Abstract
Context
Previous studies have examined the associations of individual clinical risk factors with risk of peripheral artery disease (PAD), but the combined effects of these risk factors are largely unknown.
Objective
To estimate the degree to which four conventional cardiovascular risk factors, smoking, hypertension, hypercholesterolemia and type 2 diabetes, are associated with the risk of PAD among men.
Design, settings and participants
We prospectively followed 44,985 men from the Health Professionals Follow-up Study without a history of cardiovascular disease at baseline for 25 years (1986-2011). The presence of risk factors was updated biennially during follow-up.
Main outcome measure
Clinically significant PAD (defined as limb amputation/revascularization, angiogram reporting vascular obstruction of ≥50%, ankle-brachial index<0.90 or physician-diagnosed PAD).
Results
During a median follow-up of 24.2 years (interquartile range 20.8-24.7 years), 537 PAD cases occurred. Each risk factor was significantly and independently associated with a higher risk of PAD after adjustment for the other three risk factors and confounders. The age-adjusted incidence rates per 100,000 person years were 6 cases for 0 risk factors, 18 cases for 1 risk factor, 39 cases for 2 risk factors, 76 cases for 3 risk factors and 139 cases for 4 risk factors. The multivariable-adjusted hazard ratio (HR) for each additional risk factor compared was 2.06 (95% confidence interval [95% CI], 1.92-2.32). Men without any of the four risk factors had a HR of PAD of 0.23 (95% CI, 0.14-0.36) compared with all other men in the cohort. In 96% (95% CI, 94-98%) of PAD cases, at least one of the four risk factors was present at the time of PAD diagnosis. The population-attributable risk associated with these four risk factors was 75% (95% CI, 64-87%). The incidence of PAD among men with all four risk factors was 1.4/1,000.
Conclusion
Among men in this cohort, smoking, hypertension, hypercholesterolemia and type 2 diabetes account for most of the risk associated with development of clinically significant PAD.
Peripheral artery disease (PAD) is a distinct atherosclerotic syndrome marked by stenosis or occlusion of the arteries, particularly of the lower extremities. PAD affects 8-10 million Americans,1,2 and is associated with reduced functional capacity3,4 and increased risk for cardiovascular morbidity and mortality.5-7 Despite its widespread prevalence and negative associations with quality of life, morbidity, and mortality, PAD remains underdiagnosed and undertreated.2,8,9
Preventable or treatable risk factors for PAD are generally thought to mirror other forms of cardiovascular disease and include cigarette smoking, diabetes, and clinically elevated levels of blood pressure and cholesterol, which are the main therapeutic targets in clinical and prevention guidelines.10,11 However, their respective associations with risk of PAD and the extent to which they are jointly associated with the incidence of PAD are not well established. Furthermore, despite the ongoing identification of novel risk factors for PAD,12,13 PAD may have a less prominent component of thrombosis than does ischemic stroke or myocardial infarction (MI), raising the possibility that traditional atherosclerotic risk factors may be even more important in this form of cardiovascular disease.
To estimate the individual and cumulative associations of four conventional and preventable risk factors with risk of PAD and their associated population-attributable risk(PAR), we studied a large, well-characterized, prospective sample of American men with more than two decades of follow-up.
METHODS
Participants
The Health Professionals Follow-up Study (HPFS) is a prospective investigation of 51,529 U.S. male health professionals aged 40 to 75 years at baseline in 1986 who returned a mailed questionnaire about disease history, including an item that specifically queried intermittent claudication, and lifestyle factors.14 Questionnaires were mailed biennially to update information on newly diagnosed disease and potential risk factors. We excluded men with missing data on age, height or family history, or with diagnosed cardiovascular disease (MI, stroke, coronary artery bypass grafting, coronary angioplasty, and intermittent claudication) at baseline. All participants gave written informed consent, and the Harvard School of Public Health Human Subjects Committee Review Board approved the study protocol.
