Abstract
Fibrocytes are unique, fibroblast-like cells with diverse functions and the potential for immunomodulation which prompted investigation of their previously unexplored role in sepsis. Specifically, the study goals were to determine if adoptive transfer of fibrocytes would affect outcome in sepsis and to define relevant immunopathologic changes associated with the outcomes. Initial in vitro studies demonstrated that naïve T cell proliferation was significantly increased in co-cultures with tissue-derived fibrocytes as compared to culture either alone or with fibroblasts. In vivo, the adoptive transfer of fibrocytes at the time of cecal ligation and puncture (CLP) significantly improved survival of mice compared to transfer of fibroblasts or saline. Septic mice had lower blood levels of IL-6 and markers of organ injury after fibrocyte transfer as well as a reduced bacterial burden. Locally, peritoneal lavage fluid yielded lower bacterial counts, lower IL-6, and reduced inflammatory cell counts when fibrocyte transfer was compared to saline. This was also accompanied by significant increases in splenic CD4+ and CD8+ T cells. In vitro stimulation of the splenic T cells demonstrated that, after CLP and adoptive transfer, the percentages of both CD4+ and CD8+ T cells with intracellular IFN-γ were increased while those with IL-4 remained similar between the groups. Therefore, it appears the adoptive transfer of fibrocytes improves sepsis survival, lowered bacterial burden, and promotes the proliferation of splenic T cells with a Th1 phenotype. These results confirm the immunomodulatory effects of exogenous, tissue-derived fibrocytes in sepsis and suggest their potential in cell therapy.
Keywords: Th1/Th2 cytokines, immunomodulation, sepsis, cell therapy
INTRODUCTION
The term sepsis denotes a complex systemic inflammatory response to infection that can produce serious consequences. In spite of improvements in intensive care, the incidence of sepsis increases annually (1) and has prompted investigations of the immune mechanisms associated with poor outcomes. Consequently, sepsis outcomes have been linked to several changes in immune function including reduced bacterial clearance, hyperinflammatory states, cytokine imbalances and functional loss of certain cell types (2–5). Specifically, sharp decreases in T cells numbers have been shown in experimental and clinical studies. In fact, loss of T cells has been definitively linked to reduced survival (3, 4) while preserving or restoring T cells improved survival (6–8). In addition, CD4+ T cells from septic mice have shown impaired proliferation, poor antigenic stimulation, and decreased production of Th1 cytokines (9). A shift from the protective Th1 cytokine response (IL-12, IFN-γ) to a predominantly Th2 response (IL-4, IL-5, IL-13) can significantly impair sepsis outcomes (2, 10). Therefore, preservation of T cell numbers and the Th1 phenotype have become significant targets for the treatment of sepsis (6–8).
Theoretically, fibrocytes could counteract many of the immune defects of sepsis. First described in 1994, fibrocytes are fibroblast-like cells of bone marrow origin that display both hematopoeitic and mesenchymal characteristics. They are blood-borne cells that comprise ~0.5–1.0% of the non-erythroid pool in peripheral blood (11). Functionally, fibrocytes are renowned for physiological and pathological collagen production (12–14). However, given their diverse characteristics, it is not surprising that fibrocytes have been described as pluripotent (15, 16) and have the potential for immunomodulatory functions. Fibrocytes migrate toward areas of acute inflammation where they may represent up to 15% of the cells (17, 18). They are known to express Toll-like receptors (19) and produce pro-inflammatory cytokines (12, 19) as well as antimicrobial peptides (16). Of particular interest, fibrocytes express adhesion, costimulatory, MHC class I and MHC class II molecules, all of which are pertinent to T cell interactions (14, 18). In fact, fibrocytes have the ability to stimulate naive T cells and promote viral antigen-specific T cell proliferation in an MHC class I dependent interaction, independent of macrophage function (17, 20). In addition, studies have shown that fibrocytes secrete chemoattractants for CD4+T cells (14, 17), suggesting the potential to recruit T cells to sites of inflammation. Even more intriguing, recent studies show reprogramming of mature fibrocytes may occur by a novel transdifferentiation (21) that ultimately rebalances cytokine production by CD4+ T cells to favor Th1 responses (22). The sum of these findings would suggest that fibrocytes have immunomodulatory capabilities that would be beneficial but this has not been studied in a model of bacterial sepsis.
Therefore, the purpose of this study was to perform adoptive transfer of fibrocytes and examine the effects in the cecal ligation and puncture (CLP) model of sepsis. The results of survival experiments demonstrated a dramatic improvement in survival and decrease in bacterial load after CLP accompanied by the adoptive transfer of fibrocytes. Also noteworthy, the adoptive transfer of fibrocytes increased both CD4+ and CD8+ T cell numbers in the spleen and increased their potential to launch a Th1 response. Overall, the adoptive transfer of fibrocytes demonstrated significant immunomodulatory effects that may have mechanisms that are distinct from those seen with other types of cell transfers in similar sepsis models.
MATERIAL AND METHODS
Animals
Specific-pathogen free, male, C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were used as donors and recipients in the in vivo experiments. Specific-pathogen-free, female, ICR mice (23–25g; Harlan Laboratories, Inc, Indianapolis, IN) were also used in the experiments tracking CFSE labeled cells and those included as Supplemental Digital Content. The mice were housed in a temperature-controlled room with a 12-hour dark/light cycle. Food and water were given ad libitum. The Institutional Animal Care and Use Committee approved all of the procedures and studies were adherent to animal use guidelines.
Fibrocyte culture and purification
Normal mouse lung was used as the tissue source to provide fibrocytes as described in past studies (13). All of the lung lobes were removed from a mouse and gently minced. The tissues were placed in a culture flask containing DMEM with 10% FBS, 1% L-glutamine, and 1% Antibiotic/Antimycotic (Invitrogen; Carlsbad, CA). The lung cells were cultured for 2 weeks with media changes every 3–4 days. After 2 weeks, at nearly 100% confluency, the cells were removed from the flask by mechanical scraping after addition of trypsin. Cells were washed with PBS and resuspended. Fibrocytes were isolated from the suspension based on expression of CD45 by using antibody coupled magnetic beads (Miltenyi, Bergisch Gladbach, Germany) per the manufacturer’s instructions. This consistently yields a CD45+ and collagen 1+ population that is 95% pure (13). The remaining CD45- population (fibroblasts) were also processed for use in some experiments as control cells. The cell populations were counted on a hemacytometer (Hausser Scientific Horsham, PA) and their viability confirmed with trypan blue (Invitrogen). For immediate transfer, 1.0 × 106 cells were resuspended in 200 μl saline.
