||||
| Others4 | = | = | |||||||||||
| Spleen | Immune cell populations | Total MDSCs | ↓ | ↓ | ↓ (n.s.) | = | ↑ | ||||||
| MO-MDSC | = | ||||||||||||
| PMN-MDSCs | ↓ | ||||||||||||
| Macrophages | = | ||||||||||||
| CD8+ T cells | = | ↑ | ↓ | ||||||||||
| CD4+ T cells | = | ↑ | ↓ | ||||||||||
| NK | ↑ (n.s.) | = | |||||||||||
| Tregs | = | = | ↑ | ||||||||||
Data were obtained from Markiewski et al. [36], Corrales et al. [35], Nunez-Cruz et al.[59] and Gunn et al.[60]. The first three references correspond to models of complement inhibition. In contrast, the last study analyzed the effects of an increased expression of C5a (shaded boxes).
Cell lines inoculated in mice are indicated in parenthesis.
Abreviations: KO: knockout; AP: antagonist peptide; AA: antagonist antibody; Het.: heterozygous; MDSCs: myeloid-derived suppressor cells; MO: mononuclear; PMN: polymorphonuclear; Tregs: regulatory T cells; NK: natural killers; RNS: reactive nitrogen species; ROS: reactive oxygen species; n.s.: differences did not reach statistical significance; n.a.: statistical differences no available.
IDO, PD1, CCL17, CCL22, GITR, MCP1 and granzyme A.
4.3. Complement and angiogenesis
Neoplastic tissues need to be able to grow new blood vessels in order to receive adequate oxygen and nutrients. Importantly, the aggressiveness of tumors is directly related to the density of their tumor microvasculature [104], and emerging cancer treatments are based on the suppression of angiogenesis [105]. The role of complement in angiogenesis has been studied in a wide range of diseases and experimental models. However, whether complement is pro- or anti-angiogenic is controversial, and the reported results have depended on the specific pathological features of the disease being studied.
On the one hand, activation of C5aR polarizes macrophages toward an angiogenesisinhibitory phenotype in a murine model of retinopathy of prematurity [106]. This phenotype is characterized by the upregulation of anti-angiogenic soluble vascular endothelial growth factor receptor-1. The secretion of this factor has also been observed in monocytes in an antibody-independent model of spontaneous miscarriage and intrauterine growth restriction [107].
On the other hand, C5a enhances endothelial cell migration and tube-like formation in vitro [35, 59, 108]. C5a triggers human umbilical endothelial cell activation through the upregulation of genes involved in endothelial adhesion, migration, and angiogenesis [109, 110]. In addition, C5a and C3a stimulate choroidal neovascularization, a common and severe complication of age-related macular degeneration (AMD) [111]. Also, laserinduced choroidal neovascularization is accompanied by the presence of C3 and MAC deposits and the upregulation of the angiogenic factors TGF-β2, vascular endothelial growth factor, and basic fibroblast growth factor [80]. The importance of complement in choroidal neovascularization is strongly supported by the involvement of the complement regulator factor H in AMD. Factor H is present in ocular tissues, and a polymorphic variation (Y402H) has been shown to be associated with an increased risk for AMD [112, 113].
Few studies have addressed the role of complement in tumor angiogenesis in vivo. No effect on tumor angiogenesis was observed after the blockade of C5aR in a murine cervical tumor model [36]. Similarly, in a lung cancer model, there were no differences in tumor vascular density when animals were treated with a C5aR antagonist, although the expression of basic fibroblast growth factor, a potent mitogen and chemotactic factor for endothelial cells, was significantly reduced within the tumors [35]. In contrast, genetic C3 and C5aR deficiencies have been found to be associated with reduced vascularization in a mouse model of ovarian cancer [59]. It is clear that the effect of complement on tumor angiogenesis is under the influence of uncharacterized variables that merit further investigation.
4.4. Complement and cancer cell signaling
Complement participates in several transduction pathways involved in tumor progression. Tumor cells evade lysis by the use of protective mechanisms that limit the formation of functional MAC pores [114]. Non-lytic complement activation leads to the generation of bioactive products that may be involved in pro-oncogenic signaling pathways [115]. Deposition of sublytic doses of MACs induces ion shifts that promote proliferation, differentiation, and resistance to apoptosis [116]. Sublytic MACs activate cancer-associated signaling pathways involving MAPK, phosphatidylinositol 3-kinase, Ras, and p70 S6 kinase [117–119]. In addition, sublytic doses of complement inhibit apoptosis by blocking apoptosis drivers such as FLIP, caspase-8, Bid, and inducing the phosphorylation of Bad [116]. In oligodendrocytes, sublytic complement activation induces RGC-32 [120], a molecule upregulated in several human malignancies that complexes with CDC2/cyclin Bl to positively regulate cell cycle activation [121, 122].
Other complement components can participate in the activation of relevant signaling pathways. Clq binding to frizzled receptors activates canonical Wnt signaling in a murine model of aging [123], and Wnt signaling pathways regulate various processes that are important in the pathogenesis of cancer [124]. The anaphylatoxins C5a and C3a trigger signaling pathways that mediate survival and antiapoptotic responses in a wide range of cells. They activate MAPK and AKT signaling in leukocytes [125, 126] and protect neurons against cell death [127, 128]. C5a upregulates the expression of the hepatocyte growth factor-c-Met axis in injured liver [129]. The activation of this pathway has mitogenic and anti-apoptotic effects and is deregulated in many tumors [130]. The C5a/C5aR axis also upregulates phospholipase C β2 [131], phospholipase D [132], and cyclins E and Dl [129]. In addition, C5a enhances the transactivation of epidermal growth factor receptor [109], a therapeutic target in many tumors [133].
