Abstract
Glioblastoma (GBM) is the most common and deadliest primary brain tumor in adults, with current treatments having limited impact on disease progression. Therefore the development of alternative treatment options is greatly needed. Gene therapy is a treatment strategy that relies on the delivery of genetic material, usually transgenes or viruses, into cells for therapeutic purposes, and has been applied to GBM with increasing promise. We have included selectively replication-competent oncolytic viruses within this strategy, although the virus acts directly as a complex biologic anti-tumor agent rather than as a classic gene delivery vehicle. GBM is a good candidate for gene therapy because tumors remain locally within the brain and only rarely metastasize to other tissues; the majority of cells in the brain are post-mitotic, which allows for specific targeting of dividing tumor cells; and tumors can often be accessed neurosurgically for administration of therapy. Delivery vehicles used for brain tumors include nonreplicating viral vectors, normal adult stem/progenitor cells, and oncolytic viruses. The therapeutic transgenes or viruses are typically cytotoxic or express prodrug activating suicide genes to kill glioma cells, immunostimulatory to induce or amplify anti-tumor immune responses, and/or modify the tumor microenvironment such as blocking angiogenesis. This review describes current preclinical and clinical gene therapy strategies for the treatment of glioma.
Treating malignant gliomas, of which glioblastoma (GBM) is the most common and least curable, remains a daunting challenge even after substantial efforts to develop alternative therapies. The current standard of care is maximal surgical resection followed by radiation and temozolomide chemotherapy; however, the median survival still remains less than 15 months.1,2 This poor survival is due to the aggressive and invasive nature of the tumor cells, resistance to treatment, and the challenges of delivering therapeutics into the brain.3 GBM is thought to be derived from a small population of glioblastoma stem cells (GSCs), so-called because of their stem cell-like properties of self-renewal and multilineage differentiation while being highly tumorigenic.4-6 GSCs have become an important model for studying GBM because their xenografts mimic the heterogeneous histopathology of the patient’s tumor from which they were derived7,8 and they remain genotypically similar to the patient’s tumor, in contrast to serum-cultured cell lines.9 These cells have provided insights into the origin of tumor-initiating cells and new targets for therapy. Other GBM animal models include established glioma cell lines (human and rodent) implanted intracranially into immunodeficient or immunocompetent animals, and genetically engineered mice that spontaneously develop brain tumors or are induced with viral vectors.10-12
Gene therapy for GBM is rapidly evolving, with the ultimate goal being specific delivery of therapeutic genes or oncolytic viruses to eliminate the tumor.13-17 This can result not only in tumor cell death, but also enhanced immune responses to tumor antigens and disruption of the tumor microenvironment, including inhibition of angiogenesis/neovascularization.18-20 GBM is a good candidate for gene therapy for several reasons: (1) tumors remain within the brain with only rare metastases to other tissues; (2) the majority of cells in the brain are post-mitotic, which allows for specific targeting of dividing tumor cells; (3) tumors are often accessible neurosurgically for vector administration and sophisticated imaging paradigms are available; and (4) standard therapies are minimally effective. A range of gene therapy strategies has been examined in GBM preclinical models and clinical trials. There are 2 basic questions when developing a gene therapy strategy; what gene(s) or sequence(s) to deliver and how to deliver them (vector and route of administration)? Current strategies include the use of nonreplicating viral vectors, selective replication-competent oncolytic viruses, or normal adult stem/progenitor cells for the delivery of cytotoxic genes, immunostimulatory genes, and genes modulating the tumor microenvironment. Here we discuss current preclinical and clinical gene therapy strategies for the treatment of GBM. Although this review focuses on GBM, because of its dire prognosis and the target for most clinical trials, it is important to recognize that there are many other brain tumors, both primary (ie, medulloblastoma) and metastatic from other organs,21 which are also targets for gene therapy.22-32 Similar strategies against other tumors are discussed in recent reviews.20,33-39
GENE DELIVERY VEHICLES FOR GLIOBLASTOMA
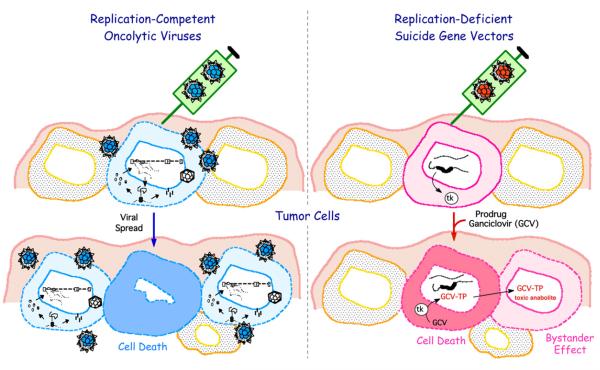
Most gene therapies for GBM use biologic vectors such as viruses or cells. Replication-defective or non-replicating virus vectors are generated by deleting genes important for viral replication or in some cases all open reading frames, to limit anti-vector immune responses and replacing them with therapeutic transgenes. The administered dose of a nonreplicating virus vector represents the maximum number of possible infected gene-transduced cells, although efficiency of delivery and infection is usually very low. Thus, any effects of the delivered and expressed gene must be amplified and affect nontransduced cells, so-called ‘bystander effects’ (Fig 1). Oncolytic viruses are selectively replication-competent in cancer cells and, thus, able to amplify themselves in situ in the tumor and continue to infect cancer cells well after initial administration39-41 (Fig 1). Cancer selectivity is often due to defects commonly found across many tumor types, such as lack of antiviral responses, activation of Ras pathways, loss of tumor suppressors, and defective apoptosis.42 In addition to their inherent cytotoxicity, oncolytic viruses can also be used as vectors for gene delivery.40,41,43,44 Several viruses (Newcastle disease virus [NDV], reovirus, and measles virus [MV]) have an inherent ability to specifically target cancer cells and upon virus replication, cause significant cell death and tumor regression. Other viruses (herpes simplex virus [HSV], adenovirus [Ad], vaccinia virus [VV], vesticular stomatitis virus [VSV], and poliovirus [PV]) need to be genetically engineered to engender oncolytic activity and safety. Viruses tested for GBM include both DNA (HSV, Ad, VV) and RNA viruses (NDV, MV, reovirus, VSV, PV) (Table I).45,46
Fig 1.
Gene therapy strategies for brain tumors.
Table I.
Oncolytic viruses against GBM
| Oncolytic virus | Virus | Mutations (for tumor selectivity and safety) | Animal model(s) | Reference |
|---|---|---|---|---|
| G207 | HSV | γ34.5Δ, ICP6−, LacZ+ | Nude, i.c. U87 | [171] |
| G47Δ | HSV | γ34.5Δ, ICP6−, ICP47Δ, LacZ+ | Nude, i.c. U87, GSC | [7, 51] |
| 1716 | HSV | γ34.5Δ | [172] | |
| MG18L | HSV | US3−, ICP6− | Nude, i.c. GSC | [53] |
| Δ68H-6 | HSV | γ34.5 BDDΔ, ICP6− | Nude, i.c. U87, GSC | [52] |
| Ad-Δ24RGD | Ad | E1AD24Δ, RGD-4C | Nude, i.c. U87 | [63] |
| ICOVIR-5 | Ad | E1AD24Δ, RGD-4C, E2Fpromoter-E1A | Nude, i.c. U87MG | [173] |
| CRAd-S-pk7 | Ad | polylysine modified fiber knob, survivin promoter-E1A | Nude, i.c. U87 | [65] |
| Ad5/35.GΔ-Ki | Ad | GFAP promoter-E1A, Ki67 promoter-E4, Ad 35 fiber knob | Nude, i.c. U251 | [68] |
| Ad5/35.IR-E1A/TRAIL | Ad | Ad 35 fiber knob, E1BΔ, TRAIL+ | SCID, s.c. U87 | [174] |
| vvDD | VV | TKΔ, VGFΔ | Rat, i.c. F98 & RG2 | [78] |
| GLV-1h68 | VV | F14.5L−, TK−, HA− | Nude, s.c. C6 | [79] |
| Nude, i.c. U87 | [80] | |||
| LIVP 1.1.1 | VV | TK− | Nude, i.c. U87 | [80] |
| PVS-RIPO | PV | HRV2 IRES Sabin vaccine strain |
Rat, i.c. U87MGDEGFR; Nude, s.c. U-118 |
[84, 175] |
| MTH68/H | NDV | mesogenic vaccine variant | [176] | |
| V4UPM | NDV | V4 vaccine variant | Nude, s.c. U87 | [89] |
| Hitchner B1 | NDV | SCID, s.c. U87 | [177] | |
| MV FmiR7 | MV | Edmonston-B vaccine strain, miR7+ | NOD/SCID, s.c. U87 | [92] |
| MV-GFP-HAA-IL-13 | MV | Edmonston-B vaccine strain, hIL-13+, HAA (CD46− & SLAM−) | Nude, i.c. GSC | [93] |
| MV-CEA | MV | Edmonston-B vaccine strain, CEA+ | Nude, i.c. GSC | [97] |
| MV-NIS | MV | Edmonston-B vaccine strain, NIS+ | Nude, s.c. U251 | [96] |
| Nude, i.c. primary GBM | ||||
| VSVΔM51 | VSV | ΔM51 | Nude, i.c. U87 | [100] |
| VSV-M51R | VSV | M51R | Nude, s.c. U87 | [101] |
| VSV-rp30 | VSV | Pmut, Lmut (VSVwt serial passage) |
SCID, i.c. U87 | [178] |
| srVSV | VSV | VSV-ΔL + VSVΔG | SCID, s.c. G62 | [104] |
Abbreviations:Ad, adenovirus; BDD, γ34.5 beclin-1 binding domain; CEA, carcinoembryonic antigen; CD, cytosine deaminase; EGFR, epidermal growth factor receptor; GBM, glioblastoma; GSC, glioblastoma stem cells; HA, hemagglutinin; HRV2, human rhinovirus type 2; HSV, herpes simplex virus; i.c., intracranial; IRES, internal ribosomal entry site; MV, measles virus; NDV, Newcastle disease virus; NIS, sodium iodide symporter; PNP, purine nucleoside phosphorylase; PV, poliovirus; PVS-RIPO, recombinant poliovirus; RCR, replication competent retrovirus; s.c., subcutaneous; TK, thymidine kinase; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; VGF, vaccinia growth factor; VSV, vesicular stomatitis virus; VV, vaccinia virus.
