Abstract
Sensitization and challenge using dinitrofluorobenzene (DNFB) induce contact hypersensitivity (CHS) with predominant type 1 helper (Th1) cell infiltration, while those using fluorescein isothiocyanate (FITC) generate CHS with Th2 cell infiltration. CHS results from inflammatory cell infiltration, a process highly regulated by expression of multiple adhesion molecules. To determine a role of L-selectin and intercellular adhesion molecule-1 (ICAM-1) in Th1- and Th2-type CHS, CHS induced by DNFB or FITC in mice lacking L-selectin, ICAM-1 (ICAM-1−/−), or both. Th1-type CHS induced by DNFB was inhibited by L-selectin and/or ICAM-1 deficiency, which was associated with reduced IFN-γ expression. Similarly, Th2-type CHS was induced by FITC was inhibited by L-selectin deficiency. In contrast, Th2-type CHS was increased by ICAM-1 deficiency, which was accompanied by increased Th2 cytokine expression. Infiltration of in vitro-generated Th1 cells into the FITC-challenged skin decreased in ICAM-1−/− mice, while in vitro-generated Th2 cell infiltration increased, suggesting that ICAM-1 mediates Th1 cell migration and that in the absence of ICAM-1, Th1 cell recruitment decreased and relatively Th2 cell migration increased. These results suggest that ICAM-1 mediates Th1 cell recruitment irrespective of DNFB or FITC and that L-selectin recruits Th1 cells in Th1-type CHS, whereas it recruits Th2 cells in Th2-type CHS.
Introduction
Leukocyte recruitment into inflammatory sites is achieved using constitutive or inducible families of cell adhesion molecules (Ley et al., 2007). Leukocytes first tether and roll on vascular cells, before they are activated to adhere firmly and subsequently to immigrate into the extravascular space. The selectin family, L-selectin (CD62L), E-selectin (CD62E), and P-selectin (CD62P), primarily mediate leukocyte capture and rolling on the endothelium (Ley et al., 2007). Intercellular adhesion molecule (ICAM)-1 (CD54) is a member of the immunoglobulin (Ig) superfamily that is constitutively expressed on endothelial cells (Dustin et al., 1986). ICAM-1 forms the counterreceptor for the lymphocyte β2 integrins, such as leukocyte function-associated antigen (LFA)-1 (Ley et al., 2007). The ICAM-1/LFA-1 interaction predominantly mediates firm adhesion and transmigration of leukocytes at sites of inflammation (Ley et al., 2007). Previous studies using mice lacking both L-selectin and ICAM-1 (selectin/ICAM-1−/− mice) demonstrate a direct role of ICAM-1 in leukocyte rolling as the frequency of rolling leukocytes in L-selectin−/− mice treated with tumor necrosis factor (TNF)-α is decreased significantly by the additional loss of ICAM-1 expression (Steeber et al., 1998). Furthermore, the loss of both L-selectin and ICAM-1 expression reduces leukocyte recruitment into sites of inflammation beyond what is observed with loss of either receptor alone (Nagaoka et al., 2000; Steeber et al., 1999). Therefore, L-selectin and ICAM-1 mediate optimal leukocyte accumulation during inflammation through overlapping as well as synergistic functions.
Contact hypersensitivity (CHS) is an inflammatory, T cell-mediated skin reaction to a hapten, such as dinitrofluorobenzene (DNFB), which is associated with the activation of type 1 helper T (Th1) cells. Upon challenging the skin with DNFB in mice sensitized with DNFB, DNFB-specific T cells are recruited to the skin and produce the Th1 cytokines, including interferon (IFN)-γ and interleukin (IL)-2, between 12–24 hours after challenge, indicating that Th1 cells are important in CHS response (Takeshita et al., 2004a). By contrast, previous studies have shown that fluorescein isothiocyanate (FITC)-induced CHS was Th2-dominant (Tang et al., 1996; Dearman and Kimber, 2000; Takeshita et al., 2004a; Takeshita et al., 2004b). When FITC was used as a hapten, Th2-like response is observed with an IL-4/IFN-γ ratio of 25, while more IFN-γ than IL-4-secreting cells are found in draining lymph nodes from DNFB-sensitized mice with an IL-4/INF-γ ratio of 0.026 (Tang et al., 1996). Thus, FITC can induce a selective Th2-type effector T cells that cause CHS in skin-sensitized mice.
Previous studies have shown that L-selectin-deficient (L-selectin−/−) mice and ICAM-1-deficient (ICAM-1−/−) mice exhibit reduced edema and inflammatory infiltration in CHS induced by DNFB or oxazolone, another Th1-inducing hapten (Dearman et al., 1994), indicating that L-selectin and ICAM-1 mediate Th1 cell recruitment to the inflamed skin. However, a role of L-selectin and ICAM-1 in Th2-type CHS induced by FITC remained unknown. Therefore, we investigated contribution of L-selectin and ICAM-1 to Th2 cell recruitment during FITC-induced CHS using L-selectin−/− mice, ICAM-1−/− mice, or mice lacking both L-selectin and ICAM-1 (L-selectin/ICAM-1−/− mice) in comparison with DNFB-induced CHS. The results of this study suggest that ICAM-1 mediates Th1 cell recruitment irrespective of DNFB or FITC and that L-selectin recruits Th1 cells in Th1-type CHS, whereas it recruits Th2 cells in Th2-type CHS.
