Abstract
In a birth cohort study, we have assessed the dose-response relationship between individual measurements of prenatal airborne PAH exposure and specific PAH-DNA adducts in cord blood adjusted for maternal blood adducts and season of birth. The study uses data from an earlier established birth cohort of children in Krakow. The final analysis included 362 pregnant women who gave birth to term babies and had complete data on personal exposure in the second trimester of pregnancy to eight airborne polycyclic aromatic hydrocarbons (PAH) including benzo[a]pyrene (B[a]P), as well as DNA adducts, both in maternal and cord blood.
The relation between cord blood PAH-DNA adducts and airborne prenatal PAH exposure was non-linear. While cord blood PAH-DNA adducts were significantly associated with the B[a]P exposure categorized by tertiles (nonparametric trend z = 3.50, p < 0.001), the relationship between B[a]P and maternal blood adducts was insignificant (z = 1.63, p = 0.103). Based on the multivariable linear regression model we estimated the effect of the prenatal airborne B[a]P on the level of cord blood adducts. In total, 14.8% of cord blood adducts variance was attributed to the level of maternal adducts and 3% to a higher prenatal B[a] exposure above 5.70 ng/m3. The calculated fetal/maternal blood adducts ratio (FMR) linearly increased with the B[a]P exposure (z = 1.99, p = 0.047) and was highest at B[a]P concentrations exceeding 5.70 ng/m3.
In conclusion, the results support other findings that transplacental exposure to B[a[P from maternal inhalation produces DNA damage in the developing fetus. It also confirms the heightened fetal susceptibility to prenatal PAH exposure that should be a matter of public health concern particularly in the highly polluted areas because DNA adducts represent a pro-carcinogenic alteration in DNA The continuation of this birth cohort study will assess the possible health effects of fetal DNA damage on health of children and help in establishing new protective guidelines for newborns.
Keywords: prenatal exposure, polycyclic aromatic hydrocarbons, biomarkers of exposure, PAH-DNA adducts, birth cohort study
Introduction
Polycyclic aromatic hydrocarbons (PAH) belong to a group of chemical compounds formed in the incomplete combustion of organic material; the best known member of this class of compounds is benzo[a]pyrene (B[a]P). The PAH compounds are ubiquitous and have been found in polluted air, occupational and urban environments, in tobacco smoke and broiled foods 1–3. PAH compounds are some of the most important health hazards since they have been found to be carcinogenic 4,5. Moreover, both research involving laboratory animals and human studies have reported their harmful impact on fetal development 6–9. Prenatal exposure to PAH has also been shown in epidemiologic studies to be associated with adverse mental development in very young children 10, as well as reduced intelligence scores at later age11,12.
PAH compounds readily cross the placenta 13–15, however, the estimated transplacental dose of genotoxic compounds such as PAH is about 10 times lower than the dose to maternal tissues. The transplacental exposure to PAHs from maternal inhalation of PAH leads to the formation of DNA adducts 16–18, which may be considered as an molecular dosimeter of the PAH absorbed over the prenatal period and represent the biologically effective dose. PAH-DNA adducts seem to constitute a reliable biomarker of individual PAH exposure, reflecting individual differences in exposure, absorption and distribution of the chemical, its metabolism into DNA reactive forms, detoxification to reactive intermediates, as well as cell turnover and repair of DNA damage 19, 20. In health risk assessments, the monitoring of PAH-DNA adducts may lead to identification of potentially hazardous exposures and susceptible populations before the adverse health effects occur 21. In this context the issue of increased susceptibility of fetuses and children to environmental genotoxic agents was also addressed 22–24.
The main purpose of this analysis was to assess the relationship between individual measurements of prenatal airborne PAH exposure in the second pregnancy trimester and the level of PAH-DNA adducts in cord blood as the endpoint. The second trimester marks the halfway period of pregnancy, when the fetus starts to grow very quickly and the brain undergoes its most important growth. From now on, the fully developed placenta provides all the fetus’ needs until birth and this may also create good conditions for toxicants absorbed by mother to cross placenta and eventually put fetus at risk. Beside the level of DNA adducts in maternal blood, the analysis took into consideration concomitant exposures, such as fine particles (PM2.5) and environmental tobacco smoke (ETS). The secondary purpose of the study was to assess increased susceptibility of fetuses to genotoxic PAH compounds by computing the fetal/maternal blood adducts ratios (FMRs) of the exposure-specific biomarker (PAH-DNA adducts) at various exposure levels.
Material and Methods
The present study uses data from a previously established birth cohort of children in Krakow that is a collaborative research project with Columbia University in New York. The design of the study and the detailed selection of the population have been described in a previous paper 25. In short, pregnant women were recruited from ambulatory prenatal clinics in the first or second trimester of pregnancy. The study included women between 18 and 35 years of age, who claimed to be non-smokers, with singleton pregnancies, without illicit drug use and HIV infection, free from chronic diseases such as diabetes or hypertension, and resident in Krakow for at least one year prior to pregnancy. All women participating in the study had read and signed an informed consent. The study was reviewed and approved by the Bioethical Committee of the Jagiellonian University in Krakow, Poland.
