Abstract
Basophils are increasingly recognized as playing important roles in the immune response towards helminths. In this study, we evaluated the role of basophils in vaccine-mediated protection against filariae, tissue-invasive parasitic nematodes responsible for diseases such as elephantiasis and river blindness. Protective immunity and immunological responses were assessed in BALB/c mice vaccinated with irradiated L3 stage larvae and depleted of basophils with weekly injections of anti-CD200R3 antibody. Depletion of basophils after administration of the vaccination regimen but before challenge infection did not alter protective immunity. In contrast, basophil depletion initiated prior to vaccination and continued after challenge infection significantly attenuated the protective effect conferred by vaccination. Vaccine-induced cellular immune responses to parasite antigen were substantially decreased in basophil-depleted mice, with significant decreases in CD4+ T-cell production of IL-4, IL-5, IL-10, and IFN-γ. Interestingly, skin mast cell numbers, which increased significantly after vaccination with irradiated L3 larvae, were unchanged after vaccination in basophil-depleted mice. These findings demonstrate that basophils help establish the immune responses responsible for irradiated L3 vaccine protection.
Keywords: filariasis, vaccine, Litomosoides sigmodontis, helminth, basophil, type 2 immunity
1. Introduction
Filariae are vector-borne tissue-invasive parasitic nematodes. As the agents of diseases such as elephantiasis and river blindness, they cause significant pain and suffering worldwide. Lymphatic filariasis causes lymphedema in 15 million people and urogenital swelling in 25 million [1], and onchocerciasis is the 4th leading cause of preventable blindness [2]. While there are important ongoing efforts to control these diseases through mass drug administration, development of anti-filarial vaccines would greatly aid our ability to decrease the prevalence of these infections [3].
A significant obstacle to rational development of filaria vaccines is an incomplete understanding of the immunogical mechanisms capable of eliminating these parasites. To date, one of the most effective approaches used to induce protective immunity in animal models of filariasis is vaccination with irradiated L3 larvae. First demonstrated effective in Brugia malayi infection of rhesus monkeys in 1969 [4], vaccination with radiation-attenuated L3 stage larvae has been shown to be effective in numerous animal models of filariasis [5–10]. Vaccination with irradiated larvae results in development of type 2 immune responses, with production of parasite-specific IgE, increased release of IL-4 and IL-5, and enhanced eosinophilia after infection [11–13].
Recently, basophils have become increasingly recognized as being important amplifiers of type 2 immune responses during helminth infections [14–16]. Basophils are circulating granulocytes that are major contributors of IL-4 and are primarily activated by cross-linking of IgE antibodies bound to their cell surface by high affinity IgE receptors [17]. By upregulating CD40L on their cell surface and releasing IL-4 upon activation, basophils are capable of both driving CD4+ T-cells towards a Th2 phenotype and of triggering IgE isotype switching in B cells [18–19]. Basophils are also thought to amplify type 2 responses by release of both IL-13 and TSLP [20–21]. Basophils are a major source of IL-4 in patients infected with filariasis [22], and depletion of basophils during primary infection of mice infected with the rodent filaria Litomo-soides sigmodontis results in decreased parasite-specific IgE and parasite antigen-driven IL-4 production from CD4+ T-cells [23].
In addition to amplifying type 2 immune responses, basophils can have important effector cell functions. Activation of basophils results in the immediate release of pre-formed inflammatory mediators such as histamine, leukotriene C4, and antimicrobial peptides, as well as subsequent release of several cytokines and chemokines [24].
To date, no studies have evaluated the role basophils may have in protective vaccine regimens for filariasis. While most studies demonstrate that basophils are not protective against primary helminth infections (reviewed in [14]), a recent study demonstrated that basophil-deficient mice exhibit impaired parasite clearance after secondary infection with the intestinal nematode Nippostrongylus brasiliensis [25].
The goal of this study was to assess whether basophils are important to establish the immune response to irradiated larval vaccination in filariasis. To test this, we assessed the protective efficacy of L3 vaccination against challenge infection in mice depleted of basophils at different timepoints. We utilized L. sigmodontis, a filariasis model in which parasites develop to maturity in immunocompetent BALB/c mice [26]. Our results demonstrate that basophils are necessary at time of immunization to establish the immune responses responsible for vaccine-mediated protective immunity.
2. Materials and Methods
2.1 Mice and parasites
Female BALB/c mice (NCI Mouse Repository, Frederick, MD) were maintained at the Uniformed Services University (USU) animal facility. Experiments were performed with mice between 5–8 weeks of age under a protocol approved by the USU Institutional Animal Care and Use Committee. Infectious-stage L3 larvae from Litomosoides sigmodontis were isolated by lavage from the pleural cavity of four-day infected jirds (Meriones unguiculatus, obtained from TRS Laboratory Inc., Athens, GA) as previously described [27]. BALB/c mice were vaccinated with three weekly subcutaneous injections of 25 irradiated L3 larvae (450 Gy, cobalt 60 irradiator) in media (RPMI-1640, Mediatech, Herndon, VA). Two weeks after the last vaccination, mice were challenged by subcutaneous injection of 40 L3 larvae. Adult parasites were enumerated after physical extraction from the pleural cavity of mice that had been infected for 4 weeks.