Fibrocyte: T-cell coculture
T cells were harvested by removing the spleens of mice and gently teasing the organ apart in 10ml of PBS. Following a wash and centrifugation (8min, 150g), 5ml of RBC Lysis Buffer (ebiosciences, San Diego, CA) were added and the T cells were separated by negative isolation using magnetic depletion beads (Life Technologies, Grand Island, NY) per the manufacturer’s instructions. Total live T cells were determined by trypan blue staining and counted with a hemacytometer. For coculture experiments, isolated T cells were incubated with CFSE (Invitrogen) at 5mM per 107 cells/ml for 10min in a warm water bath (37°C). Cultured fibrocytes were plated at a 1:10 ratio with CFSE loaded T cells. Cultures of T cells alone served as controls. All cocultures were incubated for 1wk at 37°C in a 5% CO2 incubator. T cells and their activation were identified by flow cytometry after staining with fluorescently labeled monoclonal antibodies against CD69 (PE-A) and CD3 (APC-Cy7). T-cell proliferation was assessed by loss of CFSE fluorescence in CD3+ cells as previously described (23). For all of the in vitro studies, experiments were repeated in three or four independent runs. Each time point is comprised of data from independent experiments, not serial collection from the same cultures.
CLP and adoptive transfer
Mice were anesthetized with isoflurane for laparotomy. The cecum was exteriorized, ligated, and punctured twice with a 21 gauge needle using previously described methods (24). Prior to complete closure of the abdomen, 1.0 × 106 cultured cells (either fibrocytes or fibroblasts) or equal volume saline were deposited into the abdomen. After surgery, each mouse was given 1ml of warm saline and 0.1mg/kg buprenorphine, subcutaneously.
Sample harvest and processing
Mice were anesthetized and blood collected from the retroorbital plexus directly into tubes (BD Microtainer tubes with EDTA; BD Laboratories, Franklin Lakes, NJ). A complete blood count was performed using a Hemavet Mascot Multispecies Hematology System Counter (CDC Technologies, Oxford, CT). After euthanasia, peritoneal lavage was performed by injecting 10mls of HBSS into the closed abdomen and retrieving 8 mls of the solution. The fluid was centrifuged (600g, 5 min) and supernatants were stored at −20 °C for cytokine analysis. The cell pellet was resuspended, RBCs were lysed and total counts were performed with a hemacytometer. Slides were loaded with 1×105 cells, stained with Diff-Quick (Baxter) and a differential of 300 cells counted under light microscopy. The spleens were removed and processed for flow cytometry.
Bacterial burden
Samples were obtained aseptically and included blood obtained by cardiac puncture, homogenized spleen and peritoneal lavage fluid. Samples were serially diluted and plated on 5% sheep blood agar (Remel, Lenexa, KS). The plates were incubated for 24 hours at 37°C and colonies were counted.
Flow cytometry
Splenocytes were obtained from the whole spleen as indicated above. The splenocytes were resuspended (1 × 106cells/100μl) and incubated with FcγII/III reagent (BD biosciences; 0.5ug/100ul aliquot, 5–10 minutes, 4°C). The cells were stained with the appropriate antibodies against surface antigens or their appropriate isotype controls (2.5μg/ml, 30 minutes, 4°C) which included PerCP-Cy5.5 hamster anti-mouse CD3e or PerCP-Cy5.5 hamster IgG1 (BD biosciences); Alexa Fluor 700 hamster anti-mouse CD69 or Alexa Flour 700 hamster IgG1 (BD biosciences); Anti-mouse CD4 V450 or Rat IgG2b V450 (ebiosciences, San Diego CA); Pacific orange anti-mouse CD8 or Pacific orange rat IgG2b (Life Technologies). For intracellular cytokines, cells were stained with PerCpCy5.5 anti-mouse IFN-γ (BD biosciences) and FITC anti-mouse IL-4 (BD biosciences) using an established protocol (BD Cytofix/Cytoperm Kit, BD biosciences). For all flow cytometry, fluorescence was measured utilizing a BD LSR II flow cytometer (BD biosciences) with a 488nm excitation laser with peak emission ranging from 515nm to 545nm. Compensation was performed in all experiments utilizing Winlist 6.0 software (Topsham, ME).
Cytokine measurement
Cell culture and plasma samples were diluted 1:10 and peritoneal lavage fluid was diluted 1:2. IL-6 was measured with a sandwich ELISA using matched antibody pairs (R&D Systems, Minneapolis, MN) as previously described by this laboratory(24). In addition, the Th1/Th2 ELISA Ready-SET-Go!® kit (ebiosciences) was used to measure IL-2, IL-4, IL-10 and IFN-γ per manufacturer’s instructions. Optical densities were read at 450nm on a plate reader (Biotek, Winooski, VT). KC4™ Data analysis software (Biotek) was used to assess the results.
Organ function tests
Levels of aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine were evaluated through a University of Michigan core facility using the Idexx Vet Test Analyzer (Model 8008) (Westbrook, ME), a dry chemistry analyzer. The system uses a colorimetric optical system to quantify end-point and rate measurements of biochemical reactions.
Splenocyte culture and stimulation
Spleens were harvested from mice 24hrs after CLP and processed to yield cell suspensions. One million splenocytes were plated per well and incubated overnight with plate bound anti-CD3 (1ug/ml) and anti-CD28 (2ug/ml) (BD biosciences, San Jose, CA). Within 4 hours of harvest, a protein transport inhibitor (BD GolgiPlug™; BD biosciences) was added to the cultures. The cells were processed for flow cytometry of intracellular cytokines (IFN-γ and IL-4) and extracellular T cell markers (CD4 and CD8) as described below.
Statistical analysis
Multiple groups were analyzed by one way or two way ANOVA where appropriate and differences (P <0.05) were compared post hoc by the Tukey multiple comparison method. When 2 groups were evaluated, a Student’s t test was performed. For survival studies, Kaplan-Meier curves were calculated for each group and their significance analyzed by logrank tests. GraphPad Prism® version 5.01 for Windows (GraphPad Software, San Diego California, USA) was used for analysis.