Upon enrolment, a detailed questionnaire was administered to each woman to gather information on demographic data, household characteristics, medical and reproductive history, occupational hazards, and smoking practices of others present in the home. Questionnaire also elicited information on dietary PAH (frequency of consumption of broiled, fried, grilled and smoked meat) during pregnancy. A total of 505 pregnant women initially enrolled in the study gave birth between January 2001 and February 2004. The univariate statistical analysis included 438 women who gave birth between 37 and 43 weeks of gestation and had measurements of personal exposure to pollutants and DNA adducts. In the final multivariable regression models the analysis was restricted to 362 mother/newborn pairs with complete data on all variables.
Gestational age at birth was defined as the interval between the last day of the mother’s menstruation (LMP) and the date of birth. ETS exposure was assessed by detailed interviews on passive smoke over the whole pregnancy period and validated by maternal blood cotinine level. Weighted mean number of cigarettes smoked was calculated as a total number of cigarettes smoked at home over various pregnancy periods divided by the duration of pregnancy (in days). Maternal education level (elementary, secondary or higher) was treated as a proxy for socio-economic status.
Dosimetry of prenatal personal exposure to PAH and fine particles
The monitoring of pregnant women for personal exposure to airborne PAH and fine particles was carried out over a 48-hour period (working days) during the second trimester of pregnancy. The women were instructed by a trained staff member how to use a personal monitor and asked to wear the monitoring device during the daytime hours for two consecutive days and to place it near the bed at night. On the second day, the air monitoring staff assistant and interviewer visited the study participant’s home to change the battery-pack and to complete the questionnaire on household characteristics.
A Personal Environmental Monitoring Sampler (PEMS) was used to measure both PAH and particle mass collected on the PEMS Teflon membrane filter (37 mm Teflo™, Gelman Sciences). The single pump/two impactors sampling method was developed at Harvard School of Public Health (Dr J. Spengler) and is used to measure particles and gases. For the measurement of gaseous PAH compounds, the sampling pump draws air through a polyurethane (PUF) sampler. After sampling, the field samplers were frozen and shipped on dry ice to South-West Research Institute in Texas, where tests were performed to determine concentrations of eight PAH compounds (benzo(a)anthracene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(g,h,i)perylene, benzo(a)pyrene, chrysene/iso-chrysene, dibenzo(a,h)anthracene, indeno(1,2,3-c,d)pyrene, and pyrene) in extracts of particles collected on filters and polyurethane foam cartridges. As in prior studies, the eight carcinogenic PAH were summed as the PAH exposure indicator. The chemical procedures in the analysis of the collected samples are described elsewhere 26.
Dosimetry of PAH-DNA adducts
Maternal blood (30–35 mL) was collected within 1 day postpartum, and umbilical cord blood (30–60 mL) was collected at delivery. B[a]P-DNA adducts were analyzed in all maternal and umbilical cord blood samples with sufficient DNA quantities. B[a]P is widely used as a representative PAH because concentrations of individual PAHs in the urban setting are highly correlated 23. B[a]P-DNA adducts can serve as a proxy for PAH-DNA adducts and B[a]P-DNA adducts in extracted WBC DNA were analyzed using the high-performance liquid chromatography-fluorescence method of Alexandrov et al 26,27, which detects B[a]P tetraols. The method has a coefficient of variation of 12% and a lower limit of detection of 0.25 adducts per 108 nucleotides. As in prior analyses, samples below the limit of detection were assigned a value midway between the limit of detection and zero (0.125 adducts per 108 nucleotides).
Statistical analysis
The associations between cord blood PAH-DNA adducts and prenatal exposure to PAH, PM2.5 and ETS were initially examined by univariate approach. In the univariate analysis the cord and maternal blood DNA adducts were divided into three categories: 0 = undetectable level, 1 = below the median of those with detectable level, 2 = above the median of those with detectable level. The relation between blood adducts and the prenatal PAH exposure was nonlinear, but natural logarithm transformation of the variables linearized satisfactorily the relationship. In the multivariable regression of the association between cord blood adducts and main exposure variable (prenatal airborne PAH exposure) a set of potential confounders, such as maternal DNA adducts, season of birth and co-exposure to PM2.5 and ETS were initially considered. Dietary PAH exposure was not correlated with PAH-DNA adducts and was not a predictor of cord blood PAH-DNA adducts (p>0.1) and therefore not included as a covariate. In choosing the final model the linearity between predictors and the outcome variable, normality and homogeneity of variance and collinearity between covariables were considered. In addition, only relevant covariables were included, which improved the model fitting and outliers excluded (B[a]P >30 ng/m3, DNA blood adducts > 1 per 108 necleoides). Main exposure variable (airborne B[a]P exposure) was introduced in the final model as ordered variable (in tertiles of its distribution). Increased fetal susceptibility to genotoxicity of PAH compounds was assessed by computing the fetal/maternal ratio of PAH-DNA adducts (FMR) in newborn/mother pairs. The value > 1 was considered as an indicator of a higher susceptibility of fetus to DNA damage from absorbed PAH. All statistical analyses have been performed with STATA version 12.0 software.