Stem or progenitor cell-based cancer gene therapy includes the use of neural stem cells (NSCs) and adult mesenchymal stromal (stem) cells (MSCs) that have an inherent ability to home to the site of tumors.47,48 Tumor homing of MSCs relies on the expression of soluble inflammatory mediators that often accompany tumor progression.33 MSCs, often derived from bone marrow or adipose tissue, or NSCs can be transduced with therapeutic transgenes for use as gene therapy vectors.33,48 MSCs have some advantages over other stem/progenitor cells because they are easily acquired from patients (from bone marrow or adipose tissue) and expanded ex vivo. 48 Conversely, MSCs carry the risk of adversely contributing to the tumor microenvironment through promoting angiogenesis, stroma formation, and immunosuppressive effects. NSCs were the first stem/progenitor cells tested for homing to GBM tumors and are currently in clinical trial for GBM (Table II).47,49 From a safety standpoint, stem/progenitor cells must be nontumorigenic, nonimmunogenic, and should not differentiate into functional cells that could interfere with normal brain activity.
Table II.
Recent and ongoing gene therapy clinical trials in patients with GBM
| Therapy | Type | Phase | Protocol | Results | Reference |
|---|---|---|---|---|---|
| G207 | HSV | 1b | 1.5 × 108 pfu at time of biopsy; 1 × 109 pfu into tissue surrounding resected tumor 2–5 d after biopsy |
Median survival; 6.6 months from G207. No toxicity |
[167] |
| G47Δ | HSV | 1-2 | Dose escalation, × doses (n=3) | WHO JPRN-UMIN000002661 | |
| M032 (hIL-12) |
HSV | 1 | (1) Dose escalation: 1 × 105–3 × 109 pfu (n = 3–6) (2) 15% of the MTD administered through catheters implanted at the site of tumor; then 85% of MTD into the resected tumor site |
Gene transfer protocol 0801- 899 |
|
| Delta-24-RGD-4C | Ad | 1 | Dose escalation – 8 doses (n = 3) |
NCT00805376 | |
| Reolysin | RV | 1-2 | Dose escalation up to 1 × 109 TCID50 |
safe, well tolerated | [108] NCT00528684 |
| NDV-HUJ | NDV | 1-2 | (1) Dose escalation of 0.1, 0.32, 0.93, 5.9, and 11 BIU IV followed by × cycles of 55 BIU. (2) × cycles of 11 BIU and then 2 doses of 11 BIU weekly |
MTD not reached, no toxicity, 1/11 patients had complete response |
[168] |
| MV-CEA | MV | 1 | (1) MV administered to resected cavity (2) MV administered IT and then resected cavity |
NCT00390299 | |
| PVS-RIPO | PV | 1 | IT injection through catheters implanted at time of biopsy of 1 × 108 − 1 × 1010 TCID50 |
NCT01491893 | |
| Toca 511 (CD) |
MLV | 1-2 | Dose escalation: single IT injection, 3–4 wk later 6-d cycles with oral 5-FC (130 mg/kg) repeated monthly |
NCT01156584 | |
| Toca 511 (CD) |
MLV | 1 | Injection (4 dose levels) into resection cavity, 7 wk later 8-d cycle of oral 5-FC repeated ×3 |
NCT01470794 | |
| AdV-tk (Advantagene, Woburn, MA) |
RD-Ad | 1b | Dose escalation:3 × 1010 −3 × 1011 vector into tumor bed at resection followed by valacyclovir, radiation, TMZ |
Safe, quickened the effects of radiation |
[170] |
| Ad-tk | RD-Ad | 2 | Survival rate and recurrence- free survival rate |
NCT00870181 | |
| AdV-tk (Advantagene) | RD-Ad | 1b | Dose escalation followed by GCV, radiation, and TMZ |
NCT00751270 | |
| AdV-tk (Advantagene) | RD-Ad | 2a | 3 × 1011 vector into tumor bed followed by valacyclovir 1–3 days later and radiation at 3–7 days. |
NCT00589875 | |
| HB1.F3 (CD) | NSC | 1 | HB1.F3 into tumor bed following resection. Oral 5-FC every 6 h for 4–10 d |
NCT01172964 |
Abbreviations:Ad, adenovirus; BIU, billion infectious units; CD, cytosine deaminase; HIL-12, human interleukin 12; HSV, herpes simplex virus; IT, intratumoral; MLV, murine leukemia virus; MTD, maximum tolerated dose; MV, measles virus; pfu, plaque forming unit; NCT#, from clinicaltrials.gov; NDV, Newcastle disease virus; NSC, neural stem cells; PV, poliovirus; RD-Ad, replication-defective Ad; TCID50,50% tissue culture infectious dose; TK(ortk), thymidine kinase; TMZ, temozolomide.
Gene therapy strategies can be grouped according to their mechanisms of action. Cytotoxic gene therapy encompasses oncolytic viruses, as they are inherently toxic to cancer cells (with the exception of retroviruses), and/or the delivery of directly cytotoxic or prodrug activating suicide genes (Fig 1). Delivery of immune-modulatory genes should boost immune responses to tumor antigens and the activity of cytotoxic effector cells. The tumor microenvironment is composed of normal cells in the tumor and extracellular matrix and is typically targeted with antiangiogenic genes. Combinations of multiple strategies are likely to be the best approach to attack these complex tumors.
CYTOTOXIC GENE THERAPY
Oncolytic viruses. Herpes simplex virus
HSV is a human DNA virus that has great potential for GBM therapy because of its natural neurotropism.50 The HSV genome can be manipulated to introduce mutations/deletions in nonessential genes that engender cancer selectivity and for insertion of large and multiple transgenes.43 The ability to eliminate neurovirulence genes and availability of antiviral drugs means that this lethal pathogen can be used safely in the brain.50 Three oncolytic HSVs (oHSV) have been or are in clinical trial for GBM; 1716, G207, and G47Δ40 (Table I). All have both copies of the neurovirulence gene, g34.5, deleted. In addition, the UL39 gene (encodes for ICP6, the large subunit of ribonucleotide reductase) is disrupted in both G207 and G47Δ, which further enhances specificity and safety, because they can only replicate in dividing tumor cells. G207 and 1716 were very efficacious in glioma cell line models and demonstrated safety in clinical trials (Table II). Unfortunately, oHSVs lacking γ34.5 have limited or no replication in GSCs.7 In contrast to G207 and 1716, G47Δ has an additional deletion of the gene encoding ICP47, which restores GSC sensitivity.7,51 Additional oHSVs have been developed that replicate in GSCs and are safe in the brain; Δ68H-6, with a deletion of the beclin1 binding domain in γ34.5 that blocks autophagy52 and MG18L, with a deletion in Us3, an anti-apoptotic gene53 (Table I). One hallmark of GBM is the presence of a hypoxic microenvironment, which has been shown to maintain GSC stemness.54 In contrast to other therapies, G207 replicated better in glioma cell lines in vitro under hypoxic compared with normoxic conditions,55 while G47Δ replicated similarly in hypoxic and nonhypoxic regions of GSC-derived tumors.56
Adenoviruses
Adenoviruses (Ad) are human DNA viruses that have been extensively studied as gene therapy and oncolytic agents. Conditionally-replicative adenoviruses (CRAds) typically have deletions in early genes, E1A or E1B, to target tumor cells because of inactivation of cellular Rb and p53, respectively, and thus, are not necessary in cancer cells with mutations in these tumor suppressors.57 Subsequently, it was shown that E1B is necessary for late viral RNA export, which was complemented in tumor cells.58 ONYX-015, an E1B-55kD gene deleted CRAd was one of the earliest oncolytic viruses tested in GBM clinical trials, with no toxicity observed; however, there was no significant efficacy.59 Ad with deletions in E1A, especially a 24 bp deletion (Delta-24), have been developed as base vectors to accommodate additional genetic modifications to enhance anti-GBM activity.57 Additional mutations have also been incorporated into CRAds to enhance efficacy and provide space for the insertion of transgenes, for example in E3 (involved in evasion of cellular antiviral responses) and E4 (inactivates p53) genes.60-62
One of the major limitations with using Ad (oncolytic and replication-defective) for gene therapy is the limited expression of the Ad serotype 5 (Ad5) receptor, Coxsackie-adenovirus receptor, on cancer cells, including GBM. Therefore, a major avenue to improve oncolytic Ad is through altering its tropism to more selectively bind to glioma cells. Ad Delta-24 was modified by inserting an Arginine-Glycine-Aspartic Acid (RGD) peptide (binds to integrins ανβ3 and ανβ5) into the fiber knob (Delta-24-RGD), which significantly enhanced infectivity of glioma cell lines and in vivo efficacy.63 Other glioma targeting peptides introduced into the fiber include an epidermal growth factor receptor (EGFR)vIII binding peptide (Delta24-EGFR)64 and a polylysine motif (Ad5.pK7) to bind heparan sulfate proteoglycans. 65,66 Because different Ad serotypes have different cellular receptors, using different serotype or species (xenotype) fiber knobs or chimeric fibers can alter Ad tropism.67 Examples include an Ad3 fiber knob (binds to CD80, CD86, and unknown receptor) and Ad5 fiber chimera (Ad5/3), which had greatly increased glioma infectivity and cytotoxicity in vitro68,69; Ad5 with canine Ad1 (CK1) or porcine Ad (PK) fiber was more efficient than Ad5/370; and Ad16p (binds to CD46) and chimpanzee CV23 efficiently infected GSCs.71 A screen of 16 Ad5 fiber chimeras on primary glioma cell cultures identified B-group viruses (Ad11, Ad35, Ad50 [bind to CD46, overexpressed in GBM]) as having greatly increased infectivity compared with Ad5,72 with Ad5/35 extending survival in vivo.68 These same strategies can be used for targeting replication-defective Ad vectors.64 CRAd replication efficiency and specificity can be improved by using glioma-selective promoters/enhancers to drive expression of early genes (transcriptionally-targeted) or transgenes for gene delivery.73 There are a number of promoters/enhancers that are active in glioma cells (glial fibrillary acidic protein (GFAP), nestin, midkine) or cancer cells generally (telomerase reverse transcriptase (TERT), survivin, vascular endothelial growth factor receptor (VEGFR)-1, E2F, Ki67), and these have been used to drive E1A expression in CRAds and found to selectively replicate in glioma cells.60,68,74,75
Vaccinia virus
VV is the vaccine agent used in the eradication of smallpox. This DNA virus has also demonstrated success as an oncolytic virus, with a rapid lytic replication cycle that occurs in the cytoplasm and ease of genetic manipulation that allows for the incorporation of therapeutic transgenes.76 JX-594 (Table III) cytotoxicity in mouse GL261 glioma cells was significantly better than reovirus or VSVΔM51.77 Although the vaccine strains are attenuated, there is still concern about replication-competent VV in the brain. Therefore, additional mutations, such as in thymidine kinase (TK) and vaccinia growth factor, have been introduced to target cancer cells. A double-deleted VV, vvDD (Table I), was cytotoxic to human and rat glioma cell lines in vitro and prolonged the survival of immune-competent rats bearing intracerebral rat gliomas.78 GLV-1h68 (Table I), with reporter genes inserted into F14.5L, TK, and A56R, was quite attenuated for neurovirulence,79 although systemic delivery to mice with intracerebral U87 xenografts was ineffective.80 As with other oncolytic viruses, the combination with radiotherapy significantly improved survival compared with radiation alone, and radiation increased intratumoral replication of single mutant VV LIVP 1.1.1 (Table I).80,81
Table III.
