Abstract
Xenotransplantation could provide an unlimited and elective supply of grafts, once mechanisms of graft loss and vascular injury are better understood. The development of α-1,3-galactosyltransferase gene-knockout (GalT-KO) swine with the removal of a dominant xeno-antigen has been an important advance; however, delayed xenograft and acute vascular reaction in GalT-KO animals persist. These occur, at least in part, because of humoral reactions that result in vascular injury. Intrinsic molecular incompatibilities in the regulation of blood clotting and extracellular nucleotide homeostasis between discordant species may also predispose to thrombophilia within the vasculature of xenografts. Although limited benefits have been achieved with currently available pharmacological anti-thrombotics and anti-coagulants, the highly complex mechanisms of platelet activation and thrombosis in xenograft rejection also require potent immunosuppressive interventions. We will focus on recent thromboregulatory approaches while elucidating appropriate anti-platelet mechanisms. We will discuss potential benefits of additional anti-thrombotic interventions that are possible in transgenic swine and review recent developments in pharmacological anti-platelet therapy.
Keywords: anti-thrombotics, CD39, platelets, Xenotransplantation
Introduction
The generation of α-1,3-galactosyltransferase gene-knockout (GalT-KO) pig organs has dramatically improved graft survival and has been an important step in preclinical studies [1–4]. However, even after the removal of this dominant xeno-antigen, graft failure still results. Xenotransplantation will not become clinically feasible until mechanisms of rejection that limit graft survival are better understood [2,5].
Delayed xenograft or acute vascular reaction in GalT-KO animals is characterized by thrombosis together with progressive xenograft microangiopathy. These are considered due at least in part to secondary “natural” or elicited non-Gal antibodies (Abs) [6]. Consumptive coagulopathy (CC) and platelet sequestration in xenotransplantation might be further exacerbated by intrinsic molecular incompatibilities in the vascular regulation of blood clotting and extracellular nucleotide homeostasis between discordant species [7].
Because of these immunoreaction-mediated thrombotic processes and molecular barriers, limited benefits have been achieved with currently available pharmacological approaches. Established anti-thrombotic approaches might not be effectively translated to the setting of xenotransplantation. More novel and effective use of anti-thrombotics together with the development of GalT-KO animals either transgenic for defined human thromboregulatory factors or null for porcine procoagulants might provide effective alternative approaches.
The purpose of this review is to focus on complex mechanisms of platelet activation and thrombosis in xenograft rejection and detail recent discussions of thromboregulatory approaches and appropriate anti-platelet concepts. We will therefore discuss potential benefits of additional genetic interventions and list recent developments in pharmacological anti-platelet therapy.
General aspects of platelet activation and thrombosis
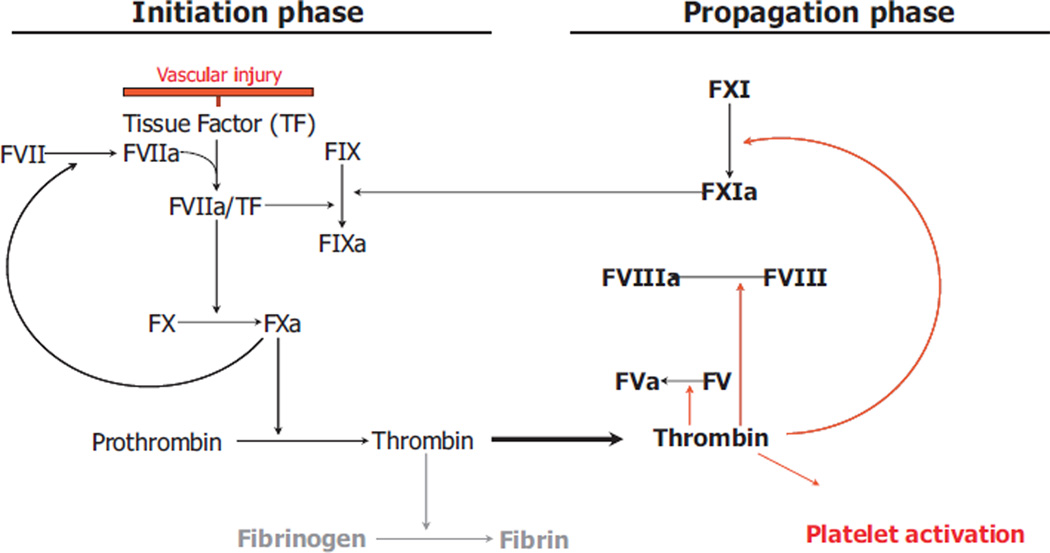
Coagulation, in general, is mediated by an interaction between activated endothelium, serum proteins, e.g. serine proteases, platelets, and leukocytes. Initially, tissue factor (TF) and collagen are exposed by the injured vessel. TF binds to activated factor VII (FVIIa) and initiates the generation of thrombin; subendothelial collagen triggers the accumulation and activation of platelets [8] (Fig. 1). Three different phases are postulated with (i) initiation, characterized by binding and activation of FVII by TF on cells or microparticles (MPs), (ii) amplification with the activation of platelets by small amounts of thrombin (as a result of the TF/FVIIa complex), and (iii) consolidation via heightened thrombin actions mediated via the prothrombinase complex [8].
Fig. 1.
Principles of the coagulation cascade in xenograft vascular injury. Tissue factor (TF) and activated factor VIIa (FVIIa) form an activated complex initiating a series of reactions, which finally leads to thrombin formation and the parallel process of platelet activation.