Results
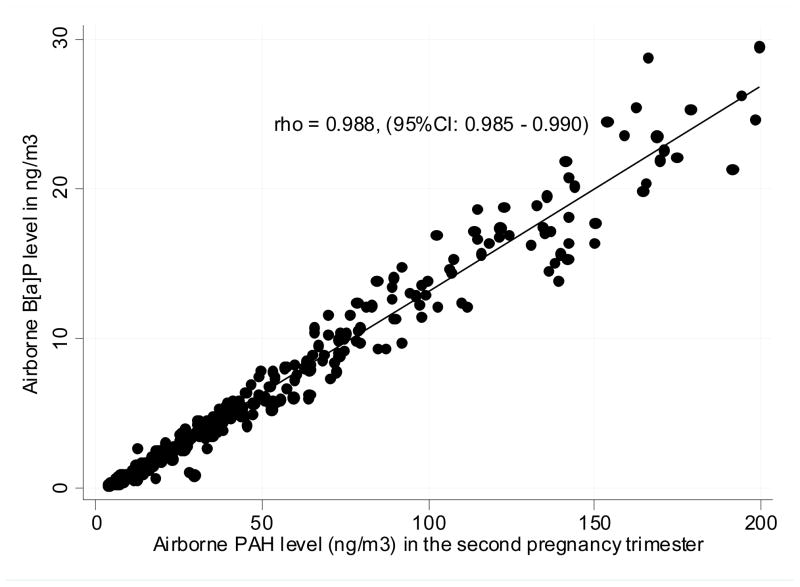
The socio-demographic characteristics of the newborns did not differ across the groups defined by various levels of the cord blood adducts (Table 1). The geometric mean of prenatal personal exposure to PAH monitored in the second pregnancy trimester was 24.2 ng/m3 (95%CI: 21.5 – 27.1 ng/m3) and that for B[a]P was 2.5 ng/m3 (95%CI: 2.2 – 2.9 ng/m3). Spearman correlation coefficient (rho) between PAH and B[a]P was 0.988 (95%CI: 0.985 – 0.990) and that for PAH and PM2.5 was 0.624 (95%CI: 0.568 – 0.680) (Figure 1).
Table 1.
Characteristics of mothers and newborns included in the analysis
| Variables | PAH-DNA adducts in cord blood
|
Total N=438 |
P-level | |||
|---|---|---|---|---|---|---|
| ≤ 0.125 | 0.126–0.318 | > 0.318 | ||||
| per 108 nucleoides n=160 | per 108 nucleoides n=139 | per 108 nucleoides n=139 | ||||
| Maternal age: | ||||||
| mean | 27.69 | 27.42 | 27.38 | 27.51 | ||
| SD | 3.53 | 3.53 | 3.77 | 3.60 | 0.7153 | |
| Maternal education: | ||||||
| elementary | n (%) | 13 (8.1) | 13 (9.4) | 18 (12.9) | 44 (10.0) | 0.1683 |
| secondary | n (%) | 33 (20.6) | 33 (23.7) | 41 (29.5) | 107 (24.4) | |
| higher | n (%) | 114 (71.2) | 93 (66.9) | 80 (57.6) | 287 (65.5) | |
| Pre-pregnancy maternal BMI: | ||||||
| mean | 21.46 | 20.83 | 21.43 | 21.25 | 0.1122 | |
| SD | 3.285 | 2.318 | 2.813 | 2.864 | ||
| Gestational age: (weeks) > 36 | ||||||
| mean | 39.56 | 39.52 | 39.59 | 39.56 | ||
| SD | 1.131 | 1.086 | 1.250 | 1.154 | 0.8718 | |
| Gender: | ||||||
| Boys | n (%) | 87 (54.4) | 68 (48.9) | 69 (49.6) | 224 (51.1) | |
| Girls | n (%) | 73 (45.6) | 71 (51.1) | 70 (50.4) | 214 (48.9) | 0.5859 |
| Birth weight (g): | ||||||
| mean | 3475.8 | 3399.8 | 3444.2 | 3441.6 | ||
| SD | 431.97 | 425.29 | 456.21 | 437.86 | 0.3255 | |
| Residence: | ||||||
| Inner city area | n (%) | 27 (16.9) | 33 (23.7) | 33 (23.7) | 93 (21.2) | 0.2390 |
| Outer city area | n (%) | 133 (83.1) | 106 (76.3) | 106 (76.3) | 345 (78.8) | |
| Prenatal ETS (+) | n (%) | 40 (25.0) | 30 (21.6) | 45 (32.4) | 115 (26.3) | 0.1116 |
| Maternal adducts | ||||||
| mean | 0.207 | 0.261 | 0.317 | 0.263 | 0.0001 | |
| SD | 0.108 | 0.111 | 0.117 | 0.144 | ||
Figure 1.
Correlation between airborne B[a]p and PAH values measured in the prenatal pepriod (Spearman correlation rho = 0.989, 95%CI: 0.987 – 0.991)
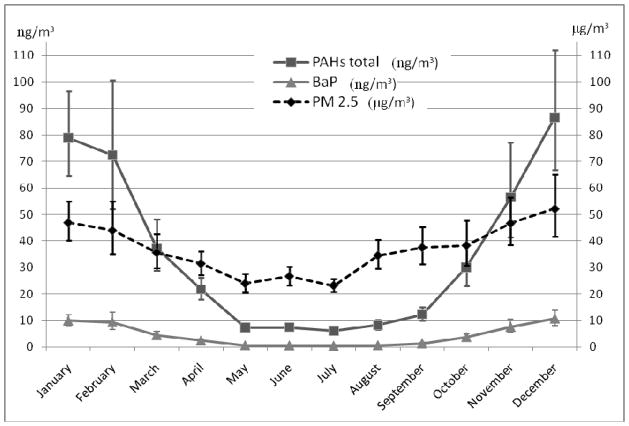
Mean airborne exposure to PAH was about threefold higher (geometric mean = 45.6 ng/m3, 95%CI: 39.6 – 52.6 ng/m3) in the heating season (October–March) than in the non-heating period (geometric mean = 14.5 ng/m3, 95%CI: 12.5 – 15.7 ng/m3); the corresponding concentrations of PM2.5 were 40.3 μg/m3 (95%CI: 37.2 – 43.7) and 33.7 μg/m3 (95%CI: 31.2 – 36.4 μg/m3). The analysis of the seasonal variability of air pollutants as measured by personal monitoring showed highest concentrations of air pollutants in the cold season, which resulted from individual domestic gas and coal stoves used in the inner city area and communal coal-fired heating generators located outside the city center (Figure 2).