Gene therapy vectors “armed” with therapeutic genes
| Gene therapy | Vector | Trangene(s) | Animal model(s) | Reference |
|---|---|---|---|---|
| ACE-CD | MLV | CD/5-FC | Nude, i.c. U87 Rat, i.c. RG2 |
[182, 183] |
| Toca 511 | MLV | CD/5-FC | Balb/c, i.c, CT26; and B6C3F1, i.c. Tu-2449 |
[116] |
| ACE-PNP | MLV | PNP/F-araAMP | Nude, i.c. U87 | [117] |
| JX-594 | VV (TK) |
Murine GM-CSF | Rat, i.c. RG2; C57BL/6, i.c. GL261 |
[157] |
| G47Δ-Flt3L | HSV | Flt3L | C57BL/6, i.c. CT-2A | [184] |
| M002 | HSV | Murine IL-12 | B6D2F1, i.c. 4C8; SCID, i.c. D54MG |
[160] |
| UCB-MSC-IL12M | MSC | IL-12p40 N-glycosylation mutant | C57BL/6, i.c. GL26 | [161] |
| AF-MSC-endostatin-sCE2 | MSC | Endostatin, sCE2 | Nude, i.c. U87MG 1 CPT11 prodrug |
[144] |
| G47Δ-mAngio | HSV | Angiostatin | Nude, i.c. U87 | [163] |
| HSVQ-Vstat120 | HSV | Vstat120 | Nude, i.c. U87DEGFR | [164] |
| G47Δ-PF4 | HSV | PF4 (CXCL4) | Nude, s.c. U87 | [165] |
| Ad-isthmin | RD-Ad | Xenopus isthmin | Nude, i.c. U251 | [166] |
| CRAdRGDflt-IL24 | Ad | mda7/IL-24 | Nude i.c. D54MG | [74] |
Abbreviations:Ad, adenovirus; GM-CSF, granulocyte macrophage colony-stimulating factor; HSV, herpes simplex virus; MLV, murine leukemia virus; MSC, mesenchymal stromal cell; RD-Ad, replication-deficient Ad; sCE2, carboxylesterase 2; UCB-MSC, umbilical cord blood-derived MSC; Vstat, vasculostatin.
Retroviruses
Retroviruses are positive-strand RNA viruses whose RNA genome is reverse transcribed into DNA that is integrated into the host genome. Replication-competent gamma-retroviruses (RCRs) have been derived from murine leukemia virus, which only integrate/replicate in mitotic cells, providing specificity for dividing tumor cells.82 RCRs are nonlytic and noncytotoxic, so to be oncolytic, they must express therapeutic transgenes, usually cytotoxic or suicide genes (Table III, discussed below). RCRs can incorporate up to 8 kb of foreign DNA and transgenes are stably expressed because of integration.82
Poliovirus
PV is a human positive-strand RNA virus that is neurotropic and whose receptor (CD155) is highly expressed on GBM cells. To eliminate neurotoxicity, its internal ribosomal entry site was replaced with a non-neurotoxic internal ribosomal entry site from human rhinovirus type 2. This recombinant poliovirus, PVS-RIPO (Table I), is derived from the Sabin polio vaccine, which further improves safety.83 It was efficacious in inhibiting subcutaneous glioma tumor growth, and importantly, in vivo serial passage in gliomas did not alter its specificity.84 PVS-RIPO replication in glioma cells is promoted by activation of Mnk1 and stimulation of cap-independent translation.85
Newcastle disease virus
NDV is an avian paramyxovirus with a negative-strand RNA genome and is non-pathogenic in humans. Cancer specificity of NDV relies on defects in antiviral immunity, resistance to apoptosis, and induction of autophagy found in many cancers, including GBM.86,87 Both pathogenic (velogenic and mesogenic; MTH68) and nonpathogenic (lentogenic; NDV-HUJ, Hitchner B1) in poultry strains have been used as oncolytic viruses against GBM88 (Table I). Another vaccine strain, V4UPM, inhibited tumor growth and induced apoptosis in U87MG subcutaneous tumor bearing mice.89
Measles virus
MVis a human paramyxovirus with similar oncolytic effects as NDV; however, MV is known to be neurotropic and in rare human cases causes encephalitis.90 The attenuated Edmonston vaccine strain is used as the backbone for most recombinant oncolytic MV. The MV hemagglutinin protein binds to CD46 receptors, which are often highly expressed in GBM.91 The MV fusion protein causes membrane fusion and syncytia formation, which leads to apoptosis. The tropism of oncolytic MV can be restricted to GBM by insertion of a brain-specific micro RNA target sequence that is downregulated in glioma.92 MV can also be retargeted to GBM by inserting IL13 into hemagglutinin protein that is ablated for binding to CD46 and SLAM.93 MVexpressing the human carcinoembryonic antigen (MV-CEA) has shown success in several GBM animal models, including intracranial GSC xenografts in nude mice.94 Serum levels of CEA are a measure of virus replication. The safety of MV-CEA has been studied after intracerebral injection in rhesus macaques95 in advance of clinical trial (Table IV). A MV expressing the sodium iodide symporter (MV-NIS) has been constructed, which allows for in vivo monitoring of 99mTc or 123I uptake or in combination with 131I for radiotherapy.96 Oncolytic MV (MV-NIS) has been shown to infect, replicate, and kill human GSCs and prolong survival of GSC tumor bearing mice.97
Table IV.
Nonreplicating cytotoxic gene therapy vectors and cells
| Gene therapy | Vector | Transgene | Animal model(s) | Additional treatment | Reference |
|---|---|---|---|---|---|
| LCMV-GP pseudo-typed | MLV | HSV-TK | Rat, i.c. GSC | GCV | [128] |
| VSV-G pseudo-typed | MLV | HSV-TK | Rat, i.c. GSC | GCV | [128] |
| Ad-TK 1 Ad-Flt3L | Ad | HSV-TK, Flt3L | Rat, i.c. CNS-1 | GCV | [123] |
| Ad-stTRAIL | Ad | TRAIL | Nude, i.c. U87, U251 | [179, 180] | |
| Ad.5/3-mda7 | Ad | mda7 /IL-24 | Nude, i.c. primary GBM | [181] | |
| Ad-mhiL-4.TRE.mhIL-13-PE | Ad | Mutated IL-13 fused to PE | Nude, i.c. U251 Rag1−/−, i.c. human primary xenograft C57/B6, i.c. GL26-H2 |
[120] | |
| NSCtk | NSC | HSV-TK | Rat, i.c. C6 | GCV | [130] |
| HB1-F3 (F3-CD) | NSC | CD | Rat, i.c. U373MG | 5-FC | [132] |
| MSCtk | MSC | HSV-TK | Rat, i.c. C6 | GCV | [143] |
| HSV/TK | MSC | HSV-TK | Nude, i.c. U87 | GCV | [140] |
| MSC-CD | MSC | CD | Rat, i.c. C6 | 5-FC | [142] |
| UCB-TRAIL | MSC | stTRAIL | Nude, i.c. U87 | [138] | |
| MSC-S-TRAIL | MSC | secreted, extracellular TRAIL domain fused to hFlt3L |
SCID, i.c. GSC | [135] | |
| hMSC S-TRAIL | MSC | secreted TRAIL | Nude, i.c. U87 | [146] | |
| hAT-MSC.TRAIL | MSC | TRAIL | Rat, i.c. F98 | [145] |
Abbreviations:Ad, adenovirus; 5-FC, 5-fluorocytosine; Flt3L, fms-like tyrosine kinase ligand; GCV, gancyclovir; HSV, herpes simplex virus; i.c., intracranial; LCMV, lymphocytic choriomeningitis virus; MLV, murine leukemia virus; MSC, mesenchymal stromal cell; NSC, neural stem cell; PE, pseudomonas exotoxin; stTRAIL, secretable trimeric TRAIL; TK, thymidine kinase; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; UCB, umbilical cord blood; VSV, vesicular stomatitis virus.
