Abstract
We developed a panel of monoclonal antibodies (MAb) against chicken β2-microglobulin (chβ2M) by fusions between SP2/0 myeloma cells and spleen cells from mice immunized with a synthesized peptide corresponding to positions 91-119 of the COOH domain of chβ2M. Two of them, 6E7 and 3D1, identified as IgG1/κ, could react with chβ2M protein from avian macrophage HD11 cells and human 293T cells transfected with pcDNA3.1-chβ2M in immunofluorescence assays. Only a 12 kDa protein band of chβ2M could be detected in the HD11 and 293T/chβ2M cell lysates by Western blot analysis. Chicken β2M in serum and plasma could be found in Western blot by MAb 3D1. Moreover, MAb 3D1 also recognized the chβ2M antigen on the cell membranes in flow cytometry. Immunohistochemical staining with these MAbs revealed that chβ2M was present in chicken thymus, spleen, and bursa. These MAbs will be good tools for analyzing the mechanism of the chicken immune system.
Introduction
β2-microglobulin (β2M) is a low molecular weight, non-glycosylated protein that is synthesized by all nucleated cells. It composes the small, invariable light chain subunit of the major histocompatibility complex (MHC) class I antigen through non-covalent linkage on the cell surface.(1) The function of β2M is interacting with and stabilizing the tertiary structure of the MHC class I α-chain to present antigenic peptides to cytotoxic (CD8+) T lymphocytes.(2) In addition, β2M is extensively involved in the functional regulation of survival, proliferation, apoptosis, and even metastasis of cancer cells.(3,4) Recent studies showed that antibodies against β2M were highly cytotoxic against some solid(5) or liquid tumors.(3,6) However, there are only a few reports focusing on chicken β2-microglobulin (chβ2M).(7–10) In this study, we developed a panel of monoclonal antibodies against chβ2M with a synthesized peptide. These monoclonal antibodies could react with both linear chβ2M and native chβ2M.
Materials and Methods
Cell lines and cell culture
HD11 cells, a replication-defective avian leukemia virus MC29-transformed macrophage-like cell line, were kindly provided by Dr. XinAn Jiao (Yangzhou University, China). HD11 cells were maintained in Dulbecco's Modified Eagle's Media (DMEM, Life Technologies-Gibco, Bethesda, MD) containing 10% fetal bovine serum (FBS) at 41°C and 5% CO2. Human renal epithelial 293T cells were cultured in DMEM supplemented with 10% FBS at 37°C and 5% CO2. The MDCC-MSB1 (MSB1) cells, a chicken T-lymphoblastoid cell line transformed with BC-1 strain of Marek's disease virus (MDV), were grown in RPMI 1640 medium (Life Technologies-Gibco) supplemented with 10% FBS and 10% tryptose phosphate broth (Sigma-Aldrich, St. Louis, MO) at 37°C and 5% CO2. Chicken embryo fibroblasts (CEF) were made from 10-day embryos supplied by Merial-Vital Laboratory Animal Technology (Beijing, China) according to conventional procedures.
Cloning of the chβ2M gene and sequences analysis
Based on the reported sequence of chβ2M (GenBank: M84767.1, AY989898, and Z48921), a pair of specific primers was designed to amplify the full-length chβ2M from the total RNA of the chicken peripheral blood mononuclear cells (PBMCs), as reported previously.(11) The primer sequences, which also contained the restriction site EcoR I in the forward primer (P1) and Xho I in the reverse primer (P2) (underlined), were as follows: forward primer (p1): 5′-TTGAATTCATGGGGAAGGCGGCGGC-3′; reverse primer (p2): 5′-TTCTCGAGTCAGAACTCGGGATCCCA-3′. PCR product of approximately 400 bp was inserted into a pGEMT-easy vector (Promega, Madison, WI), and positive clones (pGEM-T-chβ2M) from an independent PCR reaction were sequenced by the Shanghai Sangon Company (China). The sequence was analyzed by DNAstar software (Madison, WI).
