Abstract
A 46-year-old Caucasian woman with a vitelliform macular lesion secondary to desferrioxamine (DFO) retinal toxicity in the right eye was treated with brinzolamide 1% ophthalmic drops three times a day. A spectral-domain OCT (SD-OCT) unit was used to monitor any changes in the macular lesion. After 2 months of starting the eye drops, the SD-OCT showed a notable improvement in the vitelliform macular lesion’s thickness. Six months later, the patient showed a further improvement in her macular lesion’s thickness on SD-OCT testing in the right eye. Best-corrected visual acuity was initially 1.00 log MAR (20/200 on a Snellen acuity chart) OD and 0.14 log MAR (20/25−2) OS. After 6 months of treatment, visual acuity was 0.92 (20/200+1) OD and 0.08 (20/25+1) OS. In conclusion, the use of brinzolamide 1% was associated with a marked reduction in a vitelliform macular lesion on spectral-domain OCT testing secondary to DFO retinal toxicity.
Keywords: Vitelliform macular lesion, Desferrioxamine retinopathy, Carbonic anhydrase inhibitor, Spectral-domain OCT
Introduction
Desferrioxamine (DFO) is a chelating agent used for the treatment of transfusion-induced hemochromatosis in patients with thalassemia major and other hematologic diseases requiring regular blood transfusions.1
Ocular toxicity secondary to prolonged treatment with DFO may result in night blindness, visual field constriction, cataract, macular and/or peripheral pigmentary retinopathy, or optic neuropathy.2 Two previous reports showed the presence of vitelliform macular lesions in patients on DFO therapy.3,4
Topical and oral forms of carbonic anhydrase inhibitors (CAI) have been shown to be effective for the improvement of macular cysts in patients with various retinal diseases such as retinitis pigmentosa,5,6 X-linked retinoschisis,7 and enhanced S-cone syndrome.8,9
We report a patient who presented with a vitelliform lesion in the macula of the right eye, as a result of long-term treatment with DFO. The use of brinzolamide ophthalmic suspension 1% (AZOPT®, Alcon laboratories, INC., Fort Worth, Texas, USA) three times a day in her right eye was associated with a marked resolution of the vitelliform lesion.
Case report
A 46-year-old Caucasian woman of Italian ancestry had a 10 year history of progressive decrease in central vision of each eye. She had a known history for thalassemia major with regular blood transfusions and had been treated with intramuscular or subcutaneous DFO for the past 22 years. A review of the family pedigree showed no history for hereditary eye diseases.
Visual acuity (VA) was correctable to 1.00 log MAR (20/200 measured with a Snellen acuity chart) OD with a +1.25+1.25×65 and to 0.14 log MAR (20/25−2) OS with a +1.00 spherical lens. Color vision testing using Ishihara plates showed 10/17 plates read correctly OD and 17/17 OS. Anterior segment examination was unremarkable. Intraocular pressures (IOP) measured by applanation tonometry were 15 mmHg OD and 16 mmHg OS.
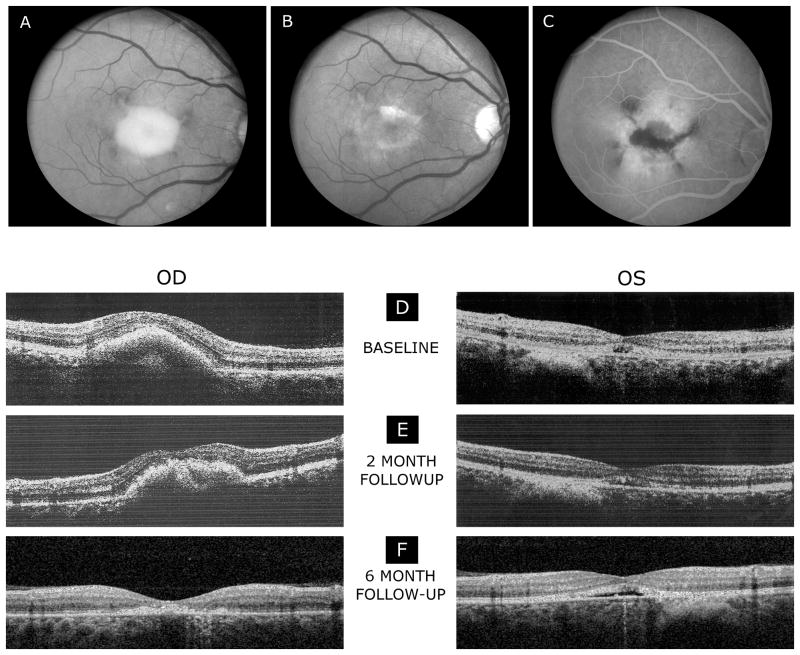
At baseline (pretreatment), the fundus examination showed normal-appearing optic discs and retinal vessels in both eyes. The right eye showed an elevated vitelliform macular lesion with hyperpigmented changes at the margins (Fig. 1A). The macula of the left eye showed a hypopigmentary lesion with mild retinal pigment epithelium (RPE) pigment mottling. There was a flat choroidal nevus, approximately 1 by 1 ½ disc diameters in size, noted inferior to the fovea of the left eye.