2.2 Basophil depletion
For in vivo basophil depletion, Ba103, a rat monoclonal IgG2b antibody that recognizes CD200R3, was obtained as described before [28] and injected weekly into mice at a concentration of 50 μg i.p. Although CD200R3 is present on both basophils and mast cells, prior studies have shown that administration of Ba103 results in almost complete depletion of basophils without affecting skin or peritoneum mast cell numbers [23, 28]. Control mice were given i.p. injections of rat IgG2b isotype control antibody (BD Biosciences, San Jose, California).
2.3 Flow cytometric detection of basophils and eosinophils
Whole blood (100 μl) was aliquoted in 5 ml polyprolylene round-bottom tubes (BD Falcon). Red blood cells were lysed and leukocytes fixed with a whole blood lysing reagent kit (Beckman Coulter, Galway, Ireland). Cells were washed twice with 2 ml of phosphate-buffered saline (PBS, Mediatech) and centrifuged at 500 x g for 5 min. Supernatants were aspirated and cells resuspended in 100 μL of 1% BSA/PBS followed by incubation at 4°C for 1hr. Cells were stained with anti-IgE FITC (R35-72), anti-CD4 PerCP (RM4-5) and anti-B220 PerCP (RA3-6B2) to identify basophils; or SiglecF PE (E50-2440), CD45 FITC (30-F11) and CD11c APC (HL3) to identify eosinophils. All the antibodies were purchased from BD Pharmingen. Cells were washed and resuspended in 200 μL of PBS for analysis using a BD LSR II Optical Bench flow cytometer.
2.4 Litomosoides sigmodontis antigen (LsAg)
Soluble LsAg was made from adult male and female L. sigmodontis parasites as previously described [23]. Although there are no L3 stage parasites used in the production of LsAg, antibody and cellular immune responses induced by L3 stage parasites are reactive to LsAg (29).
2.5 Parasite specific IgE ELISA
Blood was collected from mice by cardiac puncture and analyzed for LsAg-specific IgE by colorimetric ELISA as previously described [23].
2.6 Cytokine quantification and proliferation assays
Splenocytes were resuspended in ACK Lysing buffer (Quality Biological, Inc., Gaithers-burg, MD) to lyse red blood cells. Cells were washed and then resuspended in Iscove’s Dulbecco modified medium (Mediatech) supplemented with 10% fetal calf serum (Valley Biomedical, Winchester, VA), 1% L-glutamine (Mediatech), 1% insulin-transferrin-selenium medium (Invitrogen Inc., Carlsbad, CA) and 80 μg/ml gentamicin (Quality Biological, Inc.). CD4+ cells and CD11c+ cells were isolated from splenocytes by magnetic cell sorting (Miltenyi Biotec, Auburn, CA). CD4+ cells were plated at 2×106cells/ml along with 2×105 dendritic cells/ml isolated from naïve mice. Cells were stimulated with 20μg/ml LsAg or 5 μg/ml α-CD3 (eBioscience, San Diego, CA). After 3 days, supernatants were collected and assayed for IL-4, IL-5, IL-10 and IFN-γ using ELISA kits (eBioscience). CD4+ cell proliferation was quantified by chemiluminescence after 3 day culture utilizing a BrdU incorporation immunoassay (Roche Applied Science, Mannheim, Germany).
2.7 Skin histology
Two weeks after vaccination, mice were euthanized and a piece of skin in the area of injections removed from each mouse. Skin samples were fixed in 10% formalin (Sigma-Aldrich). Hematoxylineosin and Toluidine-blue stained slices were observed for eosinophils and mast-cells, respectively, by a pathologist (B.M) blinded to the intervention group.
2.8 Statistical analysis
Statistical analyses were performed with GraphPad Prism 4 statistics software (GraphPad Software, San Diego, CA). Differences between groups were performed by Kruskal-Wallis Test followed by Dunn’s Multiple Comparison Test. P-values < 0.05 were considered significant. Data are expressed as mean value ± SEM. All experiments were conducted twice with a minimum of four mice per group for each trial. Figures include combined data from both trials for each experiment.