5. Complement inhibition for cancer treatment
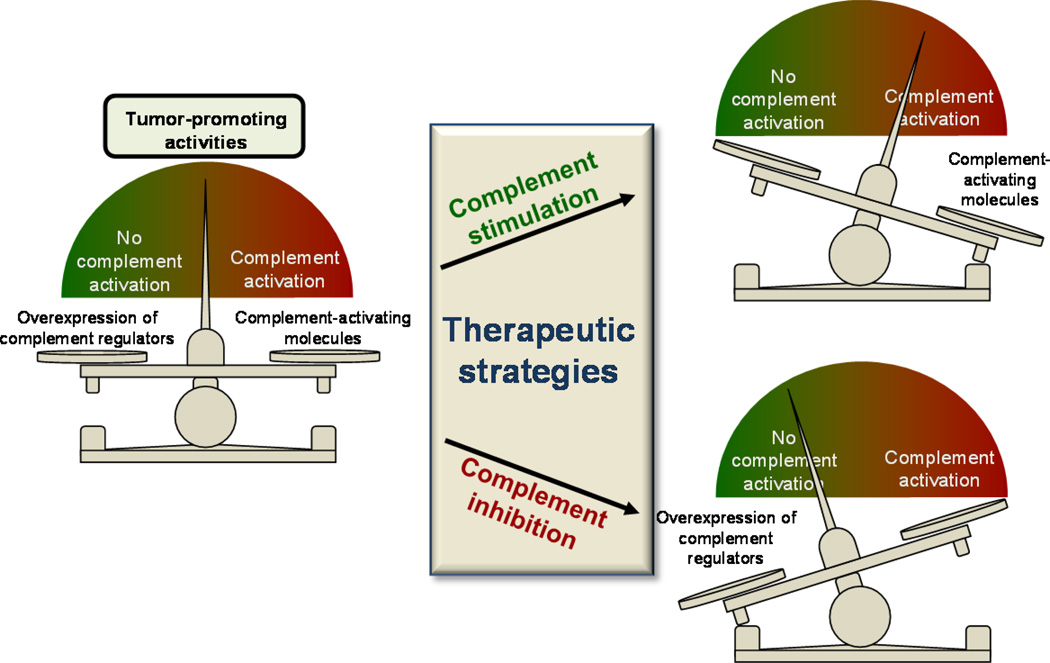
Activation of the complement system by tumor cells was long believed to act only for benefit of the host. In fact, intense research has been devoted to the use of complement to improve the efficacy of anticancer therapies based on monoclonal antibodies [29]. The Fc regions of membrane-bound therapeutic antibodies interact with the heterooligomeric Clq complex and activate the classical pathway. Complement activation leads to formation of MAC and fosters opsonization. In addition, complement can synergize with other antibody-mediated mechanisms of action. Many therapeutic strategies designed to overcome the protection mediated by complement inhibitors or to improve complement-mediated effector responses have been developed and tested experimentally in both in vitro and animal models [26, 134–136]. However, recent findings proposing novel complement-mediated roles in tumor progression provide preliminary evidence of the potential utility of a distinct therapeutic option: complement inhibition as an anticancer therapy (Fig. 4).
Fig. 4.
Potentially useful, although antagonistic, complement-based anti-cancer therapeutic strategies. Both activation and inhibition of complement can be proposed to treat cancer. In both cases, the cancer-promoting balance between activation and protection would be destroyed. For example, elimination of complement regulators would lead to an increase in tumor-control activities mediated by complement (e.g., lysis, opsonization, immunostimulation). On the other hand, complement inhibition would eliminate tumor-promoting activities, such as immunosuppression, chronic inflammation, or angiogenesis, which may be hampering other immune effector responses. In this context, combination with immunotherapies or chemotherapies would be advantageous.
Despite the ubiquitous presence of complement, relatively few side effects have been reported for complement-directed therapy [137, 138], in contrast to the high toxicity associated with traditional anticancer chemotherapeutics. A wide repertoire of chemical inhibitors targeting complement has been developed and are currently in preclinical or clinical development [138, 139]. In 2007, the US Food and Drug Administration approved the use of the first complement-specific drug eculizumab, an antibody against complement component C5. This approval was a breakthrough in the complement field that validated the complement system as a therapeutic target [139]. Since then, a multifaceted armory of therapeutic inhibitors has been proposed for the diverse array of complement-mediated pathologies [138]. However, when thinking about the best complement-related therapeutic options to treat cancer, many more questions than answers arise.
While the complexity of the complement response offers numerous potential targets, it also poses a major challenge. One of the most critical aspects to be considered is the optimal point of intervention within the complement cascade [140]. Although the effects of complement inhibition are still somewhat speculative, both advantages and disadvantages can be anticipated for the inhibition of complement at different steps of the activation cascade (Table 2). For example, inhibition of the Clq complex by the C1 inhibitor (Cl-INH), a protease inhibitor approved by the FDA for the treatment of hereditary angioedema [141], would effectively shut down the activation of the classical pathway while preserving the beneficial functions of the other initiating pathways. Alternatively, a blockade further downstream would eliminate effector activities common to different activation pathways.
Table 2.