Assessment of Medical History, Anthropometric Data and Lifestyle Factors
Smoking status and self-report of physician-diagnosed hypertension, hypercholesterolemia, and type 2 diabetes were assessed at baseline and updated biennially thereafter. At baseline, participants also reported past smoking, time since quitting, and the average number of cigarettes smoked per day. Pack-years were calculated as years of smoking multiplied by the average number of packs smoked per day and updated biennially. The validity of self-reported hypertension has been confirmed with medical record review in a sample of HPFS participants.15 Likewise, self-reported total cholesterol levels have been shown to highly correlate with measured values.16 Self-reported type 2 diabetes has been confirmed by a validated supplementary questionnaire detailing symptoms, diagnostic laboratory test results, and diabetes treatment as described previously17 and has been demonstrated to be highly accurate compared with medical record reviews in a validation study.18 Duration of hypertension, hypercholesterolemia and diabetes were calculated as years from clinical diagnosis to the return of the most recent questionnaire. Weight, physical activity and medication use were assessed biennially and alcohol every four years, using validated questionnaires.19-22 Height, geographical region of residence, and parental history of MI at age 60 years or younger were obtained from the baseline questionnaire. Body mass index (BMI) was calculated using height at baseline and updated weight.
Case Ascertainment
If a participant reported PAD of the lower extremity during follow-up, we requested permission to review all relevant medical records to confirm the diagnosis and the date of occurrence. Confirmed PAD cases required at least one of the following in the medical record: a report of revascularization (or amputation) for atherosclerotic peripheral artery occlusion in a lower limb, angiogram reporting obstruction ≥50% of at least one limb artery with ipsilateral symptoms, ankle-brachial index of <0.90 at rest, or a physician’s diagnosis of PAD.
Data Analyses
Each individual contributed person-time from the return of the 1986 questionnaire to the date of diagnosis of PAD, death, date of last questionnaire return, or end of follow-up (January 2011), whichever came first. We used Cox proportional hazards models with time-dependent covariates to calculate hazard ratios (HR) and 95% confidence intervals (CI). The assumption of proportional hazards was checked and met for each Cox model. Once a participant reported a positive history of ever smoking (past or current smoking), hypertension, hypercholesterolemia or diabetes, he was considered to have a positive history throughout follow-up. Analyses that defined hypertension and hypercholesterolemia based on either physician’s diagnosis or medication use (thiazides, β-blockers, calcium channel blockers, and angiotensin-converting enzyme/angiotensin II receptor antagonists for hypertension and statins and other cholesterol lowering agents for hypercholesterolemia) yielded similar results. We used ever rather than current smoking to ensure that the four risk factors were broadly comparable in terms of increased risk. This enabled us to sum the total number of risk factors to create a score (range, 0-4). We also developed a weighted risk score to account for the magnitude of risk observed with each risk factor.
We explored whether there were statistical interactions between the four binary risk factors by comparing the −2 log likelihood of a nested model with and without all possible two-, three- and four-way interaction terms. We found no overall evidence of interaction (χ211=11.0, P=0.45) and none of the individual interactions were statistically significant.
We examined the associations between individual risk factors and risk of PAD in several ways. We evaluated the risk of former smoking using four categories (quit for ≥20, 10-<20, 5-<10 or <5 years) and intensity of current smoking using three categories (1-14 cigarettes/day, 15-24 cigarettes/day, and ≥25 cigarettes/day). We investigated the dose-response relation between cumulative lifelong smoking exposure and incident PAD by evaluating pack-years of smoking (none, <10, 10-24, 25-44, 45-64 or ≥65 pack-years). Similarly, we modeled duration of diabetes, hypercholesterolemia, and hypertension in relation to PAD risk in three categories (0, ≤5, 6-15, or >15 years); we have previously shown a positive association of diabetes duration with PAD in this cohort.23 We also examined the number of antihypertensive medication classes used as marker of severity.
To determine the independent associations of these risk factors, we adjusted for age (continuous), height (quintiles), aspirin intake ≥2/week, parental history of MI before age 60 years, geographical region (West, Midwest, South and Northeast), BMI (<20, 20-22.9, 23-24.9, 25-29.9, and >30 kg/m2), physical activity (quintiles), and alcohol consumption (0, 0.1-4.9, 5.0-29.9, and ≥30.0 gram/day) in the multivariable models. If covariate data were missing at a given time point, the last observation was carried forward for that cycle. If data were still missing, contributions of person-time were skipped during follow-up for that specific period; participants with missing data on covariates at baseline could enter the cohort during follow-up if they had complete data on later questionnaires. We obtained similar results if we used missing indicators for covariates and in a single imputation model using the Markov Chain Monte Carlo method. We tested for linear trend in two ways: for all four factors simultaneously, we treated the number of risk factors as a continuous variable; for individual risk factors (e.g., for duration of hypertension), we used the median of each category as a continuous variable.