3. Results
3.1 Basophil depletion after vaccination of mice with irradiated L. sigmodontis larvae does not reduce protection against L. sigmodontis challenge
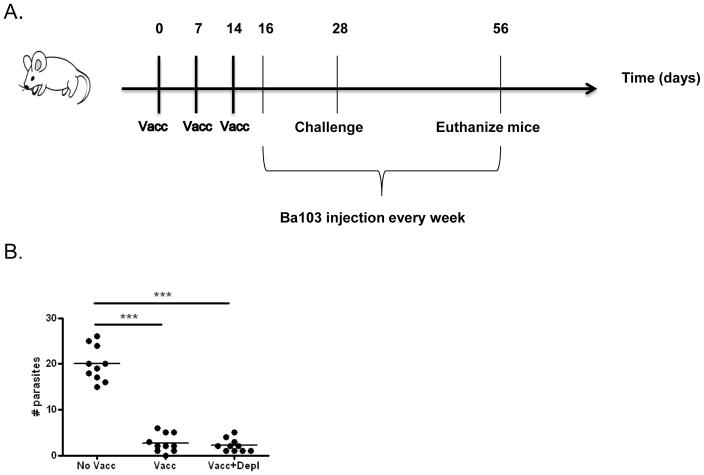
Vaccination of mice with subcutaneous injection of irradiated L. sigmodontis larvae typically confers >70% protection against subsequent challenge with infectious-stage L3 larvae [5, 11–12, 29]. To determine whether basophils function as effector cells of protective immunity after vaccination with irradiated L. sigmodontis larvae, we evaluated vaccine efficacy in mice that were depleted of basophils after receiving vaccination with three weekly i.p. injections of irradiated L. sigmodontis larvae (Fig 1A). Basophil depletion with weekly injections of Ba103 antibody was started two days after final vaccination. Effectiveness of basophil depletion was confirmed by flow cytometry, which demonstrated > 95% depletion of blood basophils (identified as CD4−B220− IgE+ cells) at time of infectious challenge (data not shown). Mice were euthanized 4 weeks p.i. and numbers of parasites residing in the pleural cavity enumerated. As seen in Fig 1B, irradiated larval vaccination resulted in significant protection against challenge infection, as a mean of 2.7 adult parasites were recovered from vaccinated mice compared to 20 adult parasites from unvaccinated controls. Basophil depletion did not diminish the protective effect of vaccination, as there was no difference in worm recovery between vaccinated mice (mean of 2.7 adult parasites) and vaccinated mice depleted of basophils (mean of 2.2 adult parasites). The protective efficacy of the vaccine was 86.5% for vaccinated mice and 89% for vaccinated and basophil-depleted mice.
Figure 1. Basophil depletion after vaccination with irradiated L3 larvae does not alter protection against L. sigmodontis challenge.
A) Timeline of experimental protocol. Both vaccinated (Vacc) and vaccinated and basophil-depleted (Vacc+Depl) groups were vaccinated with three weekly subcutaneous injections of 25 irradiated L3s. Both of these groups, plus an unvaccinated control group (No Vacc) were challenged by subcutaneous injection of 40 L3 larvae on day 28 of the protocol, two weeks after the final vaccination. From days 16 (12 days prior to challenge) until study endpoint mice were administered weekly injections of Ba103 to deplete basophils (Vacc+Depl) or isotype control antibody (No Vacc and Vacc groups). B) Number of adult parasites recovered from thoracic cavity at study endpoint four weeks after challenge infection (*** p < 0.001).
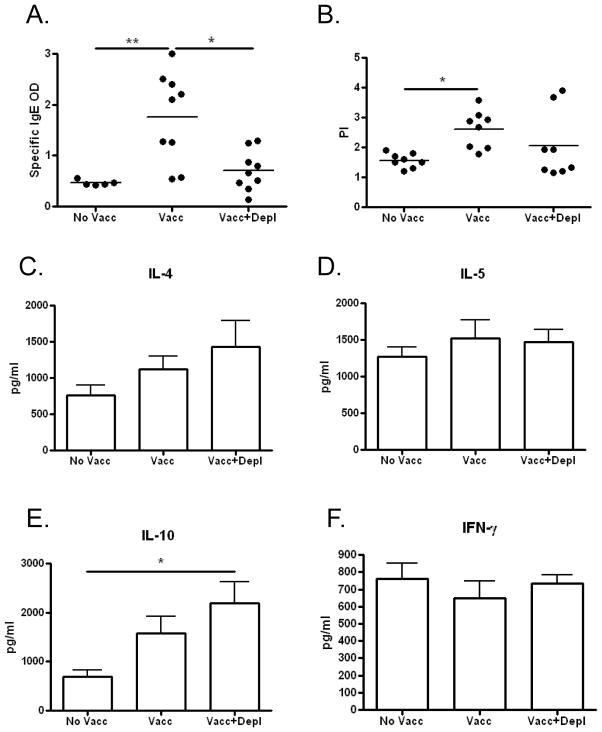
To assess whether depletion of basophils after vaccination affects immune responses to challenge infection, circulating levels of parasite-specific IgE as well as CD4+ T-cell proliferation and cytokine production in response to parasite antigen were measured 4 weeks after L. sigmodontis challenge. Consistent with prior work we have done showing that the presence of basophils is associated with increased IgE levels during filaria infection [23], circulating parasite-specific IgE concentrations were significantly higher in the vaccinated group compared to the vaccinated and basophil depleted group (Fig 2A). Proliferation of splenic CD4+ T-cells in response to parasite-antigen was significantly higher for vaccinated mice compared to control mice (Fig. 2B). In contrast, no differences in proliferation were observed when comparing vaccinated mice depleted of basophils to either control or vaccinated mice (Fig 2B). Additionally, there were no significant differences in production of IL-4, IL-5, IL-10 or IFN-γ production in response to parasite antigen between both vaccinated groups (Fig 2C–F). Of note, CD4+ cell production of IL-10 in response to parasite antigen was significantly greater in vaccinated and basophil depleted mice than in unvaccinated mice after challenge infection (Fig. 2E).
Figure 2. Immune responses measured 4 weeks after L3 challenge in mice depleted of basophils after irradiated L3 vaccination.