Potential points of therapeutic intervention to inhibit complement for cancer treatment.
| Intervention point | Drug example | Hypothetical advantages and disadvantages of each intervention point | ||||
|---|---|---|---|---|---|---|
| Pathway-specific Inhibition |
Cl-INH | Advantages: | ||||
| Preserve the beneficial functions of the other initiating pathways | ||||||
| Supported by a genetic models of cervical cancer (C4 KO) | ||||||
| Disadvantages: | ||||||
| More than one pathway may be involved in cancer progression | ||||||
| C3 inhibition | Compstatin | Advantages: | ||||
| Broadest effect | ||||||
| Supported by genetic models of cervical and ovarian cancer (C3 KO) | ||||||
| C5 inhibition | Eculizumab | Advantages: | ||||
| Intact complement deposition at the C3 level | ||||||
| Avoids the sublytic effect of MAC | ||||||
| Disadvantages: | ||||||
| No tested in in vivo cancer models | ||||||
| C5a/C5aR Inhibition |
PMX-53 | Advantages: | ||||
| Experimental data supporting the protumoral activity of C5a | ||||||
| Supported by preclinical models of cervical, ovarian, and lung cancer | ||||||
| Disadvantages: | ||||||
| Only one effector molecule is inhibited, while others may also be important (e.g., C3a) | ||||||
C5a has been shown to be a key mediator in the regulation of cancer growth by complement [35, 36, 59, 60]. Selective inhibition of the binding of C5a to its receptors could obstruct the protumoral microenvironment without depleting the defensive potential of complement activation. Drugs that inhibit C5a generation (e.g. eculizumab) or neutralize the C5a-C5aR interaction (e.g. PMX-53) are in different stages of clinical or preclinical development [142]. To date, little attention has been paid to the evaluation of their applicability against cancer. In addition, the involvement of other effector molecules, such as C3a and its receptor C3aR, has not yet been addressed and should not be neglected.
Compstatin (and its analogs) is a cyclic tridecapeptide that prevents the cleavage of C3 into its active fragments C3a and C3b and, therefore, affects the most central step in the complement cascade [143, 144]. Inhibition of complement at this level efficiently blocks all activation, amplification, and effector routes and should be seriously considered for cancer applications. Unfortunately, compstatin does not work on mouse C3 [145], restricting its evaluation in standard preclinical models of cancer. Therefore, for a comprehensive validation of this drug in the cancer field, alternative strategies need to be designed.
Irrespective of the treatment strategy, additional cancer models are needed to further validate the concept of complement inhibition for anticancer therapy. Most current in vivo models are based on heterotopic implantation of tumors cells. There is a need for testing orthotopic models, in which tumor cells are implanted into the organ of origin in models that allow a more suitable interaction between tumor cells and the surrounding stroma. Mouse models for spontaneous or chemically induced tumors can also provide more relevant information about the role of complement in the modulation of the tumor microenvironment.
Models of metastasis will also provide very useful information. Furthermore, the use of complement-directed drugs in combinatorial therapies should be considered. For example, given that complement inhibition can override tumor-dependent immunosuppression, this therapeutic approach could use to supplement antitumor vaccines. It has been hypothesized that the deposition of sublytic doses of MAC induces protection from immune clearance [146]. In this context, therapeutic inhibition of complement may increase the efficacy of cell-based tumor immunotherapies [146].
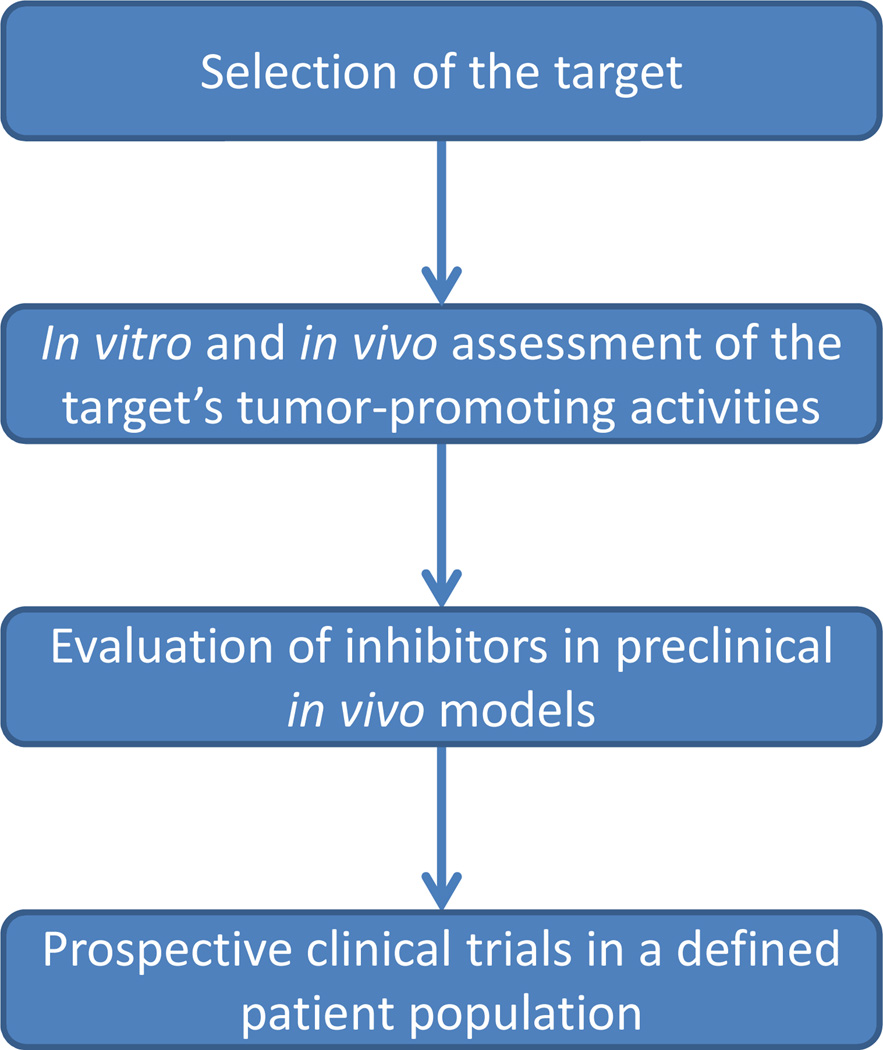
There are many challenges and limitations that can impede progress in this field. A more detailed understanding of the intricate network established between complement and cancer is crucial to bridging the gap between promising preclinical trials and effective clinical treatments. For example, despite our accumulating knowledge about the role of complement in cancer, the activating molecules that initiate the complement cascade in cancer cells are essentially unknown. Due the high heterogeneity of human cancer, it is possible that different activation pathways and mechanisms may be involved, and different strategies maybe needed to treat different tumor types. The role of complement at particular stages of tumor progression must also be considered. In addition, translational problems between mouse and human systems are expected to be also encountered. It is well-known that bench results do not necessarily translate to bedside efficacy, and truly effective applications will be only demonstrated in the clinical setting (Fig. 5).