At sites of vascular activation, platelets adhere in a highly coordinated manner. Shear forces initiate a platelet–endothelium interaction, which is characterized by the binding of glycoprotein Ib (GPIb) to von Willebrand Factor (vWF) [9,10]. This is referred to as tethering (or transient adhesion). After the distinctive stage of rolling, as a consequence of low-affinity interactions between collagen and GPVI, paracrine- and autocrine-mediated signaling leads to the stable adhesion and activation of platelets.
In this context, thromboxane A2 and the nucleotide adenosine diphosphate (ADP) are released from platelets, along with thrombin activation by TF from the vessel wall [11]. GP IIb/IIIa finally mediates thrombus formation together with fibrinogen and vWF [9]. Interested readers are referred to an excellent recent review of this process [12].
In the setting of xenotransplantation, ischemia–reperfusion and/or Ab-mediated activation result in pro-inflammatory changes in the xenograft endothelium (Fig. 2). Such vascular responses represent important factors that alter the coagulation balance. Further immunological responses to activated xenograft endothelium may lead to CC, graft thrombosis, and finally graft failure. A detailed understanding of the process of coagulation, mediated by interactions between activated endothelium, coagulation factors, and platelets, is crucial for approaches to improving graft survival by preventing thrombosis.
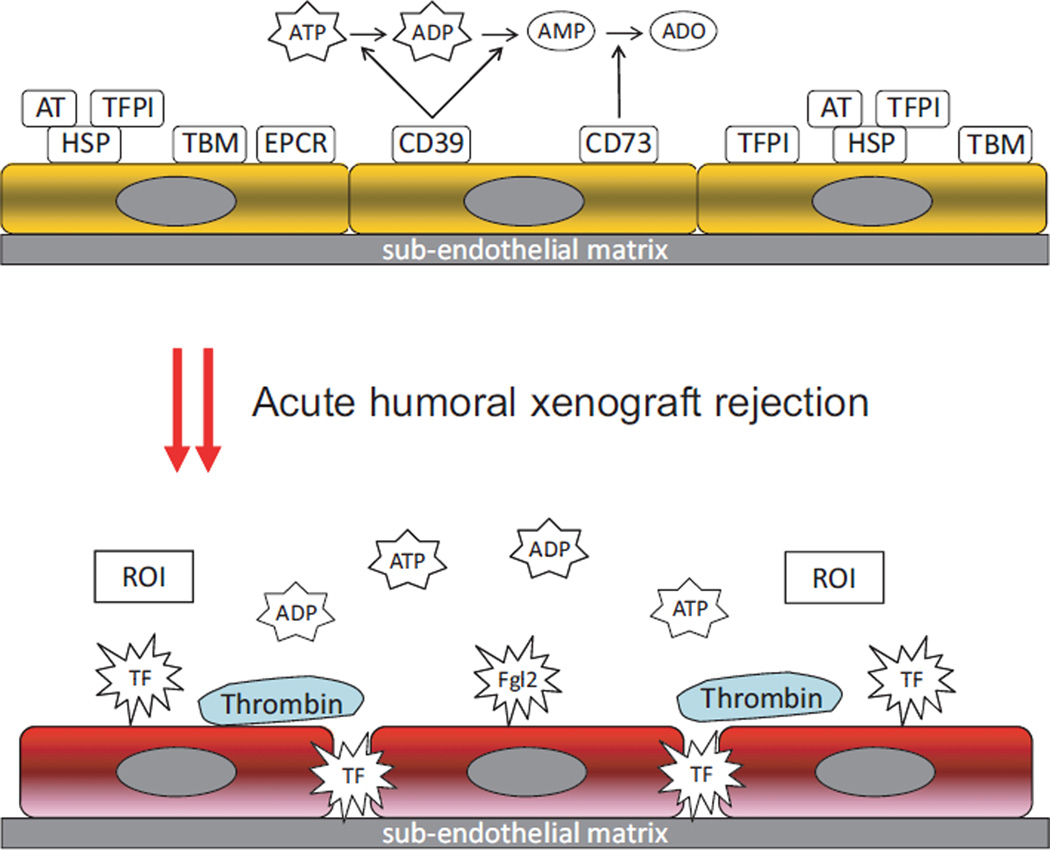
Fig. 2.
Prothrombotic changes associated with acute humoral xenograft rejection, characterized by increased vascular exposure of the immune coagulant fgl2/fibroleukin, tissue factor (TF), thrombin production and release of natural anti-coagulants viz. tissue factor pathway inhibitor (TFPI), the protein C pathway with thrombomodulin (TBM), antithrombin (AT), and endothelial protein C receptor (EPCR). CD40L-dependent thrombin generation and release of reactive oxygen intermediates (ROI) are also noted. Loss of endothelial CD39 and CD73 bioactivity leads to increased levels of extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP), with the latter acting as a major agonist for platelet aggregation.
Control points in platelet activation and thrombosis
Heparan sulfate and anti-thrombin
Coagulation factors are regulated by physiological natural anti-coagulants viz. tissue factor pathway inhibitor (TFPI-1), the protein C pathway with thrombomodulin (TBM), and anti-thrombin (AT) [13], (Fig. 2). Heparan sulfate proteoglycan is located on the surface of the vascular endothelium and inhibits intravascular coagulation by localized activation of AT, a potent inhibitor of thrombin generation [14,15]. Platt et al. have demonstrated that the exposure of cultured porcine endothelium to human serum caused cleavage and release of endothelial cell proteoglycan [15]. The loss of endothelial cell proteoglycan may be critical in other diseases involving humoral injury to vascular endothelium.
von Willebrand Factor and glycoprotein Ib
von Willebrand Factor is a multimeric glycoprotein produced by the endothelium and present in subendothelial connective tissue and αgranules of platelets. Under shear stress, at sites of injury or endothelial activation, human vWF contributes to tethering and adhesion of human platelets by interacting with platelet GPIb, VI, and Ib-V–IX, respectively, and together with GPIIb/IIIa and fibrinogen leads to thrombus formation [8,12].