A) Plasma levels of parasite antigen-specific IgE [OD]. B) Splenic CD4+ T-cell proliferation in response to parasite antigen measured after 3 days of culture. C–F) Cytokine production from splenic CD4+ T-cells in response to parasite antigen measured after 3 days of culture. (No Vacc = unvaccinated; Vacc = vaccinated; Vacc+Depl = Vaccinated and then depleted of basophils; * p < 0.05; ** p < 0.01)
3.2 Basophil depletion before vaccination diminishes protection against L. sigmodontis challenge
To investigate whether basophils are necessary for establishing the protective immune response induced by irradiated larval vaccination, vaccine efficacy was assessed when basophil depletion was initiated prior to vaccination. For these studies, weekly injections of basophil-depleting Ba103 antibody were started two weeks prior to the irradiated larvae vaccination regimen. Mice were challenged with 40 infectious-stage L3 larvae two weeks after completion of the vaccination regimen, and numbers of adult parasites retrieved from the pleural cavity enumerated 4 weeks post-infection (Fig 3A).
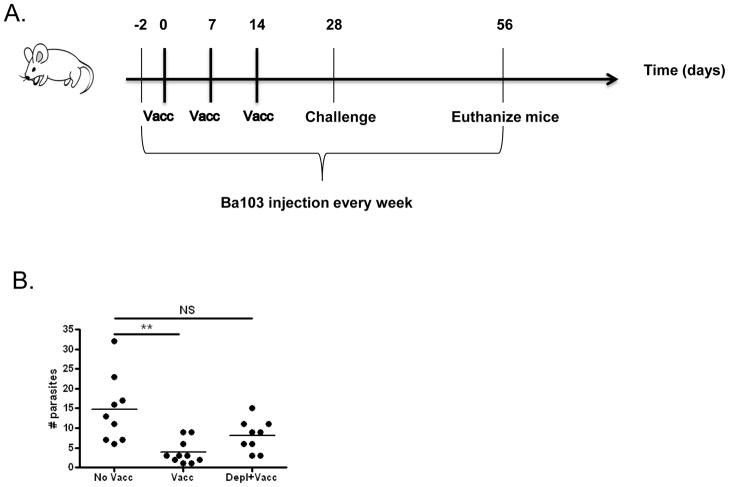
Figure 3. Basophil depletion initiated before vaccination decreases the protective effect of irradiated L3 vaccine.
A) Timeline of experimental protocol. Both vaccinated (Vacc) and vaccinated and basophil-depleted (Vacc+Depl) groups were vaccinated with three weekly subcutaneous injections of 25 irradiated L3s. Both of these groups, plus an unvaccinated control group (No Vacc) were challenged by subcutaneous injection of 40 L3 larvae on day 28 of the protocol, two weeks after the final vaccination. From days -2 (2 days prior to vaccination) until study endpoint mice were administered weekly injections of Ba103 to deplete basophils (Vacc+Depl) or isotype control antibody (No Vacc and Vacc groups). B) Number of adult worms recovered from thoracic cavity at study endpoint 4 weeks p.i. (** p < 0.01; NS no significant)
Basophil depletion before vaccination significantly decreased the protective effect conferred by irradiated L. sigmodontis larvae. Whereas vaccinated mice had a mean of 4 adult parasites (p < 0.01 compared to unvaccinated mice), mice vaccinated in the setting of basophil depletion had a mean of 8 adult worms (no significant difference compared to unvaccinated mice, Fig. 3B). As control mice in this study had a mean of 15 adult parasites at study endpoint, these values translate to protective efficacies of 73% for the vaccinated group vs. only 54% for the basophil depleted and vaccinated group.
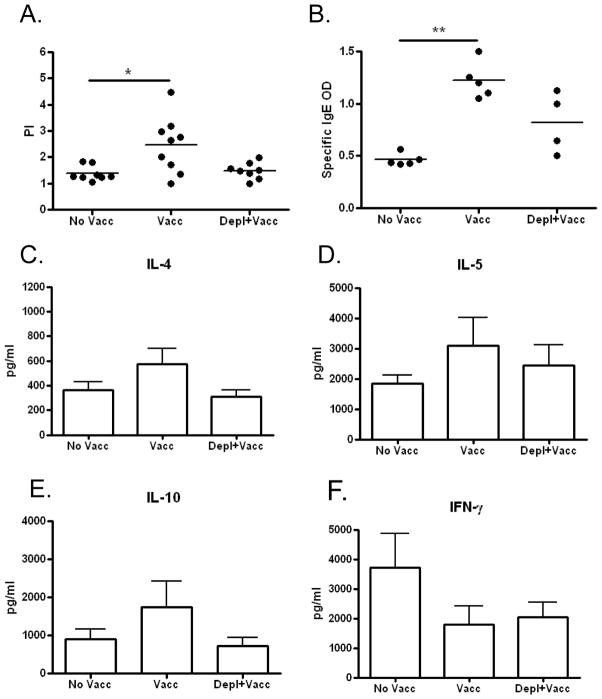
Immunological studies conducted at the end of the challenge infection demonstrated that proliferation of splenic CD4+ T-cells in response to parasite-antigen and circulating levels of parasite-specific IgE were significantly increased in the vaccinated group, but not in the vaccinated and basophil-depleted group, when compared to unvaccinated mice (Fig 4A, B). No significant differences were observed in parasite antigen-driven IL-4, IL-5, IL-10 and IFN-γ production from CD4+ T-cells between vaccination groups (Fig 4C–F), though there was a trend for lower IL-4, IL-5, and IL-10 production in vaccinated and basophil-depleted mice compared to vaccinated mice.