Fig. 5.
Sequential steps needed to validate anti-cancer targeted strategies before they can be translated to the bedside.
6. Final remarks
Based on a number of in vitro studies and preclinical models, the concept of blocking complement for the treatment of cancer is gaining recognition. However, the mechanisms and functional effects of complement-specific deregulation on the tumor microenvironment are still unclear. More work is needed to elucidate the complex processes by which cancer cells control complement activation and how complement effector activities influence tumor progression. Additional animal models have to be tested to define the best treatment strategy, and biomarkers should be development to guide the selection of patients and individualize treatment. At present, we can conclude that complement-related anticancer strategies are a promising challenge and offer hope for successfully fighting cancer.
Highlights.
-
-
Several studies suggest that cancer cells efficiently activate complement
-
-
Cancer cells establish a balance between complement activation and inhibition
-
-
Complement activation exerts tumor-promoting activities
-
-
Complement inhibition may serve as a therapeutic strategy against cancer
Acknowledgments
Work in the authors’ laboratories is funded by the UTE project CIMA, Instituto de Salud Carlos III: RTICC (RD12/0036/0040), Ministerio de Economia y Competitividad (PI1100618), the European Community's Seventh Framework Programme (HEALTH-F2-2010-258677-CURELUNG), and NIH grant AI068730. We thank Dr. Deborah McClellan for editorial assistance.
Abbreviations
- MAC
membrane attack complex
- TGF-β
transforming growth factor β
- TLR
toll-like receptor
- MDSC
myeloid-derived suppressor cells
- Tregs
regulatory T cell
- AMD
age-related macular degeneration
- C1-INH
C1 inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Tanner NT, Pastis NJ, Sherman C, Simon GR, Lewin D, Silvestri GA. The role of molecular analyses in the era of personalized therapy for advanced NSCLC. Lung Cancer. 2012;76:131–137. doi: 10.1016/j.lungcan.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxnard GR, Binder A, Janne PA. New Targetable Oncogenes in Non-Small-Cell Lung Cancer. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.42.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 6.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 7.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markiewski MM, Lambris JD. Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends Immunol. 2009;30:286–292. doi: 10.1016/j.it.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 10.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 11.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 12.Costello LC, Franklin RB. 'Why do tumour cells glycolyse?': from glycolysis through citrate to lipogenesis. Mol Cell Biochem. 2005;280:1–8. doi: 10.1007/s11010-005-8841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glunde K, Serkova NJ. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics. 2006;7:1109–1123. doi: 10.2217/14622416.7.7.1109. [DOI] [PubMed] [Google Scholar]
- 14.Chandrasekaran EV, Xue J, Neelamegham S, Matta KL. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr Res. 2006;341:983–994. doi: 10.1016/j.carres.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Miyagi T, Takahashi K, Moriya S, Hata K, Yamamoto K, Wada T, et al. Altered expression of sialidases in human cancer. Adv Exp Med Biol. 2012;749:257–267. doi: 10.1007/978-1-4614-3381-1_17. [DOI] [PubMed] [Google Scholar]
- 16.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 17.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 19.McConnell I, Klein G, Lint TF, Lachmann PJ. Activation of the alternative complement pathway by human B cell lymphoma lines is associated with Epstein-Barr virus transformation of the cells. Eur J Immunol. 1978;8:453–458. doi: 10.1002/eji.1830080702. [DOI] [PubMed] [Google Scholar]
- 20.Niculescu F, Rus HG, Retegan M, Vlaicu R. Persistent complement activation on tumor cells in breast cancer. Am J Pathol. 1992;140:1039–1043. [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas SD, Karlsson-Parra A, Nilsson B, Grimelius L, Akerstrom G, Rastad J, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol. 1996;27:1329–1335. doi: 10.1016/s0046-8177(96)90346-9. [DOI] [PubMed] [Google Scholar]
- 22.Gasque P, Thomas A, Fontaine M, Morgan BP. Complement activation on human neuroblastoma cell lines in vitro: route of activation and expression of functional complement regulatory proteins. J Neuroimmunol. 1996;66:29–40. doi: 10.1016/0165-5728(96)00015-x. [DOI] [PubMed] [Google Scholar]
- 23.Bu X, Zheng Z, Wang C, Yu Y. Significance of C4d deposition in the follicular lymphoma and MALT lymphoma and their relationship with follicular dendritic cells. Pathol Res Pract. 2007;203:163–167. doi: 10.1016/j.prp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Jurianz K, Ziegler S, Garcia-Schuler H, Kraus S, Bohana-Kashtan O, Fishelson Z, et al. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36:929–939. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 25.Gorter A, Meri S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol Today. 1999;20:576–582. doi: 10.1016/s0167-5699(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 26.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 27.Yan J, Allendorf DJ, Li B, Yan R, Hansen R, Donev R. The role of membrane complement regulatory proteins in cancer immunotherapy. Adv Exp Med Biol. 2008;632:159–174. [PubMed] [Google Scholar]