We calculated the multivariable-partial “population-attributable risk percent (PAR%)” with its 95% CI to integrate the prevalence and relative risk associated with each risk factor or set of risk factors.24 To calculate the PAR%, we estimated the prevalence of combinations of each risk factor and the relative risk from multivariable pooled logistic regression models25 using a counting process data structure.26In this approach, each 2-year interval for each participant was treated as an independent observation, and all observations were pooled into a single sample. Since smoking (both current and former) is a strong risk factor for PAD, we also assessed the impact of the other three risk factors among never smokers.
Statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc, Cary, North Carolina). All P values are 2-sided and a P value of less than .05 is considered statistically significant.
RESULTS
We excluded 3817 men with diagnosed CVD at baseline and 2727 men with missing data on confounders at baseline who did not provide this information during follow-up, leaving 44,985 participants for the analyses. During a median (interquartile range) of 24.2 (20.8-24.7) years (961,333 person-years) of follow-up, we documented 537 cases of incident PAD. Table 1 shows the baseline characteristics of the cohort according to the number of clinical risk factors. Men with more risk factors were older, had a higher BMI, exercised less frequently, and were more likely to report aspirin use and a family history of MI.
Table 1.
Characteristics of men in the Health Professional Follow-up Study according to number of clinical risk factors in 1986.*
|
No. of clinical risk factors
|
|||||
|---|---|---|---|---|---|
| 0 (n = 15,293) |
1 (n = 20,047) |
2 (n = 6571) |
3 (n = 1245) |
4 (n = 87) |
|
| Age, y | 51.6 (9.4) | 54.3 (9.5) | 57.6 (9.3) | 59.2 (8.6) | 59.3 (7.9) |
| Ever smoker, No. (%) | 0 (0) | 15,566 (77.6) | 5764 (87.7) | 1202 (96.5) | 87 (100) |
| Type 2 diabetes, No. (%) | 0 (0) | 219 (1.1) | 501 (7.6) | 320 (25.7) | 87 (100) |
| Hypertension, No. (%) | 0 (0) | 2912 (14.5) | 4679 (71.2) | 1159 (96.8) | 87 (100) |
| Hypercholesterolemia, No. (%) | 0 (0) | 1351 (6.7) | 2198 (35.5) | 1018 (81.8) | 87 (100) |
| Body mass index, kg/m2a | 25.0 (3.1) | 25.5 (3.2) | 26.2 (3.6) | 26.7 (4.3) | 27.5 (4.1) |
| Height, in | 70.2 (2.8) | 70.2 (2.7) | 70.0 (2.8) | 69.8 (2.9) | 70.0 (2.4) |
| Physical activity, METs/wk (IQR) | 13.4 (4.4-30.8) | 11.3 (3.8-27.1) | 10.2 (3.3-24.3 | 8.4 (1.1-21.0) | 7.8 (1.9-17.9) |
| Aspirin use >2 time/wk, No. (%) | 3504 (22.9) | 5812 (29.0) | 2259 (34.4) | 463 (37.2) | 42 (48.3) |
| Parental history of myocardial infarction at age 60 years or younger, No. (%) |
1685 (11.0) | 2375 (11.8) | 917 (14.0) | 187 (15.0) | 14 (16.1) |
| Alcohol consumption, g/d (IQR) | 3.1 (0-11.1) | 7.4 (1.1-16.8) | 8.1 (1.1-19.7) | 8.4 (1.1-21.0) | 3.9 (0-17.3) |
Number of men in table (N=43,243) differs from total number of men in the analysis (N=45,985). The biannual follow-up in this study allowed a staggered entry of participants with missing data in 1986 that was acquired later during follow-up. Abbreviations: IQR, interquartile range; MET, metabolic equivalent;
Calculated as weight in kilograms divided by height in meters squared.