Fig.1.
Fundus photographs of the right eye (A), demonstrating an elevated vitelliform macular lesion with hyperpigmented changes at the margins. After six months of treatment (B), the vitelliform macular lesion in the right eye was less apparent when compared to the baseline. The right eye showed a small residual yellowish lesion in the macula with pigment on the surface of the lesion. Fluorescein angiogram (late frames) of the right eye (C) shows window defects of hyperfluorescence in the macula with hypofluorescent loci centrally. Spectral-domain OCT scans (E-F) demonstrate a reduction of the vitelliform macular lesion and retinal thickness while on treatment with topical brinzolamide 1% following two (E) and six months (F) of treatment. The left eye shows a shallow RPE elevation most evident at 6 months visit.
Treatment with Azopt 1% in each eye was initiated. At her two months visit, the patient did not notice any changes in her vision. Anterior segment examination and IOP were normal in each eye. Color vision screening with the Ishihara color plates was normal at both her 2 months and 6 months visits, which was an improvement in the right eye compared to the baseline visit. After 2 months of treatment, VA was correctable to 0.96 log MAR (20/200) OD and to 0.10 log MAR (20/25) OS. VA following six months of treatment did not significantly differ from that following two months of treatment.
Fundus examination showed that the vitelliform macular lesion in the right eye was less apparent at both 2 and 6 months visits. The right eye showed a small residual yellowish lesion in the macula with pigment on the surface of the lesion at the 6 months visit (Fig. 1B). The left eye was essentially unchanged by ophthalmoscopic examination.
Fluorescein angiography obtained by another ophthalmologist after treatment of the right eye for 5 months showed a normal retinal vasculature. In the foveal region there were areas of hypofluorescence, which correlated with the hyperpigmentary changes seen by ophthalmoscopic examination. Window defects of hyperfluorescence were noted at the margins of the residual vitelliform macular lesion. There was no evidence of any fluorescein leakage or choroidal neovascularization (CNVM) in late frames of the angiogram (Fig.1C).
At the baseline and 2 months visits, a spectral-domain OCT (SD-OCT) examination with the Optovue system (Optovue Inc., Fremont, CA) was used. At the 6 months visit, a SD-OCT/SLO system (OPKO instrumentations, Miami, FL) was used as previously described.7,9 The SD-OCT exams at the baseline visit indicated a highly reflective elevated lesion with accumulation of material in the sub-RPE space associated with the vitelliform macular lesion. Focal disruption of inner segment/outer segment (IS/OS) junction of the photoreceptors over the lesion and an almost normal-appearance of all major intraretinal layers was observed, although the macular region was elevated by the material, resulting in the partial disappearance of the foveal depression. The left eye showed a small elevated RPE lesion with disruption of the normal anatomy within the macula that involved the IS/OS junction of the photoreceptors with focal foveal areas of RPE loss and disruption (Fig. 1D).
The retinal thicknesses of the central 1-mm foveal subfields of the right and left eyes at each visit are provided in the Table. At baseline, the retinal thickness of the right eye was considerably greater than normal, whereas the left eye was within the normal limits. Following two months of treatment (Fig. 1E), the retinal thickness of the right eye decreased substantially, whereas the thickness of the left eye was essentially the same as that at baseline. A further reduction in the lesion size and macular thickness were noted after 6 months of treatment.
Table 1.
Table Retinal Thickness and Visual Acuity for Each Visit
| Retinal thickness (μm) | ETDRS* log MAR visual acuity (Snellen acuity) | |||
|---|---|---|---|---|
| OD | OS | OD | OS | |
| Baseline | 342 | 242 | 1.00 (20/200) | 0.14 (20/25−2) |
| Two-month follow-up** | 236 | 229 | 0.96 (20/200) | 0.10 (20/25) |
| Six-month follow-up*** | 189 | 216 | 0.92 (20/200+1) | 0.08 (20/25+1) |
ETDRS= Early Treatment Diabetic Retinopathy Study
The Optovue OCT system was used
The OPKO OCT system was used.
The SD-OCT scans at the 6 months visit of the right eye showed a residual irregular, broad-based, foveal lesion at the level of the RPE with focal disruption of the overlying IS/OS junction of the photoreceptors. The left eye OCT scans were similar to both the prior and the baseline visits (Fig. 1F).
Discussion
Chelating therapy with desferrioxamine (DFO) is well established and widely used to remove excess iron. Unfortunately, visual disorders have been documented after DFO infusion.2,10 Several mechanisms have been proposed for DFO toxicity such as the induction of oxidation, damage to the blood-retina barrier or reduction in certain metallic ions (zinc, copper), which are essential for normal retinal function.10 Several previous studies have documented a retinopathy secondary to DFO therapy such as RPE pigmentary mottling in the macular and/or equatorial regions, bull’s-eye maculopathy, and a vitelliform macular lesion.2–4
Our case showed that topical brinzolamide 1% eye drops may be effective in the treatment of a vitelliform macular lesion associated with DFO toxicity that was, at least partially, likely responsible for the loss of central vision in our patient. We observed a notable reduction in the lesion thickness on SD-OCT testing and an improvement in color vision in the right eye after treatment initiation.