Results
Ear swelling induced by FITC or DNFB in adhesion molecule-deficient mice
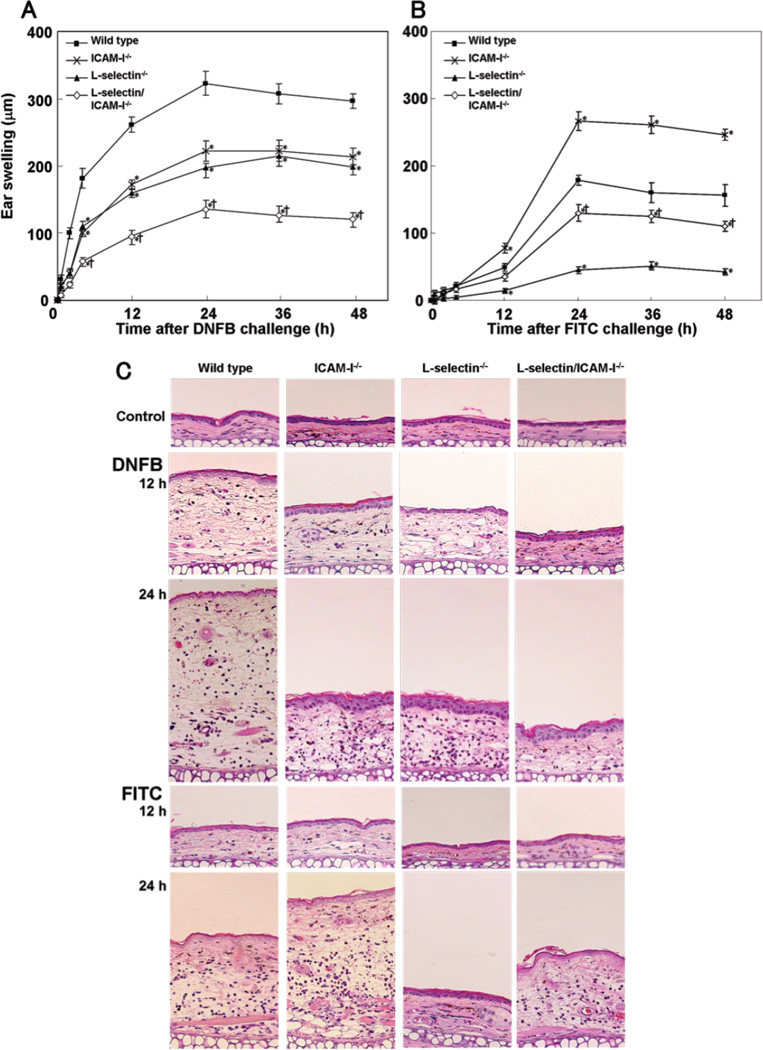
To assess a role of ICAM-1 and L-selectin in Th1-type and Th2-type CHS response, L-selectin−/−, ICAM-1−/−, and L-selectin/ICAM-1−/− mice were challenged with DNFB or FITC after sensitization. Ear swelling was similar between untreated mice and vehicle-treated mice (data not shown). Ear swelling reached peak at 24 hours after DNFB or FITC challenge, then decreased gradually (Fig 1A, B). After DNFB elicitation, L-selectin−/−, ICAM-1−/−, and L-selectin/ICAM-1−/− mice showed moderate ear swelling that was 33%, 31%, and 61% thinner than wild type mice, respectively (p<0.05, Fig 1A, C). Furthermore, L-selectin/ICAM-1−/− mice exhibited decreased ear swelling relative to either L-selectin−/− (p<0.05) or ICAM-1−/− (p<0.05) mice. Similarly, ear swelling in FITC-treated L-selectin−/− and L-selectin/ICAM-1−/− mice was significantly 75% and 27% thinner than that in FITC-treated wild type mice (p<0.05), although it was more strongly inhibited in L-selectin−/− mice than L-selectin/ICAM-1−/− mice (p<0.05; Fig 1B, C). In contrast, ear swelling was augmented by 49% in FITC-treated ICAM-1−/− mice compared with FITC-treated wild type mice. Thus, DNFB-induced Th1-type CHS was inhibited by L-selectin and/or ICAM-1 loss, while FITC-induced Th2-type CHS was suppressed by L-selectin loss but augmented by ICAM-1 loss.
Figure 1.
Ear swelling following elicitation with DNFB (A) or FITC (B) in adhesion molecule-deficient and wild type mice. Ear thickness was measured at 0, 0.5, 2, 4, 6, 9, 12, 24, and 48 hours (h) after challenge. Ear swelling was determined as the difference between the ear thickness before and after each elicitation. These results were obtained from 6 mice in each group. All values represent the mean ± SEM. *p<0.05 vs. wild type mice and †p < 0.05 vs. both L-selectin−/− mice and ICAM-1−/− mice. Representative histological sections of the ear pinnae from mutant and wild type mice before and 12 h and 24 h after DNFB or FITC challenge (C). The sections were stained with H&E (original magnification, ×40).
Leukocyte infiltration at the elicitation site
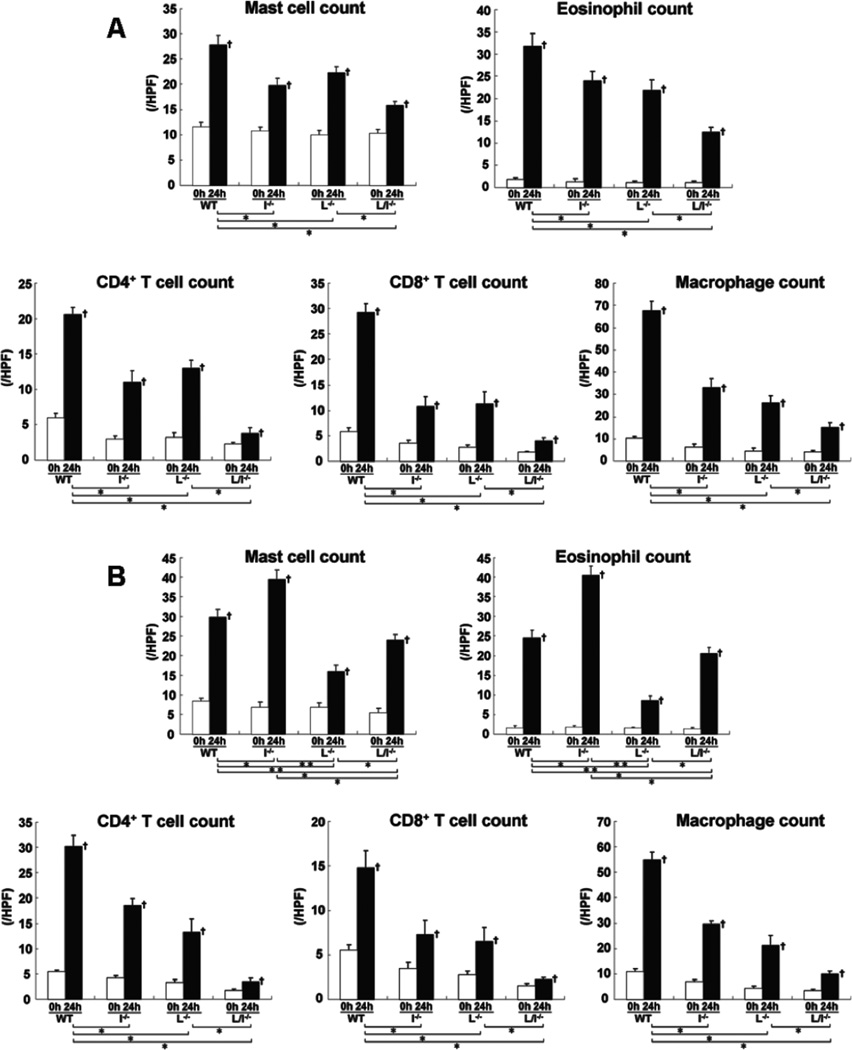
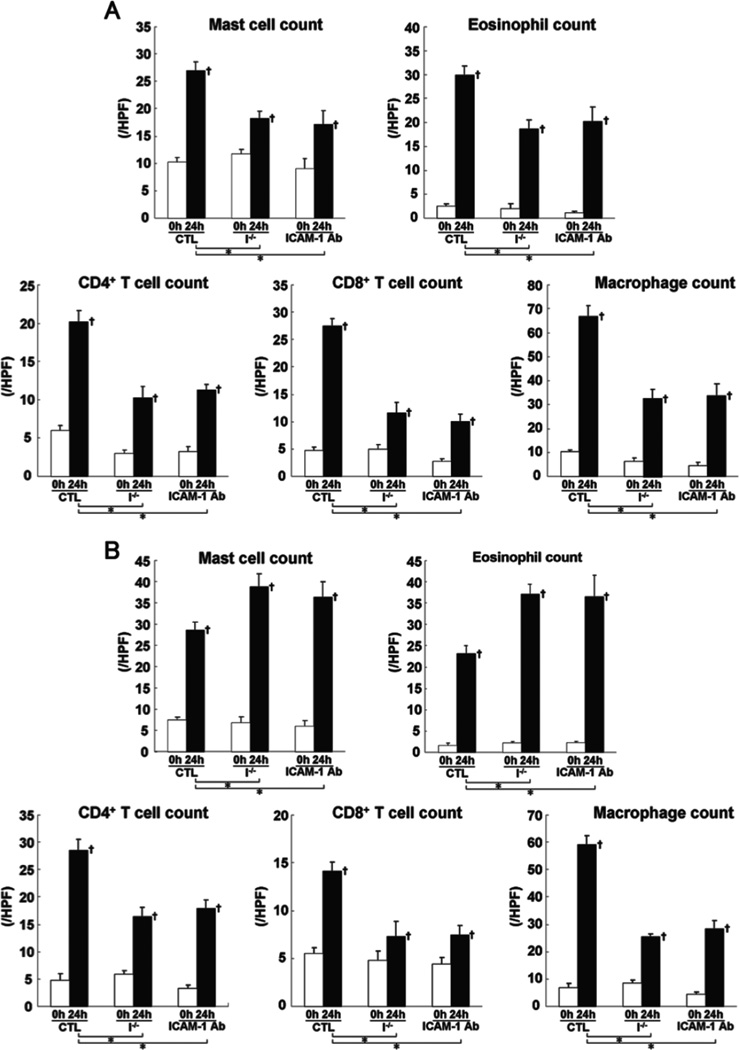