RESULTS
Fibrocytes increase splenic T cell proliferation and cytokine production in vitro
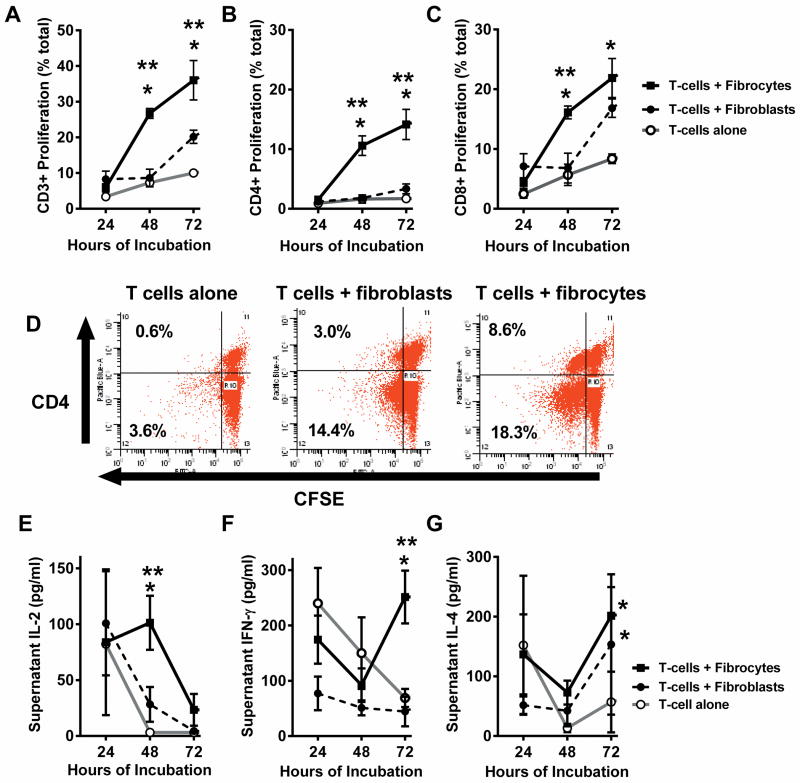
To determine the potential for interaction, cultured fibrocytes or fibroblasts were incubated with CFSE-stained T cells from normal, syngeneic mice for 3 days (4 experiments/timepoint). At 24 hour intervals, cultures were processed with antibodies to identify T cell subpopulations via flow cytometry. Cultured alone, T cells demonstrated little proliferation as evidenced by no change in the intensity of CFSE staining; however, when cultured with fibrocytes, the percentage of CD3+ cells exhibiting proliferation was significantly increased at 48 and 72 hours of culture as compared to T cells cultured alone or with fibroblasts (Figure 1A). When gated for CD3 expression and analyzed for CD4 expression and CFSE load, the percentage of CD4+ T cells demonstrating proliferation increased over time and was significantly higher when the T cells were cultured with fibrocytes as compared to T cells alone or with fibroblasts (Figure 1B). When gated for CD3 and analyzed for CD8 expression and CFSE load, the results also demonstrated increased proliferation at 48 and 72 hours of incubation when cultured with fibrocytes as compared to T cells alone (Figure 1C). When cultured with fibroblasts, the proliferation of CD8+ T cells did eventually occur but was delayed to 72 hours of culture compared to fibrocytes. In the representative dot plots (Figure 1D), gated for CD3+, the concentrated left shift of CD4+ cells on the x-axis demonstrated at least one cell division of T cells had taken place in the cocultures with fibrocytes (8.6% of CD4+T cells) as compared to cocultures with fibroblasts (3.0% of CD4+ T cells) within 72 hours.
Figure 1. Fibrocytes increase T cell proliferation and alter cytokine profiles in vitro.
CFSE-stained splenic T cells were cultured alone, with tissue derived fibroblasts or fibrocytes for 72 hours. At 24 hour intervals, cocultures were processed for flow cytometry with fluorescently labeled antibodies against CD3, CD4 and CD8, demonstrating A) Proliferation of the CD3+ cell population, B) Percentage of CD4+ T cells exhibiting proliferation, and C) Percentage of CD8 + T cells exhibiting proliferation. D) Representative plots of cells after 72 hours of incubation demonstrating flow cytometry results for CD4 and CFSE staining of cells gated for CD3. In addition, culture media were assayed for cytokines with sandwich ELISAs, demonstrating concentrations of E) IL-2 F) IFN-γ and G) IL-4. (n= 4 independent experiments/timepoint), Data are reported as mean ± SEM. * = p<0.05 as compared to saline group. ** = p<0.05 as compared to fibroblast group.
Cytokines were measured in the culture media via ELISAs. On Day 1, IL-2 levels were similar for all groups (Figure 1E). The IL-2 levels declined rapidly by 48 hours when T cells were cultured either alone or with fibroblasts, suggesting consumption of the IL-2 by the existing T cells; however, the IL-2 levels in T cell-fibrocyte cocultures remained significantly elevated as compared to the other groups at 48 hours. When fibrocytes were cultured alone, the cell media did not contain detectable amounts of IL-2 at 48 hours (lower limit of detection = <10 pg/ml, n=3), suggesting the high IL-2 seen in cocultures was more than an additive effect of the two cell types. IFN-γ levels in cocultures of T cells with either fibrocytes or fibroblasts showed decline over 48 hours (Figure 1F); however, IFN-γ levels in cocultures with fibrocytes demonstrated a significant rebound at 72 hours, suggesting an increase in production or a decrease in consumption. When fibrocytes or fibroblasts were cultured alone, IFN-γ levels were below the limit of detection of the assay (lower limit 30 pg/ml, n=3). IL-4 levels fluctuated but by 72 hours were elevated in cocultures of T cells with either fibrocytes or fibroblasts in comparison to T cells alone (Figure 1G). Overall, the results suggested stronger CD4+ T cell proliferation and a greater Th1 response when T cells were cultured with fibrocytes versus culture with fibroblasts or alone. This proliferation occurred in the absence of specific antigenic stimulation and may represent an innate effect of fibrocytes on T cells.
While cytokine production might contribute to the increased T cell proliferation seen in the presence of fibrocytes, soluble factors might not be the sole mediators of increased proliferation. To examine this, a transwell system was used to culture CFSE-loaded T cells alone, in contact with fibrocytes, or physically separated from fibrocytes. At 24 hour intervals, cells were harvested and proliferation was assessed with flow cytometry. T cells demonstrated minimal proliferation when physically separated from fibrocytes (see figure, Supplemental Digital Content 1, demonstrating lack of T cell proliferation in a transwell culture), in spite of shared culture media and soluble factors.