- 28.Gancz D, Fishelson Z. Cancer resistance to complement-dependent cytotoxicity (CDC): Problem-oriented research and development. Mol Immunol. 2009;46:2794–2800. doi: 10.1016/j.molimm.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Kolev M, Towner L, Donev R. Complement in cancer and cancer immunotherapy. Arch Immunol Ther Exp (Warsz) 2011;59:407–419. doi: 10.1007/s00005-011-0146-x. [DOI] [PubMed] [Google Scholar]
- 30.Nishioka K, Kawamura K, Hirayama T, Kawashima T, Shimada K. The complement system in tumor immunity: significance of elevated levels of complement in tumor bearing hosts. Ann N Y Acad Sci. 1976;276:303–315. doi: 10.1111/j.1749-6632.1976.tb41656.x. [DOI] [PubMed] [Google Scholar]
- 31.Maness PF, Orengo A. Serum complement levels in patients with digestive tract carcinomas and other neoplastic diseases. Oncology. 1977;34:87–89. doi: 10.1159/000225191. [DOI] [PubMed] [Google Scholar]
- 32.Gminski J, Mykala-Ciesla J, Machalski M, Drozdz M, Najda J. Immunoglobulins and complement components levels in patients with lungcancer. Rom J Intern Med. 1992;30:39–44. [PubMed] [Google Scholar]
- 33.Ytting H, Jensenius JC, Christensen IJ, Thiel S, Nielsen HJ. Increased activity of the mannan-binding lectin complement activation pathway in patients with colorectal cancer. Scand J Gastroenterol. 2004;39:674–679. doi: 10.1080/00365520410005603. [DOI] [PubMed] [Google Scholar]
- 34.Bjorge L, Hakulinen J, Vintermyr OK, Jarva H, Jensen TS, Iversen OE, et al. Ascitic complement system in ovarian cancer. Br J Cancer. 2005;92:895–905. doi: 10.1038/sj.bjc.6602334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrales L, Ajona D, Rafail S, Lasarte JJ, Riezu-Boj JI, Lambris JD, et al. Anaphylatoxin c5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189:4674–4683. doi: 10.4049/jimmunol.1201654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fust G, Miszlay Z, Czink E, Varga L, Paloczi K, Szegedi G, et al. CI and C4 abnormalities in chronic lymphocytic leukaemia and their significance. Immunol Lett. 1987;14:255–259. doi: 10.1016/0165-2478(87)90110-6. [DOI] [PubMed] [Google Scholar]
- 38.Schlesinger M, Broman I, Lugassy G. The complement system is defective in chronic lymphatic leukemia patients and in their healthy relatives. Leukemia. 1996;10:1509–1513. [PubMed] [Google Scholar]
- 39.Budzko DB, Lachmann PJ, McConnell I. Activation of the alternative complement pathway by lymphoblastoid cell lines derived from patients with Burkitt's lymphoma and infectious mononucleosis. Cell Immunol. 1976;22:98–109. doi: 10.1016/0008-8749(76)90011-3. [DOI] [PubMed] [Google Scholar]
- 40.Theofilopoulos AN, Perrin LH. Binding of components of the properdin system to cultured human lymphoblastoid cells and B lymphocytes. J Exp Med. 1976;143:271–289. doi: 10.1084/jem.143.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraut EH, Sagone AL., Jr. Alternative pathway of complement in multiple myeloma. Am J Hematol. 1981;11:335–345. doi: 10.1002/ajh.2830110402. [DOI] [PubMed] [Google Scholar]
- 42.Kalwinsky DK, Urmson JR, Stitzel AE, Spitzer RE. Activation of the alternative pathway of complement in childhood acute lymphoblastic leukemia. J Lab Clin Med. 1976;88:745–756. [PubMed] [Google Scholar]
- 43.Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, et al. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 45.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, et al. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632:71–9. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos-Casals M, Campoamor MT, Chamorro A, Salvador G, Segura S, Botero JC, et al. Hypocomplementemia in systemic lupus erythematosus and primary antiphospholipid syndrome: prevalence and clinical significance in 667 patients. Lupus. 2004;13:777–783. doi: 10.1191/0961203304lu1080oa. [DOI] [PubMed] [Google Scholar]
- 48.Palikhe A, Sinisalo J, Seppanen M, Haario H, Meri S, Valtonen V, et al. Serum complement C3/C4 ratio, a novel marker for recurrent cardiovascular events. Am J Cardiol. 2007;99:890–895. doi: 10.1016/j.amjcard.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 49.Kanmura S, Uto H, Sato Y, Kumagai K, Sasaki F, Moriuchi A, et al. The complement component C3a fragment is a potential biomarker for hepatitis C virus-related hepatocellular carcinoma. J Gastroenterol. 2010;45:459–467. doi: 10.1007/s00535-009-0160-5. [DOI] [PubMed] [Google Scholar]
- 50.Carli M, Bucolo C, Pannunzio MT, Ongaro G, Businaro R, Revoltella R. Fluctuation of serum complement levels in children with neuroblastoma. Cancer. 1979;43:2399–2404. doi: 10.1002/1097-0142(197906)43:6<2399::aid-cncr2820430634>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 51.Varga L, Czink E, Miszlai Z, Paloczi K, Banyai A, Szegedi G, et al. Low activity of the classical complement pathway predicts short survival of patients with chronic lymphocytic leukaemia. Clin Exp Immunol. 1995;99:112–116. doi: 10.1111/j.1365-2249.1995.tb03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ytting H, Christensen IJ, Thiel S, Jensenius JC, Nielsen HJ. Serum mannan-binding lectin-associated serine protease 2 levels in colorectal cancer: relation to recurrence and mortality. Clin Cancer Res. 2005;11:1441–1446. doi: 10.1158/1078-0432.CCR-04-1272. [DOI] [PubMed] [Google Scholar]