Figure 2.
Geometric means with 95% confidence intervals for PAH, B[a]P and PM2.5, by dates of measurement in the second trimester of pregnancy
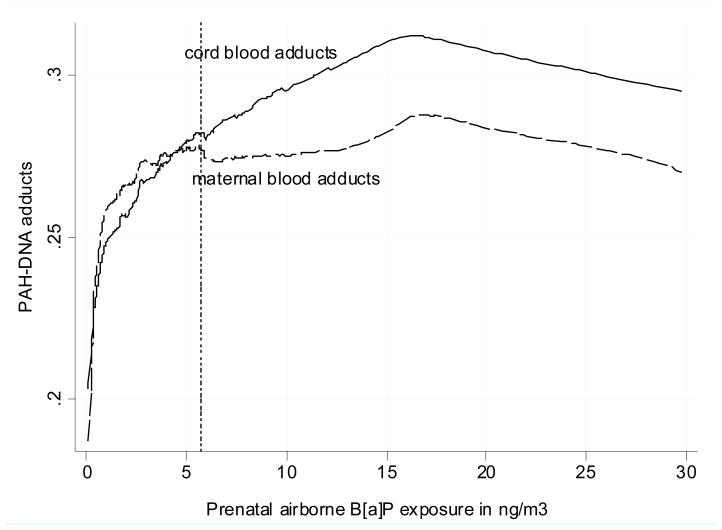
Figure 3 presents the different pattern of relationship between the prenatal B[a]P exposure and PAH-DNA adducts in cord and maternal blood. At the lower exposure the difference between the shape of the dose-response curves is negligible, however, the gap between them gradually widens at the higher exposure range. While cord blood PAH-DNA adducts significantly correlated with prenatal level of B[a{P in tertiles (non-parametric trend z = 3.50, p < 0.001), the association of B[a]P with maternal blood adducts did not reach the significance level (z = 1.63, p = 0.103), Prenatal PM2.5 concentrations were significantly associated with B[a]P, however, ETS exposure did not correlate with B[a]P (Table 2).
Figure 3.
Non-linear dose-response ralationship between prenatal airborne B[a] exposure as measured in the second trimester of pregnancy and PAH-DNA adducts in cord and maternal blood (lowess line)
Table 2.
Mean concentrations of PAH-DNA adducts in cord and maternal blood together with prenatal ambient pollutants grouped by the prenatal airborne B[a] level (in tertiles)
| Variables | Prenatal B[a]P exposure level (in tertiles) | p-level for nonparametric trend | ||
|---|---|---|---|---|
| <= 1.15 ng/m3 | 1.16 – 5.69 ng/m3 | >5.69 ng/m3 | ||
| Cord blood adducts per 108 nucleoides | 0.24 (0.22 – 0.26) | 0.25 (0.23 – 0.27) | 0.30 (0.27 – 0.32) | z = 3.50 p <0.001 |
| Maternal blood adducts per 108 nucleoides | 0.25 (0.23 – 0.27) | 0.26 (0.24 – 0.28) | 0.27 (0.25 – 0.30) | z = 1.63 p = 0.103 |
| Prenatal PAH in ng/m3 | 7.7 (7.0 – 8.3) | 26.8 (24.6 – 28.5) | 102.3 (93.4 – 111.3) | z = 17.72 p < 0.001 |
| Prenatal PM2.5 μg/m3 | 28.9 (26.1 – 31.7) | 36.1 (32.9 – 39.3) | 58.5 (53.4 – 63.7) | z = 10.17 p <0.001 |
| Weighted mean number of cigarettes smoked by others daily at home | 1.45 | 1.34 | 2.19 | z = −0.88 p =0.881 |
In brackets: 95% confidence interval
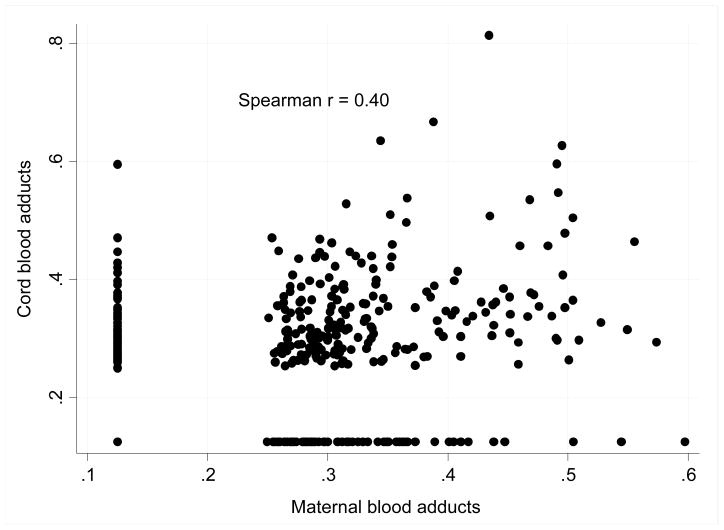
DNA adducts in mothers and newborns were significantly correlated with each other (Figure 4). The average level of cord blood adducts was slightly higher (geometric mean = 0.235 per 108 nucleotides, 95%CI: 0.224 – 247) than that found in maternal blood (mean = 0.230 per 108 nucleotides, 95%CI: 0,219 – 0.241) but the difference was insignificant (t = 0.803 p = 0.422, paired t-test). Neither maternal educational status, nor age, gestational age or pre-pregnancy weight of mothers or gestational weight gain had a significant effect on the level of DNA adducts in mothers or newborns. Frequency of consumption of broiled, fried, grilled and smoked meat in pregnancy did not correlate with DNA adducts either.