Adoptive transfer of fibrocytes enhances sepsis survival
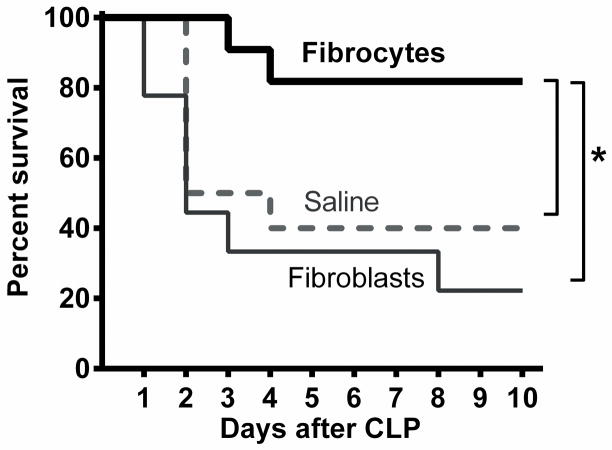
Immediately after CLP, cultured fibrocytes identified by the surface expression of CD45, a marker of bone marrow origin, were deposited in the abdomen. An equal number of cultured fibroblasts, defined by the absence of CD45 denoting their mesenchymal origin, was delivered as a control to another group of mice. A third group received an equal volume of saline. All of the mice (n=10 mice/group, 3 independent experiments) were observed for ten days after surgery. The majority of the deaths occurred within the first two days (Figure 2). The saline group had a survival rate of 40% while the fibroblast group had a 30% survival. However, the fibrocyte group had a significantly increased (p<0.05) survival of 80% compared to either control group, suggesting the adoptive transfer of fibrocytes has a beneficial effect on the outcome of sepsis. Similar results were obtained in separate experiments with female, ICR mice given either fibrocytes or saline after CLP (see figure, Supplemental Digital Content 2A, demonstrating improved survival with fibrocytes).
Figure 2. Fibrocytes enhance survival after CLP.
Male, C57BL/6 mice were anesthetized for CLP surgery followed by adoptive transfer of fibrocytes, fibroblasts or saline. They were observed for ten days after surgery. The results were plotted on a Kaplan Meier Survival Curve. n= 10 mice/group, 3 independent experiments, * = p<0.05.
Adoptive transfer of fibrocytes decreases systemic markers of organ injury
Plasma was obtained 24 and 48 hours after CLP performed in the presence or absence of fibrocyte adoptive transfer (Figure 3). The mean AST level (Figure 3A) was elevated above reference range (59–247 U/L) in both groups at 24 hours after CLP. By 48 hours, the mean AST level in the saline group was still elevated while the level in the fibrocyte group was significantly lower and within the reference range. Both the fibrocyte treated animals and the saline controls had mean BUN levels that were elevated above references ranges (18–29 mg/dl) at 24 hours post-CLP (Figure 3B). The fibrocyte treated animals showed a significant drop in BUN from 24 to 48 hours (p=0.003) to within normal limits. A similar drop was not apparent in the saline controls. Although higher in the saline group, mean creatinine levels remained within or below normal reference ranges (0.2–0.8 mg/dl) throughout the studies and no significant differences were noted between groups (Figure 3C).
Figure 3. Fibrocytes decrease organ injury.
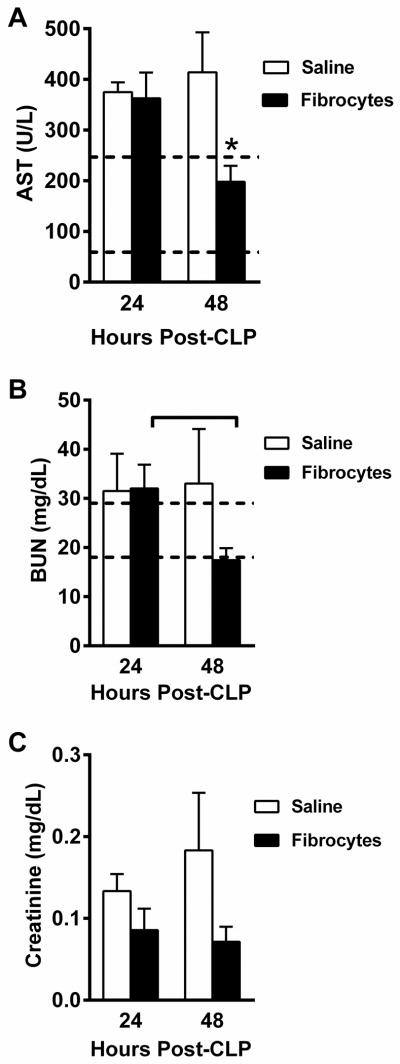
Male, C57BL/6 mice were anesthetized for CLP surgery followed by adoptive transfer of fibrocytes or saline. The mice were euthanized 24 or 48 hours later and plasma was assayed for blood chemistries. Graphs depict A) aspartate aminotransferase (AST), B) blood urea nitrogen (BUN), and C) creatinine. Dotted lines denote the reported laboratory reference ranges. Data are reported as mean ± SEM. n=6–7/group; 3 independent experiments. * = p<0.05 as compared to saline group at that time point.
Adoptive transfer of fibrocytes decreases inflammation
To examine potential mechanisms for the improved survival, we performed adoptive transfer of fibrocytes in mice as described above and compared them to mice that received saline. Since the majority of deaths were observed between 24 and 48 hours, we euthanized the animals at the early time points of 12, 24 and 48 hours after CLP.
Peripheral blood counts
Immediately prior to euthanasia, blood was obtained from the retroorbital plexus and WBC counts were assessed (n=3–6/group). Total WBC counts were below normal limits in both the fibrocyte-treated and saline control animals (Figure 4A). However, there were no differences between the groups at any time point. Peripheral neutrophil counts (Figure 4B) and monocyte counts (Figure 4C) were similar in both groups for the first 24 hours but were significantly lower (p<0.001) in the fibrocyte group as compared to the saline group at 48 hours post-CLP. The mean peripheral blood lymphocyte counts were not significantly different at any of the time points (Figure 4D).
Figure 4. Fibrocytes decrease peripheral inflammatory cell counts.