Vesicular stomatitis virus
VSV is a negative-strand RNA virus of the Rhabdoviridae family. Although VSV has not been associated with any human disease, it is neurotoxic in animal models so efforts have been made to attenuate its neuropathogenicity. VSV is highly sensitive to innate type I interferon responses, which are often lacking in tumor cells and this allows VSV to specifically target tumor cells.98 Mutations in the VSV-M protein, in particular at Met-51, renders the virus unable to block anti-viral innate responses, which improves safety but doesn’t affect replication in cancer cells.99 VSVΔM51 is efficacious in killing human glioma xenografts even after systemic delivery,100 and M51R VSV was even effective in U87 glioma cells overexpressing anti-apoptotic Bcl-XL101 (Table I). To further improve glioma specificity, a VSV mutant (VSV-rp30, with single mutations in the P and L genes) was isolated by serial passage on glioma cells in vitro followed by lack of adsorption to fibroblasts.102 Unfortunately, this virus was still quite cytotoxic to normal human glia.103 VSV-p1-GFP, a first-position reporter gene virus, was identified in a screen of VSV mutants for selective cytotoxicity in glioma cells and not normal glial cells, even in the presence of interferon-α, and found to be non-neurovirulent in mice.103 Recently, a semireplication competent VSV vector system was created (srVSV; Table I), where viruses lacking the viral polymerase (ΔL) were combined with viruses lacking the glycoprotein (ΔG) so that only cells infected with both viruses could replicate by providing proteins in trans. This virus combination was as efficacious as wild-type VSV in subcutaneous human G62 xenografts and as opposed to the wild-type virus, did not cause any neurotoxicity.104
Reovirus
Reovirus is a double-stranded RNAvirus that is nonpathogenic to humans. Serotype 3 was originally shown to have oncolytic activity, with replication dependent upon activated Ras pathways that are often present in cancer cells including GBM.105 Reovirus type 3 has had success in several in vivo studies106,107 and is one of the only genetically unmodified oncolytic viruses to enter clinical trial (Reolysin [Oncolytics Biotech Inc, Calgary, Canada]; Table II). 108 Recently, it was demonstrated that all 4 serotypes of reovirus have oncolytic activity against primary GBM cells in vitro.109
Stem cells as oncolytic virus carriers
A new approach to deliver oncolytic viruses to brain tumors is to use stem cells as a carrier system.110 Several groups have demonstrated that MSCs and NSCs can support Ad infection and replication.111-114 An advantage of this approach to oncolytic virus alone is that stem cells can be delivered intravascularly, evading anti-viral immunity, and then extravasate into the brain, or intracranially at a distance from the tumor. After intravascular administration, MSCs loaded with Ad-Δ24RGD, but not Ad-Δ24-RGD alone, migrated to and infected intracerebral gliomas, resulting in a significant increase in survival.113 When compared directly, both human MSCs and NSCs loaded with CRAd-S-pk7 (Table I) supported Ad replication and migration to tumors, however, loaded NSCs were significantly better than MSCs in extending survival.111 Additional studies with CRAd-S-pk7 loaded HB1.F3 NSCs, which are currently in clinical trial, demonstrated that the infected NSCs retained their tumor homing, supported Ad replication, and gave rise to infected tumor cells in vivo.114
Cytotoxic or suicide gene/prodrug therapy
Cytotoxic chemo- and radiotherapy have been the standards of care for GBM patients, with limited success mostly because of their negative effects on surrounding healthy tissue and small therapeutic indexes. Suicide gene therapy involves delivery of a prodrug activating enzyme (suicide gene) that converts nontoxic prodrugs to cytotoxic metabolites.35 The most common suicide gene/prodrug combination is HSV thymidine kinase (TK)/ganciclovir (GCV). Phosphorylated GCV is only toxic to dividing cells and can spread to surrounding cells (bystander effect) through gap junctions (Fig 1). Earlier TK gene therapy trials typically used replication-defective retrovirus and adenovirus vectors, and have been reviewed.115 Other suicide gene/prodrug combinations are also under investigation for GBM,18 using viral vectors and stem/progenitor cells. The yeast enzyme cytosine deaminase (CD)/5-fluorocytosine (5-FC) prodrug system is similar to the TK system. CD converts the nontoxic prodrug 5-FC into the cytotoxic metabolite 5-FU.35 Toca 511, a RCR that expresses CD, was shown to prolong survival in 2 immunocompetent mouse models of GBM.116 Purine nucleoside phosphorylase (PNP), which converts F-araAMP to diffusible toxic 2-FA, was inserted into a RCR (ACE-PNP; Table III). Treatment of intracranial gliomas with ACE-PNP had no effect on tumor growth, while administration of F-araAMP significantly extended survival with a second round of F-araAMP further extending survival, although all mice eventually succumbed to disease.117 Other cytotoxic strategies are to express secreted pro-apoptotic proteins, such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or mda-7/IL-24 that are selectively active in tumor cells118,119 or cytotoxins, such as Pseudomonas exotoxin, that are fused to a ligand (IL-13) for a glioma-specific receptor120 (Table IV).
Because TK/GCV therapy was shown to induce an antitumor immune response, combinations with immunomodulatory genes are an obvious strategy. The most promising combinations for GBM are replication-defective Ad expressing TK with the immune-stimulatory cytokine fms-like tyrosine kinase 3 ligand (Flt3L) to recruit dendritic cells. Intratumoral delivery of AdTK and AdFlt3L (Table IV) achieved increased survival in rat and dog syngeneic glioblastoma models, including with multifocal tumors.121,122 This was associated with the development of immune memory against GBM antigens.123,124 Helper-dependent gutless or high capacity adenovirus vectors have all their viral genes eliminated, so they induce only minimal anti-adenovirus immune responses and provide cloning space for up to 35 kb.125 A bicistronic gutted adenovirus vector is being prepared for clinical use.126,127
A large problem with the earlier studies with replication-defective vectors was the limited number and distribution of TK transduced glioma cells. To overcome this, lymphocytic choriomeningitis virus-pseudo-typed lentiviral vectors were generated, which had a high transduction efficiency in vivo, including GSCs, compared with a few cells with retroviral vectors, such that GCV treatment greatly prolonged survival.128 One way to improve the distribution of cytotoxic, or other therapeutic, genes is to use migratory stem or progenitor cells (NSCs and MSCs) that are attracted to the tumor.47,48 NSCs expressing TK (NSCtk; Table IV) directly implanted into intracranial C6 tumors led to complete tumor regression in 67% of treated animals.129 NSCtk were then shown to migrate to intracranial tumors when delivered to distant sites from the tumor.130 A human immortalized NSC line, HB1.F3,131 transduced with CD migrated across the brain to implanted gliomas132 and significantly reduced intracranial glioma size after treatment with 5-FC.133 The F3-CD NSCs are currently in clinical trial for GBM (Table II).
Human MSCs can migrate in the brain or after carotid artery injection in a range of GBM mouse models, including to GSC-derived and immunocompetent genetically induced (replication-competent avian leukosis virus splice acceptor (RCAS)-Ntv-a) tumors.134-136 Different viral vectors have been used to transduce MSCs including, retrovirus, lentivirus, adenovirus, and baculovirus.137,138 A number of suicide genes (TK, CD, rCE) have been introduced into MSCs and shown to be effective with prodrug in treating intracranial glioma models139-143 (Table IV). Unfortunately, some of these studies were performed by co-implanting the MSCs with the glioma cell lines,142,144 which obviates the migratory advantage of this strategy.
Cytotoxic pro-apoptotic secreted proteins, especially TRAIL, are another popular class of transgenes to introduce into stem/progenitor cells, and several studies have shown therapeutic effects and increased apoptosis in glioma xenograft models135,138,145,146 (Table IV). MSCs, as normal cells, are resistant to TRAIL,135 however many glioma cells are also resistant to TRAIL despite the expression of TRAIL receptors. To overcome this, MSC-TRAIL was combined with a lipoxygenase inhibitor MK886, which increased DR5 (TRAIL receptor) and decreased anti-apoptotic protein expression, leading to prolonged survival in vivo.147 Differentiated embryonic stem cells or induced pluripotent stem cells have some potential advantages; they can be stably genetically-modified, proliferate indefinitely, and could be established with a whole range of HLA types for immunologic matching. Mouse embryonic stem cells expressing TRAIL or mda-7 have been generated by site-specific recombination and then terminally differentiated into astrocytes, which migrate in vivo and induce apoptosis.148,149 However, treatment activity in orthotopic glioma models has not been demonstrated. Induced pluripotent stem cells were differentiated into NSCs, which were then transduced with TK using baculovirus, and shown to migrate from the contralateral hemisphere and inhibit tumor growth after GCV treatment.150
IMMUNE-STIMULATORY GENE THERAPY
The immune-privileged state of the brain is a major obstacle to immunotherapy against brain tumors.151 The brain has a limited supply of antigen-presenting cells and lacks lymphatics that impede immune cells from exiting the brain parenchyma.152 In addition, the GBM induced microenvironment is very immunesuppressed, with elevated regulatory T cells and myeloid-derived suppressor cells.153 Despite these challenges, significant progress with immune-mediated gene therapy strategies has been achieved. Replication-defective Ad expressing Flt3L was shown to enhance survival in a syngeneic rat glioma model and this was associated with increased infiltration of DCs.154 This strategy was improved when combined with tumor cell death to provide tumor antigens for the recruited DCs, as shown with the Ad-tk combination.124 A similar strategy has been described using oHSV expressing Flt3L. G47Δ-Flt3L significantly extended survival in the mouse CT2A glioma model, with about 40% long-term survivors compared with G47Δ-Empty (no transgene), which had minimal effect155 (Table III).