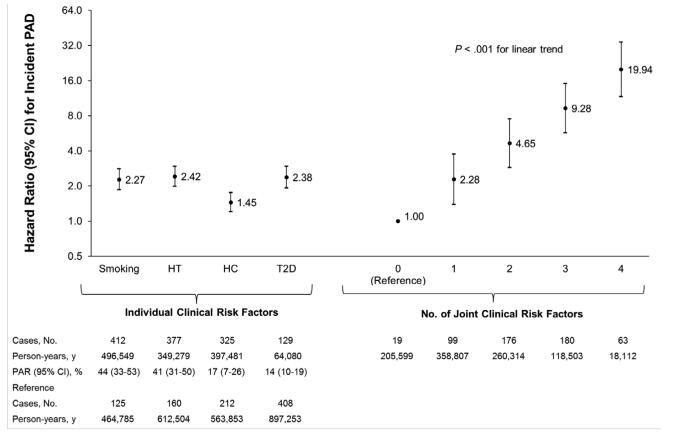
Ever smoking or having a history of hypertension, hypercholesterolemia or type 2 diabetes were all independently and significantly associated with a higher risk of PAD after multivariable adjustment (Figure 1). The differences in age-adjusted incidence of PAD per 100,000 person-years were 30 cases for ever smoking, 37 cases for hypertension, 18 cases for hypercholesterolemia, and 52 cases for diabetes. The age-adjusted incidence of PAD per 100,000 person years was 6 cases among men with 0 risk factors, 18 cases among men with 1 risk factor, 39 cases among men with 2 risk factors, 76 cases among men with 3 risk factors and 139 cases among men with 4 risk factors. Thus, the incidence of PAD was 1.4/1,000 among participants with all four risk factors. The multivariable-adjusted HR for each additional risk factor was 2.06 (95% CI, 1.88-2.26) compared with the group free of risk factors (P<.001 for linear trend). Similar results were obtained when men aged 80 years or older were censored to minimize the effects of extreme age.
Figure 1.
Hazard ratios (HRs) for incident peripheral artery disease (PAD) according to individual and joint clinical risk factors.
HRs are adjusted for age, height, aspirin use, parental history of myocardial infarction at age 60 years or younger, geographical region, body mass index, physical activity, alcohol consumption and each of the other three clinical risk factors. The reference group for each of the individual risk factors was the remainder of the cohort without the individual risk factor. Population attributable risks percent (PAR%) for each individual risk factor were calculated using pooled logistic regression models and were adjusted for the same factors mentioned above. The linear trend for the joint risk factors was obtained by treating the number of risk factors as a continuous variable.
Abbreviations: HT, Hypertension; HC, hypercholesterolemia; T2D, type 2 diabetes.
The four risk factors were not statistically identical (P<.001). Therefore, we alternatively created a weighted score that subtracted 1 from each multivariable-adjusted HR in Figure 1 and summed the values. When this weighted score was transformed to the original four-point scale and analyzed as a continuous variable, the multivariable-adjusted HR associated with a 1 point increase in weighted score was 2.07 (95% CI, 1.89-2.26).
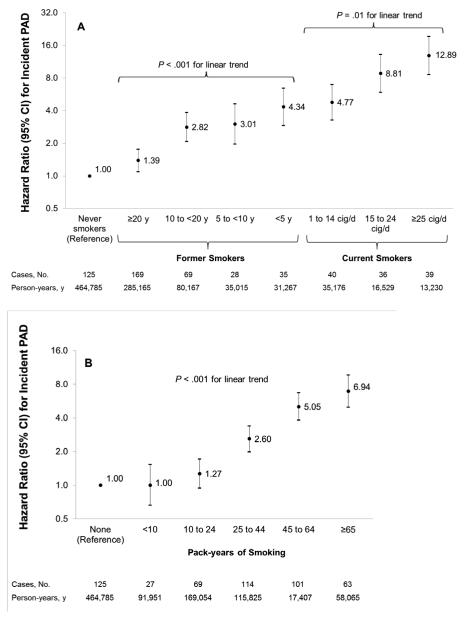
Risk of PAD was strongly and dose-dependently associated with current smoking when compared with men who never smoked, with a multivariable-adjusted HR of 12.89 (95% CI, 8.59-19.34) among the heaviest smokers compared with men who never smoked (Figure 2A). Duration of smoking cessation was also strongly associated with risk of PAD (P<.001 for linear trend), with progressively lower risk with greater duration since cessation. Even men who quit smoking more than 20 years in the past had a significantly higher risk compared with men who never smoked (HR, 1.39; 95% CI, 1.10-1.76). Compared with all former smokers, the HR for incident PAD among all current smokers was 3.81 (95% CI, 3.00-4.84). There was a strong dose-response relationship between pack-years of smoking and risk of PAD (P<.001 for linear trend) (Figure 2B).
Figure 2.
Hazard ratios (HRs) for incident peripheral artery disease (PAD) according to smoking status (A) and pack-years of smoking (B).
HRs are adjusted for age, height, positive history of type 2 diabetes, hypertension and hypercholesterolemia, aspirin use, parental history of myocardial infarction at age 60 years or younger, geographical region, body mass index, physical activity and alcohol consumption. Data on pack-years of smoking was missing for 6.0% of person-years during follow-up.