At the time of CLP surgery, fibrocytes or an equal volume of saline were transferred to the abdomen of C57BL/6 mice. The animals were euthanized at the early time points of 12, 24, and 48 hours. Blood was collected from the retroorbital plexus to obtain A) total WBC counts, B) neutrophil counts, C) monocyte counts, D) lymphocyte counts. Data are reported as mean ± SEM. n= 6–9/group, 3 independent experiments.* = p<0.005 as compared to saline group at that time point. Brackets (black = Fibrocyte, grey = Saline) indicate significance over time within a treatment group.
Plasma cytokines
Plasma cytokines were obtained from the retroorbital plexus blood samples taken at the time of euthanasia (12, 24, or 48 hours). Consistent with the CLP model, mean plasma IL-6 levels were elevated in the early time points after CLP. However, the mean plasma IL-6 levels of the fibrocyte group were significantly decreased in comparison to the saline group at 12 hours post-CLP (Figure 5A). Interestingly, the fibrocyte group also had a significantly lower (p= 0.03) level of the anti- inflammatory cytokine IL-10 as compared to the saline group, although only at the 24 hour time point (Figure 5B). The plasma IFN-γ levels in both the saline and fibrocyte groups increased between 24 and 48 hours but were not significantly different between the groups (Figure 5C). IL-4 levels significantly increased over time in both groups. The fibrocyte group actually had significantly higher levels by 24 hours (Figure 5D) which remained stable until 48 hours; however, the saline group showed continued increases of IL-4 over time which eliminated differences between the groups by 48 hours. The systemic levels of Th1 cytokines were similar between groups and would reflect the cumulative contribution of multiple cell types throughout the body. This prompted further study of localized production of Th1 CD4+ and CD8+ T cells (see below).
Figure 5. Fibrocyte transfer modulates systemic cytokines.
At the time of CLP surgery, fibrocytes or an equal volume of saline were transferred to the abdomen of C57BL/6 mice. The animals were euthanized at the early time points of 12, 24, and 48 hours and blood collected from the retroorbital sinus for cytokine analysis with ELISAs for A) IL-6, B) IL-10, C) IFN-γ, and D)IL-4. Data are reported as mean ± SEM. n= 6–9/group with 3 independent experiments, IL-6 24 hour groups n=20, 4 independent runs. * = p<0.005 as compared to saline group at that time point. Brackets (black = Fibrocyte, grey = Saline) indicate significance over time within a treatment group.
Peritoneal cell counts and IL-6
Peritoneal lavage fluid was evaluated 24 hours after CLP. The total cell counts were significantly lower in the fibrocyte group than in the saline group (Figure 6A). This appears to be the result of reduction in both neturophils and macrophages in the peritoneal cavity. In addition, the fibrocyte group had a signifcantly lower IL-6 in the peritoneal fluid than did the saline group (Figure 6B).
Figure 6. Fibrocytes decrease local inflammation.
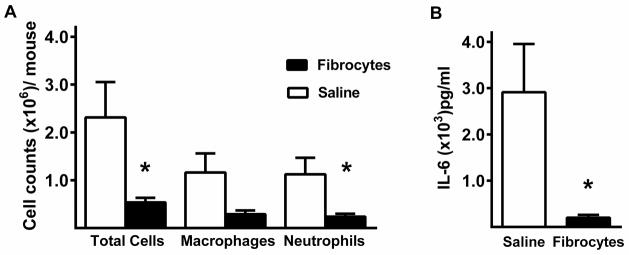
Within 24 hours of CLP with or without adoptive transfer of fibrocytes, peritoneal lavage fluid was collected. The lavage samples were centrifuged to obtain A) total and differential cell counts of the cell pellet and B) IL-6 concentrations from the supernatant. Data are reported as mean ± SEM. n= 8/group, 3 independent experiments. * = p<0.05 as compared to saline group.
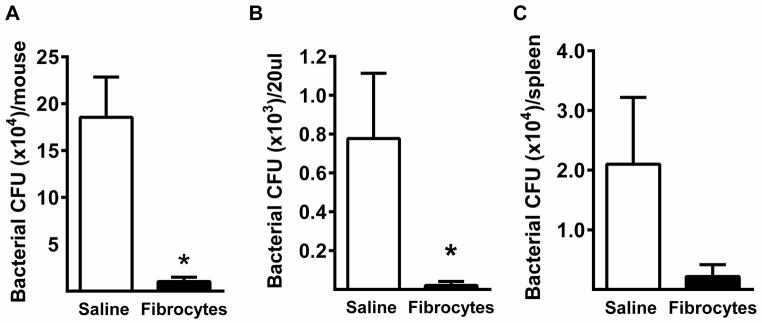
Adoptive transfer reduces bacterial burden
At 24 hours post-CLP, samples were harvested aseptically from mice and processed for assessment of bacterial counts. The mean colony counts derived from peritoneal lavage fluid (Figure 7A), blood (Figure 7B) an dspleen (Figure 7C) were lower in the fibrocyte group as compared to the saline group, with statistically significant differences seen in the peritoneal lavage fluid and blood.
Figure 7. Fibrocytes lower local and systemic bacterial burden.
CLP was performed on mice followed by transfer of either fibrocytes or an equal volume of saline into the abdomen. Samples were harvested aseptically 24 hours after surgery for serial dilution and culture on blood agar plates. After 24 hours of incubation at 37°C, the colony forming units were recorded from A) peritoneal cavity B) blood and C) spleen. n= 7–8/group, 3 independent experiments. Data are reported as mean ± SEM. * = p<0.05 as compared to saline group.