Another immunotherapy strategy is to express cytokines to enhance adaptive immune responses. JX-594, a TK-deleted VV expressing granulocyte macrophage colony stimulating factor (GM-CSF; Table III), is currently in clinical trials for peripheral tumors.156 In 2 immunocompetent GBM models, JX-594 inhibited tumor growth and increased survival, which was associated with increased GM-CSF-dependent inflammation.77 JX-594 replicated in most human GSCs tested in vitro, although considerably less than in U87, and was cytotoxic in those GSCs that supported virus replication.157 IL-12 is one of the more potent anti-tumor cytokines, driving a TH1 response.158 Several groups have delivered IL-12 using a variety of gene therapy vectors. A γ34.5-deleted HSV-1 expressing mouse IL-12 (M002; Table III) was shown to retain its oncolytic activity and perform better than other oHSVs in rodent models of GBM.159 In addition, M002 was tested in nonhuman primates and demonstrated no toxicity, but increased activation of nonhuman primates lymphocytes.160 The same oHSV construct expressing human IL-12 has been produced for human trial (M032; Table II). MSCs expressing IL-12 (UCB-MSC-IL12M; Table III) inhibited GL26 intracranial tumor growth and prolonged survival when administered in the contralateral brain hemisphere. 161 The surviving mice were protected from re-challenge with GL26 cells in both contralateral and ipsilateral sides of the brain, indicating a memory response against tumor antigens.161
DISRUPTING TUMOR MICROENVIRONMENT
Targeting the tumor microenvironment is an attractive approach because it consists of normal cells that should not develop resistance to the therapy. Normally, neovascularization is a tightly regulated balance between naturally occurring angiogenesis activators and inhibitors, however, in tumors the dividing cancer cells outgrow the normal vasculature, increasing hypoxia and the expression of proangiogenic factors.162 GBM is a highly vascularized tumor, but there are limitations to current antiangiogenic drugs such as bevacizumab (Avastin [Genentech, South San Francisco, CA]), an anti-VEGF monoclonal antibody, which have a negative effect of increasing glioma invasiveness and don’t significantly improve overall survival.162 Developing alternative strategies such as combination therapies, including targeting multiple angiogenic pathways, might be a better strategy, especially since inhibiting angiogenesis is cytostatic and not cytotoxic. A number of antiangiogenic factors have been expressed from oHSV. Angiostatin, an endogenous inhibitor of angiogenesis, was inserted into G47Δ.163 A single treatment of G47Δ-mAngio (Table III) significantly extended survival of glioma-bearing mice over nontransgene containing G47Δ, and this was associated with decreased microvascular density and VEGF expression.163 Combining a lower dose of G47Δ-mAngio with a low (non-invasive) dose of bevacizumab further improved survival.163 Expression of other naturally occurring angiogenesis inhibitors, vasculostatin and CXCL4 (PF4), have also been shown to improve the efficacy of oHSV in human glioma models 164,165 (Table III). IL-12, in addition to its immunostimulatory activity is also antiangiogenic in GSC-derived gliomas (Cheema T, Rabkin SD; unpublished results). An adenovirus expressing isthmin, an angiogenesis inhibitor derived from the brain of Xenopus, inhibited angiogenesis and intracranial tumor growth.166 Antiangiogenesis in combination with suicide gene therapy, using MSCs transduced with endostatin (an endogenous antiangiogenic substance) and the prodrug activated enzyme, carboxylesterase 2 (Table III), exhibited antitumor activity in an intracranial model of GBM by inhibiting angiogenesis and cytotoxicity.144
STATUS OF CLINICAL TRIALS FOR GBM
In the past, gene therapy clinical trials for GBM patients have been promising as far as safety, with no maximum tolerated dose reached in any trial; however, overall benefits were marginal compared with the standard of care. Preclinical success of newer gene therapy strategies for GBM has led to greater optimism. Oncolytic viruses, nonreplicative viral vectors and NSCs are all currently being investigated in clinical trials for patients suffering from GBM (Table II). Oncolytic viruses make up the majority of the currently active clinical trials for GBM. Second generation oncolytic viruses have demonstrated safety in humans in previous clinical trials, and these viruses and third generation viruses are being further pursued.40 oHSV G207, which exhibited safety and anecdotal efficacy in an early phase 1 clinical trial, was examined in a phase 1b trial (Table II). This trial demonstrated only a marginal increase in survival of patients; however, it was the first study to demonstrate oHSV replication in vivo.167 A clinical trial using G47Δ, a third-generation derivative of G207, has been initiated in Japan (Table II). M032, an oHSV expressing hIL-12, is entering clinical trial for patients with recurrent/progressive GBM (Table II). A retargeted and tumor specific CRAd, AdV-delta24-RGD, is the only oncolytic Ad currently in phase 1 clinical trials for GBM patients (Table II).
Several RNA viruses are being assessed in the clinic. Reolysin, a nonengineered reovirus was well-tolerated and safe in a phase 1 trial and is being assessed in a phase 2 (Table II).108 NDV-HUJ was well-tolerated in GBM patients in a phase 1 study, no toxicity was observed, and a maximum tolerated dose was not achieved, and one out of 11 patients had a complete response to treatment (Table II). 168 Other ongoing phase 1/2 trials with RNA viruses include, MV-CEA and PVS-RIPO (Table II). The RCR, Toca 511, expressing CD is also being analyzed in GBM patients (Table II).
Several groups are investigating the effects of nonreplicating Ad expressing TK (Ad-tk) (Table II). Cerepro, the commercial name for the Ad-tk developed by Ark Therapeutics (London, UK), has made it through phase 3 clinical trial, and the results are currently being examined by European officials, with the fate of this therapy remaining uncertain.169 A phase 1B trial with Ad-tk/valacyclovir as an adjuvant therapy at the time of resection followed by radiation and temozolomide treatment demonstrated no toxicity in GBM patients,170 and efficacy is being examined in phase 2 trials (Table II). The majority of past and present clinical trials involve the use of replication-defective or replication-competent viral vectors for direct delivery of therapeutic genes to the tumor. However, a feasibility study is currently being performed using the genetically-modified NSC line, HB1.F3 expressing CD delivered intracerebrally (Table II).
CURRENT CHALLENGES FACING GBM GENE THERAPY AND FUTURE DIRECTIONS
Malignant tumors within the brain remain a therapeutic challenge; however, current strategies being tested in animal models as well as in the clinic show promise. The brain environment differs greatly from other organs in the body and is an obstacle in treating brain tumors. The blood-brain barrier restricts access to the brain, including gene therapies directed at GBM. Thus, delivery of gene therapy vectors often requires direct injection into the tumor bed at the time of biopsy or surgery, or the complicated setup of catheters into the brain. The lack of lymphocytes and the overall immunosuppressive nature of GBM make it a difficult target for immunotherapy. Specificity and spread of gene therapy vectors also remains a challenge in treating GBM. Replication-competent viruses and tumor-homing stem or progenitor cell therapy have the added benefit of motility and spread throughout the tumor, while replication-defective vectors must rely on an efficient bystander effect. Designing vectors that combine cytotoxic, immune-stimulatory, and antiangiogenic genes is a future direction of gene therapy for brain tumors. It will also be important and likely therapeutically beneficial to combine gene therapy with other therapeutic modalities, including the standards-of-care.
Acknowledgments
The authors thank past and current members of the laboratory for contributing to our research, which has been supported by grants from the National Institutes of Health (National Cancer Institute, National Institute of Neurologic Disorders and Stroke), Department of Defense, and the American Brain Tumor Association.
Abbreviations
- Ad
adenoviruses
- Ad5
adenovirus serotype 5
- CD
cytosine deaminase
- CEA
carcinoembryonic antigen
- CRAds
conditionally-replicative adenoviruses
- 5-FC
5-fluorocytosine
- Flt3L
fms-like tyrosine kinase ligand
- GBM
glioblastoma
- GCV
ganciclovir
- GM-CSF
granulocyte macrophage colony stimulating factor
- GSCs
glioblastoma stem cells
- HSV
herpes simplex virus
- MSCs
mesenchymal stromal (stem) cells
- MV
measles virus
- MV-CEA
measles virus expressing the human carcinoembryonic antigen
- MV-NIS
measles virus expressing the sodium iodide symporter
- NDV
Newcastle disease virus
- NSCs
neural stem cells
- oHSV
oncolytic herpes simplex virus
- PNP
purine nucleoside phosphorylase
- PV
poliovirus
- RCRs
replication-competent gamma-retroviruses
- tk or TK
thymidine kinase
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- VEGF
vascular endothelial growth factor
- VSV
vesticular stomatitis virus
- VV
vaccinia virus
Footnotes
Conflict of interest: The authors have no financial or personal relationships that could be perceived as influencing the described research. All authors have read the journal’s policy on conflicts of interest and have none to declare.