Porcine vWF has been observed to both aggregate and activate human platelets in an enhanced manner via GPIb interactions, in vitro. However, in contrast to human vWF, these enhanced reactions are independent of shear stress and of further platelet stimulation [16–18]. Schulte am Esch et al. have demonstrated that such spontaneous interactions of porcine vWF can be ascribed to the GPIb-binding vWF-A1 domain [19,20]. Interestingly, loss of O-linked glycosylation seems to abolish differences in aggregatory responses between isolated human and porcine vWF-A1 domains [19].
Using a left single-lung transplant model, where baboons were depleted of anti-GalT Ab, Cantu et al. [21] could show that vWF plays a major role mainly in delayed dysfunction of these grafts. In this setting, where around 2/3 of pulmonary intravascular macrophages are depleted, vWF-deficient grafts survived longer than any previous reported pulmonary xenograft [21]. These data further underline the importance of porcine vWF as a regulatory protein in the setting of xenotransplantation and show vWF as a promising target for modification or for deletion.
Glycoprotein IIB/IIIa
The glycoprotein complex GPIIb/IIIa is located on the platelet membrane. GPIIb/IIIa functions as a receptor for adhesive proteins like fibrinogen, fibronectin, and vWF, and activation represents the final pathway for platelet aggregation. Interestingly, ADP acts as a major agonist by initiating intracellular signaling and imparting ligand competency to the extracellular domain [22]. Among the large family of integrin adhesion receptors, the GPIIb/IIIa complex is of major clinical importance as inhibitors are already used in the clinic [23]. The role of GPIIb/IIIa inhibitors in xenotransplantation will be discussed later.
Thromboregulation, extracellular nucleotides, and ectonucleotidases
The endothelium contains at least three thromboregulatory mechanisms, namely nitric oxide, prostacyclin, and the ectonucleotidase CD39/ectonucleoside triphosphate diphosphohydrolase1 [24]. The latter ectoenzyme hydrolyzes extracellular adenosine triphosphate (ATP) to ADP and adenosine monophosphate that in tandem with CD73 generates ultimately adenosine. Extracellular nucleotides (e.g. ATP, UTP, ADP) are released by both activated endothelium and platelets and bind to specific cell-surface type-2 purinergic (P2) receptors. CD39 plays a major thromboregulatory role by decreasing the amount of extracellular ADP, which in turn reduces the activation of platelet ADP receptors P2X1, P2Y1, and P2Y12 [25].
In xenotransplantation, ischemia–reperfusion and Ab-mediated activation of the xenograft endothelium lead to important pro-inflammatory changes in the expression of thromboregulatory factors, e.g. with the loss of CD39 bioactivity [26]. Our group showed that the anti-thrombotic effects of the ATPDase, like heparan sulfate and TBM, are lost after EC activation and that the loss of the ATPDase is crucial for the progression of vascular injury [26].
Using a heterotopic cardiac xenotransplantation model from GalT-KO swine to baboons, Shimizu et al. [27] showed that thrombotic microangiopathy develops in parallel with decreased levels of CD39 and an increased expression of TF and vWF. This study confirmed changes after pig-to-baboon kidney transplantation, characterized by decreased levels of CD39 and an upregulation of TF and vWF; these changes were closely associated with the formation of multiple fibrin–platelet microthrombi [28].
Ashwell–Morell asialoglycoprotein receptor
The Ashwell–Morell asialoglycoprotein receptor is located on the surface of hepatocytes and to a lesser extent on sinusoidal endothelial cells. This lectin removes circulating glycoproteins and has recently been shown to play a major role in the clearance of aged or altered platelets in mice [29,30]. Rumjantseva and co-workers [30] showed that both the density and concentration of galactose residues on membranes are dramatically increased after refrigeration of donor platelets, as mediated by the loss of sialic acid; this results in rapid clearance from the circulation of stored platelets after transfusion. Additionally, the increased vWF plasma half-life and higher levels of circulating vWF in Asgr-1-deficient mice indicate that the receptor might be involved in vWF-linked hemostasis [29]. Desialylated platelets are also recognized and cleared by the receptors, which thereby demonstrate a protective effect against disseminated intravascular coagulation [29].
Cyclooxygenase
Thromboxane A2 is produced by activated platelets and has pro-thrombotic properties increasing platelet aggregation. Expression of platelet GPIIb/IIIa, which interacts with vWF, is mediated by thromboxane A2. Non-selective inhibition of the enzyme cyclooxygenase (COX) or selective inhibition of the iso-enzyme COX-1 prevents the formation of prostaglandin H2 and finally thromboxane A2. Pharmacologically targeting COX-1 is therefore widely used to inhibit platelet aggregation and thrombus formation [31].
Complement
The complement system may be activated in the GalT-KO xenograft setting via three different pathways: (i) the classical pathway, by binding of non-Gal antibodies and/or ischemia–reperfusion injury (IRI), (ii) the alternative pathway, and (iii) the lectin pathway and represents an important mechanism of acute humoral xenograft reactions (AHXR) [32]. Influences of complement on B-cell responses and the importance of complement-dependent T-cell recognition, expansion, and differentiation have recently been reviewed by Sacks [33].