- 53.Durrant LG, Chapman MA, Buckley DJ, Spendlove I, Robins RA, Armitage NC. Enhanced expression of the complement regulatory protein CD55 predicts a poor prognosis in colorectal cancer patients. Cancer Immunol Immunother. 2003;52:638–642. doi: 10.1007/s00262-003-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu C, Jung M, Burkhardt M, Stephan C, Schnorr D, Loening S, et al. Increased CD59 protein expression predicts a PSA relapse in patients after radical prostatectomy. Prostate. 2005;62:224–232. doi: 10.1002/pros.20134. [DOI] [PubMed] [Google Scholar]
- 55.Watson NF, Durrant LG, Madjd Z, Ellis IO, Scholefield JH, Spendlove I. Expression of the membrane complement regulatory protein CD59 (protectin) is associated with reduced survival in colorectal cancer patients. Cancer Immunol Immunother. 2006;55:973–980. doi: 10.1007/s00262-005-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michlmayr A, Bachleitner-Hofmann T, Baumann S, Marchetti-Deschmann M, Rech-Weichselbraun I, Burghuber C, et al. Modulation of plasma complement by the initial dose of epirubicin/docetaxel therapy in breast cancer and its predictive value. Br J Cancer. 2011;103:1201–1208. doi: 10.1038/sj.bjc.6605909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shearer WT, Atkinson JP, Frank MM, Parker CW. Humoral immunostimulation IV Role of complement. J Exp Med. 1975;141:736–752. [PMC free article] [PubMed] [Google Scholar]
- 58.Markiewski MM, Lambris JD. Unwelcome complement. Cancer Res. 2009;69:6367–6370. doi: 10.1158/0008-5472.CAN-09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunez-Cruz S, Gimotty PA, Guerra MW, Connolly DC, Wu YQ, Deangelis RA, et al. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 2012;14:994–1004. doi: 10.1593/neo.121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gunn L, Ding C, Liu M, Ma Y, Qi C, Cai Y, et al. Opposing roles for complement component C5a in tumor progression and the tumor microenvironment. J Immunol. 2012;189:2985–2994. doi: 10.4049/jimmunol.1200846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 62.Sharma P, Old LJ, Allison JP. Immunotherapeutic strategies for high-risk bladder cancer. Semin Oncol. 2007;34:165–172. doi: 10.1053/j.seminoncol.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanca T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology. 2012;1:717–125. doi: 10.4161/onci.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dumont P, Berton A, Nagy N, Sandras F, Tinton S, Demetter P, et al. Expression of galectin-3 in the tumor immune response in colon cancer. Lab Invest. 2008;88:896–906. doi: 10.1038/labinvest.2008.54. [DOI] [PubMed] [Google Scholar]
- 65.Ong SM, Tan YC, Beretta O, Jiang D, Yeap WH, Tai JJ, et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol. 2012;42:89–100. doi: 10.1002/eji.201141825. [DOI] [PubMed] [Google Scholar]
- 66.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 68.Boffetta P. Epidemiology of environmental and occupational cancer. Oncogene. 2004;23:6392–6403. doi: 10.1038/sj.onc.1207715. [DOI] [PubMed] [Google Scholar]
- 69.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 70.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013 doi: 10.4049/jimmunol.1203487. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarma VJ, Huber-Lang M, Ward PA. Complement in lung disease. Autoimmunity. 2006;39:387–394. doi: 10.1080/08916930600739456. [DOI] [PubMed] [Google Scholar]
- 72.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 73.Kachur JF, Won-Kim S, Anglin C, Gaginella TS. Eicosanoids and histamine mediate C5a-induced electrolyte secretion in guinea pig ileal mucosa. Inflammation. 1995;19:717–725. doi: 10.1007/BF01534574. [DOI] [PubMed] [Google Scholar]
- 74.Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ballanti E, Perricone C, di Muzio G, Kroegler B, Chimenti MS, Graceffa D, et al. Role of the complement system in rheumatoid arthritis and psoriatic arthritis: relationship with anti-TNF inhibitors. Autoimmun Rev. 2011;10:617–623. doi: 10.1016/j.autrev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 76.Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Parsa AT. Cancer and the complement cascade. Mol Cancer Res. 2010;8:1453–1465. doi: 10.1158/1541-7786.MCR-10-0225. [DOI] [PubMed] [Google Scholar]
- 77.Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, et al. The regulation of liver cell survival by complement. J Immunol. 2009;182:5412–5418. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Sayah S, Ischenko AM, Zhakhov A, Bonnard AS, Fontaine M. Expression of cytokines by human astrocytomas following stimulation by C3a and C5a anaphylatoxins: specific increase in interleukin-6 mRNA expression. J Neurochem. 1999;72:2426–2436. doi: 10.1046/j.1471-4159.1999.0722426.x. [DOI] [PubMed] [Google Scholar]
- 80.Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- 81.Gionanlis L, Alexopoulos E, Papagianni A, Leontsini M, Memmos D. Fibrotic mechanisms in idiopathic rapidly progressive glomerulonephritis: the role of TGF-betal and C5b-9. Ren Fail. 2008;30:239–246. doi: 10.1080/08860220701804979. [DOI] [PubMed] [Google Scholar]