REFERENCES
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Rinne ML, Wykosky J, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26:756–84. doi: 10.1101/gad.187922.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng L, Bao S, Rich JN. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem Pharmacol. 2010;80:654–65. doi: 10.1016/j.bcp.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13:e83–9. doi: 10.1016/S1470-2045(11)70257-1. [DOI] [PubMed] [Google Scholar]
- 6.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 7.Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69:3472–81. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakimoto H, Mohapatra G, Kanai R, et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 2012;14:132–44. doi: 10.1093/neuonc/nor195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 10.de Vries NA, Beijnen JH, van Tellingen O. High-grade glioma mouse models and their applicability for preclinical testing. Cancer Treat Rev. 2009;35:714–23. doi: 10.1016/j.ctrv.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Munoz DM, Guha A. Mouse models to interrogate the implications of the differentiation status in the ontogeny of gliomas. Oncotarget. 2011;2:590–8. doi: 10.18632/oncotarget.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rankin SL, Zhu G, Baker SJ. Review: insights gained from modelling high-grade glioma in the mouse. Neuropathol Appl Neurobiol. 2012;38:254–70. doi: 10.1111/j.1365-2990.2011.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germano IM, Binello E. Gene therapy as an adjuvant treatment for malignant gliomas: from bench to bedside. J Neurooncol. 2009;93:79–87. doi: 10.1007/s11060-009-9869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwami K, Natsume A, Wakabayashi T. Gene therapy for high-grade glioma. Neurol Med Chir (Tokyo) 2010;50:727–36. doi: 10.2176/nmc.50.727. [DOI] [PubMed] [Google Scholar]
- 15.Kroeger KM, Muhammad AK, Baker GJ, et al. Gene therapy and virotherapy: novel therapeutic approaches for brain tumors. Discov Med. 2010;10:293–304. [PMC free article] [PubMed] [Google Scholar]
- 16.Kyritsis AP, Sioka C, Rao JS. Viruses, gene therapy and stem cells for the treatment of human glioma. Cancer Gene Ther. 2009;16:741–52. doi: 10.1038/cgt.2009.52. [DOI] [PubMed] [Google Scholar]
- 17.Asadi-Moghaddam K, Chiocca EA. Gene- and viral-based therapies for brain tumors. Neurotherapeutics. 2009;6:547–57. doi: 10.1016/j.nurt.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro MG, Candolfi M, Kroeger K, et al. Gene therapy and targeted toxins for glioma. Curr Gene Ther. 2011;11:155–80. doi: 10.2174/156652311795684722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assi H, Candolfi M, Baker G, et al. Gene therapy for brain tumors: basic developments and clinical implementation. Neurosci Lett. 2012;527:71–7. doi: 10.1016/j.neulet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samaranayake H, Maatta AM, Pikkarainen J, Yla-Herttuala S. Future prospects and challenges of antiangiogenic cancer gene therapy. Hum Gene Ther. 2010;21:381–96. doi: 10.1089/hum.2010.017. [DOI] [PubMed] [Google Scholar]
- 21.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. IARC; Lyon: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aboody KS, Najbauer J, Schmidt NO, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8:119–26. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seol HJ, Jin J, Seong DH, et al. Genetically engineered human neural stem cells with rabbit carboxyl esterase can targetbrain metastasis from breast cancer. Cancer Lett. 2011;311:152–9. doi: 10.1016/j.canlet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Natsume A, Lee HJ, et al. Neural stem cell-based dual suicide gene delivery for metastatic brain tumors. Cancer Gene Ther. 2012;19:796–801. doi: 10.1038/cgt.2012.63. [DOI] [PubMed] [Google Scholar]
- 25.Yi BR, Kim SU, Kim YB, et al. Antitumor effects of genetically engineered stem cells expressing yeast cytosine deaminase in lung cancer brain metastases via their tumor-tropic properties. Oncol Rep. 2012;27:1823–8. doi: 10.3892/or.2012.1721. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Martuza RL, Rabkin SD. Intracarotid delivery of oncolytic HSV vector G47Delta to metastatic breast cancer in the brain. Gene Ther. 2005;12:647–54. doi: 10.1038/sj.gt.3302445. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DL, Doty R, Tosic V, et al. Myxoma virus combined with rapamycin treatment enhances adoptive T cell therapy for murine melanoma brain tumors. Cancer Immunol Immunother. 2011;60:1461–72. doi: 10.1007/s00262-011-1045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lun XQ, Zhou H, Alain T, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67:8818–27. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Studebaker AW, Kreofsky CR, Pierson CR, et al. Treatment of medulloblastoma with a modified measles virus. Neuro Oncol. 2010;12:1034–42. doi: 10.1093/neuonc/noq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu L, Baxter PA, Zhao X, et al. A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro Oncol. 2011;13:14–27. doi: 10.1093/neuonc/noq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutova M, Shackleford GM, Khankaldyyan V, et al. Neural stem cell-mediated CE/CPT-11 enzyme/prodrug therapy in transgenic mouse model of intracerebellar medulloblastoma. Gene Ther. 2012 doi: 10.1038/gt.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pu K, Li SY, Gao Y, et al. Bystander effect in suicide gene therapy using immortalized neural stem cells transduced with herpes simplex virus thymidine kinase gene on medulloblastoma regression. Brain Res. 2011;1369:245–52. doi: 10.1016/j.brainres.2010.10.107. [DOI] [PubMed] [Google Scholar]
- 33.Cihova M, Altanerova V, Altaner C. Stem cell based cancer gene therapy. Mol Pharm. 2011;8:1480–7. doi: 10.1021/mp200151a. [DOI] [PubMed] [Google Scholar]
- 34.Fukazawa T, Matsuoka J, Yamatsuji T, et al. Adenovirus-mediated cancer gene therapy and virotherapy (Review) Int J Mol Med. 2010;25:3–10. [PubMed] [Google Scholar]
- 35.Duarte S, Carle G, Faneca H, Lima MC, Pierrefite-Carle V. Suicide gene therapy in cancer: where do we stand now? Cancer Lett. 2012;324:160–70. doi: 10.1016/j.canlet.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 36.Thomas SM, Grandis JR. The current state of head and neck cancer gene therapy. Hum Gene Ther. 2009;20:1565–75. doi: 10.1089/hum.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vachani A, Moon E, Wakeam E, Albelda SM. Gene therapy for mesothelioma and lung cancer. Am J Respir Cell Mol Biol. 2010;42:385–93. doi: 10.1165/rcmb.2010-0026RT. [DOI] [PubMed] [Google Scholar]
- 38.Zhao L, Wu J, Zhou H, et al. Local gene delivery for cancer therapy. Curr Gene Ther. 2011;11:423–32. doi: 10.2174/156652311797415854. [DOI] [PubMed] [Google Scholar]
- 39.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zemp FJ, Corredor JC, Lun X, Muruve DA, Forsyth PA. Oncolytic viruses as experimental treatments for malignant gliomas: using a scourge to treat a devil. Cytokine Growth Factor Rev. 2010;21:103–17. doi: 10.1016/j.cytogfr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Parker JN, Bauer DF, Cody JJ, Markert JM. Oncolytic viral therapy of malignant glioma. Neurotherapeutics. 2009;6:558–69. doi: 10.1016/j.nurt.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–33. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker JN, Zheng X, Luckett W, Markert JM, Cassady KA. Strategies for the rapid construction of conditionally-replicating HSV-1 vectors expressing foreign genes as anticancer therapeutic agents. Mol Pharm. 2011;8:44–9. doi: 10.1021/mp100230y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cody JJ, Douglas JT. Armed replicating adenoviruses for cancer virotherapy. Cancer Gene Ther. 2009;16:473–88. doi: 10.1038/cgt.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer J. 2012;18:69–81. doi: 10.1097/PPO.0b013e31824671c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohyeldin A, Chiocca EA. Gene and viral therapy for glioblastoma: a review of clinical trials and future directions. Cancer J. 2012;18:82–8. doi: 10.1097/PPO.0b013e3182458b13. [DOI] [PubMed] [Google Scholar]
- 47.Binello E, Germano IM. Stem cells as therapeutic vehicles for the treatment of high-grade gliomas. Neuro Oncol. 2012;14:256–65. doi: 10.1093/neuonc/nor204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bexell D, Scheding S, Bengzon J. Toward brain tumor gene therapy using multipotent mesenchymal stromal cell vectors. Mol Ther. 2010;18:1067–75. doi: 10.1038/mt.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim SU. Neural stem cell-based gene therapy for brain tumors. Stem Cell Rev. 2011;7:130–40. doi: 10.1007/s12015-010-9154-1. [DOI] [PubMed] [Google Scholar]
- 50.Shen Y, Nemunaitis J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006;13:975–92. doi: 10.1038/sj.cgt.7700946. [DOI] [PubMed] [Google Scholar]
- 51.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanai R, Zaupa C, Sgubin D, et al. Effect of gamma34.5 deletions on oncolytic herpes simplex virus activity in brain tumors. J Virol. 2012;86:4420–31. doi: 10.1128/JVI.00017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanai R, Wakimoto H, Martuza RL, Rabkin SD. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin Cancer Res. 2011;17:3686–96. doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bar EE. Glioblastoma, cancer stem cells and hypoxia. Brain Pathol. 2011;21:119–29. doi: 10.1111/j.1750-3639.2010.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aghi MK, Liu TC, Rabkin S, Martuza RL. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol Ther. 2009;17:51–6. doi: 10.1038/mt.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sgubin D, Wakimoto H, Kanai R, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus counteracts the hypoxia-induced modulation of glioblastoma stem-like cells. Stem Cells Transl Med. 2012;1:322–32. doi: 10.5966/sctm.2011-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–7. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–23. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–66. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 60.Horst M, Brouwer E, Verwijnen S, et al. Targeting malignant gliomas with a glial fibrillary acidic protein (GFAP)-selective oncolytic adenovirus. J Gene Med. 2007;9:1071–9. doi: 10.1002/jgm.1110. [DOI] [PubMed] [Google Scholar]
- 61.Lamfers ML, Gianni D, Tung CH, et al. Tissue inhibitor of metalloproteinase-3 expression from an oncolytic adenovirus inhibits matrix metalloproteinase activity in vivo without affecting antitumor efficacy in malignant glioma. Cancer Res. 2005;65:9398–405. doi: 10.1158/0008-5472.CAN-04-4264. [DOI] [PubMed] [Google Scholar]