Figure 4. Immune responses measured 4 weeks after challenge infection of mice in which basophil depletion was started before irradiated L3 vaccination.
A) Proliferation of CD4+ T-cells in response to parasite antigen measured after 3 days of culture. B) Circulating parasite-specific IgE levels [OD]. C–F) Cytokine production from splenic CD4+ T-cells in response to parasite antigen measured after 3 days of culture. (No Vacc = unvaccinated; Vacc = vaccinated; Vacc+Depl = Vaccinated and then depleted of basophils; * p < 0.05; ** p < 0.01)
3.3 Basophil depletion before vaccination of mice with irradiated L. sigmodontis larvae results in decreased antibody and cellular immune responses to parasite antigen
To better understand the effects basophil depletion has on the development of protective immune responses induced by irradiated larval vaccination, we examined immune responses from vaccinated mice and mice vaccinated in the setting of basophil depletion two weeks after final vaccination without challenge infection (Fig 5A).
Figure 5. Basophil depletion attenuates cellular responses induced by irradiated L3 vaccine.
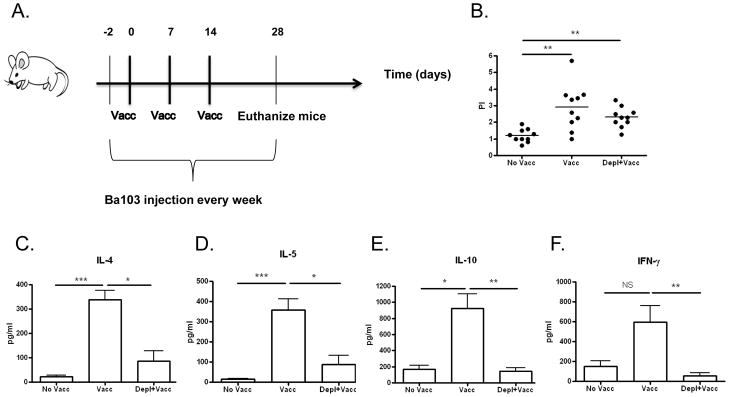
A) Timeline of experimental protocol. Both vaccinated (Vacc) and vaccinated and basophil-depleted (Vacc+Depl) groups were vaccinated with three weekly subcutaneous injections of 25 irradiated L3s. A control group of mice was not vaccinated (No Vacc). From days -2 (2 days prior to vaccination) to day 28 mice were administered weekly injections of Ba103 to deplete basophils (Vacc+Depl) or isotype control antibody (No Vacc and Vacc groups). No mice were challenged with infective L3 larvae in this study. B) Proliferation and C–F) cytokine production of splenic CD4+ T-cells in response to parasite antigen measured after 3 days of culture. (PI = proliferative index; * p < 0.5; ** p < 0.01; *** p < 0.001; NS not significant).
As seen in Figure 5, vaccine-induced cellular immunity against parasite antigen is decreased when vaccination is administered to basophil-depleted mice. While basophil depletion did not alter splenic CD4+ T-cell proliferation in response to parasite antigen (Fig. 5B), it significantly decreased CD4+ T-cell cytokine responses (Fig 5C–F). Whereas mean concentrations of IL-4 and IL-5 released by CD4+ T-cells in response to LsAg were 339 and 357.25pg/ml in vaccinated mice, respectively, vaccinated mice depleted of basophils produced means of only 86.5 pg/ml of IL-4 and 87.37 pg/ml of IL-5 (p < 0.05 for both, Fig. 5C–D). Similarly, CD4+ production of IL-10 was decreased in response to in vitro stimulation with parasite antigen (from 923 pg/ml in vaccinated mice to 144.5 pg/ml in basophil-depleted vaccinated mice, p < 0.01, Fig. 5E). Production of IFN-γ, a Th1 cytokine which has been reported as being increased by irradiated larval vaccination, was also decreased in basophil-depleted mice (from 595.87 pg/ml in vaccinated mice to 53.4 pg/ml in basophil depleted mice, p <0.01).
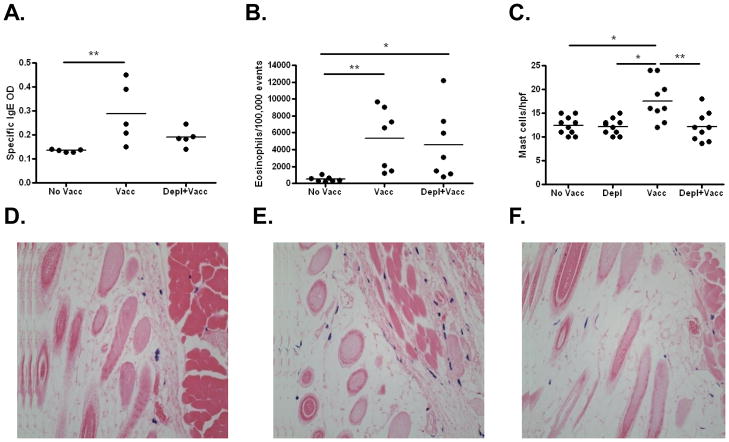
As seen in Figure 6A, LsAg-specific IgE responses were similar in basophil-depleted mice compared to vaccinated mice. Circulating eosinophil numbers (Figure 6B) and parasite-specific IgG1 concentrations (data not shown) were also similar in both vaccinated and basophil-depleted and vaccinated mice.