Adoptive transfer of fibrocytes preserves splenic T cells
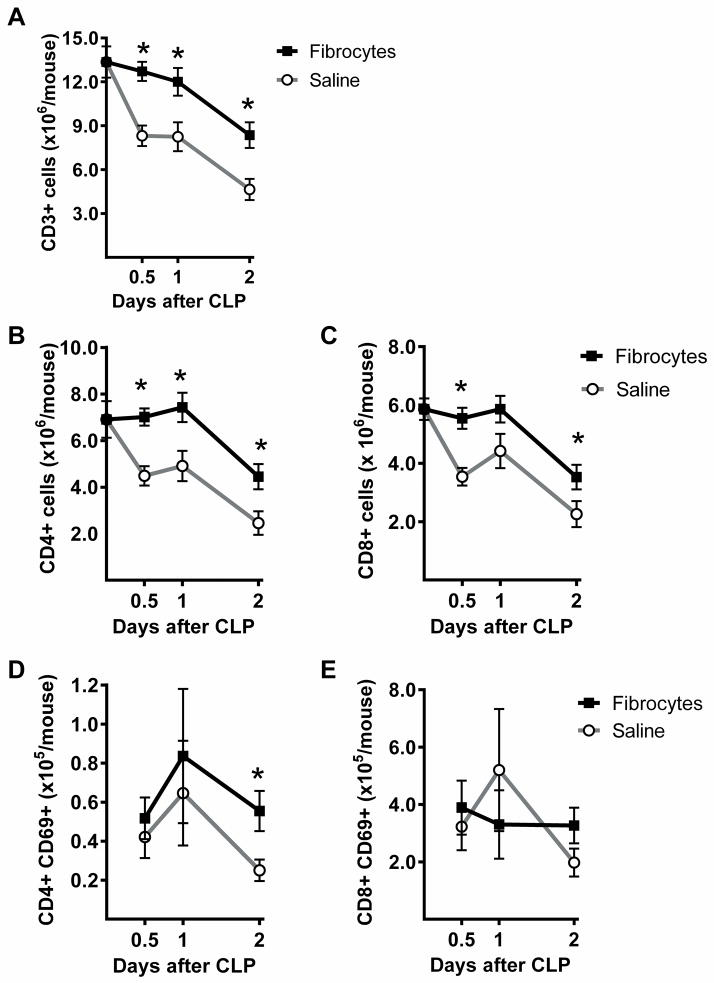
The spleens of mice were harvested 0, 12, 24, and 48 hours after CLP and total cell counts were obtained. Flow cytometry was used to identify T cells by pattern of expression of CD3 and forward scatter. The CD3+ cells demonstrated that the fibrocyte group had significantly greater splenic T cell counts than the saline group at all of the early time points (Figure 8A). Further evaluation was done by gating for CD3 and evaluating for expression of CD4 (Figure 8B) or CD8 (Figure 8C). The results demonstrated that counts of both cell types remained within normal limits for the first 24 hours in the fibrocyte treated group and were significantly increased as compared to the saline treated animals. Expression of CD69, a marker of activation, was significantly increased on the CD4+ T cells at 48 hours and remained above normal limits in the fibrocyte group as compared to the saline group (Figure 8D). The numbers of CD8 + T cells expressing CD69 did not change significantly over time or by treatment group (Figure 8E). In separate experiments, female, ICR mice also had significantly higher splenic T cell counts after CLP, approaching normal limits, when treated with fibrocytes as compared to saline (see figure, Supplemental Digital Content 2B, demonstrating restoration of T cell numbers with adoptive transfer after CLP). These studies demonstrate that fibrocytes can influence splenic T cell numbers in vivo, a phenomenon that required direct contact in vitro. To determine if contact could potentially happen in vivo, the dispersion of transferred fibrocytes was examined. Fibrocytes from culture were labeled with CFSE and 1 × 106 cells were deposited in the abdomen at the time of CLP. The mice were euthanized 24 hours later and frozen sections of spleen were observed with a fluorescent microscope. Fluorescence was demonstrated in the spleen of mice after adoptive transfer as compared to normal spleen, suggesting contact between the fibrocytes and T cells was possible in vivo (Figure 9).
Figure 8. Fibrocytes enhance splenic T cell counts during sepsis.

At the time of CLP, fibrocytes or an equal volume of saline were transferred to the abdomen of C57BL/6 mice. The mice were euthanized at the early time points of 0, 12, 24, and 48 hours. Spleens were harvested and processed for flow cytometry with antibodies against A) CD3, B) CD4, and C) CD8. For analysis of flow cytometry, Cells were identified by gating for CD3 and then for either CD4 or CD8. The activation status of the cells was also evaluated by identification of CD69 expression on D) CD4+ T cells and E) CD8+ cells T cells. n= 7–12/group, 3 independent experiments. Data are reported as mean ± SEM. * = p<0.05 as compared to saline group at that time point.
Figure 9. Transferred fibrocytes gain access to splenic tissues.
Female, ICR mice were anesthetized and CLP was performed with a 21g double puncture. A) Spleen of unmanipulated mouse, A) Spleen of mouse 24 hours after CLP and adoptive transfer of CFSE labeled cells. Photomicrographs are representative of results for 3 mice/group.
Adoptive transfer of fibrocytes enhances IFN-γ production by splenic T cells
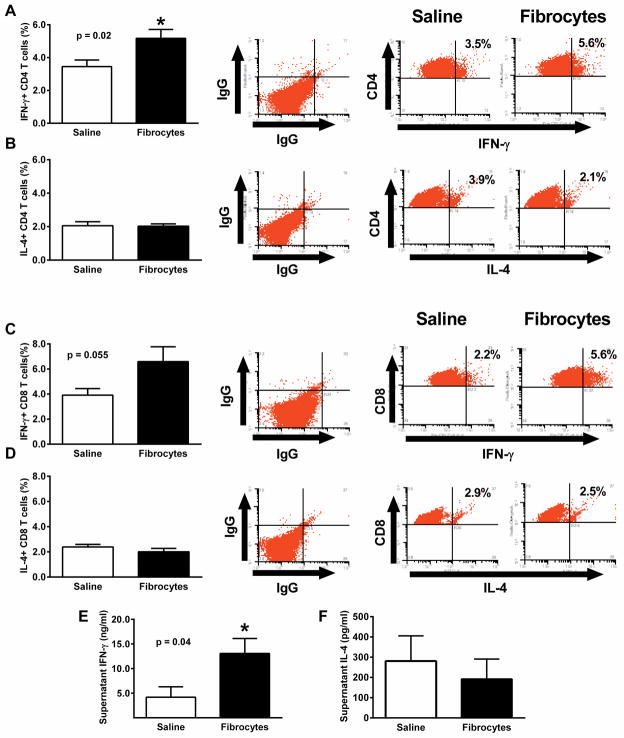
To determine the effects of fibrocyte transfer on IFN-γ production by localized T cells, CLP was performed with or without adoptive transfer of cells (n = 9 mice/group, 3 independent experiments) and spleens were harvested after 24 hours. Splenocytes were stimulated overnight with anti-CD3 and anti-CD28 and then processed for detection of intracellular cytokines via flow cytometry. T cells were gated for either CD4 or CD8 expression and then evaluated for intracellular cytokines. Compared to the saline treated animals, the fibrocyte treated animals had a significantly increased percentage of their CD4+ T cells that stained for intracellular IFN-γ (Figure 10A) while the percentage of CD4+ T cells with intracellular staining for IL-4 was not different between the groups (Figure 10B). The percentage of CD8+ positive T cells with intracellular staining for IFN-γ showed similar trends as the CD4+ T cells, although the values did not quite reach statistical significance (p = 0.055; Figure 10C). However, the mean fluorescence intensity of the intracellular IFN-γ in CD8+ T cells was significantly higher (p=0.02) in the fibrocyte group (707.0 ± 18.0) than in the saline group (653.9 ± 10.8). As with the CD4+ T cells, intracellular staining for IL-4 in the CD8+ cells showed no differences between groups (Figure 10D). The culture media from the splenocyte cultures were also examined for cytokines. The levels of IFN-γ found in the cultures from animals treated with fibrocytes was significantly higher (Figure 10E) than cultures of splenocytes from animals given saline. As with the intracellular IL-4 results, there were no differences in the amount of extracellular IL-4 measured in the culture media from either group (Figure 10F).