Extravascular leukocytes were assessed in skin tissue sections 24 hours after elicitation by DNFB or FITC. Before challenge, there was no significant difference in cutaneous leukocyte numbers between mutant and wild type mice (Fig 2). After DNFB challenge, the number of mast cells, eosinophils, CD4+ T cells, CD8+ T cells, and macrophages was significantly reduced in L-selectin−/−, ICAM-1−/−, and L-selectin/ICAM-1−/− mice compared with wild type mice (p<0.05, Fig 2A). The combined loss of L-selectin and ICAM-1 led to greater reduction in leukocyte accumulation than loss of each adhesion molecule alone (p<0.05). Similar results with the number of CD4+ T cells, CD8+ T cells, and macrophages were obtained by FITC challenge (Fig 2B). The number of eosinophils and mast cells after FITC challenge was also reduced in L-selectin−/− (p<0.005) and L-selectin/ICAM-1−/− (p<0.05) mice compared with wild type mice, although it was further decreased in L-selectin−/− mice relative to L-selectin/ICAM-1−/− mice (p<0.05). In contrast, ICAM-1−/− mice showed the increased number of eosinophils and mast cells after FITC challenge compared with wild type mice (p<0.05) as well as L-selectin−/− (p<0.005) and L-selectin/ICAM-1−/− (p<0.05) mice. Thus, although leukocyte infiltration was generally inhibited by loss of L-selectin and/or ICAM-1, mast cell and eosinophil number after FITC challenge increased by ICAM-1 loss.
Figure 2.
The number of mast cells, eosinophils, CD4+ T cells, CD8+ T cells, and macrophages in the skin from wild type mice (WT), L-selectin−/− mice (L−/−), ICAM-1−/− mice (I−/−), and L-selectin/ICAM-1−/− mice (L/I−/−) treated with either DNFB (A) or FITC (B) on the ears before and 24 hours (h) after challenge. Mast cells were identified by toluidine blue staining, whereas eosinophils were identified by H&E staining. Macrophages, CD4+ T cells, and CD8+ T cells were stained with F4/80, anti-CD4 mAb, and anti-CD8 mAb, respectively. The number of cells was counted in 10 random grids under magnification of ×400 high-power fields (HPF) and averaged. Each histogram shows the mean (± SEM) results obtained from 6 mice of each group. †p<0.05 vs. before elicitation in each group. *p<0.05 and **p<0.01.
Cytokine expression and IgE production
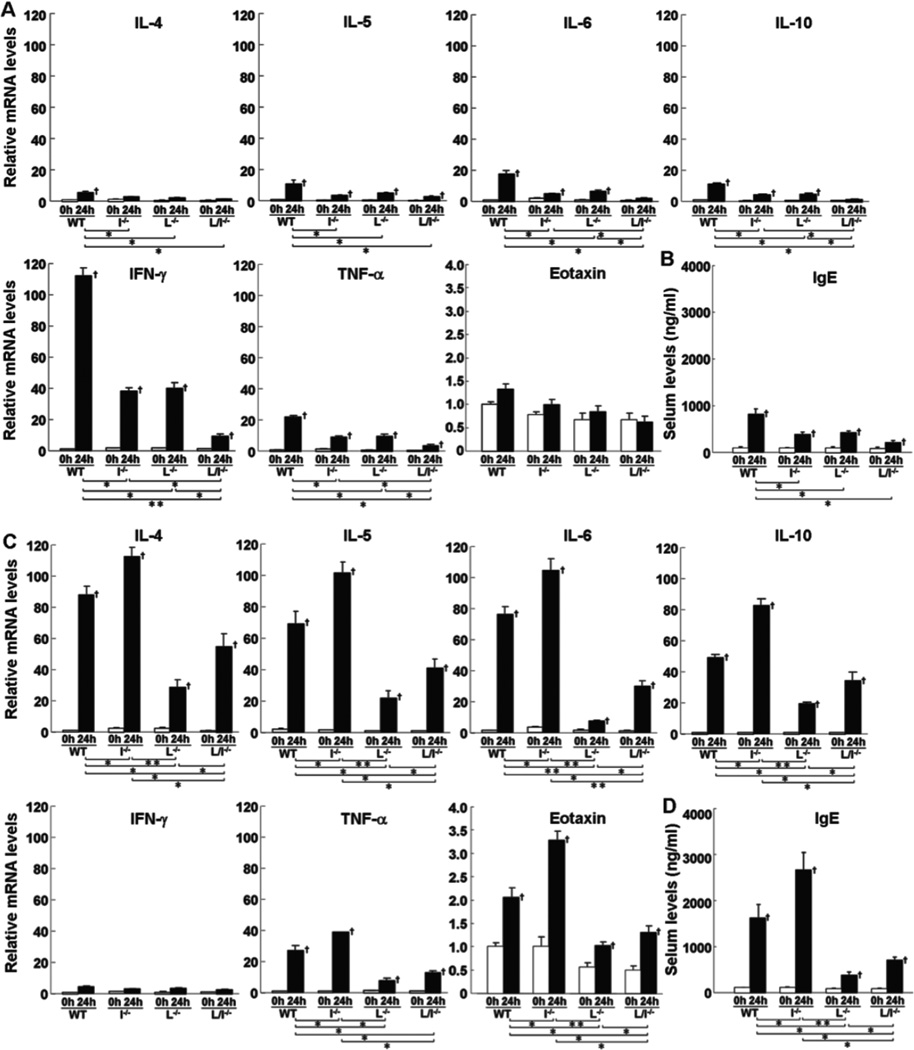
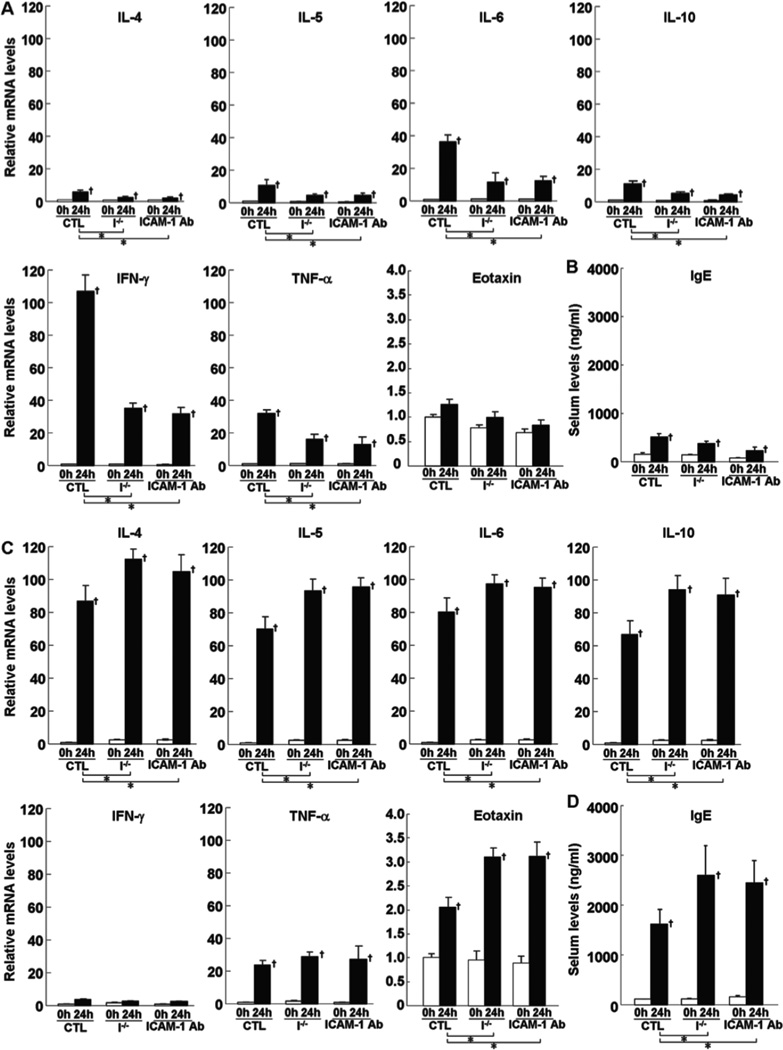
Cytokine expression in the challenged ear was assessed by real-time polymerase chain reaction (PCR) before and 24 hours after DNFB or FITC challenge. Before challenge, there was no significant difference in each cytokine expression between mutant and wild type mice (Fig 3A, C). In wild type mice, DNFB challenge predominantly increased IFN-γ expression compared with FITC challenge, while FITC elicitation mainly augmented expression of IL-4, IL-5, IL-6, IL-10, and eotaxin relative to DNFB treatment (Fig 3A, C). TNF-α expression was up-regulated by DNFB challenge to a similar level by FITC challenge. After DNFB challenge, expression of all cytokines, except eotaxin, significantly decreased in