Figure 4.
Correlation between maternal and cord blood DNA adducts (per 108 nucleoides)
Table 3 presents the final multivariable linear regression for cord blood PAH-DNA adducts (ln-transformed) where the main exposure variable (B[a]P) was introduced in tertiles together with only the relevant covariates (maternal adducts and season of birth). The traditional table summarizing the results of multivariable regression was supplemented with information of the variance inflation factor (VIF) and Eta2 coefficient measuring the effect size of individual covariates. In total, all the covariates included in the model explained 18.3% of the variance and most of it (14.8%) was attributed to maternal adducts and about 3% to higher B[a]P exposure (> 5.69 ng/m3).
Table 3.
Adjusted effect of personal airborne exposure to B[a]P measured in the second trimester of pregnancy in mother/newborn pairs on the cord blood (ln-transformed) concentrations of PAH-DNA adducts (per 108 nucleotides). Multivariable regression model (N = 362, R-squared = 0.183
| Predictors | Coef. | t | P>t | [95% Conf. Interval] | VIF* | Partial Eta2** | |
|---|---|---|---|---|---|---|---|
| Maternal adducts*** | 0.380 | 7.88 | 0.000 | 0.285 | 0.474 | 1.01 | 0.148 |
|
| |||||||
| Season of birth**** | −0.067 | −1.29 | 0.197 | −0.168 | 0.035 | 1.02 | 0.005 |
| Prenatal airborne B[a]P level | |||||||
| <1.15 ng/m3 | reference | ||||||
| 1.16 – 5.69 ng/m3 | 0.049 | 0.82 | 0.411 | −0.068 | 0.166 | 1.32 | 0.002 |
| >5.69 ng/m3 | 0.192 | 3,14 | 0.002 | 0.072 | 0.312 | 1.34 | 0.027 |
VIF = variance inflation factor
Eta2 = effect size
ln-transformed values
1 = non-heating season (April – September), 2 = heating season (October – March)
In the sample under study we found 39.0% of newborns (95%CI: 33.9 – 44.0%) with FMR above 1.0, which may suggest an increased susceptibility to the DNA fetal damage. There was a significant trend of FMR values with increasing B[a]P exposure (nonparametric trend z = 1.99, p = 0.047). Even at the moderate exposure level (1.16 – 5.69 ng/m3) FMR was moderately elevated (FMR = 1.15, 95%CI: 1.03 – 1.28) and reached 1.28 mean vlaue (95%CI: 1.10 – 1.43) at the B[a]P level above 5.69 ng/m3 (Table 4).
Table 4.
Mean FMR values for each mother/newborn pair related to the level of prenatal airborne PAH exposure (in tertiles)
| Prenatal airborne B[a] level | FMR | 95%Confidence Interval | |
|---|---|---|---|
| <=1.15 ng/m3 | 1.10 | 0.99 | 1.21 |
| 1.16 – 5,69 ng/m3 | 1.15 | 1.03 | 1.28 |
| >5.69 ng/m3 | 1.28 | 1.13 | 1.43 |
| Total | 1.18 | 1.10 | 1.25 |
Non-paranetric trend z = 1.99, p = 0.047
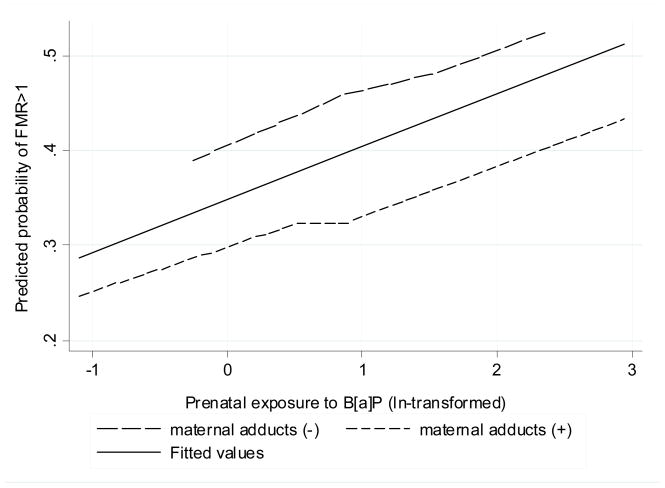
Odds ratio of a heightened susceptibility to fetal DNA damage (FMR>1) remained significant after adjustment for maternal adducts (Table 5). Probability of the heightened susceptibility to the DNA fetal damage (estimated from logistic model) increased linearly with ln-units of exposure, but was at the lower level in newborns with higher maternal blood adducts (Figure 5).