CD154/CD40L
CD154/CD40 ligand is primarily expressed on activated T cells; however, engagement of CD154/CD40L on platelets has been implicated in thrombosis. We recently noted that patients with IRI with orthotopic liver transplantation (OLT) develop parameters of platelet activation 48 h after OLT, characterized by an increased surface presentation of P-selectin, thrombospondin-1, TF, and CD154/CD40L [34].
Possible therapeutic approaches
Heparan sulfate and anti-thrombin
Heparan sulfate is shed from the endothelial cell membrane after activation, which leads to a loss of anti-coagulant and anti-inflammatory properties of the endothelium [15]. Dextran sulfate (DXS) is a semisynthetic glycosaminoglycan analog and an efficient inhibitor of complement activation [35–37]. Several studies have shown that DXS binds to xenograft endothelial cells in vitro and in vivo and acts as an endothelial cell protectant by at least functionally replacing shed endothelial heparan sulfate [35–37].
Studies investigating the use of recombinant AT failed to show significant benefits. Even high daily doses of recombinant human AT (500 U/kg twice daily), administered in a pig-to-primate model, did not influence survival or prevent fibrin formation and deposition in the graft [38]. Similarly, co-administration of AT with recombinant human-activated protein C did not prevent the eventual occurrence of AHXR [39].
von Willebrand Factor and anti-glycoprotein Ib antibodies
Porcine vWF plays a major role in the pathogenesis of xenograft dysfunction and aggregates human platelets independent of further stimulation [16–20,40]. Gaca et al. [41] have targeted the GPIb-A1 domain interaction by administering mouse anti-human GPIb monoclonal antibody in baboons before orthotopic pulmonary xenotransplantation. Although anti-GPIb Abs prevented platelet deposition, there was no improvement in the vasoconstriction associated with the loss of xenograft function, compared to controls [41].
Another group has investigated the inhibitory effect of the GPIb-binding protein Trimeresurus mucrosquamatus venom activator (TMVA) on platelet aggregation in a guinea pig-to-rat cardiac xenograft model [42]. This approach was effective in preventing platelet microthrombi formation and fibrin deposition and resulted in complete inhibition of platelet aggregation. Furthermore, the authors also reported a significant prolongation of xenograft survival [42].
The effects of aurintricarboxylic acid (ATA), an inhibitor of platelet adhesion, were recently investigated in an ex vivo model of porcine lung perfusion with fresh human whole blood [43]. Although ATA could be shown to abolish thrombin-induced vWF secretion and improve pulmonary function, additional in vitro experiments, using porcine endothelial cells (PECs) incubated with human serum, confirmed detrimental effect on the induction of endothelial TF and platelet activation [43]. Our studies indicated that clinical use might be precluded by severe disturbances of coagulation [44].
Glycoprotein IIb/IIIa inhibitors
In xenotransplantation, several platelet GPIIb/IIIa inhibitors have been shown to inhibit platelet aggregation and prolong xenograft survival [45,46]. Candinas et al. [46] noted that Lewis rats receiving heterotopic guinea pig cardiac xenografts benefit from therapy with a putative GPIIb/IIIa antagonist in a dose-dependent manner. Furthermore, our group showed that high-dose GPIIb/IIIa antagonist led to a significant increase in graft survival in both acute and delayed rejection groups [46].
In a working-heart model with non-transgenic and human complement regulator (hDAF) pig hearts, Brandl et al. [47] confirmed a certain impact for tirofiban by showing that this GPIIb/IIIa inhibitor leads to an improvement in heart performance and reduction in myocardial damage and intravascular thrombosis. Depletion of fibrinogen was equally diminished by tirofiban and hDAF, but the combination of tirofiban and GPIIb/IIIa showed additional benefits with respect to AT consumption and intravascular fibrin deposition [47].
A beneficial effect of combination therapy could also be shown for ATA (GPIb–vWF inhibitor) and SC52012A, a synthetic GPIIb/IIIa-inhibiting peptide [48]. In a model using isolated piglet lungs, perfused with (heparinized) whole human blood, Pfeiffer et al. [48] showed that addition of ATA or SC before lung perfusion significantly attenuated the rise in pulmonary vascular resistance, diminished histamine release, and prolonged survival from 8 ± 3 min to 31 ± 11 and 31 ± 22 min, respectively. Interestingly, when therapies were combined, mean survival was significantly longer compared to both agents given as monotherapy [48].
CD39
The activity of CD39, the dominant vascular ectonucleotidase modulating hemostasis and thrombotic reactions [49], rapidly decreases in xenografts after transplantation [50]. Consequently, decreased expression of CD39 is noted with the sequestration of platelet-rich fibrin thrombi in the xenograft microvasculature [27,28]. Recombinant adenoviral-mediated Cd39 gene transfer ex vivo provides survival benefits in a small animal model in vivo [51], and intrinsic CD39 upregulation in renal xenografts may have associated protective effects in the pig-to-primate model [27,52]. These promising results suggest that Cd39 should be one of the next genes added to the GalT-KO background [52].
In addition to genetic interventions, the soluble form of CD39 (solCD39) might also be a drug candidate as this causes complete inhibition of ADP-induced platelet aggregation when added ex vivo to human platelet-rich plasma [53]. Administration of solCD39 increases the bleeding time to a maximal extent, similar to aspirin, and inhibited platelet aggregation by >90% in a swine model [53].
CD39 is also contained within plasma MPs that exhibit modulatory roles in the exchange of regulatory signals between leukocytes and endothelium [54]. Work is ongoing to analyze the nature and role of MPs in the xenotransplantation context.