L-selectin−/−, ICAM-1−/−, and L-selectin/ICAM-1−/− mice relative to wild type mice (p<0.05, Fig 3A). In general, L-selectin−/− and ICAM-1−/− mice exhibited similar expression levels of all cytokines, which were higher than those of L-selectin/ICAM-1−/− mice except eotaxin (p<0.05, Fig 3A). By contrast, after FITC challenge, expression of IL-4, IL-5, IL-6, IL-10, eotaxin, and TNF-α increased in ICAM-1−/− mice relative to wild type mice (p<0.05) as well as L-selectin/ICAM-1−/− mice (p<0.05) and L-selectin−/− mice (p<0.05 for TNF-α and p<0.01 for cytokines other than TNF-α; Fig 3C). Expression of these cytokines in L-selectin/ICAM-1−/− and L-selectin−/− mice decreased compared with wild type mice (p<0.05), although it was further inhibited in L-selectin−/− mice relative to L-selectin/ICAM-1−/− mice except TNF-α (p<0.05).
Figure 3.
Levels of mRNA expression of IL-4, IL-5, IL-6, IL-10, IFN-γ, TNF-α, and eotaxin (A, C) in the skin and serum total IgE levels in the serum (B, D) from wild type mice (WT), L-selectin−/− mice (L−/−), ICAM-1−/− mice (I−/−), and L-selectin/ICAM-1−/− mice (L/I−/−) treated with either DNFB (A, B) or FITC (C, D). Levels of mRNA expression, which were quantified by real-time PCR, were determined as mRNA expression levels relative to those in wild type mice before elicitation. Serum total IgE levels were determined by ELISA. All values represent the mean ± SEM. These results were obtained from 6 mice in each group. †p<0.05 vs. before elicitation in each group. *p<0.05 and **p<0.01.
Before elicitation, serum total IgE levels were not significantly different between mutant and wild type mice (Fig 3B, D). Although both DNFB and FITC elicitation increased serum IgE levels compared with those before elicitation (p<0.05), FITC challenge induced higher IgE levels than DNFB challenge (p<0.05). After DNFB elicitation, IgE levels were significantly lower in ICAM-1−/−, L-selectin−/−, and L-selectin/ICAM-1−/− mice than those found in wild type mice (p<0.05, Fig 3B). Similarly, IgE levels were lower in L-selectin−/− and L-selectin/ICAM-1−/− mice after FITC elicitation relative to wild type mice (p<0.05), although they were further decreased in L-selectin−/− mice relative to L-selectin/ICAM-1−/− mice (p<0.05; Fig 3C). By contrast, IgE levels increased in ICAM-1−/− mice compared to wild type mice (p<0.05) as well as L-selectin/ICAM-1−/− (p<0.01) and L-selectin−/− (p<0.05) mice. Thus, up-regulated Th1 cytokine expression by DNFB challenge was reduced by L-selectin and/or ICAM-1 loss. By contrast, while up-regulated Th2 cytokine expression and IgE production were suppressed by L-selectin loss, they were increased by ICAM-1 loss.