To our knowledge, our current study is the first to demonstrate a beneficial effect likely from the use of a CAI in a patient with desferrioxamine vitelliform retinopathy. Of note, our case showed that the vitelliform macular lesion was apparent for at least 1 year without any gross-anatomical changes in the lesion morphology and/or thickness until treatment was initiated, which lessens the possibility that the notable improvement in the lesion’s thickness was due to a natural history of spontaneous resolution in the macular lesion. The vitelliform macular lesion seen in our patient had a phenotype simulating the vitelliform lesion seen in Best disease and some forms of pattern dystrophy, which are also characterized by an abnormal accumulation of a yellow pigment (mainly lipofuscin).11 The patient’s history and clinical exam, including a late-onset decrease in her central acuity (fourth decade of life), prolonged use of desferrioxamine, and negative family history of hereditary ocular diseases, favor an acquired cause for her maculopathy and is likely related to the use of desferrioxamine.
Of note, we decided to treat the left eye in our case, which showed a small degree of yellowish material in the macula 7 years previously, for determining any possible effects of the brinzolamide drops on macular thickness in the absence of a vitelliform lesion. There was no change in the retinal thickness after 6 months of treatment.
The current report will be added to the list of other retinal diseases 5–9 that have shown an improvement in macular thickness after treatment with a CAI. Our patient showed no clinically significant change in her visual acuity during the follow-up visits, which raises the possibility of irreversible RPE and photoreceptor toxicity in the macula associated with an atrophic-appearing lesion from the prolonged use of DFO. The lack of any significant change in VA did not correlate well with the improvement of the vitelliform macular lesion on SD-OCT.
One of our report’s limitations was using two different SD-OCT instruments. However, the differences in retinal thickness between both SD-OCT units used in our study were minimal and not likely to be clinically relevant as previously described.12
In summary, the use of brinzolamide 1% was associated with a marked reduction in a vitelliform macular lesion on spectral-domain OCT testing secondary to DFO retinopathy. This finding supports a recommendation that a trial of this topical CAI should be considered in the treatment of a vitelliform macular lesion in patients with DFO retinal toxicity.
Acknowledgments
Supported by funds from the Foundation Fighting Blindness, Columbia, Maryland; Pangere Corporation; Grant Healthcare Foundation, Lake Forest, Illinois; NIH core grant EYO1792; and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
None of the authors have any proprietary interest in this report.
References
- 1.Chaston TB, Richardson DR. Iron chelators for the treatment of iron overload disease: relationship between structure, redox activity, and toxicity. Am J Hematol. 2003;73 (3):200–210. doi: 10.1002/ajh.10348. [DOI] [PubMed] [Google Scholar]
- 2.Haimovici R, D’Amico DJ, Gragoudas ES, Sokol S. The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology. 2002;109(1):164–171. doi: 10.1016/s0161-6420(01)00947-2. [DOI] [PubMed] [Google Scholar]
- 3.Gonzales CR, Lin AP, Engstrom RE, Kreiger AE. Bilateral vitelliform maculopathy and deferoxamine toxicity. Retina. 2004;24(3):464–467. doi: 10.1097/00006982-200406000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Genead MA, Fishman GA, Anastasakis A, Lindeman M. Macular vitelliform lesion in desferrioxamine-related retinopathy. Doc Ophthalmol. 2010;121(2):161–166. doi: 10.1007/s10633-010-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grover S, Apushkin MA, Fishman GA. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 2006;141(5):850–858. doi: 10.1016/j.ajo.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Genead MA, Fishman GA. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with retinitis pigmentosa and Usher syndrome. Arch Ophthalmol. 2010;128(9):1146–1150. doi: 10.1001/archophthalmol.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genead MA, Fishman GA, Walia S. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch Ophthalmol. 2010;128(2):190–197. doi: 10.1001/archophthalmol.2009.398. [DOI] [PubMed] [Google Scholar]
- 8.Iannaccone A, Fung KH, Eyestone ME, Stone EM. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am J Ophthalmol. 2009;147(2):307–312. doi: 10.1016/j.ajo.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genead MA, Fishman GA, McAnany JJ. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc Ophthalmol. 2010;121(3):231–240. doi: 10.1007/s10633-010-9247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Virgiliis S, Congia M, Turco MP, Frau F, Dessi C, Argiolu F. Depletion of trace elements and acute ocular toxicity induced by desferrioxamine in patients with thalassaemia. Arch Dis Child. 1998;63(3):250–255. doi: 10.1136/adc.63.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marmorstein AD, Cross HE, Peachey NS. Functional roles of bestrophins in ocular epithelia. Prog Retin Eye Res. 2009;28(3):206–226. doi: 10.1016/j.preteyeres.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50(7):3432–3437. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]