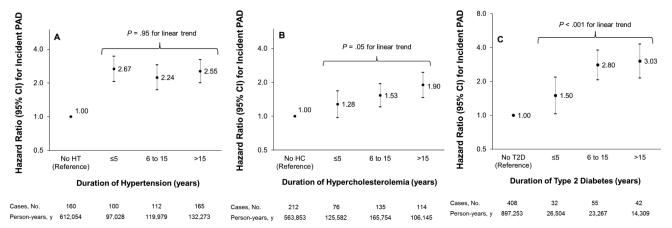
Figure 3 displays the association between duration of hypertension (A), hypercholesterolemia (B) and diabetes (C), and risk of PAD. Regardless of duration category, all men with a risk factor had higher risks of developing PAD compared to men without the risk factors. Risk of PAD tended to increase with duration of both diabetes (P<.001 for linear trend) and hypercholesterolemia (P=0.05 for linear trend), although duration of hypertension did not clearly modify risk. Among men with a positive history of hypertension, risk of PAD was higher among men who reported use of one (HR, 1.40, 95%; CI, 1.09-1.78) or two or more antihypertensive drugs (HR, 2.07; 95% CI, 1.455-2.78) compared to hypertensive men who did not report current use of antihypertensive drugs.
Figure 3.
Hazard ratios (HRs) for incident peripheral artery disease (PAD) according to duration of hypertension (A), hypercholesterolemia (B) and diabetes (C).
HRs are adjusted for age, height, smoking status, history of diabetes, hypertension and hypercholesterolemia, aspirin use, parental history of myocardial infarction at age 60 years or younger, geographical region, body mass index, physical activity and alcohol consumption. Linear trends are based only on participants with the disease and are tested by using the median of each duration category as a continuous variable.
Abbreviations: HT, Hypertension; HC, hypercholesterolemia; T2D, type 2 diabetes.
Table 2 provides the PAR%s associated with combinations of two, three or all four clinical risk factors. The PAR% of all four risk factors together was 75% (95% CI, 61%-84%). The PAR% increased slightly (PAR%, 78%; 95% CI, 66%-86%) when we did not adjust for potentially modifiable variables (i.e. BMI, physical activity level, aspirin use and alcohol consumption). Men who did not have any of the four risk factors had a HR of 0.23 (95% CI, 0.14-0.36) as compared with all other men in the cohort. Finally, the proportion of cases with at least one risk factor at the time of PAD diagnosis was 96% (95% CI, 94-98%).
Table 2.
Hazard ratio (HR) and population attributable risk percent (PAR%) of peripheral artery disease (PAD) by combinations of clinical risk factors.a
| Combinations of clinical risk factors exposed to |
Exposed group |
Unexposed group |
HR (95% CI)b | PAR% (95% CI), %c |
||
|---|---|---|---|---|---|---|
| Person-years No. (%) |
PAD cases No. (%) |
Person-years, No. (%) |
PAD cases, No. (%) |
|||
| Combinations of 2 risk factors: | ||||||
| Hypercholesterolemia, diabetes | 421,803 (44) | 361 (67) | 539,530 (56) | 176 (33) | 2.08 (1.71-2.52) | 28 (18-37) |
| Hypertension, hypercholesterolemia | 550,313 (57) | 449 (84) | 411,020 (43) | 88 (16) | 2.61 (2.05-3.32) | 48 (37-58) |
| Hypertension ,diabetes | 370,415 (39) | 403 (75) | 590,918 (61) | 134 (25) | 3.28 (2.67-4.03) | 50 (41-58) |
| Smoking, diabetes | 522,607 (54) | 441 (82) | 438,729 (46) | 96 (18) | 2.91 (2.32-3.65) | 53 (42-61) |
| Smoking, hypercholesterolemia | 680,275 (71) | 490 (91) | 281,058 (29) | 47 (9) | 3.00 (2.21-4.07) | 57 (44-68) |
| Smoking, hypertension | 652,259 (68) | 491 (91) | 309,074 (32) | 46 (9) | 3.29 (2.42-4.48) | 60 (47-71) |
| Combinations of 3 risk factors: | ||||||
| Hypertension, hypercholesterolemia, diabetes |
561,786 (58) | 461 (86) | 399,547 (42) | 76 (14) | 2.92 (2.26-3.76) | 56 (46-64) |
| Smoking, hypercholesterolemia, diabetes |
690,551 (72) | 498 (93) | 270,782 (28) | 39 (7) | 3.40 (2.44-4.73 | 63 (50-73) |
| Smoking, hypertension, hypercholesterolemia |
750,808 (78) | 513 (96) | 210,524 (22) | 24 (4) | 3.59 (2.37-5.44) | 67 (52-78) |
| Smoking, hypertension, diabetes | 660,985 (69) | 501 (93) | 300,348 (31) | 36 (7) | 4.08 (2.89-5.76) | 70 (58-78) |
| Combinations of all 4 risk factors: | ||||||
| Smoking, hypertension, hypercholesterolemia, diabetes |
755,734 (79) | 518 (96) | 205,599 (21) | 19 (4) | 4.37 (2.75-6.94) | 75 (64-87) |
There were 961,333 person-years in the cohort and 537 cases of PAD.