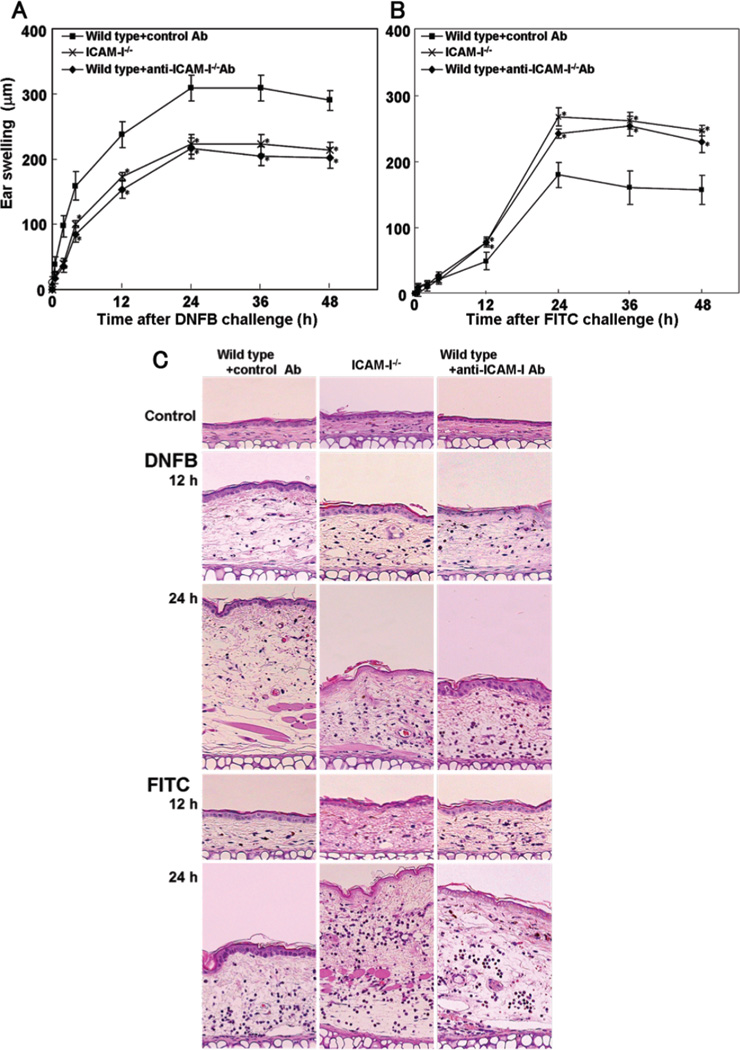
Ear swelling, leukocyte infiltration, and cytokine expression in mice treated with anti-ICAM-1 antibody (Ab)
The augmented CHS response induced by FITC in ICAM-1−/− mice suggests compensatory up-regulation of other cell adhesion molecules. However, mRNA expression levels of E-selectin and P-selectin were similar between ICAM-1−/− and wild type mice (data not shown). To further clarify the role of ICAM-1 during Th2-type CHS response, a blocking study using anti-ICAM-1 monoclonal Ab (mAb) was performed. Wild type mice were intravenously injected with anti-ICAM-1 mAb immediately before elicitation. Wild type mice injected with irrelevant isotype-matched control Ab served as control. There was no significant difference in all parameters examined in this study between untreated wild type mice and wild type mice treated with control Ab (data not shown). In DNFB-induced Th1-type CHS response, there was no significant difference in ear swelling (Fig 4A, C), leukocyte infiltration (Fig 5A), cytokine expression (Fig 6A), and IgE production (Fig 6B) between mice treated with anti-ICAM-1 mAb and ICAM-1−/− mice. Similarly, in FITC-induced Th2-type CHS response, ear swelling (Fig 4B, C), leukocyte infiltration (Fig 5B), cytokine expression (Fig 6C), and IgE production (Fig 6D) did not significantly differ between mice treated with anti-ICAM-1 mAb and ICAM-1−/− mice. Thus, results similar to ICAM-1−/− mice were obtained by anti-ICAM-1 mAb treatment in wild type mice.
Figure 4.
The effect of anti-ICAM-1 mAb on ear swelling response to DNFB (A) or FITC (B) challenge. Wild type mice treated with mAb to ICAM-1 intravenously 1 hour (h) before elicitation. Wild type mice injected with irrelevant isotype-matched control Ab served as control. All values represent the mean ± SEM. These results were obtained from 6 mice in each group. *p<0.05 vs. wild type mice. Histological sections of their ear pinnae treated with either DNFB or FITC challenge (C). The sections were stained with H&E (original magnification, ×40).
Figure 5.
The number of mast cells, eosinophils, CD4+ T cells, CD8+ T cells, and macrophages in the ear skin from wild type mice treated with control mAb (CTL), ICAM-1−/− mice (I−/−), and wild type mice treated with anti-ICAM-1 mAb (ICAM-1 Ab). These mice were challenged with either DNFB (A) or FITC (B) on the ears before and 24 hours (h) after challenge. The blocking study by mAb was performed as described in the legend of Figure 4. Methods for counting the number of each immune cell were described in the legend of Figure 2. Each histogram shows the mean (± SEM) results obtained from 6 mice of each group. †p<0.05 vs. before elicitation in each group and *p<0.05 vs. CTL.
Figure 6.
Levels of mRNA expression of IL-4, IL-5, IL-6, IL-10, IFN-γ, TNF-α, and eotaxin (A, C) in the skin and serum total IgE levels (B, D) of wild type mice treated with control mAb (CTL), ICAM-1−/− mice (I−/−), and wild type mice treated with anti-ICAM-1 mAb (ICAM-1 Ab) before and 24 hours (h) after either DNFB (A, B) or FITC (C, D) challenge. Methods of mRNA quantification and IgE measurement were described in the legend of Figure 3. All values represent the mean ± SEM of results obtained from 10 mice in each group. †p<0.05 vs. before elicitation in each group and *p<0.05 vs. CTL.
Trafficking of Th1- or Th2-polarized cells into the skin challenged with hapten
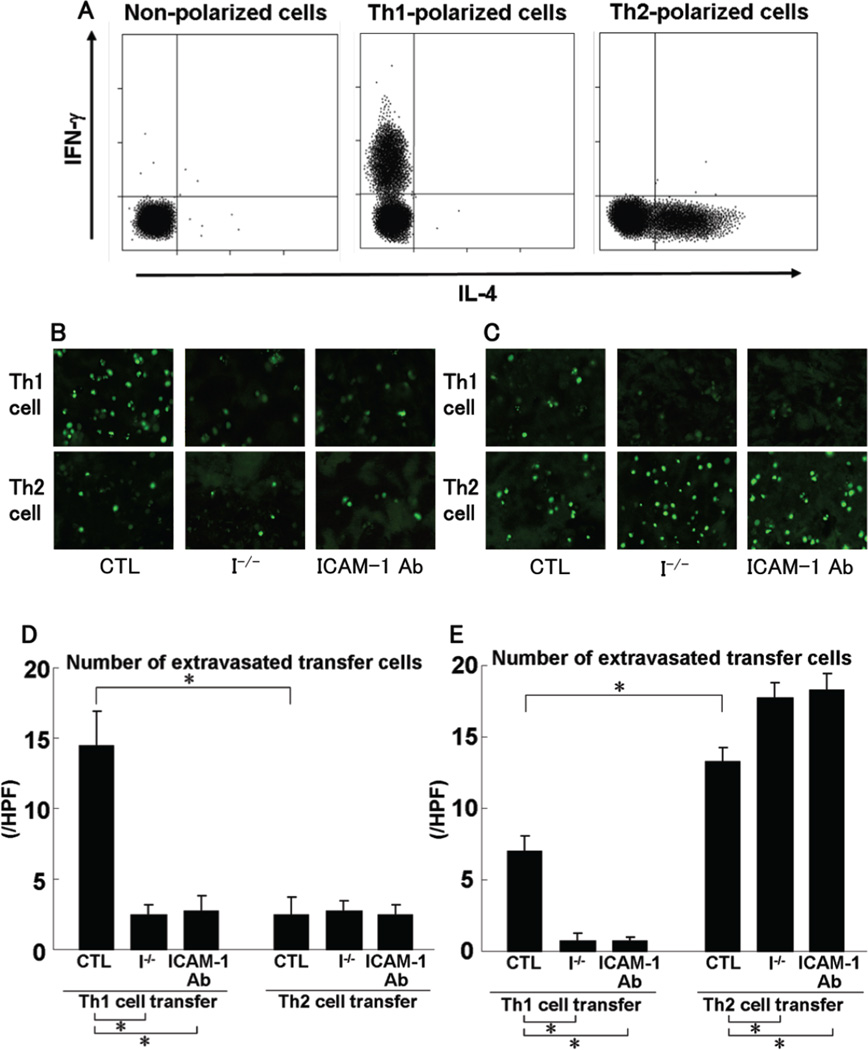
To investigate selective recruitment of Th1 and Th2 cells during CHS response, in vitro-generated, antigen-nonspecific, Th1- or Th2- polarized cells that were labeled with calcein-acetoxymethyl ester (C-AM) were adoptively transferred into ICAM-1−/− mice and wild type mice with or without anti-ICAM-1 mAb treatment before DNFB elicitation. Intracellular Th1 or Th2 cytokine production by in vitro-generated polarized cells was confirmed by flow cytometry (Fig 7A). Skin infiltration of Th1- or Th2-polarized cells was detected by confocal laser microscopy. Wild type mice injected with irrelevant isotype-matched control Ab served as control. There was no significant difference in polarized cell infiltration between untreated wild type mice and wild type mice treated with control Ab (data not shown). At 24 hours after DNFB elicitation, many Th1 cells infiltrated to the skin from wild type mice, while the number of Th1 cells in the skin from ICAM-1−/− mice was reduced compared with wild type mice (p<0.05; Fig 7B, D). The number of infiltrating Th2 cells was lower than that of Th1 cells (p<0.05) and was similar between wild type and ICAM-1−/− mice. At 24 hours after FITC challenge, although the small number of Th1 cells infiltrated into the skin from wild type mice, the Th1 cell number was further reduced in ICAM-1−/− mice relative to wild type mice (p<0.05; Fig 7C, E). The number of Th2 cells in the skin from wild type mice was higher than that of Th1 cells and was increased in ICAM-1−/− mice relative to wild type mice (p<0.05). Results similar to ICAM-1−/− mice were obtained for wild type mice treated with anti-ICAM-1 mAb (Fig 7). Thus, Th1 cell recruitment appeared to be dependent upon ICAM-1 irrespective of hapten.