Table 5.
Odds ratio of the increased susceptibility to fetal DNA damage related to the prenatal exposure to B[a]P adjusted for maternal adducts
| Predictors | Odds Ratio | z | P>z | 95% Conf. Interval | |
|---|---|---|---|---|---|
| Maternal adducts (by median) | 0.621 | −2.15 | 0.031 | 0.40 | 0.96 |
| B[a]P ln-transformed | 1.266 | 2.85 | 0.004 | 1.08 | 2.03 |
Figure 5.
Fitted probability of an increased susceptibility to fetal DNA damage (FMR>1) by the prenatal B[a]P exposure and maternal adduct levels (dichotomized by median)
Discussion
The study confirmed a significant trend for cord blood PAH-DNA adducts with increasing prenatal exposure to airborne PAH, but not PM2.5 or ETS. Neither maternal educational status, nor age, gestational age or pre-pregnancy weight of mothers or gestational weight gain had a significant effect on the level of adducts in mothers or newborns. The dose-response relationship between the airborne PAH exposure measured in the second trimester of pregnancy and cord blood DNA adducts was non-linear and 14.8% of cord blood adducts variance was explained by maternal blood adducts and about 3% by exposure to B[a]P values. Moreover, prenatal airborne B[a]P exposure above 5.70 ng/m3 increased significantly the risk of delivering a “susceptible” newborns (FMR>1). It seems worthwhile to mention that newborns with higher maternal adducts (above median) had a 37% reduced risk of being in the susceptible group compared with those whose mothers had a lower level of blood adducts.
These findings provide additional evidence that fetal DNA is prone to more DNA damage per unit of exposure of PAH due either to a higher rate of adduct formation and/or weaker DNA repair capacity. Our study did not show the association between dietary PAH in pregnancy (frequency of consumption of broiled, fried, grilled and smoked meat) and cord blood PAH-DNA adducts. DNA adduct formation is a subject to a greater variability than external exposure. As this variability results from differences in metabolic phenotypes related to genetic polymorphisms in a variety of enzymes involved in the activation or detoxification of PAH or repair of PAH-DNA adducts, individuals with a similar level of external exposure, might happen to have different PAH-DNA adduct levels or vice versa 20. Moreover, the recent study by Kelvin et al 28 showed that plasma antioxidants may also modulate the effect of prenatal PAH exposure on PAH-DNA adducts in cord blood.
The study results support the hypothesis that cord blood PAH-DNA adducts levels reflecting past exposure to airborne PAH may persist over a relatively long period (about 4 months). This observation would be in agreement with the studies on persistence of DNA adducts in smokers who stopped smoking, and in patients after their treatment with coal-tar ointments. The calculated half-life of aromatic-DNA adducts in peripheral white blood cells (monocytes and lymphocytes) was approximately 10 – 12 weeks 29–31.
The effect of urban pollution on the level of DNA damage (PAH-DNA adducts) was earlier investigated in Krakow by Whyatt et al 32. Although on a smaller scale, the previous study showed higher DNA damage for newborns from high and medium polluted areas compared with the less polluted area. Ambient air pollution was significantly associated with the amount of PAH-DNA adducts in both maternal and infant cord white blood cells. The results of a cross-sectional study carried out in a small sample of healthy pregnant women in Denmark (Copenhagen) was recently published by Pedersen et al 33. In the latter study bulky DNA adducts from 75 maternal and 69 cord blood samples were collected at the time of the planned Cesarean section. Modeled traffic data, validated in the subsample of subjects by indoor nitrogen dioxide and polycyclic aromatic hydrocarbons, was used to approximate the traffic related residential air pollution. All participants had detectable DNA levels and they were highest among mother-newborns pairs who lived near medium traffic density places. As in our study the authors found significantly higher DNA adducts in maternal and cord blood of newborns delivered in the summer season (April – September) compared with the winter period.
The effect of urban pollution on the level of DNA damage (Comet assay) was also investigated in a newborn-mother population from two areas of the Czech republic differing in air pollutants level. As the authors found no differences between polluted areas in Comet assay parameters they concluded on an equivalence of DNA damage in mothers and newborns34. It is very difficult to compare the results of that study with our data as the Comet assay may be insufficiently sensitive to determine the effects of environmental pollution on the DNA damage in peripheral white blood cells. Moreover, the contrast in airborne PAH exposure between the exposed and the control area was approximated and based on the routinely collected data from urban sampling stations.
Our findings on the relationship between airborne PAH exposure and DNA adducts would be consistent with the conclusion of a systematic review performed by Castano et al35. The authors identified thirty five studies, with 410 subjects, which evaluated environmental air pollution to PAHs in relation to metabolites of PAH, PAH-DNA adducts or protein adducts. PAH metabolites and, to a lesser extent, PAH-DNA adducts correlated well at the group level with exposure to B[a]P, even at low levels of air pollution.