Purinergic receptor inhibitors
Thienopyridines represent a class of ADP receptor inhibitors and are widely used for thromboregulatory purposes [55]. Clopidogrel (Plavix), a specific and irreversible P2Y12 antagonist, affects the ADP-dependent activation of the GPIIb/IIIa pathway. Prasugrel (Effient), a third-generation thienopyridine, is a specific and irreversible P2Y12 antagonist that is characterized by potent antiplatelet effects but also by a higher risk for severe bleeding. Whereas prasugrel has not been added to anti-platelet regimes in xenotransplantation, clopidogrel as a part of anti-platelet approaches has been investigated in two comprehensive pig-to-baboon studies [6,56]. Recent studies have suggested that other medications metabolized by cytochrome P450 (CYP450), e.g. proton pump inhibitors, could influence and expand the effect of clopidogrel [57,58]. Whether possible CYP450 alterations in swine and respective changes of pharmacokinetics might further influence the function of the pro-drug clopidogrel remain to be investigated.
Schirmer et al. [6] have investigated the effect of combined anti-platelet treatment with aspirin and clopidogrel in a human CD46 transgenic pig heart transplantation model in baboons. Combination therapy of aspirin (80 mg/day) and clopidogrel (75 mg/day) starting 2 days after transplantation and in addition to immunosuppression resulted in the inhibition of platelet aggregation. However, this had no impact on the development of microvascular thrombosis or on the length of xenograft survival, when compared to a group of animals that received similar immunosuppression but no anti-platelet therapy [6].
Using CD46 transgenic pig hearts in baboons, it has been demonstrated that significant prolongation in xenograft survival was related to stronger immunosuppression rather than to anti-platelet therapy [56,59]. Comparable median survival for group 1, receiving low-dose tacrolimus and sirolimus maintenance therapy, with splenectomy, anti-CD20, and daily alpha-Gal polymer, is also seen in groups 2–4, which received additionally either aspirin and clopidogrel, enoxaparin or warfarin, respectively [56]. Changing immunosuppressive regime to include high-dose tacrolimus and sirolimus maintenance therapy (Group 5) increased the survival independently of administered enoxaparin (Group 6). Neither calcineurin inhibitors nor mTOR inhibitors have effects on adhesion molecule expression of human micro- and macrovascular endothelial cells and are unlikely to contribute to EC activation and vasculopathy [60]. However, data showing that angiogenesis can be blocked by rapamycin demonstrate that the inhibition of mTOR is characterized by complex effects, which needs further evaluation in the setting of xenotransplantation [61].
Ashwell–Morell asialoglycoprotein receptor
Injection of refrigerated platelets with a competitor for the Ashwell–Morell receptor into mice markedly improved the recovery and survival time of platelets [30]. The role of the Ashwell–Morell receptor and differential sialic acid substitution of platelet glycoproteins in the setting of platelet consumption seen during xenotransplantation has not been investigated to date. Whether Ashwell–Morell receptor competitors, given as a component of multimodal therapy, could also decrease Ab-mediated microangiopathy in the setting of xenotransplantation is worth investigation.
Cyclooxygenase inhibitors
In two human CD46 transgenic pig-to-baboon heart xenotransplantation models, combination therapy of the unselective COX inhibitor aspirin alone or with either clopidogrel, enoxaparin, or warfarin led to an inhibition of platelet aggregation [6,56]. This approach had little impact on the development of microvascular thrombosis or on the length of xenograft survival, when compared to a group of animals that received the same immunosuppression but no anti-platelet therapy [6,56].
In another setting, heterotopic transplantation of GalT-KO pig hearts in eight baboons resulted in graft function for a period approaching 6 months [3,61]. Thrombotic microangiopathy developed in all cases; however, the onset of vascular injury was delayed in two baboons that received aspirin in addition to the anti-human CD154 monoclonal antibody-based immunosuppressive regimen. The clinical course of one baboon was particularly promising, as graft survival was noted for 179 days. This animal received aspirin (40 mg on alternate days) from day 4 in addition to heparin, to be discussed as a possible factor in the delay of thrombotic microangiopathy [3,62].
Complement pathways
Beneficial effects of complement inhibition and/or depletion have been described in various studies. Infiltration of macrophages and CD4 (+) T cells was markedly delayed in a pig-to-mouse corneal xenograft model [63]. However, as described in previous studies investigating the effects of various soluble complement receptor type 1 and CVF regimens, rejection could not be effectively prevented [63–65].
Anti-CD154/CD40L
Choi et al. [66] showed that the interaction between human CD40L and porcine CD40 contributes to acute vascular rejection in xenotransplantation by activating PECs through NF-kappaB signaling. However, we showed previously that anti-CD40L mAb had no effect on purified baboon platelet aggregation profiles; the therapeutic benefit of such a therapy is therefore controversial [67].
Transgenesis
Kolber-Simonds et al. [68] have generated GalT null inbred miniature swine by nuclear transfer techniques. Putative molecular incompatibilities have been shown between primate coagulation factors and porcine natural anti-coagulants that would exacerbate the inflammatory and thrombotic state within the xenograft vasculature of GalT-KO grafts [69] that could be addressed by transgenic approaches.
Transgenic animals over-expressing human natural anti-coagulants and the thromboregulatory factor CD39 may be effective in the prevention of graft rejection and thrombosis [69–71]. Experiments with mice transgenic for human CD39 recently showed that both systemic and local strategies to increase levels of extracellular adenosine have beneficial effects in humoral rejection of mouse cardiac allografts and renal IRI [71,72]. This is in agreement with previous studies and may be applied to future strategies [71,72].
Others have proposed the use of anti-coagulant fusion proteins based on human TFPI and the leech anti-coagulant hirudin and demonstrated functional efficacy in vitro; modification with P-selectin sequences for localization within Weibel-Palade bodies was used to restrict cell-surface expression to activated endothelium [73]. Transgenic mice expressing these anti-coagulant fusion proteins have been developed and benefits demonstrated in small animal models [74], with ongoing work in pigs [75].