Figure 7.
Cytokine profile of in vitro-generated polarized Th1 or Th2 cell populations (A). Polarized Th1 and Th2 cells were double-stained for intracellular IFN-γ and IL-4 after activation with anti-CD3 and anti-CD28 mAb and were analyzed by flow cytometry. C-AM-labeled polarized Th1 or Th2 cells were injected into wild type mice treated with control Ab (CTL), ICAM-1−/− mice (I−/−), and wild type mice treated with anti-ICAM-1 mAb (ICAM-1 Ab) at 1 hour before DNFB (B, D) or FITC (C, E) elicitation. Mice were sacrificed 24 hours after elicitation. Cell infiltration in the skin was scanned by confocal laser microscopy (B, C). The number of extravasated transfer cells was counted in 10 random grids under magnification of high-power fields (HPF, ×400) and averaged (D, E). Each histogram shows the mean (± SEM) results obtained from 5 mice of each group. *p<0.05.
Discussion
Consistent with the previous studies (Wang et al., 2001), Th1-type CHS induced by DNFB was inhibited by L-selectin and/or ICAM-1 deficiency, which was associated with reduced Th1 cytokine expression, indicating that L-selectin and ICAM-1 mediate Th1 cell recruitment into the challenged skin. This is further confirmed by the finding that infiltration of in vitro-generated Th1 cells into the DNFB-challenged skin was suppressed by ICAM-1 deficiency. Th2-type CHS induced by FITC was inhibited by L-selectin deficiency, suggesting that unlike DNFB challenge, L-selectin is involved in Th2 cell migration in this inflammatory situation. In contrast, Th2-type CHS induced by FITC was augmented by ICAM-1 deficiency, which was accompanied by increased Th2 cytokine expression. Our observation that ICAM-1 deficiency suppressed infiltration of in vitro-generated Th1 cells into the FITC-challenged skin and augmented that of in vitro-generated Th2 cells suggests that ICAM-1 primarily mediates Th1 cell migration and that in the absence of ICAM-1, Th1 cell recruitment was reduced and relatively Th2 cell migration increased. This increased Th2 cell migration was not due to compensatory up-regulated expression of other cell adhesion molecules, such as E- and P-selectins, since expression of E- and P-selectins was not altered in ICAM-1−/− mice (data not shown). Furthermore, it is unlikely that other factors susceptible to genetic ICAM-1 deficiency would affect the results, since results similar to ICAM-1−/− mice were obtained from wild type mice treated with anti-ICAM-1 mAb. Collectively, this study is the first to reveal the differential contribution of L-selectin and ICAM-1 to Th1-type and Th2-type CHS response.
The results of this study suggest that ICAM-1 preferentially mediates Th1 cell recruitment irrespective of DNFB or FITC. Consistent with this finding, in vivo adherence of adoptively transferred alloreactive Th1 cells to the lung is decreased in ICAM-1−/− mice (Dixon et al., 2000). By contrast, allergic lung disease is reduced in mice deficient for LFA-1, an ICAM-1 counterreceptor, which is due to markedly impaired recruitment of Th2 cells to the lungs (Lee et al., 2008). Similarly, ICAM-1 deficiency attenuates skin fibrosis and leukocyte infiltration with decreased Th2 cytokine expression in the affected skin from tight-skin mouse, a model of systemic sclerosis (Matsushita et al., 2007). On the other hand, in experimental autoimmune uveoretinnitis, trafficking of both Th1 and Th2 cells across the blood-retina barrier is mediated by ICAM-1 and LFA-1 interaction (Xu et al., 2004). These findings suggest that contribution of ICAM-1 to Th1 and/or Th2 cell recruitment varies according to the tissue site of inflammation and the nature of the inflammatory stimuli. Nonetheless, our results suggest that ICAM-1 predominantly mediates Th1 cell migration into the skin in both Th1-type and Th2-type CHS.
The results of the current study suggest that L-selectin recruits Th1 cells in Th1-type CHS response, whereas it recruits Th2 cells in Th2-type CHS. Therefore, it is possible that L-selectin may mediate specific effector T cells according to local inflammatory environment. Although the number of studies, which investigated a role of L-selectin in Th1/Th2 cell recruitment, is limited, several studies have shown that L-selectin+ memory CD4+ T cells produce Th2 cytokines, while L-selectin− memory CD4+ T cells produce Th1 cytokines (Kanegane et al., 1996; Matsuzaki et al., 2005). Therefore, L-selectin may preferentially mediate Th2 cell migration, which is consistent with our finding that ear swelling was reduced in Th2-type CHS. In contrast, the previous observation that IL-12 can maintain L-selectin expression has implications for a role of L-selectin in Th1 cell migration to sites of inflammation (van Wely et al., 1999). This could explain our observation that L-selectin also recruits Th1 cells in the setting of Th1-type CHS. Although the mechanisms for this phenomenon remain unknown, L-selectin may recruit both Th1 and Th2 cells according to the local inflammatory environment.