A strength of our study is the prospective birth cohort design that also enabled us to limit measurement error in estimating prenatal exposure to PAH compounds by assigning an individual prenatal personal exposure level to each child. The personal monitoring of ambient exposure is a relevant measure incorporating outdoor and indoor exposures. Another strong point of our study stems from the fact that we were able to monitor both the level of PAH-DNA adducts in cord blood and maternal blood at delivery. Since the mobility of the subjects under study was very moderate and mainly restricted to the same urban air pollution area, this gave us an additional confidence that the estimates of exposure were unbiased. On the other hand, we are aware of the limitations of our study, which are mainly related to a relatively small sample size and the lack of the better data on dietary PAH exposure in pregnancy.
In conclusion, the study results confirm that transplacental exposure to PAH from maternal inhalation produces some DNA damage in the developing fetus. Our study has also evidenced a significantly stronger association between fetal DNA damage and prenatal exposure to PAH than that observed in mothers. The heightened fetal susceptibility to prenatal PAH exposure is a matter of growing public health concern because DNA adducts not only represent a pro-carcinogenic alteration in DNA, but are associated with adverse child behavior, potentially affecting school performance 36. If the findings are confirmed by other studies, we believe that these results should be considered in reestablishing health guidelines for newborns. We hope that the continuation of this birth cohort study should allow us to assess the possible delayed health and developmental effects of fetal DNA damage in children at later age.
Acknowledgments
The study received funding from an R01 grant entitled “Vulnerability of the Fetus/Infant to PAH, PM2.5 and ETS” (5 R01 ES10165 NIEHS; 02/01/00 – 01/31/04) and from the NIEHS (R01 ES010165-0451) the Lundin Foundation, the John and Wendy Neu foundation, and the Gladys T. and Roland Harriman Foundation. Principal investigator: Prof. FP Perera, co-investigator: Prof. WA Jedrychowski.
References
- 1.WHO. WHO Air Quality Guidelines for Europe. 2. World Health Organization, Regional Office for Europe; Copenhagen: 2001. Polynuclear aromatic hydrocarbons (PAH) [Google Scholar]
- 2.Lijinsky W. The formation and occurrence of polynuclear aromatic hydrocarbons associated with food. Mutat Res. 1991 Mar-Apr;259(3–4):251–261. doi: 10.1016/0165-1218(91)90121-2. [DOI] [PubMed] [Google Scholar]
- 3.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999 Jul 15;443(1–2):139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 4.US EPA. Supplemental Guidance for Assessing Cancer Susceptibility from Early-Life Exposure to Carcinogens. Environmental Protection Agency; 2005. [Google Scholar]
- 5.IARC. IARC Monographs on the evaluation of carcinogenic risks in humans. Vol. 92. IARC; Lyon, France: 2010. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. [PMC free article] [PubMed] [Google Scholar]
- 6.Perera FP, Whyatt RM, Jedrychowski W, Rauh V, Manchester D, Santella RM, et al. Recent developments in molecular epidemiology: A study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am J Epidemiol. 1998 Feb 1;147(3):309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- 7.Dejmek J, Solansky I, Benes I, Lenicek J, Sram RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000 Dec;108(12):1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perera FP, Tang D, Rauh V, Lester K, Tsai WY, Tu YH, et al. Relationships among polycyclic aromatic hydrocarbon-DNA adducts, proximity to the World Trade Center, and effects on fetal growth. Environ Health Perspect. 2005 Aug;113(8):1062–1067. doi: 10.1289/ehp.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi H, Jedrychowski W, Spengler J, Camann DE, Whyatt RM, Rauh V, et al. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect. 2006 Nov;114(11):1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006 Aug;114(8):1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009 Aug;124(2):e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect. 2010 Sep;118(9):1326–1331. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kihlstrom I. Placental transfer of benzo(a)pyrene and its hydrophilic metabolites in the guinea pig. Acta Pharmacol Toxicol (Copenh) 1986 Apr;58(4):272–276. doi: 10.1111/j.1600-0773.1986.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava VK, Chauhan SS, Srivastava PK, Kumar V, Misra UK. Fetal translocation and metabolism of PAH obtained from coal fly ash given intratracheally to pregnant rats. J Toxicol Environ Health. 1986;18(3):459–469. doi: 10.1080/15287398609530885. [DOI] [PubMed] [Google Scholar]
- 15.Neubert D, Tapken S. Transfer of benzo(a)pyrene into mouse embryos and fetuses. Arch Toxicol. 1988;62(2–3):236–239. doi: 10.1007/BF00570149. [DOI] [PubMed] [Google Scholar]
- 16.Schoket B. DNA damage in humans exposed to environmental and dietary polycyclic aromatic hydrocarbons. Mutat Res. 1999 Mar 8;424(1–2):143–153. doi: 10.1016/s0027-5107(99)00015-9. [DOI] [PubMed] [Google Scholar]