- 82.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ridnour LA, Cheng RY, Switzer CH, Heinecke JL, Ambs S, Glynn S, et al. Molecular Pathways: Toll-like Receptors in the Tumor Microenvironment—Poor Prognosis or New Therapeutic Opportunity. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shay JE, Celeste Simon M. Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol. 2012;23:389–394. doi: 10.1016/j.semcdb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Okroj M, Corrales L, Stokowska A, Pio R, Blom AM. Hypoxia increases susceptibility of non-small cell lung cancer cells to complement attack. Cancer Immunol Immunother. 2009;58:1771–1780. doi: 10.1007/s00262-009-0685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang E, Worschech A, Marincola FM. The immunologic constant of rejection. Trends Immunol. 2008;29:256–262. doi: 10.1016/j.it.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 90.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. The dendritic cell-regulatory T lymphocyte crosstalk contributes to tumor-induced tolerance. Clin Dev Immunol. 2011;2011:430394. doi: 10.1155/2011/430394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 94.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 95.Kim AH, Dimitriou ID, Holland MC, Mastellos D, Mueller YM, Altman JD, et al. Complement C5a receptor is essential for the optimal generation of antiviral CD8+ T cell responses. J Immunol. 2004;173:2524–2529. doi: 10.4049/jimmunol.173.4.2524. [DOI] [PubMed] [Google Scholar]
- 96.Fang C, Miwa T, Shen H, Song WC. Complement-dependent enhancement of CD8+ T cell immunity to lymphocytic choriomeningitis virus infection in decayaccelerating factor-deficient mice. J Immunol. 2007;179:3178–3186. doi: 10.4049/jimmunol.179.5.3178. [DOI] [PubMed] [Google Scholar]
- 97.Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, et al. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood. 2008;111:2452–2461. doi: 10.1182/blood-2007-06-095018. [DOI] [PubMed] [Google Scholar]
- 98.Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, et al. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood. 2008;112:5084–5094. doi: 10.1182/blood-2008-05-156646. [DOI] [PubMed] [Google Scholar]
- 99.Kwan WH, van der Touw W, Paz-Altai E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257–268. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-betal signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:162–171. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 102.Sohn JH, Bora PS, Suk HJ, Molina H, Kaplan HJ, Bora NS. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Nat Med. 2003;9:206–212. doi: 10.1038/nm814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsieh CC, Chou HS, Yang HR, Lin F, Bhatt S, Qin J, et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013 doi: 10.1182/blood-2012-06-440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giatromanolaki A, Sivridis E, Koukourakis MI. Angiogenesis in colorectal cancer: prognostic and therapeutic implications. Am J Clin Oncol. 2006;29:408–417. doi: 10.1097/01.coc.0000221317.56731.4e. [DOI] [PubMed] [Google Scholar]
- 105.Schneider BP, Shen F, Miller KD. Pharmacogenetic biomarkers for the prediction of response to antiangiogenic treatment. Lancet Oncol. 2012;13:e427–e436. doi: 10.1016/S1470-2045(12)70275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Langer HF, Chung KJ, Orlova VV, Choi EY, Kaul S, Kruhlak MJ, et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116:4395–4403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurihara R, Yamaoka K, Sawamukai N, Shimajiri S, Oshita K, Yukawa S, et al. C5a promotes migration, proliferation, and vessel formation in endothelial cells. Inflamm Res. 2010;59:659–666. doi: 10.1007/s00011-010-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schraufstarter IU, Trieu K, Sikora L, Sriramarao P, DiScipio R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002;169:2102–2110. doi: 10.4049/jimmunol.169.4.2102. [DOI] [PubMed] [Google Scholar]
- 110.Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, et al. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004;164:849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. ProcNatl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mandal MN, Ayyagari R. Complement factor H: spatial and temporal expression and localization in the eye. Invest Ophthalmol Vis Sci. 2006;47:4091–4097. doi: 10.1167/iovs.05-1655. [DOI] [PubMed] [Google Scholar]
- 114.Cole DS, Morgan BP. Beyond lysis: how complement influences cell fate. Clin Sci (Lond) 2003;104:455–466. doi: 10.1042/CS20020362. [DOI] [PubMed] [Google Scholar]
- 115.Vlaicu SI, Tegla CA, Cudrici CD, Danoff J, Madani H, Sugarman A, et al. Role of C5b-9 complement complex and response gene to complement-32 (RGC-32) in cancer. Immunol Res. 2012 doi: 10.1007/s12026-012-8381-8. [DOI] [PubMed] [Google Scholar]
- 116.Tegla CA, Cudrici C, Patel S, Trippe R, 3rd, Rus V, Niculescu F, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res. 2011;51:45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kraus S, Seger R, Fishelson Z. Involvement of the ERK mitogen-activated protein kinase in cell resistance to complement-mediated lysis. Clin Exp Immunol. 2001;123:366–374. doi: 10.1046/j.1365-2249.2001.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niculescu F, Badea T, Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999;142:47–56. doi: 10.1016/s0021-9150(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 119.Niculescu F, Rus H, van Biesen T, Shin ML. Activation of Ras and mitogen-activated protein kinase pathway by terminal complement complexes is G protein dependent. J Immunol. 1997;158:4405–4412. [PubMed] [Google Scholar]