Figure 6. Effects of basophil depletion prior to vaccination on IgE levels, circulating eosinophils, and numbers of skin mast cells.
A) Plasma levels of parasite-specific IgE [OD] and B) circulating eosinophil numbers two weeks after final irradiated L3 vaccination in unvaccinated mice, vaccinated mice, and mice vaccinated in the setting of basophil depletion. No mice were challenged with infective L3 larvae in this study. C) Numbers of mast cells observed per high powered field in unvaccinated mice, mice depleted of basophils, vaccinated mice, and mice vaccinated in the setting of basophil depletion two weeks after final vaccination. Representative histological skin sections of D) control mice, E) vaccinated mice, and F) vaccinated + basophil depleted mice. Sections were stained with toluidine blue, which results in blue staining of mast cells (400X). (No Vacc = unvaccinated; Vacc = vaccinated; Vacc+Depl = Vaccinated and then depleted of basophils; * p < 0.05; ** p < 0.01)
Finally, as mast cells have been shown to be important in the clearance of some helminth infections [30–31], we also examined mast cell numbers in skin tissue at sites of vaccination. For these studies, we added another group of mice that received Ba103 to confirm that this antibody does not depleted mast cells from the skin. As seen in figure 6C, administration of Ba103 did not decrease baseline skin mast cell numbers. Interestingly, vaccination with irradiated larvae results in significant increases in the number of mast cells present in the dermis and subcutaneous tissue prior to challenge infection (Figs. 6C–F). Basophil depletion prior to vaccination, however, prevents this increase in tissue mast cell numbers. Of note, vaccination did not result in increased skin basophil numbers, as no basophils were observed by histology in the skin of either control or vaccinated mice.
4. Discussion
This study demonstrates that basophils contribute to the protective immunity which develops against filarial parasites after vaccination with irradiated L3-stage larvae. This study rules out a biologically significant effector role for basophils in the clearance of filarial parasites, since depletion of basophils after vaccination does not alter protection against challenge infection. Rather, as depletion prior to vaccination diminishes protective efficacy, basophils are important for the development of anti-filarial immune responses induced by vaccination.
The findings of this study confirm that basophils function to amplify type 2 immune responses. Consistent with our prior work evaluating the role of basophils during primary filaria infection [23], depletion of basophils blunted vaccine-induced IL-4 and IL-5 production towards parasite antigen by CD4+ T-cells, as well as production of parasite-specific IgE. This decrease in type 2 immunity is the most obvious explanation for the loss of vaccine efficacy observed in basophil-depleted mice, as type 2 immune responses are generally considered protective against helminth infections [32]. Indeed, natural resistance of C57BL/6 mice to L. sigmodontis infection is dependent on IL-4 [33]. While IL-4 deficiency does not affect L. sigmodontis development after primary infection of susceptible BALB/c mice [33], IL-5 deficiency in BALB/c mice is associated with increased worm burdens and prolonged adult worm survival [34].
Since irradiated L3 vaccination fails to enhance protective immunity in either BALB/c mice treated with an IL-5 neutralizing antibody [35] or C57BL/6 mice deficient in IL-5 [36], the lack of vaccine-induced IL-5 responses to parasite antigen observed in basophil-depleted mice in this study suggests that basophil amplification of IL-5 production from CD4+ cells may be the key mechanism by which basophils help establish protective immunity. Transgenic overexpression of IL-5 is associated with increased eosinophilia and results in substantial reductions in L. sigmodontis worm burdens [37]. Although eosinophil numbers were similar four weeks after challenge infection in basophil-depleted and non-depleted mice administered L3 vaccination, it is possible that wild-type vaccinated mice exhibited a more rapid eosinophilic response, or that their eosinophils became more activated in the setting of increased IL-5 levels. Dissecting the contribution individual components of the type 2 immune response make with regards to protective efficacy conferred by the irradiated L3 vaccine may be the subject of future studies using mice genetically deficient in IgE, IL-4, and eosinophils.
A caveat to this study is that we utilized antibody-mediated depletion of basophils using the Ba103 clone. While we routinely observed greater than 95% depletion of basophils with this technique, it never completely eliminated all basophils. Thus, while this study suggests basophils are not necessary for clearance of filarial parasites at time of challenge, it does not completely rule out the possibility that a small number of basophils could act as effector cells.