Synthesis of COOH-terminal chβ2M fragments
The peptide was synthesized by GL Biochem (Shanghai, China) with a solid-phase method and purified by high performance liquid chromatography. The purity of the peptide was greater than 99%.
Preparation of monoclonal antibodies
BALB/c mice were immunized with 100 μg synthetic peptide in complete Freund's adjuvant through a peritoneal injection, boosted with 150 μg in incomplete Freund's adjuvant 2 weeks later and 200 μg in 0.01 M/L PBS (pH 7.2) after another 3 weeks. Three days after the final injection, the spleen cells from the immunized mice were fused with SP2/0 myeloma cells according to standard procedures.(12) The hybridoma cell supernatants were screened for specific antibodies. Positive hybridomas were subcloned twice by conventional limiting-dilution.
Construct of chβ2M expression plasmids and transfection
The pcDNA3.1-chβ2M plasmid was constructed by chβ2M removed from PGEM-T-chβ2M with EcoR I and Xho I inserted into a pcDNA3.1 plasmid vector (Life Technologies-Invitrogen, Carlsbad, CA). The recombinant expression plasmid pcDNA3.1-chβ2M was transfected into 293T cells with Lipofectamine 2000 (Life Technologies-Invitrogen) according to the manufacturer's instruction. Then the cells were cultured for 48 h to express chβ2M proteins. pcDNA3.1 vector DNA was as control.
Western blot analysis
Cell lysates were prepared from HD11 cells and 293T cells transfected with pcDNA3.1-chβ2M or pcDNA3.1 by a lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS, 65 mM DTT) in the presence of a protease inhibitor (Fermentas, Burlington, Canada) for 30 min at 4°C. The lysates were pelleted by centrifugation at 12,000 r/min for 10 min at 4°C and the supernatants were collected. The samples of the supernatants, chicken serum, and plasma were respectively diluted 1:5 in sample buffer (0.5 M Tris [pH 7.0], with 10% sodium dodecyl sulfate, 20% glycerol, and 5% β-mercaptoethanol) and heat denatured for 5 min at 100°C, then subjected to a 10% SDS-polyacrylamide gel and Western blot assay according to conventional procedures. Bovine serum and duck serum maintained in our laboratory were used as controls and treated in the same manner.
Immunofluorescence
HD11 cells, CEF cells, and 293T cells transfected with pcDNA3.1-chβ2M or pcDNA3.1 were fixed with chilled acetone-alcohol (3:2) for 10 min and washed three times with PBS. The cells were then incubated with MAb diluted 1:200 in PBS at 37°C for 45 min and further incubated with goat anti-mouse IgG conjugated with FITC (Sigma-Aldrich) at 37°C for 45 min. For double staining, the specimens were counterstained with 4′, 6′-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) at 10 mg/L for 2 min at room temperature. After each incubation step, the cells on the coverslip were washed five times with PBS. Immunofluorenscence was observed under confocal laser scanning microscope (Leica, Wetzlar, Germany). Normal mouse serum was used for negative control.
Flow cytometry
To detect chβ2M antigen on the cell membrane, MSB1 cells cultured in logarithmic growth phase were collected by centrifugation at 800 rpm for 5 min. Then the cells were washed three times with cold PBS and incubated for 45 min at 4°C with 200 μL chβ2M MAb 3D1 at a 1:100 dilution in PBS with 1% BSA. The excess primary antibody was removed by three washes with cold PBS, and the cells were incubated for an additional 45 min with FITC-conjugated goat anti-mouse IgG (Sigma-Aldrich). After three washes, the cells were resuspended in PBS and 1.0×104 cells were analyzed by flow cytometry (FACScan Flow cytometer, BD Biosciences, San Jose, CA) using the CellQuest (BD Biosciences) software package. The cells incubated with equal concentrations of an irrelevant isotype MAb were used for control.