- 120.Badea TC, Niculescu FI, Soane L, Shin ML, Rus H. Molecular cloning and characterization of RGC-32, a novel gene induced by complement activation in oligodendrocytes. J Biol Chem. 1998;273:26977–2681. doi: 10.1074/jbc.273.41.26977. [DOI] [PubMed] [Google Scholar]
- 121.Badea T, Niculescu F, Soane L, Fosbrink M, Sorana H, Rus V, et al. RGC-32 increases p34CDC2 kinase activity and entry of aortic smooth muscle cells into S-phase. J Biol Chem. 2002;277:502–508. doi: 10.1074/jbc.M109354200. [DOI] [PubMed] [Google Scholar]
- 122.Fosbrink M, Cudrici C, Niculescu F, Badea TC, David S, Shamsuddin A, et al. Overexpression of RGC-32 in colon cancer and other tumors. Exp Mol Pathol. 2005;78:116–122. doi: 10.1016/j.yexmp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 123.Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, et al. Complement Clq activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–1313. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 125.la Sala A, Gadina M, Kelsall BL. G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and INK. J Immunol. 2005;175:2994–2999. doi: 10.4049/jimmunol.175.5.2994. [DOI] [PubMed] [Google Scholar]
- 126.Wrann CD, Tabriz NA, Barkhausen T, Klos A, van Griensven M, Pape HC, et al. The phosphatidylinositol 3-kinase signaling pathway exerts protective effects during sepsis by controlling C5a-mediated activation of innate immune functions. J Immunol. 2007;178:5940–5948. doi: 10.4049/jimmunol.178.9.5940. [DOI] [PubMed] [Google Scholar]
- 127.van Beek J, Nicole O, Ali C, Ischenko A, MacKenzie ET, Buisson A, et al. Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. Neuroreport. 2001;12:289–293. doi: 10.1097/00001756-200102120-00022. [DOI] [PubMed] [Google Scholar]
- 128.Mukherjee P, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through mitogen-activated protein kinase-dependent inhibition of caspase 3. J Neurochem. 2001;77:43–49. doi: 10.1046/j.1471-4159.2001.00167.x. [DOI] [PubMed] [Google Scholar]
- 129.Daveau M, Benard M, Scotte M, Schouft MT, Hiron M, Francois A, et al. Expression of a functional C5a receptor in regenerating hepatocytes and its involvement in a proliferative signaling pathway in rat. J Immunol. 2004;173:3418–3424. doi: 10.4049/jimmunol.173.5.3418. [DOI] [PubMed] [Google Scholar]
- 130.Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 131.Jiang H, Kuang Y, Wu Y, Smrcka A, Simon MI, Wu D. Pertussis toxin-sensitive activation of phospholipase C by the C5a and fMet-Leu-Phe receptors. J Biol Chem. 1996;271:13430–1344. doi: 10.1074/jbc.271.23.13430. [DOI] [PubMed] [Google Scholar]
- 132.Mullmann TJ, Siegel MI, Egan RW, Billah MM. Complement C5a activation of phospholipase D in human neutrophils. A major route to the production of phosphatidates and diglycerides. J Immunol. 1990;144:1901–1908. [PubMed] [Google Scholar]
- 133.Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009;6:352–366. doi: 10.1038/nrclinonc.2009.62. [DOI] [PubMed] [Google Scholar]
- 134.Ajona D, Castano Z, Garayoa M, Zudaire E, Pajares MJ, Martinez A, et al. Expression of complement factor H by lung cancer cells: effects on the activation of the alternative pathway of complement. Cancer Res. 2004;64:6310–6318. doi: 10.1158/0008-5472.CAN-03-2328. [DOI] [PubMed] [Google Scholar]
- 135.Ajona D, Hsu YF, Corrales L, Montuenga LM, Pio R. Down-regulation of human complement factor H sensitizes non-small cell lung cancer cells to complement attack and reduces in vivo tumor growth. J Immunol. 2007;178:5991–5998. doi: 10.4049/jimmunol.178.9.5991. [DOI] [PubMed] [Google Scholar]
- 136.Hsu YF, Ajona D, Corrales L, Lopez-Picazo JM, Gurpide A, Montuenga LM, et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol Cancer. 2010;9:139. doi: 10.1186/1476-4598-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kohl J. Drug evaluation: the C5a receptor antagonist PMX-53. Curr Opin Mol Ther. 2006;8:529–538. [PubMed] [Google Scholar]
- 138.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013 doi: 10.4049/jimmunol.1203200. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ricklin D, Lambris JD. Progress and trends in complement therapeutics. Adv Exp Med Biol. 2013;735:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Davis AE, Lu F, Mejia P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost. 2010;104:886–893. doi: 10.1160/TH10-01-0073. [DOI] [PubMed] [Google Scholar]
- 142.Woodruff TM, Nandakumar KS, Tedesco F. Inhibiting the C5-C5a receptor axis. Mol Immunol. 2011;48:1631–1642. doi: 10.1016/j.molimm.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 143.Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- 144.Janssen BJ, Halff EF, Lambris JD, Gros P. Structure of compstatin in complex with complement component C3c reveals a new mechanism of complement inhibition. J Biol Chem. 2007;282:29241–29247. doi: 10.1074/jbc.M704587200. [DOI] [PubMed] [Google Scholar]
- 145.Sahu A, Morikis D, Lambris JD. Compstatin, a peptide inhibitor of complement, exhibits species-specific binding to complement component C3. Mol Immunol. 2003;39:557–566. doi: 10.1016/s0161-5890(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 146.Kempshall E, Thebault S, Morgan BP, Harris CL, Gallimore A. Complement-induced protection: an explanation for the limitations of cell-based tumour immunotherapies. Immunol Cell Biol. 2012;90:869–871. doi: 10.1038/icb.2012.30. [DOI] [PubMed] [Google Scholar]