A potential and long-term solution to the xenograft vasculopathy in porcine kidneys transplanted into primates will likely be the development of GalT-KO, transgenic pigs over-expressing a hDAF, an anti-coagulant (TBM or TFPI), an anti-thrombotic and anti-inflammatory factor (CD39), and potentially other cytoprotective genes.
Conclusions
Several approaches have been suggested to address the delayed xenograft rejection responses in GalT-KO animals. Gene-knock outs have been developed, and several transgenic swine are now becoming available for study, respectively. Procoagulant and anti-coagulant genes can be targeted for deletion or modification. Antibody-based immunosuppressive regimens have been tested, and new mechanisms in thrombosis with the potential for the administration of soluble anti-coagulants and anti-thrombotics have been proposed.
The application of molecular genetic approaches to the pathobiology of delayed rejection in xenotransplantation is becoming increasingly important. Although some of the proposed approaches may have been beneficial, none of the pharmacological modalities to date have been validated in terms of significantly prolonged graft survivals on a consistent basis. The major task in the next years will be to find the appropriate thromboregulatory and anti-inflammatory genes that should be added to the GalT-KO background and whether such proposed approaches are adequate alone or require further pharmacological interventions.
Acknowledgments
Authors acknowledge ongoing collaborations and multiple inputs from colleagues in this area of research. MS acknowledges grant support from DFG SCHM 2661/1-1 and 2661/1-2. PJC acknowledges grant support from the National Health and Medical Research Council of Australia (NHMRC). SCR acknowledges grant support from R01 HL094400-01, U01 AI066331, and P01 AI045897.
References
- 1.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hisashi Y, Yamada K, Kuwaki K, et al. Rejection of cardiac xenografts transplanted from alpha1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant. 2008;8:2516–2526. doi: 10.1111/j.1600-6143.2008.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng YL, Kuwaki K, Dor FJ, et al. Alpha1,3-Galactosyltransferase geneknockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 4.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal- knockout pig kid-neys. Nat Med. 2005;11:1295. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schirmer JM, Fass DN, Byrne GW, Tazelaar HD, Logan JS, McGregor CG. Effective antiplatelet therapy does not prolong transgenic pig to baboon cardiac xenograft survival. Xenotransplantation. 2004;11:436–443. doi: 10.1111/j.1399-3089.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmelzle M, Schulte Esch J, II, Robson SC. Coagulation, platelet activation and thrombosis in xenotransplantation. Curr Opin Organ Transplant. 2010;15:212–218. doi: 10.1097/MOT.0b013e3283373ccc. [DOI] [PubMed] [Google Scholar]
- 8.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 9.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5509. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 10.Dopheide SM, Maxwell MJ, Jackson SP. Shear-dependent tether formation during platelet translocation on von Willebrand factor. Blood. 2002;99:159–167. doi: 10.1182/blood.v99.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28:403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 12.Kiefer TL, Becker RC. Inhibitors of platelet adhesion. Circulation. 2009;120:2488–2495. doi: 10.1161/CIRCULATIONAHA.109.886895. [DOI] [PubMed] [Google Scholar]
- 13.Lee KF, Salvaris EJ, Roussel JC, et al. Recombinant pig TFPI efficiently regulates human tissue factor pathways. Xenotransplantation. 2008;15:191–197. doi: 10.1111/j.1399-3089.2008.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcum JA, Atha DH, Fritze LMS, Nawroth P, Stern D, Rosenberg RD. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986;261:7507. [PubMed] [Google Scholar]
- 15.Platt JL, Vercellotti GM, Lindman BJ, Oegema TR, Jr, Bach FH, Dalmasso AP. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990;171:1363–1368. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans RJ, Austen DE. Assay and characterization of the factor in porcine and bovine plasma which aggregates human platelets. Br J Haematol. 1977;36:117. doi: 10.1111/j.1365-2141.1977.tb05761.x. [DOI] [PubMed] [Google Scholar]
- 17.Pareti FI, Mazzucato M, Bottini E, Mannucci PM. Interaction of porcine von Willebrand factor with the platelet glycoproteins Ib and IIb/IIIa complex. Br J Haematol. 1992;82:81. doi: 10.1111/j.1365-2141.1992.tb04597.x. [DOI] [PubMed] [Google Scholar]
- 18.Mazzucato M, De Marco L, Pradella P, et al. Porcine von Willebrand Factor binding to human platelet GPIb induces transmembrane calcium influx. Thromb Haemost. 1996;75:655. [PubMed] [Google Scholar]
- 19.Schulte Am Esch J, II, Robson SC, Knoefel WT, et al. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation. 2005;12:30–37. doi: 10.1111/j.1399-3089.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 20.Schulte Am Esch J, II, Cruz MA, Siegel JB, et al. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood. 1997;90:4425–4437. [PubMed] [Google Scholar]
- 21.Cantu E, Balsara KR, Li B, et al. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7:66–75. doi: 10.1111/j.1600-6143.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma YQ, Qin J, Plow EF. Platelet integrin alpha(IIb) beta(3): activation mechanisms. J Thromb Haemost. 2007;5:1345–1352. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 23.Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580–587. doi: 10.1056/NEJMra066469. [DOI] [PubMed] [Google Scholar]