Although the molecular mechanisms for the differential role of L-selectin and ICAM-1 in Th1-type and Th2-type CHS remain unknown in this study, it may be related to the generation of memory T cells in the lymph nodes, since ICAM-1 functions as a costimulatory molecule on the surface of antigen-presenting cells and L-selectin functions as a homing receptor of naive T cells to the lymph nodes (Ley and Kansas, 2004). However, this is unlike regarding ICAM-1, since the adoptive transfer of in vitro-polarized Th cells into the hapten-challenged ear from ICAM-1−/− mice in this study was antigen-nonspecific. Rather, altered local expression of cytokines and chemokines induced by specific hapten might be relevant as chemokine can modulate affinity of LFA-1 to ICAM-1 (Shulman et al., 2009). Further studies will be required to clarify the underlying mechanisms of differential regulation of Th cell recruitment by L-selectin and ICAM-1.
In the FITC-induced CHS model, an inflammatory skin pathology reproducing all the key features of human atopic dermatitis develops, including what appears to be Th2-driven immune response involving mast cell and eosinophil infiltration, and elevation of total serum IgE levels (Takeshita et al., 2004a). Since a lack of ICAM-1 augments Th2-type immune response in the FITC-induced CHS model, this may represent a suitable mouse model with which to analyze therapeutics developed for the human disease, such as atopic dermatitis. Furthermore, understanding differential regulation of Th2-type immune response by ICAM-1 and L-selectin could provide useful information for the therapy of atopic dermatitis by blockade of adhesion molecules.
Materials and methods
Mice
L-selectin−/− mice were generated as described previously (Arbones et al., 1994). ICAM-1−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME). L-selectin/ICAM-1−/− mice were generated as described previously (Steeber et al., 1998). All mice were healthy, fertile, and did not display evidence of infection or disease. All mice were backcrossed 10 generations onto the C57BL/6 genetic background. Mice used for experiments were 8 weeks old. All mice were housed in a pathogen-free barrier facility and screened regularly for pathogens. All studies and procedures were approved by the Committee on Animal Experimentation of Nagasaki University Graduate School of Biomedical Sciences.
Sensitization and elicitation of CHS
Mice were sensitized with 25 µl of 0.5% DNFB (Sigma-Aldrich, St. Louis, MO), in acetone and olive oil (4:1), onto shaved abdominal skin on days 0 and 1, respectively. Six days later, CHS was elicited by applying 20 µl of 0.25% DNFB on the right ear. The left ear was treated with acetone/olive oil alone. Ear thickness was measured in a blinded manner with a dial thickness gauge (Ozaki Seisakusho, Tokyo, Japan) under light ether anesthesia. For detailed time-course analysis of ear swelling reaction, ear thicknesses were measured before the elicitation and 0.5, 2, 4, 12, 24, 36, and 48 hours after the elicitation. The degree of CHS was determined as swelling of the hapten-challenged ear compared with that of the vehicle-treated ear. As for sensitization with FITC (Sigma-Aldrich), 400 µl of 0.5% FITC in acetone and dibutylphthalate (1:1) was applied on shaved abdominal skin on days 0 and 1, followed by loading 20 µl of 0.5% FITC on the right ear and acetone/dibutylphthalate alone on the left ear after 6 days.
Histological examination
A central strip of the ear was fixed in 10% formalin, then paraffin embedded. Six-micrometer sections were stained using hematoxylin and eosin (H&E) for general histological evaluation and toluidine blue for mast cell staining. Dermal leukocyte infiltration was evaluated by averaging the number of leukocytes that was counted in 10 random grids under high-magnification (×400) power fields of a light microscope. Each section was examined independently by two investigators in a blinded fashion.
Immunohistochemical staining
For immunohistochemistry, frozen tissue sections of skin biopsies were acetone-fixed and then incubated with 10% normal rabbit serum in phosphate-buffered saline (10 minutes, 37°C) to block nonspecific staining. Sections were then incubated with rat mAbs specific for mouse macrophages (F4/80, Serotec, Oxford, UK), CD4 (Clone RM4–5, BD PharMingen, San Diego, CA), and CD8 (clone 53-6.7, BD PharMingen). Rat IgG (Southern Biotechnology Associates, Birmingham, AL) was used as a control for nonspecific staining. Sections were then incubated sequentially (20 minutes, 37°C) with biotinylated rabbit anti-rat IgG (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). Stained cells were counted in 10 random grids under high-magnification (×400) power fields of a light microscope. Each section was examined independently by two investigators in a blinded fashion.
RNA isolation and real-time PCR
Total RNA was isolated from the ear with RNeasy spin columns (Qiagen, Crawley, UK). Total RNA from each sample was reverse-transcribed into cDNA. Expression of IL-4, IL-5, IL-6, IL-10, IFN-γ, TNF-α, and eotaxin was analyzed by an ABI Prism 7000 Sequence Detector (Applied Biosystems, Foster City, CA), according to the manufacturer’s instructions. Sequence-specific primers and probes were designed by Pre-Developed TaqMan assay reagents or Assay-On-Demand (Applied Biosystems). Glyceraldehyde-3-phosphate was used to normalize mRNA. Relative expression of real-time PCR products was determined by using the ΔΔCt method (Meijerink et al., 2001) to compare target gene and housekeeping gene mRNA expression.
ELISA for serum IgE
Total serum IgE levels were quantified by sandwich ELISA according to the manufacturer’s protocol (BD PharMingen). Rat anti-mouse IgE mAb (clone R35–72) was used for coating the plates and rat anti-mouse IgE mAb (clone R35–92) was used for detection. The relative concentration of total IgE levels in individual samples was calculated by comparing the mean OD to a semilog standard curve of the titrated purified mouse IgE using linear regression analysis.
Blocking study by mAb
For a blocking study, anti-ICAM-1 mAb (3E2, Armenian hamster IgG, 5 mg/kg; BD PharMingen) (Scheynius et al., 1993) was intravenously injected 1 hour before elicitation. This was the mAb concentration required to inhibit ICAM-1-dependent leukocyte recruitment in vivo as previously described (Kumasaka et al., 1996; Kunkel et al., 1996). Irrelevant isotype-matched, purified Armenian hamster IgG mAb (Ha4/8) served as control (5 mg/kg; BD PharMingen).
Cell preparation and culture
Naive CD4+ T cells were isolated from lymph node cells using MACS positive selection protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). A total of 92–95% of these cells were CD4+ when tested with anti-CD4 mAb (BD Biosciences, Oxford, U.K.). A modified version of the method described by Siveke and Hamann (Siveke and Hamann, 1998) was used for the establishment of polarized T cells. CD4+ cells were treated for 2 days in six-well plates precoated with 2 µg/ml anti-mouse CD3e mAb (clone 145-2C11; BD Biosciences) in RPMI 1640 plus 10% FCS supplemented either with anti-mouse CD28 mAb (2 µg/ml, 37.51 clone; BD Bioscience), IL-12 (5 ng/ml; BD Biosciences), IFN-γ (20 ng/ml; BD Biosciences), and anti-IL-4 mAb (1 µg/ml, 11B11 clone; BD Biosciences) for generation of IFN-γ-producing Th1 cells or with anti-mouse CD28 mAb (2 µg/ml, 37.51 clone; BD Biosciences), IL-2 (5 ng/ml; BD Biosciences), IL-4 (10 ng/ml), and anti-IFN-γ mAb (2 µg/ml, clone XMG1.2; BD Biosciences) for IL-4-producing Th2 cells. The cells were then transferred onto uncoated plates without a change of medium and were cultured for an additional 4–6 days to allow the cells to return to a resting state (Siveke and Hamann, 1998). The return to a resting state is important because resting cells have been shown to actively traffic to a much greater extent than fully activated cells (Austrup et al., 1997). These cells are thought to resemble mature effector T cells in vivo, which re-enter the circulation after a sessile, proliferative phase (Austrup et al., 1997). Intracellular staining of polarized T cell subsets was performed after restimulation on a plate precoated with 2 µg/ml of anti-CD3 and anti-CD28 mAb overnight. Stained cells were analyzed using a FACScan flow cytometer (BD PharMingen).