Hazard ratio (HR) compares individuals exposed to a specified combination of clinical risk factors to all unexposed individuals (reference group) in this population. HRs (95% CIs) were estimated from Cox proportional hazard models adjusted for age, height, regular aspirin use, parental history of myocardial infarction at age 60 years or younger, geographical region, body mass index, physical activity, and alcohol consumption, and each of the other clinical risk factor not included in the exposed group.
The population attributable risk percent (PAR%) was calculated using pooled logistic regression models and were adjusted for age, time period, height, regular aspirin use, parental history of myocardial infarction at age 60 years or younger, geographical region, body mass index, physical activity and alcohol consumption.
Given the strong relationship between both current and past smoking and risk of PAD, we explored the association of the remaining three clinical risk factors separately among never smokers. Among never smokers, the adjusted HRs for PAD were 2.55 (95% CI, 1.60-4.06) for diabetes, 1.84 (95% CI, 1.23-2.76) for hypertension, and 1.80 (95% CI, 1.20-2.72) for hypercholesterolemia. Never smokers with no other risk factors (44.6% of all never smokers) had a relative risk of 0.37 (95% CI, 0.23-0.62) compared to all other never smokers in this cohort. Among never smokers, the PAR% associated with the three remaining clinical risk factors was 53% (95% CI, 29%-71%).
COMMENT
In this large, prospective cohort study of men, the combination of four clinical risk factors - smoking, hypertension, hypercholesterolemia and diabetes - was strongly and independently associated with risk of confirmed and clinically significant PAD. Duration of diabetes and hypercholesterolemia, severity of hypertension, and cumulative intensity of smoking all demonstrated graded relationships with risk. Participants without these four traditional risk factors had 77% lower risk of PAD compared with all other men.
The overall incidence of PAD was extremely low in this cohort (537 of 44,985 particiapnts, or an incidence of 1%). This low incidence of PAD is likely related to the definition of a new PAD diagnosis. Most people with PAD will never require lower extremity revascularization or even undergo angiography. In addition, previous study shows that PAD is frequently underdiagnosed.8 Thus, our findings regarding risk factors for PAD are generalizable to people with clinically significant PAD diagnosed in a medical practice setting.
In addition, the absolute risk of our PAD outcome was low. Among individuals with one risk factor, the absolute risk of PAD was 0.18 per 1,000. Even among individuals with all four risk factors, the incidence of PAD was 1.4 per 1,000, likely because of the definition used for the PAD outcome.
All of the four risk factors appeared to confer increased risk within short periods following recognition and, in the case of smoking, associated risk remained elevated even 20 years after cessation. At the same time, the joint effect of multiple risk factors combined was independent and graded, with no evidence that these risk factors became less important among those already at high risk. This observation highlights the need for aggressive risk factor modification as the number of risk factors increases, consistent with ATP III27 and JNC 728 efforts to set more aggressive targets in higher-risk individuals. Intense risk factor modification in the form of correcting blood pressure29, optimizing diabetic control30 and normalizing LDL-cholesterol levels31 has been shown to reduce the risk of morbidity or mortality among high-risk PAD patients. Nevertheless, lowering LDL-cholesterol, for instance, does not necessarily improve functional capacity, such as calf muscle perfusion or exercise performance among PAD patients, and impairment of quality of life associated with the disease remains.32 Unfortunately, millions of US adults with prevalent but undiagnosed PAD do not receive secondary prevention therapies.2
Taken together, these four risk factors had PAR% of 75% for incident PAD, roughly comparable to the PAR seen for other cardiovascular diseases,33,34 and cardiovascular and noncardiovascular mortality.35 Despite this similarity, there are distinct and important differences between their relationships with PAD and other forms of cardiovascular disease. For example, at least one of these risk factors is present in some 80% of patients with coronary heart disease (CHD)34 compared with over 95% of cases of PAD.