Figure 10. Fibrocytes enhance IFN-γ production by splenic T cells.
At the time of CLP, fibrocytes or an equal volume of saline were transferred to the abdomen of C57BL/6 mice. The mice were euthanized at 24 hours and spleens were harvested. Splenocytes were incubated overnight with platebound anti-CD3 and anti-CD28. Flow cytometery was used to identify cells gated for either CD4 or CD8 expression and then analyzed for intracellular cytokines. The results are presented in representative dot plots and graphs of T cell phenotypes including A) IFN-γ+, CD4+; B)IL-4+, CD4+; C)IFN-γ+, CD8+; and D) IL-4+, CD8+. In addition, culture media were analyzed by ELISA for E) INF-γ and F) IL-4. Data expressed as mean ± SEM. n= 7–12/group, 3 independent experiments. * = p<0.05 as compared to saline group at that time point.
DISCUSSION
The studies presented here demonstrate that the adoptive transfer of the fibrocytes results in immunomodulation and improved outcome in the CLP model. Most notably, the adoptive transfer of fibrocytes resulted in reduced bacterial load and increased proliferation of CD4+ and CD8+ splenic T cells. This was shown in the male mice of our current studies as well as the female mice used in supplemental studies. Improved survival after CLP has been soundly associated with changes in bacterial load and CD4+ T cell counts in previous studies (6–8). In this study, it appears that increased T cell numbers are either directly or indirectly a major effect of the adoptive transfer of fibrocytes.
Our in vitro studies demonstrate that fibroyctes in direct contact with T cells significantly impact their proliferation. Further, we demonstrated that fibrocytes deposited in the abdomen at the time CLP can be found in the spleen within 24 hours. It is well-established that fibrocytes do migrate into sites of inflammation (17, 18) and studies have also shown they have an affinity for tissues with large T cell populations. For instance, endogenous fibrocytes will accumulate in the spleen within 24 hours of an injection of LPS or infection with Listeria monocytogenes (16). Histological studies of chronic pancreatitis have also demonstrated higher numbers of fibrocytes in areas of tissues where T cells have also accumulated (25). Likewise, exogenous fibrocytes loaded with viral antigen will migrate to local popliteal lymph nodes within 24 hours of footpad injection (17). In the CLP model, it is unknown at this time whether the appearance of exogenous fibrocytes in the spleen is secondary to passive peritoneal drainage and/or due to active recruitment via chemokine receptors as is seen in fibrotic lung disease (13, 14). Either mechanism would provide the direct contact necessary for fibrocyte and T cell interaction.
Fibrocytes exhibit many of the phenotypic characteristics of antigen presenting cells including the expression of adhesion molecules (CD11b, CD13, CD54) and costimulatory molecules (CD80 and CD86) (11, 17, 18). They may also constitutively express MHC class I and MHC class II molecules, in contrast to tissue fibroblasts that require activation by IFN-γ to express measurable quantities of MHC class II molecules (17). In fact, studies have shown that fibrocytes exposed to viral antigens could elicit proliferation of naïve CD4+ T cells (17) and naïve CD8+ T cells (20). However, our in vitro studies demonstrated that the proliferative response of presumably naïve T cells cultured with fibrocytes occurred without exposure to a specific antigen. Previous studies have documented antigen-independent proliferation of CD4+ and CD8+ T cells (26–29). Although not completely understood, antigen-independent CD4+ T cell proliferation may occur through the stimulation of specialized subpopulations of memory cells that are not derived from traditional antigen-driven clonal expansion (26). In our studies, the proliferation of both the CD4+ and CD8+ T cells may prove to be the result of independent molecular mechanisms or the CD8+ T cell proliferation may have been dependent upon the CD4+ T cells. Regardless, the antigen-independent T cell proliferation could confer a host advantage over pathogens (26).
To further characterize the proliferative T cells in our in vivo studies, we examined intra and extracellular cytokine profiles. The overall systemic response of the fibrocyte treated and control animals determined in plasma suggested a mixed Th1 and Th2 response that increased over the first 48 hours after CLP. However, T cells localized in the spleen demonstrated greater potential for early IFN-γ production at 24 hours after CLP when the animals had adoptive transfer of fibrocytes compared to controls. The production of IL-4 from stimulated splenic T cells remained the same in both groups. These results are similar to work that showed mature fibrocytes may undergo a transdifferentiation or reprogramming, driving T cell responses toward a predominantly Th1 response (21, 22) characterized by stable IL-4 production but increased IFN-γ. From our results, it appears that the adoptive transfer of fibrocytes can result in localized phenotypic changes to T cells within 24 hours of CLP.
As further evidence of their immunomodulatory capacity, fibrocyte transfer resulted in lower systemic and local IL-6 concentrations. Fibrocytes are capable of IL-6 and IL-10 production in response to inflammatory stimuli (12, 19, 30) and their central role in proinflammatory processes has been suggested (16). Therefore, it is interesting that the adoptive transfer of fibrocytes into the septic environment caused reductions in both cytokines in comparison to the controls. These results were somewhat different from those seen with adoptive transfer of bone marrow stromal cells (BMSCs) in the CLP model (31). Although transfer of either cell type caused an improvement in survival and a decrease in plasma IL-6, BMSC transfer was associated with a significantly higher plasma IL-10 at 12 hours post CLP as compared to controls, with no differences seen at 24 hours. In that study, the higher IL-10 may have contributed to survival through its anti-inflammatory effects on other cell types (31). In contrast, our model demonstrated similar levels of IL-10 in control and fibrocyte-treated mice at 12 hours, with a significant decrease in IL-10 at 24 hours in the fibrocyte treated mice. Since IL-10 levels have been linked to decreased Th1 responses and immunosuppression (32, 33), the reduction in IL-10 may have contributed to the benefits of adoptive transfer of fibrocytes.