In vivo leukocyte trafficking assay
A total of 2 × 107 in vitro-generated polarized Th1 or Th2 cells in 10 ml were incubated with 40 µg/ml of C-AM (Molecular Probes, Leiden, The Netherlands) at 37°C for 30 minutes. C-AM (green) is nontoxic and has no effect on cell adhesion (Abbitt et al., 2000). Then, 1 × 107 (150 µl) of C-AM-labeled polarized Th1 or Th2 cells were injected via the tail vein at 1 hour before DNFB or FITC elicitation. At 24 hours after elicitation, the ears were harvested and fixed in 2% (w/v) paraformaldehyde (Agar Scientific, Cambridge, U.K.) for 1 hour. Cell infiltration in the ear was assessed by confocal laser microscopy.
Statistical analysis
All data are expressed as mean values ± SD. The Mann-Whitney U test was used to determine the level of significance of differences between sample means, and Bonferroni’s test was used for multiple comparisons.
Acknowledgements
We thank Ms. M. Yozaki, A. Usui and K. Shimoda for technical assistance. This work was supported by a grant of Research on Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (to S. Sato) and National Institutes of Health, USA grants CA96547, CA105001, and AI56363 (to T. F. Tedder).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- Abbitt KB, Rainger GE, Nash GB. Effects of fluorescent dyes on selectin and integrin-mediated stages of adhesion and migration of flowing leukocytes. J Immunol Methods. 2000;239:109–119. doi: 10.1016/s0022-1759(00)00189-7. [DOI] [PubMed] [Google Scholar]
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Dearman RJ, Kimber I. Role of CD4(+) T helper 2-type cells in cutaneous inflammatory responses induced by fluorescein isothiocyanate. Immunology. 2000;101:442–451. doi: 10.1046/j.1365-2567.2000.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearman RJ, Ramdin LS, Basketter DA, Kimber I. Inducible interleukin-4-secreting cells provoked in mice during chemical sensitization. Immunology. 1994;81:551–557. [PMC free article] [PubMed] [Google Scholar]
- Dixon AE, Mandac JB, Martin PJ, Hackman RC, Madtes DK, Clark JG. Adherence of adoptively transferred alloreactive Th1 cells in lung: partial dependence on LFA-1 and ICAM-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:L583–L591. doi: 10.1152/ajplung.2000.279.3.L583. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- Kanegane H, Kasahara Y, Niida Y, Yachie A, Sughii S, Takatsu K, et al. Expression of L-selectin (CD62L) discriminates Th1- and Th2-like cytokine-producing memory CD4+ T cells. Immunology. 1996;87:186–190. doi: 10.1046/j.1365-2567.1996.446530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumasaka T, Quinlan WM, Doyle NA, Condon TP, Sligh J, Takei F, et al. Role of the intercellular adhesion molecule-1(ICAM-1) in endotoxin-induced pneumonia evaluated using ICAM-1 antisense oligonucleotides, anti-ICAM-1 monoclonal antibodies, and ICAM-1 mutant mice. J Clin Invest. 1996;97:2362–2369. doi: 10.1172/JCI118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Jung U, Bullard DC, Norman KE, Wolitzky BA, Vestweber D, et al. Absence of trauma-induced leukocyte rolling in mice deficient in both P-selectin and intercellular adhesion molecule 1. J Exp Med. 1996;183:57–65. doi: 10.1084/jem.183.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Prince JE, Rais M, Kheradmand F, Ballantyne CM, Weitz-Schmidt G, et al. Developmental control of integrin expression regulates Th2 effector homing. J Immunol. 2008;180:4656–4667. doi: 10.4049/jimmunol.180.7.4656. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Hasegawa M, Matsushita T, Fujimoto M, Horikawa M, Fujita T, et al. Intercellular adhesion molecule-1 deficiency attenuates the development of skin fibrosis in tight-skin mice. J Immunol. 2007;179:698–707. doi: 10.4049/jimmunol.179.1.698. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Shinozaki K, Kobayashi N, Agematsu K. Polarization of Th1/Th2 in human CD4 T cells separated by CD62L: analysis by transcription factors. Allergy. 2005;60:780–787. doi: 10.1111/j.1398-9995.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol. 2000;157:237–247. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheynius A, Camp RL, Pure E. Reduced contact sensitivity reactions in mice treated with monoclonal antibodies to leukocyte function-associated molecule-1 and intercellular adhesion molecule-1. J Immunol. 1993;150:655–663. [PubMed] [Google Scholar]
- Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- Steeber DA, Campbell MA, Basit A, Ley K, Tedder TF. Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc Natl Acad Sci U S A. 1998;95:7562–7567. doi: 10.1073/pnas.95.13.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeber DA, Tang ML, Green NE, Zhang XQ, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and ICAM-1 pathways. J Immunol. 1999;163:2176–2186. [PubMed] [Google Scholar]
- Takeshita K, Yamasaki T, Akira S, Gantner F, Bacon KB. Essential role of MHC II-independent CD4+ T cells, IL-4 and STAT6 in contact hypersensitivity induced by fluorescein isothiocyanate in the mouse. Int Immunol. 2004a;16:685–695. doi: 10.1093/intimm/dxh073. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Yamasaki T, Nagao K, Sugimoto H, Shichijo M, Gantner F, et al. CRTH2 is a prominent effector in contact hypersensitivity-induced neutrophil inflammation. Int Immunol. 2004b;16:947–959. doi: 10.1093/intimm/dxh096. [DOI] [PubMed] [Google Scholar]
- Tang A, Judge TA, Nickoloff BJ, Turka LA. Suppression of murine allergic contact dermatitis by CTLA4Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J Immunol. 1996;157:117–125. [PubMed] [Google Scholar]
- van Wely CA, Beverley PC, Brett SJ, Britten CJ, Tite JP. Expression of L-selectin on Th1 cells is regulated by IL-12. J Immunol. 1999;163:1214–1221. [PubMed] [Google Scholar]
- Wang B, Feliciani C, Freed I, Cai Q, Sauder DN. Insights into molecular mechanisms of contact hypersensitivity gained from gene knockout studies. J Leukoc Biol. 2001;70:185–191. [PubMed] [Google Scholar]
- Xu H, Manivannan A, Jiang HR, Liversidge J, Sharp PF, Forrester JV, et al. Recruitment of IFN-gamma-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by P-selectin glycoprotein ligand 1:P/E-selectin interactions. J Immunol. 2004;172:3215–3224. doi: 10.4049/jimmunol.172.5.3215. [DOI] [PubMed] [Google Scholar]