Similarly, active smoking is 2 to 3 times more strongly associated with PAD than with CHD36 and elevated risk among former smokers does not appear to return to baseline, in contrast to our findings on both CHD37 and stroke.38 Although risk remained elevated among former smokers, it was nearly three-fold higher among current smokers, emphasizing that smoking cessation is never too late, as it lowers the risk of cardiovascular disease morbidity and mortality even among individuals with diagnosed PAD.39
Our findings also highlight the importance of hypertension as a major risk factor for PAD, with a PAR% of more than 40%. Among men with hypertension, the number of different antihypertensive medications used, as a proxy for hypertension severity, was also associated with risk of PAD. We have previously reported the importance of healthy lifestyle practices in prevention of CHD among men with hypertension40, and our current findings support the need for lifestyle measures to prevent hypertension and reduce its severity where possible.
The four risk factors were not of equal magnitude in their association with PAD risk. We found a somewhat lower increase in risk of PAD associated with hypercholesterolemia compared with the other three risk factors. This may reflect the importance of effective treatment for hypercholesterolemia. While treatment for hypertension does not appear to fully reduce its associated risk for CHD,41 statin therapy treats hypercholesterolemia extremely effectively, essentially fully eliminating its associated risk. It is also possible that hypercholesterolemia is simply less important in the arterial beds that characterize PAD, much as it seems to be less important for stroke than for CHD.42
The strengths of our study include its prospective design, large sample size, long-term and updated follow-up, validated questionnaires to assess large numbers of potential confounders, homogeneity in gender and professions, and many clinically confirmed endpoints. Nevertheless, several potential limitations warrant clarification. First, we used symptomatic PAD as our outcome. Subclinical or asymptomatic PAD, which may have otherwise been detected by abnormal pulse examination or ankle-brachial index, may have been missed, much as individuals with asymptomatic carotid stenosis or silent stroke are missed in studies of clinical stroke. If so, our results represent only the most severe manifestations of a much more common problem, although these risk factors also appear to increase risk of subclinical PAD.43 Moreover, endpoints included in this analysis were confirmed by medical records, reducing the likelihood of false positive cases albeit at the risk of a falsely low incidence rate. Perhaps most importantly, this definition – similar to that used in most large cohort studies – captures clinically meaningful cases and a level of disease severity that is of unequivocal importance to both patients and physicians.
We used self-reported measures of smoking, and self-report of physician-diagnosed diabetes, hypertension and hypercholesterolemia. While generally valid and clearly associated with risk, these self-reported measures may underestimate the risk if nondifferential misclassification is present. We also evaluated duration of risk factors but not detailed measures of severity, which may be important for the relationships of blood pressure44 and diabetes30 with risk of PAD. Nevertheless, the simplicity of binary cut points of clinically elevated levels may help provide discrete guidance for patients in the clinical setting.
The cohort included mainly Caucasian men, and our findings may not be generalizable to other groups, although we have no reason to suspect that standard risk factors are any less important in other populations. Finally, as with any observational study, unmeasured or residual confounding may be present, despite the substantial number of potentially confounding factors for which we adjusted. For example, these risk factors co-occur with elevations in inflammatory and hemostatic biomarkers, although they appear to represent independent statistical factors.45
In conclusion, in this well-characterized cohort of American men followed for over two decades, smoking, hypertension, hypercholesterolemia and diabetes each demonstrated strong, graded, and independent associations with risk of clinically significant PAD.
Acknowledgement
The authors thank Lydia Liu, MSc of the Department of Nutrition of the Harvard School of Public Health, for statistical and programming assistance.
Funding/Support: This work was supported by grants R01 HL091874, HL35464 and CA55075 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the Sponsor: The funding source had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Joosten had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosure: None of the authors reported disclosures.