The effects of fibrocyte transfer on peritoneal inflammatory cell counts were dramatic while systemically there were modest reductions in neutrophils and monocytes by 48 hours. The results in peripheral blood were different than those seen with BMSC transfer (increased neutrophil and decreased monocyte counts) (31). However, the results of our study are in keeping with studies of adoptive transfer of fibrocytes in a model of collagen antibody-induced arthritis, in which blood counts of Gr1+ cells were decreased (34). That study suggested an indirect effect of fibrocytes through modulation of other cells to cause recruitment of neutrophils into tissues. Regarded together, the cytokine and cell data from our study suggest that the beneficial effects of exogenous fibrocytes included a decrease in inflammation.
In summary, this is the first report of either the basic or applied biology of fibrocytes in the context of sepsis. The studies presented here further demonstrate the diverse nature of fibrocytes and their capacity for immunomodulation. The adoptive transfer of fibrocytes at the time of CLP resulted in a decreased bacterial load, increased splenic Th1 T cells and improved survival. Consequently, it is conceivable that fibrocytes have an important role in host defense and a potential application as cell therapy for sepsis.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/HLB R21 108196-01-A1 (JN) and HL087846 (BM)
Footnotes
The authors have no relevant conflicts of interest.
References
- 1.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;(62):1–8. [PubMed] [Google Scholar]
- 2.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24(7):1125–8. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1(6):496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162(7):4148–56. [PubMed] [Google Scholar]
- 5.Efron P, Moldawer LL. Sepsis and the dendritic cell. Shock. 2003;20(5):386–401. doi: 10.1097/01.SHK.0000092698.10326.6f. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci. 1999;96(25):14541–6. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184(7):3768–79. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36(1):38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scumpia PO, Delano MJ, Kelly-Scumpia KM, Weinstein JS, Wynn JL, Winfield RD, Xia C, Chung CS, Ayala A, Atkinson MA, Reeves WH, Clare-Salzler MJ, Moldawer LL. Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood. 2007;110(10):3673–81. doi: 10.1182/blood-2007-04-087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, Kunkel SL. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 2005;105(9):3588–95. doi: 10.1182/blood-2004-08-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–25. [PubMed] [Google Scholar]
- 13.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166(3):675–84. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117(3):549–56. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curnow SJ, Fairclough M, Schmutz C, Kissane S, Denniston AK, Nash K, Buckley CD, Lord JM, Salmon M. Distinct types of fibrocyte can differentiate from mononuclear cells in the presence and absence of serum. PLoS ONE [Electronic Resource] 2010;5(3):e9730. doi: 10.1371/journal.pone.0009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisseleva T, von Kockritz-Blickwede M, Reichart D, McGillvray SM, Wingender G, Kronenberg M, Glass CK, Nizet V, Brenner DA. Fibrocyte-like cells recruited to the spleen support innate and adaptive immune responses to acute injury or infection. J Mol Med. 2011;89(10):997–1013. doi: 10.1007/s00109-011-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94(12):6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60(7):1342–50. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balmelli C, Alves MP, Steiner E, Zingg D, Peduto N, Ruggli N, Gerber H, McCullough K, Summerfield A. Responsiveness of fibrocytes to toll-like receptor danger signals. Immunobiology. 2007;212(9–10):693–9. doi: 10.1016/j.imbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Balmelli C, Ruggli N, McCullough K, Summerfield A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol. 2005;77(6):923–33. doi: 10.1189/jlb.1204701. [DOI] [PubMed] [Google Scholar]
- 21.Medina A, Ghahary A. Fibrocytes can be reprogrammed to promote tissue remodeling capacity of dermal fibroblasts. Mol Cell Biochem. 2010;344(1–2):11–21. doi: 10.1007/s11010-010-0524-4. [DOI] [PubMed] [Google Scholar]
- 22.Medina A, Ghahary A. Reprogrammed fibrocytes induce a mixed Th1/Th2 cytokine response of naive CD4(+) T cells. Mol Cell Biochem. 2011;346(1–2):89–94. doi: 10.1007/s11010-010-0595-2. [DOI] [PubMed] [Google Scholar]
- 23.De Clerck LS, Bridts CH, Mertens AM, Moens MM, Stevens WJ. Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. J Immunol Methods. 1994;172(1):115–24. doi: 10.1016/0022-1759(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 24.Hugunin KM, Fry C, Shuster K, Nemzek JA. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock. 2010;34(3):250–60. doi: 10.1097/shk.0b013e3181cdc412. [DOI] [PubMed] [Google Scholar]
- 25.Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramaswamy A. CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch. 2002;440(2):128–33. doi: 10.1007/s00428-001-0551-3. [DOI] [PubMed] [Google Scholar]
- 26.Younes SA, Punkosdy G, Caucheteux S, Chen T, Grossman Z, Paul WE. Memory phenotype CD4 T cells undergoing rapid, nonburst-like, cytokine-driven proliferation can be distinguished from antigen-experienced memory cells. PLoS Biol. 2011;9(10):e1001171. doi: 10.1371/journal.pbio.1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong P, Pamer EG. Cutting edge: antigen-independent CD8 T cell proliferation. J Immunol. 2001;166(10):5864–8. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- 28.Lu J, Giuntoli RL, 2nd, Omiya R, Kobayashi H, Kennedy R, Celis E. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8(12):3877–84. [PubMed] [Google Scholar]
- 29.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286(5443):1381–3. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 30.Peng H, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Curr Opin Pharmacol. 2012;12(4):491–6. doi: 10.1016/j.coph.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caux C, Massacrier C, Vanbervliet B, Barthelemy C, Liu YJ, Banchereau J. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int Immunol. 1994;6(8):1177–85. doi: 10.1093/intimm/6.8.1177. [DOI] [PubMed] [Google Scholar]
- 33.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78(4):1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galligan CL, Fish EN. Circulating fibrocytes contribute to the pathogenesis of arthritis. Arthritis Rheum. 2012 doi: 10.1002/art.34589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.