It is interesting to note that dermal and subcutaneous mast cell numbers were elevated following irradiated L3 vaccination, and that this increase was not present when mice were depleted of basophils prior to vaccination. While mast cells have been implicated in the clearance of intestinal helminths in some animal models [30–31], to date it remains unclear whether mast cells play a protective role in either primary or vaccine-induced immunity against filariasis. As L3 vaccination induces parasite-specific IgE which can sensitize tissue mast cells, it is likely that the increased number of tissue mast cells induced by vaccination results in the release of greater amounts of pro-inflammatory mediators from mast cells as L3 larvae begin their invasion into the body. Whether mast cell degranulation is damaging to filariae, however, is simply unknown. Indeed, a recent report found that high levels of dermal mast cells observed in mice deficient in the chemokine CCL17 are associated with increased susceptibility to L. sigmodontis, raising the possibility that mast cells may actually enhance permissiveness for filarial invasion [38]. Thus, our observation that basophil depletion decreases vaccine-mediated skin mast cell numbers as well as vaccine-mediated protection does not necessarily mean that mast cells are key effector cells in this vaccination model. The role of mast cells in vaccine-mediated immunity will be investigated in future studies.
Finally, a surprising observation in this study was that basophil depletion resulted in almost complete abrogation of parasite antigen-driven IFN gamma production from CD4+ cells after irradiated larval vaccination. This finding suggests that basophils may at times function to enhance Th1 as well as Th2 responses. As C57BL/6 mice, which are naturally resistant to L. sigmodontis, produce greater amounts of both Th1 and Th2 cytokines when infected than BALB/c mice [39], it is possible that the principal driver of vaccine mediated protection is simply the overall magnitude of the cellular immune response, or even just IFN-γ production on its own. Indeed, BALB/c mice deficient in IFN-γ exhibit higher parasite burdens and prolonged parasite survival when infected with L. sigmodontis than wild type controls [40].
In conclusion, this study demonstrates that basophils help establish the protective immune responses against filariasis conferred by vaccination with irradiated L3 larvae. Our results suggest that basophils likely function to amplify vaccine-induced parasite-specific type 2 immune responses, but they also may be enhancing immunity by increasing tissue mast cell numbers and increasing IFN-γ production. Determining the exact pathways by which irradiated larval vaccination enables clearance of filarial worms will be the focus of future studies.
Highlights.
Basophils help establish protective immunity induced by irradiated larval vaccination for filariasis
Basophil depletion attenuates protective efficacy of irradiated L3 vaccination in filariasis.
Basophil depletion decreases both IgE and cellular immune responses induced by irradiated L3 vaccination.
Acknowledgments
This work was supported by grant R01AI076522 from NIAID/NIH.
We thank Yinghui Shi for her technical assistance. We also thank Karen Wolcott and Kateryna Lund at the Uniformed Services University Biomedical Instrumentation Center for their valuable assistance with flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.W.H.O. Global programme to eliminate lymphatic filariasis: progress report, 2011. Weekly epidemiological record. 2012;87:346–356. [PubMed] [Google Scholar]
- 2.W.H.O. Onchocerciasis – the disease and its impact. African Programme for Onchocerciasis Control. 2012 [cited March 5, 2013]; Available from: http://www.who.int/apoc/onchocerciasis/disease/en/index.html.
- 3.Babayan SA, Allen JE, Taylor DW. Future prospects and challenges of vaccines against filariasis. Parasite Immunol. 2012;34(5):243–53. doi: 10.1111/j.1365-3024.2011.01350.x. [DOI] [PubMed] [Google Scholar]
- 4.Wong MM, Fredericks HJ, Ramachandran CP. Studies on immunization against Brugia malayi infection in the rhesus monkey. Bull World Health Organ. 1969;40(4):493–501. [PMC free article] [PubMed] [Google Scholar]
- 5.Le Goff L, et al. Early reduction of the challenge recovery rate following immunization with irradiated infective larvae in a filaria mouse system. Trop Med Int Health. 1997;2(12):1170–4. doi: 10.1046/j.1365-3156.1997.d01-218.x. [DOI] [PubMed] [Google Scholar]
- 6.Lucius R, et al. Acanthocheilonema viteae: vaccination of jirds with irradiation attenuated stage-3 larvae and with exported larval antigens. Exp Parasitol. 1991;73(2):184–96. doi: 10.1016/0014-4894(91)90021-n. [DOI] [PubMed] [Google Scholar]
- 7.Oothuman P, et al. Successful vaccination of cats against Brugia pahangi with larvae attenuated by irradiation with 10 krad cobalt 60. Parasite Immunol. 1979;1(3):209–16. doi: 10.1111/j.1365-3024.1979.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 8.Schrempf-Eppstein B, et al. Acanthocheilonema viteae: vaccination with irradiated L3 induces resistance in three species of rodents (Meriones unguiculatus, Mastomys coucha,Mesocricetus auratus) Trop Med Int Health. 1997;2(1):104–10. doi: 10.1046/j.1365-3156.1997.d01-129.x. [DOI] [PubMed] [Google Scholar]
- 9.Yates JA, Higashi GI. Brugia malayi: vaccination of jirds with 60cobalt attenuated infective stage larvae protects against homologous challenge. Am J Trop Med Hyg. 1985;34(6):1132–7. doi: 10.4269/ajtmh.1985.34.1132. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida M, et al. Immunologic protection against canine heartworm infection. J Vet Med Sci. 1997;59(12):1115–21. doi: 10.1292/jvms.59.1115. [DOI] [PubMed] [Google Scholar]