Immunohistochemical staining
Immunohistochemistry was performed using a Roly-One kit (XiYa JinQiao Co., Beijing, China) according to the manufacturer's protocol. Briefly, frozen sections of thymus, spleen, and bursa from 28-day-old chickens (a White Leghorn specific-pathogen-free line) were fixed in chilled acetone for 30 min. The sections were then blocked with 5% horse serum in PBS and incubated with MAb diluted 1:500 with dilution buffer (provided in the Roly-One kit) in a moist chamber for 4 h at 37°C, followed by incubation with HRP-conjugated anti-mouse IgG (XiYa JinQiao Co.) for 2 h at 37°C. The sections were then developed with diaminobenzidine substrate and hydrogen peroxide, which produced a brown dark precipitate. The slides were counterstained with hematoxylin and observed under a microscope (Olympus, Tokyo, Japan).
Results
Chβ2M genes analysis and peptide selected
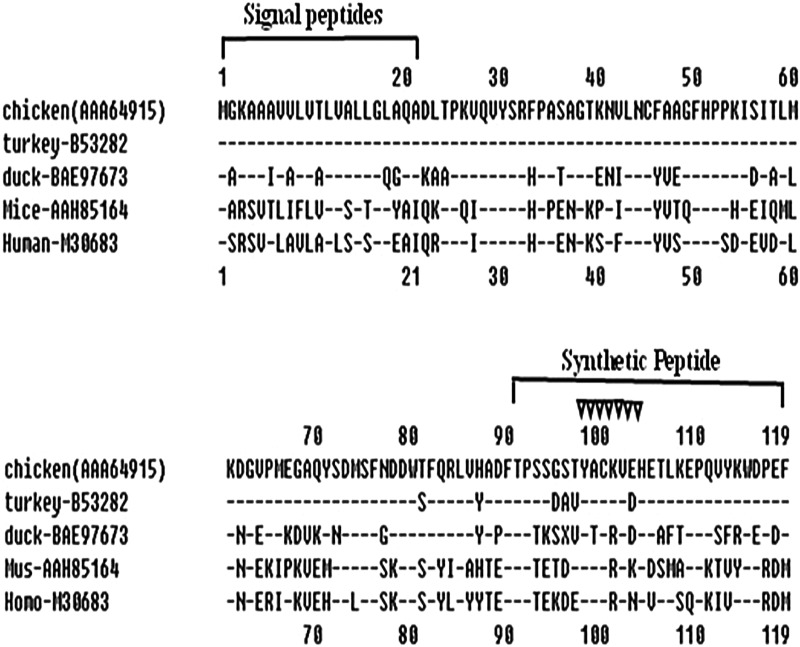
The chβ2M gene was amplified from chicken PBMCs by RT-PCR. Sequence analysis showed that the sequence of the cloned chβ2M was the same as that previously reported (M84767, AY989898, Z48921). It consists of 360 bp encoding 119 amino acids (aa) with a molecular weight of 12 kDa (Fig. 1). There are two conserved cysteines at positions 45 and 100. The immunoglobulin (Ig) motif YXCXVXH (X indicates any amino acid) were located at positions 98 to 104. Chβ2M showed 94.1% amino acid homology to the turkey β2M gene (B53282) with only seven amino acid substitutions at positions 81, 86, 95, 96, 97, 99, and 103. There is a relatively low homology of 65.9% between duck β2M and chicken β2M. Multiple sequence alignment showed that chβ2M shared only 44.2% and 42.5% amino acid identity with human and mouse β2M, respectively. Most of the amino acid substitutions were focused on the signal peptide domain and the C terminus, which suggest that the C-terminus' amino acids are specific to chβ2M. Antigenic analysis of chβ2M by DNAstar software indicated that the 29 amino acids of the chβ2M COOH-terminus (NH2-TPSSGSTYACKVEHETLKEPQVYKWDPEF-COOH, named TF29) is different from the mouse β2M (Fig. 1). TF29 was selected.
FIG. 1.
Amino acid comparison between the chicken β2M allele sequences with that of turkey (GenBank protein accession no. B53282), duck (BAE97673), human (M30683), and mouse (AAH85164). Nos. 1–119 above line, β2M aa sequence numbers; nos. 1–20, site of signal peptide; nos. 91–119, site of synthetic peptide; dashes, similarity to chicken β2M sequence;  above the chicken β2M sequences, immunoglobulin (Ig) motif; nos. 90–119, site of chβ2M COOH-terminal 29 amino acids used as antigen.
above the chicken β2M sequences, immunoglobulin (Ig) motif; nos. 90–119, site of chβ2M COOH-terminal 29 amino acids used as antigen.