References
- 1.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. doi: 10.1161/CIRCULATIONAHA.110.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Guralnik JM, Tian L, et al. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study) J Am Coll Cardiol. 2009;53:1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 7.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral Arterial Disease Detection, Awareness, and Treatment in Primary Care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 9.Cacoub PP, Abola MT, Baumgartner I, et al. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Atherosclerosis. 2009;204:e86–92. doi: 10.1016/j.atherosclerosis.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic) Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 11.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Journal of Vascular Surgery. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Ho DY, Cook NR, Britton KA, et al. High-Molecular-Weight and Total Adiponectin Levels and Incident Symptomatic Peripheral Artery Disease in Women / Clinical Perspective. Circulation. 2011;124:2303–2311. doi: 10.1161/CIRCULATIONAHA.111.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlstein TS, Pande RL, Beckman JA, Creager MA. Serum Total Bilirubin Level and Prevalent Lower-Extremity Peripheral Arterial Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:166–172. doi: 10.1161/ATVBAHA.107.153262. [DOI] [PubMed] [Google Scholar]
- 14.Colditz GA, Giovannucci E, Rimm EB, et al. Alcohol intake in relation to diet and obesity in women and men. Am J Clin Nutr. 1991;54:49–55. doi: 10.1093/ajcn/54.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Ascherio A, Rimm E, Giovannucci E, et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86:1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 16.Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology. 2007;69:1688–1695. doi: 10.1212/01.wnl.0000271883.45010.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joosten MM, Chiuve SE, Mukamal KJ, Hu FB, Hendriks HFJ, Rimm EB. Changes in Alcohol Consumption and Subsequent Risk of Type 2 Diabetes in Men. Diabetes. 2011;60:74–79. doi: 10.2337/db10-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical Activity and Television Watching in Relation to Risk for Type 2 Diabetes Mellitus in Men. Arch Intern Med. 2001;161:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 23.Al-Delaimy WK, Merchant AT, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. Am J Med. 2004;116:236–240. doi: 10.1016/j.amjmed.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18:571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9:1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 26.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer-Verlag; New York, New York: 2000. [Google Scholar]
- 27.Expert Panel on Detection E, Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.Mehler PS, Coll JR, Estacio R, Esler A, Schrier RW, Hiatt WR. Intensive Blood Pressure Control Reduces the Risk of Cardiovascular Events in Patients With Peripheral Arterial Disease and Type 2 Diabetes. Circulation. 2003;107:753–756. doi: 10.1161/01.cir.0000049640.46039.52. [DOI] [PubMed] [Google Scholar]
- 30.Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25:894–899. doi: 10.2337/diacare.25.5.894. [DOI] [PubMed] [Google Scholar]
- 31.Hussein AA, Uno K, Wolski K, et al. Peripheral Arterial Disease and Progression of Coronary Atherosclerosis. J Am Coll Cardiol. 2011;57:1220–1225. doi: 10.1016/j.jacc.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 32.West AM, Anderson JD, Epstein FH, et al. Low-density lipoprotein lowering does not improve calf muscle perfusion, energetics, or exercise performance in peripheral arterial disease. J Am Coll Cardiol. 2011;58:1068–1076. doi: 10.1016/j.jacc.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenland P, Knoll MD, Stamler J, et al. Major Risk Factors as Antecedents of Fatal and Nonfatal Coronary Heart Disease Events. JAMA. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 34.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of Conventional Risk Factors in Patients With Coronary Heart Disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 35.Stamler J, Stamler R, Neaton JD, et al. Low Risk-Factor Profile and Long-term Cardiovascular and Noncardiovascular Mortality and Life Expectancy. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 36.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344–353. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- 37.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking Cessation and Time Course of Decreased Risks of Coronary Heart Disease in Middle-Aged Women. Arch Intern Med. 1994;154:169–175. [PubMed] [Google Scholar]
- 38.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation and decreased risk of stroke in women. Jama. 1993;269:232–236. [PubMed] [Google Scholar]
- 39.Jonason T, Bergstrom R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221:253–260. [PubMed] [Google Scholar]
- 40.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy Lifestyle Factors in the Primary Prevention of Coronary Heart Disease Among Men. Circulation. 2006;114:160–167. doi: 10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]
- 41.Psaty BM, Furberg CD, Kuller LH, et al. Association Between Blood Pressure Level and the Risk of Myocardial Infarction, Stroke, and Total Mortality: The Cardiovascular Health Study. Arch Intern Med. 2001;161:1183–1192. doi: 10.1001/archinte.161.9.1183. [DOI] [PubMed] [Google Scholar]
- 42.Gentil A, Béjot Y, Lorgis L, et al. Comparative epidemiology of stroke and acute myocardial infarction: the Dijon Vascular project (Diva) Journal of Neurology, Neurosurgery & Psychiatry. 2009;80:1006–1011. doi: 10.1136/jnnp.2009.172551. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy M, Solomon C, Manolio TA, et al. Risk factors for declining ankle-brachial index in men and women 65 years or older: the Cardiovascular Health Study. Arch Intern Med. 2005;165:1896–1902. doi: 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 44.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33:13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 45.Sakkinen PA, Wahl P, Cushman M, Lewis MR, Tracy RP. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol. 2000;152:897–907. doi: 10.1093/aje/152.10.897. [DOI] [PubMed] [Google Scholar]