- 62.Soria C, Estermann FE, Espantman KC, O’Shea CC. Heterochromatin silencing of p53 target genes by a small viral protein. Nature. 2010;466:1076–81. doi: 10.1038/nature09307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–60. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 64.Piao Y, Jiang H, Alemany R, et al. Oncolytic adenovirus retargeted to Delta-EGFR induces selective antiglioma activity. Cancer Gene Ther. 2009;16:256–65. doi: 10.1038/cgt.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ulasov, Zhu ZB, Tyler MA, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 66.Van Houdt WJ, Wu H, Glasgow JN, et al. Gene delivery into malignant glioma by infectivity-enhanced adenovirus: in vivo versus in vitro models. Neuro Oncol. 2007;9:280–90. doi: 10.1215/15228517-2007-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranki T, Hemminki A. Serotype chimeric human adenoviruses for cancer gene therapy. Viruses. 2010;2:2196–212. doi: 10.3390/v2102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann D, Meyer B, Wildner O. Improved glioblastoma treatment with Ad5/35 fiber chimeric conditionally replicating adenoviruses. J Gene Med. 2007;9:764–78. doi: 10.1002/jgm.1076. [DOI] [PubMed] [Google Scholar]
- 69.Nandi S, Ulasov, Rolle CE, Han Y, Lesniak MS. A chimeric adenovirus with an Ad 3 fiber knob modification augments glioma virotherapy. J Gene Med. 2009;11:1005–11. doi: 10.1002/jgm.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paul CP, Everts M, Glasgow JN, et al. Characterization of infectivity of knob-modified adenoviral vectors in glioma. Cancer Biol Ther. 2008;7:786–93. doi: 10.4161/cbt.7.5.5421. [DOI] [PubMed] [Google Scholar]
- 71.Skog J, Edlund K, Bergenheim AT, Wadell G. Adenoviruses 16 and CV23 efficiently transduce human low-passage brain tumor and cancer stem cells. Mol Ther. 2007;15:2140–5. doi: 10.1038/sj.mt.6300315. [DOI] [PubMed] [Google Scholar]
- 72.Brouwer E, Havenga MJ, Ophorst O, et al. Human adenovirus type 35 vector for gene therapy of brain cancer: improved transduction and bypass of pre-existing anti-vector immunity in cancer patients. Cancer Gene Ther. 2007;14:211–9. doi: 10.1038/sj.cgt.7701010. [DOI] [PubMed] [Google Scholar]
- 73.Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61:554–71. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Kaliberova LN, Krendelchtchikova V, Harmon DK, et al. CRAdRGDflt-IL24 virotherapy in combination with chemotherapy of experimental glioma. Cancer Gene Ther. 2009;16:794–805. doi: 10.1038/cgt.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ulasov, Tyler MA, Zhu ZB, et al. Oncolytic adenoviral vectors which employ the survivin promoter induce glioma oncolysis via a process of beclin-dependent autophagy. Int J Oncol. 2009;34:729–42. doi: 10.3892/ijo_00000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 77.Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol Ther. 2010;18:1927–36. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lun XQ, Jang JH, Tang N, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–88. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Q, Liang C, Yu YA, et al. The highly attenuated oncolytic recombinant vaccinia virus GLV-1h68: comparative genomic features and the contribution of F14.5L inactivation. Mol Genet Genomics. 2009;282:417–35. doi: 10.1007/s00438-009-0475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Advani SJ, Buckel L, Chen NG, et al. Preferential replication of systemically delivered oncolytic vaccinia virus in focally irradiated glioma xenografts. Clin Cancer Res. 2012;18:2579–90. doi: 10.1158/1078-0432.CCR-11-2394. [DOI] [PubMed] [Google Scholar]
- 81.Touchefeu Y, Vassaux G, Harrington KJ. Oncolytic viruses in radiation oncology. Radiother Oncol. 2011;99:262–70. doi: 10.1016/j.radonc.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 82.Tai CK, Kasahara N. Replication-competent retrovirus vectors for cancer gene therapy. Front Biosci. 2008;13:3083–95. doi: 10.2741/2910. [DOI] [PubMed] [Google Scholar]
- 83.Goetz C, Dobrikova E, Shveygert M, Dobrikov M, Gromeier M. Oncolytic poliovirus against malignant glioma. Future Virol. 2011;6:1045–58. doi: 10.2217/fvl.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dobrikova EY, Broadt T, Poiley-Nelson J, et al. Recombinant oncolytic poliovirus eliminates glioma in vivo without genetic adaptation to a pathogenic phenotype. Mol Ther. 2008;16:1865–72. doi: 10.1038/mt.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goetz C, Gromeier M. Preparing an oncolytic poliovirus recombinant for clinical application against glioblastoma multiforme. Cytokine Growth Factor Rev. 2010;21:197–203. doi: 10.1016/j.cytogfr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 2012;7:347–67. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meng C, Zhou Z, Jiang K, et al. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch Virol. 2012;157:1011–8. doi: 10.1007/s00705-012-1270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lam HY, Yeap SK, Rasoli M, et al. Safety and clinical usage of newcastle disease virus in cancer therapy. J Biomed Biotechnol. 2011;2011:718710. doi: 10.1155/2011/718710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zulkifli MM, Ibrahim R, Ali AM, et al. Newcastle diseases virus strain V4UPM displayed oncolytic ability against experimental human malignant glioma. Neurol Res. 2009;31:3–10. doi: 10.1179/174313208X325218. [DOI] [PubMed] [Google Scholar]
- 90.Galanis E. Therapeutic potential of oncolytic measles virus: promises and challenges. Clin Pharmacol Ther. 2010;88:620–5. doi: 10.1038/clpt.2010.211. [DOI] [PubMed] [Google Scholar]
- 91.Allen C, Paraskevakou G, Liu C, et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin Biol Ther. 2008;8:213–20. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leber MF, Bossow S, Leonard VH, et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol Ther. 2011;19:1097–106. doi: 10.1038/mt.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Allen C, Paraskevakou G, Iankov I, et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol Ther. 2008;16:1556–64. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–9. [PubMed] [Google Scholar]
- 95.Myers R, Harvey M, Kaufmann TJ, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19:690–8. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Opyrchal M, Allen C, Iankov I, et al. Effective radiovirotherapy for malignant gliomas by using oncolytic measles virus strains encoding the sodium iodide symporter MV-NIS. Hum Gene Ther. 2012;23:419–27. doi: 10.1089/hum.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen C, Opyrchal M, Aderca I, et al. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther. 2012 doi: 10.1038/gt.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–9. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 99.LB Stojdl DF, tenOever BR, Paterson JM, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–75. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 100.Lun X, Senger DL, Alain T, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(delta-M51);on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98:1546–57. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 101.Cary ZD, Willingham MC, Lyles DS. Oncolytic vesicular stomatitis virus induces apoptosis in U87 glioblastoma cells by a type II death receptor mechanism and induces cell death and tumor clearance in vivo. J Virol. 2011;85:5708–17. doi: 10.1128/JVI.02393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.TP Wollmann G, van den Pol AN. Targeting human glioblastoma cells: comparison of nine viruses with oncolytic potential. J Virol. 2005;79:6005–22. doi: 10.1128/JVI.79.10.6005-6022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.RV Wollmann G, Simon I, Rose JK, van den Pol AN. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J Virol. 2010;84:1563–73. doi: 10.1128/JVI.02040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muik A, Dold C, Geiss Y, et al. Semireplication-competent vesicular stomatitis virus as a novel platform for oncolytic virotherapy. J Mol Med (Berl) 2012;90:959–70. doi: 10.1007/s00109-012-0863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Comins C, Heinemann L, Harrington K, et al. Reovirus: viral therapy for cancer ‘as nature intended’. Clin Oncol (R Coll Radiol) 2008;20:548–54. doi: 10.1016/j.clon.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 106.Yang WQ, Lun X, Palmer CA, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–76. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 107.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–12. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 108.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–32. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 109.Alloussi SH, Alkassar M, Urbschat S, Graf N, Gartner B. All reovirus subtypes show oncolytic potential in primary cells of human high-grade glioma. Oncol Rep. 2011;26:645–9. doi: 10.3892/or.2011.1331. [DOI] [PubMed] [Google Scholar]
- 110.Nakashima H, Kaur B, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21:119–26. doi: 10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed AU, Tyler MA, Thaci B, et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharm. 2011;8:1559–72. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tyler MA, Ulasov, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16:262–78. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yong RL, Shinojima N, Fueyo J, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–40. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thaci B, Ahmed AU, Ulasov, et al. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther. 2012;19:431–42. doi: 10.1038/cgt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–98. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 116.Ostertag D, Amundson KK, Lopez Espinoza F, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14:145–59. doi: 10.1093/neuonc/nor199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tai CK, Wang W, Lai YH, et al. Enhanced efficiency of prodrug activation therapy by tumor-selective replicating retrovirus vectors armed with the Escherichia coli purine nucleoside phosphorylase gene. Cancer Gene Ther. 2010;17:614–23. doi: 10.1038/cgt.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dent P, Yacoub A, Hamed HA, et al. MDA-7/IL-24 as a cancer therapeutic: from bench to bedside. Anticancer Drugs. 2010;21:725–31. doi: 10.1097/CAD.0b013e32833cfbe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yerbes R, Palacios C, Lopez-Rivas A. The therapeutic potential of TRAIL receptor signalling in cancer cells. Clin Transl Oncol. 2011;13:839–47. doi: 10.1007/s12094-011-0744-4. [DOI] [PubMed] [Google Scholar]