- 24.Marcus AJ, Broekman MJ, Pinsky DJ. COX inhibitors and thromboregulation. N Engl J Med. 2002;347:1025–1026. doi: 10.1056/NEJMcibr021805. [DOI] [PubMed] [Google Scholar]
- 25.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 26.Robson SC, Kaczmarek E, Siegel JB, et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3- galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172:1471–1481. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu A, Yamada K, Yamamoto S, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-tobaboon kidney xenografts. J Am Soc Nephrol. 2005;16:2732–2745. doi: 10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- 29.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14:648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15:1273–1280. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 32.Miyagawa S, Yamamoto A, Matsunami K, et al. Complement regulation in the GalT KO era. Xenotransplantation. 2010;17:11–25. doi: 10.1111/j.1399-3089.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 33.Sacks SH. Complement fragments C3a and C5a: the salt and pepper of the immune response. Eur J Immunol. 2010;40:668–670. doi: 10.1002/eji.201040355. [DOI] [PubMed] [Google Scholar]
- 34.Esch JS, Jurk K, Knoefel WT, et al. Platelet activation and increased tissue factor expression on monocytes in reperfusion injury following orthotopic liver transplantation. Platelets. 2010;21:348–359. doi: 10.3109/09537101003739897. [DOI] [PubMed] [Google Scholar]
- 35.Laumonier T, Walpen AJ, Maurus CF, et al. Dextran sulfate acts as an endothelial cell protectant and inhibits human complement and natural killer cell-mediated cytotoxicity against porcine cells. Transplantation. 2003;76:838–843. doi: 10.1097/01.TP.0000078898.28399.0A. [DOI] [PubMed] [Google Scholar]
- 36.Fiorante P, Banz Y, Mohacsi PJ, et al. Low molecular weight dextran sulfate prevents complement activation and delays hyperacute rejection in pig-to-human xenotransplantation models. Xenotransplantation. 2001;8:24–35. doi: 10.1046/j.0908-665x.2000.00088.x. [DOI] [PubMed] [Google Scholar]
- 37.Laumonier T, Mohacsi PJ, Matozan KM, et al. Endothelial cell protection by dextran sulfate: a novel strategy to prevent acute vascular rejection in xenotransplantation. Am J Transplant. 2004;4:181–187. doi: 10.1046/j.1600-6143.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- 38.Cozzi E, Simioni P, Boldrin M, et al. Effects of long-term administration of high-dose recombinant human antithrombin in immunosuppressed primate recipients of porcine xenografts. Transplantation. 2005;80:1501–1510. doi: 10.1097/01.tp.0000178377.55615.8b. [DOI] [PubMed] [Google Scholar]
- 39.Simioni P, Boldrin M, Gavasso S, et al. Effects of long-term administration of recombinant human protein c in xenografted primates. Transplantation. 2011;91:161–168. doi: 10.1097/TP.0b013e318200ba0e. [DOI] [PubMed] [Google Scholar]
- 40.Lau CL, Cantu E, III, Gonzalez-Stawinski GV, et al. The role of antibodies and von Willebrand factor in discordant pulmonary xenotransplantation. Am J Transplant. 2003;3:1065–1075. doi: 10.1034/j.1600-6143.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 41.Gaca JG, Lesher A, Aksoy O, Ruggeri ZM, Parker W, Davis RD. The role of the porcine von Willebrand factor: baboon platelet interactions in pulmonary xenotransplantation. Transplantation. 2002;74:1596–1603. doi: 10.1097/00007890-200212150-00018. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Wei Q, Wang XM, Wang WY, Xiong YL, Chen S. TMVA, a novel GPIb-binding protein, significantly prevents platelet microthrombi formation and prolongs discordant cardiac xenograft survival. Xenotransplantation. 2004;11:203–209. doi: 10.1111/j.1399-3089.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim HK, Kim JE, Wi HC, et al. Aurintricarboxylic acid inhibits endothelial activation, complement activation, and von Willebrand factor secretion in vitro and attenuates hyperacute rejection in an ex vivo model of pig-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15:246–256. doi: 10.1111/j.1399-3089.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 44.Alwayn IP, Appel JZ, Goepfert C, Buhler L, Cooper DK, Robson SC. Inhibition of platelet aggregation in baboons: therapeutic implications for xenotransplantation. Xenotransplantation. 2000;7:247–257. doi: 10.1034/j.1399-3089.2000.00965.x. [DOI] [PubMed] [Google Scholar]
- 45.Alwayn IP, Buhler L, Appel JZ, III, et al. Mechanisms of thrombotic microangiopathy following xenogeneic hematopoietic progenitor cell transplantation. Transplantation. 2001;71:1601–1609. doi: 10.1097/00007890-200106150-00020. [DOI] [PubMed] [Google Scholar]
- 46.Candinas D, Lesnikoski BA, Hancock WW, et al. Inhibition of platelet integrin GPIIbIIIa prolongs survival of discordant cardiac xenografts. Transplantation. 1996;62:1–5. doi: 10.1097/00007890-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 47.Brandl U, Jöckle H, Erhardt M, et al. Reduced fibrin deposition and intravascular thrombosis in hDAF transgenic pig hearts perfused with tirofiban. Transplantation. 2007;84:1667–1676. doi: 10.1097/01.tp.0000295742.45413.dc. [DOI] [PubMed] [Google Scholar]
- 48.Pfeiffer S, Zorn GL, III, Zhang JP, et al. Hyperacute lung rejection in the pig-to-human model. III. Platelet receptor inhibitors synergistically modulate complement activation and lung injury. Transplantation. 2003;75:953–959. doi: 10.1097/01.TP.0000058517.07194.90. [DOI] [PubMed] [Google Scholar]