- 11.Hubner MP, Torrero MN, Mitre E. Type 2 immune-inducing helminth vaccination maintains protective efficacy in the setting of repeated parasite exposures. Vaccine. 2010;28(7):1746–57. doi: 10.1016/j.vaccine.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Goff L, et al. Parasitology and immunology of mice vaccinated with irradiated Litomosoides sigmodontis larvae. Parasitology. 2000;120(Pt 3 Pt 3):271–80. doi: 10.1017/s0031182099005533. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MJ, et al. Protective immunity against Onchocerca volvulus and O. lienalis infective larvae in mice. Trop Med Parasitol. 1994;45(1):17–23. [PubMed] [Google Scholar]
- 14.Karasuyama H, et al. Emerging roles of basophils in protective immunity against parasites. Trends Immunol. 2011;32(3):125–30. doi: 10.1016/j.it.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Leon-Cabrera S, Flisser A. Are basophils important mediators for helminth-induced th2 immune responses? A debate. J Biomed Biotechnol. 2012;2012(20):274150. doi: 10.1155/2012/274150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voehringer D. Microbes Infect. 2011. Basophils in immune responses against helminths. [DOI] [PubMed] [Google Scholar]
- 17.Mitre E, Nutman TB. Basophils, basophilia and helminth infections. Chem Immunol Allergy. 2006;90:141–56. doi: 10.1159/000088886. [DOI] [PubMed] [Google Scholar]
- 18.Oh K, et al. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109(7):2921–7. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 19.Gauchat JF, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365(6444):340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 20.Siracusa MC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;14(10) doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Sim TC, Alam R. IL-13 released by and localized in human basophils. J Immunol. 1996;156(12):4833–8. [PubMed] [Google Scholar]
- 22.Mitre E, et al. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172(4):2439–45. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 23.Torrero MN, et al. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J Immunol. 2010;185(12):7426–34. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- 24.Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci. 2011;1217:166–77. doi: 10.1111/j.1749-6632.2010.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184(1):344–50. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann W, et al. Litomosoides sigmodontis in mice: reappraisal of an old model for filarial research. Parasitol Today. 2000;16(9):387–9. doi: 10.1016/s0169-4758(00)01738-5. [DOI] [PubMed] [Google Scholar]
- 27.Hubner MP, et al. Litomosoides sigmodontis: a simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus) Exp Parasitol. 2009;123(1):95–8. doi: 10.1016/j.exppara.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obata K, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110(3):913–20. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 29.Martin C, et al. B-cell deficiency suppresses vaccine-induced protection against murine filariasis but does not increase the recovery rate for primary infection. Infect Immun. 2001;69(11):7067–73. doi: 10.1128/IAI.69.11.7067-7073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newlands GF, et al. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N brasiliensis infection. Blood. 1995;86(5):1968–76. [PubMed] [Google Scholar]
- 31.Grencis RK, et al. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 1993;15(1):55–9. doi: 10.1111/j.1365-3024.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 32.Anthony RM, et al. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7(12):975–87. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Goff L, et al. IL-4 is required to prevent filarial nematode development in resistant but not susceptible strains of mice. Int J Parasitol. 2002;32(10):1277–84. doi: 10.1016/s0020-7519(02)00125-x. [DOI] [PubMed] [Google Scholar]
- 34.Volkmann L, et al. Murine filariasis: interleukin 4 and interleukin 5 lead to containment of different worm developmental stages. Med Microbiol Immunol (Berl) 2003;192(1):23–31. doi: 10.1007/s00430-002-0155-9. [DOI] [PubMed] [Google Scholar]
- 35.Martin C, et al. IL-5 is essential for vaccine-induced protection and for resolution of primary infection in murine filariasis. Med Microbiol Immunol. 2000;189(2):67–74. doi: 10.1007/pl00008258. [DOI] [PubMed] [Google Scholar]
- 36.Le Goff L, et al. Interleukin-5 is essential for vaccine-mediated immunity but not innate resistance to a filarial parasite. Infect Immun. 2000;68(5):2513–7. doi: 10.1128/iai.68.5.2513-2517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin C, et al. Drastic reduction of a filarial infection in eosinophilic interleukin-5 transgenic mice. Infect Immun. 2000;68(6):3651–6. doi: 10.1128/iai.68.6.3651-3656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Specht S, et al. CCL17 Controls Mast Cells for the Defense against Filarial Larval Entry. J Immunol. 2011 doi: 10.4049/jimmunol.1000612. [DOI] [PubMed] [Google Scholar]
- 39.Babayan S, et al. Resistance and susceptibility to filarial infection with Litomosoides sigmodontis are associated with early differences in parasite development and in localized immune reactions. Infect Immun. 2003;71(12):6820–9. doi: 10.1128/IAI.71.12.6820-6829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saeftel M, et al. Lack of interferon-gamma confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 2001;3(3):203–13. doi: 10.1016/s1286-4579(01)01372-7. [DOI] [PubMed] [Google Scholar]