- 120.Candolfi M, Xiong W, Yagiz K, et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc Natl Acad Sci U S A. 2010;107:20021–6. doi: 10.1073/pnas.1008261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.King GD, Kroeger KM, Bresee CJ, et al. Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumorinduced behavioral deficits. Mol Ther. 2008;16:682–90. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.King GD, Muhammad AK, Curtin JF, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.King GD, Muhammad AK, Larocque D, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol Ther. 2011;19:1793–801. doi: 10.1038/mt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghulam Muhammad AK, Xiong W, Puntel M, et al. Safety profile of gutless adenovirus vectors delivered into the normal brain parenchyma: implications for a glioma phase 1 clinical trial. Hum Gene Ther Methods. 2012;23:271–84. doi: 10.1089/hgtb.2012.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Puntel M, Muhammad AK, Candolfi M, et al. A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics. J Virol. 2010;84:6007–17. doi: 10.1128/JVI.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muhammad AK, Puntel M, Candolfi M, et al. Study of the efficacy, biodistribution, and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin Pharmacol Ther. 2010;88:204–13. doi: 10.1038/clpt.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huszthy PC, Giroglou T, Tsinkalovsky O, et al. Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. PLoS One. 2009;4:e6314. doi: 10.1371/journal.pone.0006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li S, Tokuyama T, Yamamoto J, et al. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;12:600–7. doi: 10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- 130.Li S, Gao Y, Tokuyama T, et al. Genetically engineered neural stem cells migrate and suppress glioma cell growth at distant intracranial sites. Cancer Lett. 2007;251:220–7. doi: 10.1016/j.canlet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 131.Kim SU, Nagai A, Nakagawa E, et al. Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol Biol. 2008;438:103–21. doi: 10.1007/978-1-59745-133-8_10. [DOI] [PubMed] [Google Scholar]
- 132.Kim JH, Lee JE, Kim SU, Cho KG. Stereological analysis on migration of human neural stem cells in the brain of rats bearing glioma. Neurosurgery. 2010;66:333–42. doi: 10.1227/01.NEU.0000363720.07070.A8. discussion 342. [DOI] [PubMed] [Google Scholar]
- 133.Kim JH, Kim JY, Kim SU, Cho KG. Therapeutic effect of genetically modified human neural stem cells encoding cytosine deaminase on experimental glioma. Biochem Biophys Res Commun. 2012;417:534–40. doi: 10.1016/j.bbrc.2011.11.155. [DOI] [PubMed] [Google Scholar]
- 134.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 135.Sasportas LS, Kasmieh R, Wakimoto H, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106:4822–7. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doucette T, Rao G, Yang Y, et al. Mesenchymal stem cells display tumor-specific tropism in an RCAS/Ntv-a glioma model. Neoplasia. 2011;13:716–25. doi: 10.1593/neo.101680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bak XY, Yang J, Wang S. Baculovirus-transduced bone marrow mesenchymal stem cells for systemic cancer therapy. Cancer Gene Ther. 2010;17:721–9. doi: 10.1038/cgt.2010.32. [DOI] [PubMed] [Google Scholar]
- 138.Kim SM, Lim JY, Park SI, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–23. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 139.Choi SA, Lee JY, Wang KC, et al. Human adipose tissue-derived mesenchymal stem cells: characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas. Eur J Cancer. 2012;48:129–37. doi: 10.1016/j.ejca.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 140.Bak XY, Lam DH, Yang J, et al. Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum Gene Ther. 2011;22:1365–77. doi: 10.1089/hum.2010.212. [DOI] [PubMed] [Google Scholar]
- 141.Kosaka H, Ichikawa T, Kurozumi K, et al. Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther. 2012;19:572–8. doi: 10.1038/cgt.2012.35. [DOI] [PubMed] [Google Scholar]
- 142.Altanerova V, Cihova M, Babic M, et al. Human adipose tissue-derived mesenchymal stem cells expressing yeast cytosinedeaminase: uracil phosphoribosyltransferase inhibit intracerebral rat glioblastoma. Int J Cancer. 2012;130:2455–63. doi: 10.1002/ijc.26278. [DOI] [PubMed] [Google Scholar]
- 143.Amano S, Li S, Gu C, et al. Use of genetically engineered bone marrow-derived mesenchymal stem cells for glioma gene therapy. Int J Oncol. 2009;35:1265–70. doi: 10.3892/ijo_00000443. [DOI] [PubMed] [Google Scholar]
- 144.Yin J, Kim JK, Moon JH, et al. hMSC-mediated concurrent delivery of endostatin and carboxylesterase to mouse xenografts suppresses glioma initiation and recurrence. Mol Ther. 2011;19:1161–9. doi: 10.1038/mt.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Choi SA, Hwang SK, Wang KC, et al. Therapeutic efficacy and safety of TRAIL-producing human adipose tissue-derived mesenchymal stem cells against experimental brainstem glioma. Neuro Oncol. 2011;13:61–9. doi: 10.1093/neuonc/noq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Menon LG, Kelly K, Yang HW, et al. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells. 2009;27:2320–30. doi: 10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- 147.Kim SM, Woo JS, Jeong CH, et al. Effective combination therapy for malignant glioma with TRAIL-secreting mesenchymal stem cells and lipoxygenase inhibitor MK886. Cancer Res. 2012;72:4807–17. doi: 10.1158/0008-5472.CAN-12-0123. [DOI] [PubMed] [Google Scholar]
- 148.Uzzaman M, Keller G, Germano IM. In vivo gene delivery by embryonic-stem-cell-derived astrocytes for malignant gliomas. Neuro Oncol. 2009;11:102–8. doi: 10.1215/15228517-2008-056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Germano IM, Emdad L, Qadeer ZA, Binello E, Uzzaman M. Embryonic stem cell (ESC)-mediated transgene delivery induces growth suppression, apoptosis and radiosensitization, and overcomes temozolomide resistance in malignant gliomas. Cancer Gene Ther. 2010;17:664–74. doi: 10.1038/cgt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee EX, Lam DH, Wu C, et al. Glioma gene therapy using induced pluripotent stem cell derived neural stem cells. Mol Pharm. 2011;8:1515–24. doi: 10.1021/mp200127u. [DOI] [PubMed] [Google Scholar]
- 151.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery. 2012;71:201–23. doi: 10.1227/NEU.0b013e31824f840d. [DOI] [PubMed] [Google Scholar]
- 154.Ali S, Curtin JF, Zirger JM, et al. Inflammatory and antiglioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–84. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barnard Z, Wakimoto H, Zaupa C, et al. Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma. Neurosurgery. 2012;71:741–8. doi: 10.1227/NEU.0b013e318260fd73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Breitbach CJ, Thorne SH, Bell JC, Kirn DH. Targeted and armed oncolytic poxviruses for cancer: the lead example of JX-594. Curr Pharm Biotechnol. 2012;13:1768–72. doi: 10.2174/138920112800958922. [DOI] [PubMed] [Google Scholar]
- 157.Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol Ther. 2010;18:1927–36. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–85. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 159.Hellums EK, Markert JM, Parker JN, et al. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol. 2005;7:213–24. doi: 10.1215/S1152851705000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Markert JM, Cody JJ, Parker JN, et al. Preclinical evaluation of a genetically engineered herpes simplex virus expressing interleukin-12. J Virol. 2012;86:5304–13. doi: 10.1128/JVI.06998-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ryu CH, Park SH, Park SA, et al. Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum Gene Ther. 2011;22:733–43. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 162.Gerstner ER, Batchelor TT. Antiangiogenic therapy for glioblastoma. Cancer J. 2012;18:45–50. doi: 10.1097/PPO.0b013e3182431c6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang W, Fulci G, Buhrman JS, et al. Bevacizumab With Angiostatin-armed oHSV increases antiangiogenesis and decreases bevacizumab-induced invasion in U87 glioma. Mol Ther. 2012;20:37–45. doi: 10.1038/mt.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hardcastle J, Kurozumi K, Dmitrieva N, et al. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol Ther. 2010;18:285–94. doi: 10.1038/mt.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu TC, Zhang T, Fukuhara H, et al. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther. 2006;14:789–97. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 166.Yuan B, Xian R, Ma J, et al. Isthmin inhibits glioma growth through antiangiogenesis in vivo. J Neurooncol. 2012;109:245–52. doi: 10.1007/s11060-012-0910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13:221–8. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 169.van Putten EH, Dirven CM, van den Bent MJ, Lamfers ML. Sitimagene ceradenovec: a gene-based drug for the treatment of operable high-grade glioma. Future Oncol. 2010;6:1691–710. doi: 10.2217/fon.10.134. [DOI] [PubMed] [Google Scholar]
- 170.Chiocca EA, Aguilar LK, Bell SD, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–9. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–43. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 172.Harrow S, Papanastassiou V, Harland J, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–58. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 173.Alonso MM, Cascallo M, Gomez-Manzano C, et al. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007;67:8255–63. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- 174.Wohlfahrt ME, Beard BC, Lieber A, Kiem HP. A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL leads to enhanced cancer cell killing in human glioblastoma models. Cancer Res. 2007;67:8783–90. doi: 10.1158/0008-5472.CAN-07-0357. [DOI] [PubMed] [Google Scholar]
- 175.Ochiai H, Campbell SA, Archer GE, et al. Targeted therapy for glioblastoma multiforme neoplastic meningitis with intrathecal delivery of an oncolytic recombinant poliovirus. Clin Cancer Res. 2006;12:1349–54. doi: 10.1158/1078-0432.CCR-05-1595. [DOI] [PubMed] [Google Scholar]
- 176.Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol. 2004;67:83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 177.Alkassar M, Gartner B, Roemer K, et al. The combined effects of oncolytic reovirus plus Newcastle disease virus and reovirus plus parvovirus on U87 and U373 cells in vitro and in vivo. J Neurooncol. 2011;104:715–27. doi: 10.1007/s11060-011-0606-5. [DOI] [PubMed] [Google Scholar]
- 178.Wollmann G, Rogulin V, Simon I, Rose JK, van den Pol AN. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J Virol. 2010;84:1563–73. doi: 10.1128/JVI.02040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jeong M, Kwon YS, Park SH, et al. Possible novel therapy for malignant gliomas with secretable trimeric TRAIL. PLoS One. 2009;4:e4545. doi: 10.1371/journal.pone.0004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu Y, Lang F, Xie X, et al. Efficacy of adenovirally expressed soluble TRAIL in human glioma organotypic slice culture and glioma xenografts. Cell Death Dis. 2011;2:e121. doi: 10.1038/cddis.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hamed HA, Yacoub A, Park MA, et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18:1130–42. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 182.Wang W, Tai CK, Kershaw AD, et al. Use of replication-competent retroviral vectors in an immunocompetent intracranial glioma model. Neurosurg Focus. 2006;20:E25. doi: 10.3171/foc.2006.20.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tai CK, Wang WJ, Chen TC, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12:842–51. doi: 10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barnard Z, Wakimoto H, Zaupa C, et al. Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma. Neurosurgery. 2012;71:741–8. doi: 10.1227/NEU.0b013e318260fd73. [DOI] [PMC free article] [PubMed] [Google Scholar]