- 17.Shuker DE. The enemy at the gates? DNA adducts as biomarkers of exposure to exogenous and endogenous genotoxic agents. Toxicol Lett. 2002 Aug 5;134(1–3):51–56. doi: 10.1016/s0378-4274(02)00162-5. [DOI] [PubMed] [Google Scholar]
- 18.Perera F, Tang D, Whyatt R, Lederman SA, Jedrychowski W. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol Biomarkers Prev. 2005 Mar;14(3):709–714. doi: 10.1158/1055-9965.EPI-04-0457. [DOI] [PubMed] [Google Scholar]
- 19.Schulte PA, Perera FP. Principles and Practices. Academic Press; San Diego, California: 1993. Molecular Epidemiology. [Google Scholar]
- 20.Godschalk RW, Feldker DE, Borm PJ, Wouters EF, van Schooten FJ. Body mass index modulates aromatic DNA adduct levels and their persistence in smokers. Cancer Epidemiol Biomarkers Prev. 2002 Aug;11(8):790–793. [PubMed] [Google Scholar]
- 21.Whyatt RM, Jedrychowski W, Hemminki K, Santella RM, Tsai WY, Yang K, et al. Biomarkers of polycyclic aromatic hydrocarbon-DNA damage and cigarette smoke exposures in paired maternal and newborn blood samples as a measure of differential susceptibility. Cancer Epidemiol Biomarkers Prev. 2001 Jun;10(6):581–588. [PubMed] [Google Scholar]
- 22.Anderson LM, Diwan BA, Fear NT, Roman E. Critical windows of exposure for children’s health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ Health Perspect. 2000 Jun;108( Suppl 3):573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera FP, Tang D, Tu YH, Cruz LA, Borjas M, Bernert T, et al. Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environ Health Perspect. 2004 Jul;112(10):1133–1136. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winder C, Bonin T. The genotoxicity of lead. Mutat Res. 1993 Jan;285(1):117–124. doi: 10.1016/0027-5107(93)90059-o. [DOI] [PubMed] [Google Scholar]
- 25.Jedrychowski W, Whyatt RM, Camann DE, Bawle UV, Peki K, Spengler JD, et al. Effect of prenatal PAH exposure on birth outcomes and neurocognitive development in a cohort of newborns in Poland. Study design and preliminary ambient data. Int J Occup Med Environ Health. 2003;16(1):21–29. [PubMed] [Google Scholar]
- 26.US EPA. Compendium method to-13a in compendium of methods for the determination of toxic organic compounds in ambient air. 2. Center for Environmental Research Information Office of Research and Development U.S. Environmental Protection Agency; Cincinnati: 1999. [Google Scholar]
- 27.Alexandrov K, Rojas M, Geneste O, Castegnaro M, Camus AM, Petruzzelli S, et al. An improved fluorometric assay for dosimetry of benzo(a)pyrene diol-epoxide-DNA adducts in smokers’ lung: comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Res. 1992 Nov 15;52(22):6248–6253. [PubMed] [Google Scholar]
- 28.Kelvin EA, Edwards S, Jedrychowski W, Schleicher RL, Camann D, Tang D, et al. Modulation of the effect of prenatal PAH exposure on PAH-DNA adducts in cord blood by plasma antioxidants. Cancer Epidemiol Biomarkers Prev. 2009 Aug;18(8):2262–2268. doi: 10.1158/1055-9965.EPI-09-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mooney LA, Santella RM, Covey L, Jeffrey AM, Bigbee W, Randall MC, et al. Decline of DNA damage and other biomarkers in peripheral blood following smoking cessation. Cancer Epidemiol Biomarkers Prev. 1995 Sep;4(6):627–634. [PubMed] [Google Scholar]
- 30.Paleologo M, van Schooten FJ, Pavanello S, Kriek E, Zordan M, Clonfero E, et al. Detection of benzo[a]pyrene-diol-epoxide-DNA adducts in white blood cells of psoriatic patients treated with coal tar. Mutat Res. 1992 Jan;281(1):11–16. doi: 10.1016/0165-7992(92)90030-l. [DOI] [PubMed] [Google Scholar]
- 31.Godschalk RW, Van Schooten FJ, Bartsch H. A critical evaluation of DNA adducts as biological markers for human exposure to polycyclic aromatic compounds. J Biochem Mol Biol. 2003 Jan 31;36(1):1–11. doi: 10.5483/bmbrep.2003.36.1.001. [DOI] [PubMed] [Google Scholar]
- 32.Whyatt RM, Santella RM, Jedrychowski W, Garte SJ, Bell DA, Ottman R, et al. Relationship between ambient air pollution and DNA damage in Polish mothers and newborns. Environ Health Perspect. 1998 Jun;106( Suppl 3):821–826. doi: 10.1289/ehp.98106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen M, Wichmann J, Autrup H, Dang DA, Decordier I, Hvidberg M, et al. Increased micronuclei and bulky DNA adducts in cord blood after maternal exposures to traffic-related air pollution. Environ Res. 2009 Nov;109(8):1012–1020. doi: 10.1016/j.envres.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Sram RJ, Podrazilova K, Dejmek J, Mrackova G, Pilcik T. Single cell gel electrophoresis assay: sensitivity of peripheral white blood cells in human population studies. Mutagenesis. 1998 Jan;13(1):99–103. doi: 10.1093/mutage/13.1.99. [DOI] [PubMed] [Google Scholar]
- 35.Castano-Vinyals G, D’Errico A, Malats N, Kogevinas M. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup Environ Med. 2004 Apr;61(4):e12. doi: 10.1136/oem.2003.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perera FP, Wang S, Vishnevetsky J, Zhang B, Cole KJ, Tang D, et al. Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ Health Perspect. 2011 Aug;119(8):1176–1181. doi: 10.1289/ehp.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]