- 49.Enjyoji K, Sévigny J, Lin Y, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 50.Imai M, Takigami K, Guckelberger O, et al. Modulation of nucleoside [correction of nucleotide] triphosphate diphosphohydrolase-1 (NTPDase-1)cd39 in xenograft rejection. Mol Med. 1999;5:743–752. [PMC free article] [PubMed] [Google Scholar]
- 51.Imai M, Takigami K, Guckelberger O, et al. Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplantation. 2000;70:864–870. doi: 10.1097/00007890-200009270-00003. [DOI] [PubMed] [Google Scholar]
- 52.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buergler JM, Maliszewski CR, Broekman MJ, et al. Effects of SolCD39, a novel inhibitor of platelet aggregation, on platelet deposition and aggregation after PTCA in a porcine model. J Thromb Thrombolysis. 2005;19:115–122. doi: 10.1007/s11239-005-1381-y. [DOI] [PubMed] [Google Scholar]
- 54.Banz Y, Beldi G, Wu Y, et al. CD39 is incorporated into plasma microparticles where it maintains functional properties and impacts endothelial activation. Br J Haematol. 2008;142:627–637. doi: 10.1111/j.1365-2141.2008.07230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berger JS. Platelet-directed therapies and coronary artery bypass grafting. Am J Cardiol. 2009;104(5) Suppl:44C–48C. doi: 10.1016/j.amjcard.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Byrne GW, Davies WR, Oi K, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006;82:1787–1791. doi: 10.1097/01.tp.0000251387.40499.0f. [DOI] [PubMed] [Google Scholar]
- 57.Ma TK, Lam YY, Tan VP, Kiernan TJ, Yan BP. Impact of genetic and acquired alteration in cytochrome P450 system on pharmacologic and clinical response to clopidogrel. Pharmacol Ther. 2010;125:249–259. doi: 10.1016/j.pharmthera.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Evanchan J, Donnally MR, Binkley P, Mazzaferri E. Recurrence of acute myocardial infarction in patients discharged on clopidogrel and a proton pump inhibitor after stent placement for acute myocardial infarction. Clin Cardiol. 2010;33:168–171. doi: 10.1002/clc.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGregor CG, Davies WR, Oi K, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130:844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 60.Lehle K, Schreml S, Kunz-Schughart LA, et al. mTOR inhibitors and calcineurin inhibitors do not affect adhesion molecule expression of human macro- and microvascular endothelial cells. J Vasc Res. 2008;45:333–342. doi: 10.1159/000119199. [DOI] [PubMed] [Google Scholar]
- 61.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dor FJ, Kuwaki K, Tseng YL, et al. Potential of aspirin to inhibit thrombotic microangiopathy in alpha1,3-galactosyltransferase gene-knockout pig hearts after transplantation in baboons. Transplant Proc. 2005;37:489–490. doi: 10.1016/j.transproceed.2004.12.235. [DOI] [PubMed] [Google Scholar]
- 63.Oh JY, Kim MK, Lee HJ, et al. Complement depletion with cobra venom factor delays acute cell-mediated rejection in pig-to-mouse corneal xenotransplantation. Xenotransplantation. 2010;17:140–146. doi: 10.1111/j.1399-3089.2010.00574.x. [DOI] [PubMed] [Google Scholar]
- 64.Candinas D, Lesnikoski BA, Robson SC, et al. Effect of repetitive high-dose treatment with soluble complement receptor type 1 and cobra venom factor on discordant xenograft survival. Transplantation. 1996;62:336–342. doi: 10.1097/00007890-199608150-00006. [DOI] [PubMed] [Google Scholar]
- 65.Wu W, Wang HD, Zhu XX, Lan G, Yang K. Prolonged cardiac allograft survival in mouse model after complement depletion with Yunnan cobra venom factor. Transplant Proc. 2009;41:4321–4327. doi: 10.1016/j.transproceed.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 66.Choi I, Kim SD, Cho B, et al. Xenogeneic interaction between human CD40L and porcine CD40 activates porcine endothelial cells through NF-kappaB signaling. Mol Immunol. 2008;45:575–580. doi: 10.1016/j.molimm.2007.06.161. [DOI] [PubMed] [Google Scholar]
- 67.Appel JZ, III, Alwayn IP, Buhler L, DeAngelis HA, Robson SC, Cooper DK. Modulation of platelet aggregation in baboons: implications for mixed chimerism in xenotransplantation. I. The roles of individual components of a transplantation conditioning regimen and of pig peripheral blood progenitor cells. Transplantation. 2001;72:1299–1305. doi: 10.1097/00007890-200110150-00020. [DOI] [PubMed] [Google Scholar]
- 68.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robson SC, Schulte am Esch J, II, Bach FH. Factors in xenograft rejection. Ann N Y Acad Sci. 1999;875:261–276. doi: 10.1111/j.1749-6632.1999.tb08509.x. [DOI] [PubMed] [Google Scholar]
- 70.Sachs DH, Sykes M, Robson SC, Cooper DK. Xenotransplantation. Adv Immunol. 2001;79:129–223. doi: 10.1016/s0065-2776(01)79004-9. [DOI] [PubMed] [Google Scholar]
- 71.Dwyer KM, Robson SC, Nandurkar HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crikis S, Lu B, Murray-Segal LM, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10:2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen D, Riesbeck K, McVey JH, et al. Regulated inhibition of coagulation by porcine endothelial cells expressing P-selectin-tagged hirudin and tissue factor pathway inhibitor fusion proteins. Transplantation. 1999;68:832–839. doi: 10.1097/00007890-199909270-00016. [DOI] [PubMed] [Google Scholar]
- 74.Chen D, Weber M, McVey JH, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004;4:1958–1963. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 75.Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]