MAb production
The mice were immunized with the synthesized peptide TF29. Fusions between SP2/0 cells and the mice spleen cells were made according to the conventional hybridoma procedure. A panel of monoclonal antibodies screened by ELISA, termed 3D1, 6E7, 1E2, 2E9, 7D5, was obtained. Immunogluobin subtypes were identified as IgM for MAbs 1E2, 2E9, 7D5, and IgG1/κ for MAbs 6E7 and 3D1.
MAbs recognized a 12 kDa of chβ2M
SDS-PAGE and Western blot results are shown in Figure 2. MAbs 6E7 and 3D1 specifically recognized a unique protein band, with a molecular weight of approximately 12 kDa, from the lysates of 293T/chβ2M but did not react with the lysates of untransfected 293T cells. These MAbs also reacted with chβ2M antigen from HD11 cells. The same band was found in chicken sera and plasma in the Western blot analysis (Fig. 2B).
FIG. 2.
Western blot analysis of monoclonal antibodies against chβ2M. (A) Untransfected 293T cells lysate was not detectable with MAbs 6E7 and 3D1 (lanes a, d). 293T cells lysate transfected with chβ2M were detected (lanes b, e). 12 kDa protein in the HD11 cells lysates recognized by antibodies 6E7 and 3D1 (lanes c, f, respectively). (B) MAb 3D1 could react with the chβ2M in chicken serum (lane a) and plasma (lane b). No reactions with bovine serum (lane c) and duck serum (lane d) were observed.
MAbs reacted chβ2M in immunofluorescence
MAb 6E7 demonstrated specific binding to 293T/chβ2M and HD11 cells in immunofluorescence (Fig. 3A, c). There was not any fluorescence signal observed in untransfected 293T cells (Fig. 3A, d). The staining pattern by confocal positioning indicated that the MAb 3D1 reacted with chβ2M epitopes on both the cell membrane and in the cytoplasm of HD11 cells (Fig. 3B). HD11 cells (Fig. 3A, a) and CEF cells (Fig. 3A, b) demonstrated different expression levels of chβ2M. Chβ2M in HD11 cells produced a significantly stronger reaction than that in CEF cells. Flow cytometry analysis results showed that MAb 3D1 specifically could bind to the chβ2M antigen on the membrane surface of MSB1 cells (<1.0×102) (Fig. 4).
FIG. 3.
Indirect immunofluorescence imaging of MAb 6E7 binding to different cells. (A) HD11 cells (a); CEF cells (b); 293T/chβ2M (c); pcDNA3.1 transfected 293T cells (d). 200×(B) chβ2M on the cell membrane and cytoplasm of HD11 cells (green, a); nuclei stained with DAPI (blue, c); merged images (b). 650×
FIG. 4.
Analysis of chβ2M antigen on the cell membrane by flow cytometry with MAb 3D1. MSB1 cells incubated with the mouse isotype control antibody (A) and MAb 3D1 (B). Y-axis, cell counts; X-axis, relative fluorescence level.
chβ2M locations in tissues
Frozen tissue sections from chicken thymus, spleen, and bursa were immunostained with the MAbs. We found that MAb 6E7 reacted weakly with the bursal cortex and medulla and very weakly or not at all with bursal cortical cells. However the thymus sections showed much stronger staining in medullary cells and mesenchyme, but weak staining in the cortical area. Spleen sections were strongly positive, but in a patchy fashion, with the most positive patches corresponding to lymphocyte-rich areas (Fig. 5).
FIG. 5.
Immunohistochemical stain of frozen thymus (a), spleen (b), and bursa (c) sections with antibody 6E7. (d–f) Same tissue types as in a–c, negative controls stained with hematoxylin nuclear dye. Arrows show borders between cortex (top) and medulla (bottom). The tissues were from a 28-day-old SPF chicken. The bar represents 200 μm.
Discussion
The current knowledge of chicken MHC class I structure and biological function has been advanced by the extensive development of MAb reagents.(13–15) In previous studies, MAbs against chicken MHC I and II were generated using chicken peripheral blood lymphocytes.(16,17) Herein, we analyzed β2M sequences of different species and selected a polypeptide (TF29) as immunogen for developing monoclonal antibodies. There is relatively high homology between mammalian β2M sequences but much lower homology between the mammalian and avian β2M sequences. The 29 amino acid residues of the chβ2M C-terminus have only 12 and 13 aa homology with the mouse and human sequences, respectively. Among them, most amino acid substitutions are dispersed and discontinuous. It suggests TF29 is a better immunogen than chicken PBMC. The MAbs 6E7 and 3D1 are specific to the chβ2M and no cross-reaction with human β2M. Moreover, despite previous suggestions that β2M was relatively conserved between the different poultry species, we found that antibody 6E7 and 3D1 could not react with duck serum β2M.
During the last several years, the two-dimensional (2D) and three-dimensional (3D) structures of chβ2M and MHC class I proteins have been frequently discussed. As originally noted,(8) chβ2M contains a typical β-sheet with no α-helix. Although the 20 amino acid residues of the C-terminus of chβ2M consist of two discontinuous β-sheets, they may be freely exposed to the external environment.(18) In our study, MAbs 6E7 and 3D1 could react both with the linear chβ2M antigen in Western blot and the native chβ2M antigen in flow cytometry. It suggests that antibody recognition of the chβ2M antigen at C-terminal acid residues should be less influenced by MHC 1 conformation.
β2M is particularly plentiful on the surface of white blood cells, and white blood cell membrane turnover is the principal source of serum β2M. Serum β2M is a significant diagnostic predictor, and increased levels of β2M have been found to be of prognostic relevance in a wide variety of diseases.(19–21) Serum Western blot immunoassays have been developed as a confirmatory test for many diseases, including Mycoplasma hyopneumoniae,(22) Toxocara vitulorum,(23) and severe acute respiratory syndrome (SARS).(24) Perhaps detection of chicken serum β2M levels by serum blots or other immunological methods will prove highly useful for future study.
With our MAbs, the strongest staining was found in medullary cells and the mesenchyme in thymus. The staining pattern of lymphocytes in the bursa mesenchyme was much weaker than those in the thymus. We found that the chβ2M antigen's tissue distribution, especially on sections of thymus and spleen, corresponded to that reported for Class I MHC antigens.(16) The biological functions of chβ2M still need to be investigated, especially in immune regulation and cell-cell interaction. These MAbs will be good tools for analyzing the mechanism of the chicken immune system.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant no. 31072135 and 30871873), the Major Basic Research of Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant no. 12KJA23001), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT0978).
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Bjorkman PJ. Saper MA. Samraoui B. Bennett WS. Strominger JL. Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. J Immunol. 2005;174:6–19. [PubMed] [Google Scholar]
- 2.Shi C. Zhu Y. Su Y. Chung LW. Cheng T. Beta2-microglobulin: emerging as a promising cancer therapeutic target. Drug Discov Today. 2009;14:25–30. doi: 10.1016/j.drudis.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Huang WC. Wu D. Xie Z. Zhau HE. Nomura T. Zayzafoon M. Pohl J. Hsieh CL. Weitzmann MN. Farach-Carson MC. Chung LW. Beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66:9108–9116. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 4.Nomura T. Huang WC. Zhau HE. Wu D. Xie Z. Mimata H. Zayzafoon M. Young AN. Marshall FF. Weitzmann MN. Chung LW. Beta2-microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-responsive element-binding protein, and vascular endothelial growth factor axis. Clin Cancer Res. 2006;12:7294–7305. doi: 10.1158/1078-0432.CCR-06-2060. [DOI] [PubMed] [Google Scholar]
- 5.Nomura T. Huang WC. Seo S. Zhau HE. Mimata H. Chung LW. Targeting beta2- microglobulin mediated signaling as a novel therapeutic approach for human renal cell carcinoma. J Urol. 2007;178:292–300. doi: 10.1016/j.juro.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Huang WC. Havel JJ. Zhau HE. Qian WP. Lue HW. Chu CY. Nomura T. Chung LW. Beta2-microglobulin signaling blockade inhibited androgen receptor axis and caused apoptosis in human prostate cancer cells. Clin Cancer Res. 2008;14:5341–5347. doi: 10.1158/1078-0432.CCR-08-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan RQ. Li XS. Yang TY. Xia C. Structures and homology modeling of chicken major histocompatibility complex protein class I (BF2 and beta2m) Mol Immunol. 2006;43:1040–1046. doi: 10.1016/j.molimm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Li XS. Fang QM. Yan RQ. Gao FS. Hao HF. Jia ZH. Lin CY. Xia C. Extensive analysis of different allelelic structures of the chicken BF2 and beta2m proteins. Vet Immunol Immunopathol. 2006;113:215–223. doi: 10.1016/j.vetimm.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Yan RQ. Li XS. Yang TY. Xia C. Characterization of BF2 and beta2m in three Chinese chicken lines. Vet Immunol Immunopathol. 2005;108:417–425. doi: 10.1016/j.vetimm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Riegert P. Andersen R. Bumstead N. Dohring C. Dominguez-Steglich M. Engberg J. Salomonsen J. Schmid M. Schwager J. Skjodt K. Kaufman J. The chicken beta 2-microglobulin gene is located on a non-major histocompatibility complex microchromosome: a small, G+C-rich gene with X and Y boxes in the promoter. Proc Natl Acad Sci USA. 1996;93:1243–1248. doi: 10.1073/pnas.93.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S. Miska KB. Jenkins MC. Fetterer RH. Cox CM. Stuard LH. Dalloul RA. Molecular cloning and functional characterization of the avian macrophage migration inhibitory factor (MIF) Dev Comp Immunol. 2010;34:1021–1032. doi: 10.1016/j.dci.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Smith KA. Favata MF. Oroszlan S. Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol. 1983;131:1808–1815. [PubMed] [Google Scholar]
- 13.Antalikova J. Simon M. Horovska L. Valentovicova J. Monoclonal antibody produced against bovine MHC class I antigens. Folia Biol. 2004;50:29–31. [PubMed] [Google Scholar]
- 14.Pleskova I. Sedlak J. Duraj J. Festin R. Munozova H. Polakova K. Chorvath B. Monoclonal antibody directed to MHC class I antigen (Bra23/9): characterization and utilization for study of antigen expression in differentiation of U 937 cell line. Neoplasma. 1988;35:657–664. [PubMed] [Google Scholar]
- 15.Mescher MF. Stallcup KC. Sullivan CP. Turkewitz AP. Herrmann SH. Purification of murine MHC antigens by monoclonal antibody affinity chromatography. Methods Enzymol. 1983;92:86–109. doi: 10.1016/0076-6879(83)92011-6. [DOI] [PubMed] [Google Scholar]
- 16.Pink JR. Kieran MW. Rijnbeek AM. Longenecker BM. A monoclonal antibody against chicken MHC class I (B-F) antigens. Immunogenetics. 1985;21:293–297. doi: 10.1007/BF00375381. [DOI] [PubMed] [Google Scholar]
- 17.Crone M. Simonsen M. Skjodt K. Linnet K. Olsson L. Mouse monoclonal antibodies to class I and class II antigens of the chicken MHC. Evidence for at least two class I products of the B complex. Immunogenetics. 1985;21:181–187. doi: 10.1007/BF00364870. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman J. Andersen R. Avila D. Engberg J. Lambris J. Salomonsen J. Welinder K. Skjodt K. Different features of the MHC class I heterodimer have evolved at different rates. Chicken B-F and beta 2-microglobulin sequences reveal invariant surface residues. J Immunol. 1992;148:1532–1546. [PubMed] [Google Scholar]
- 19.Bien E. Rapala M. Krawczyk M. Balcerska A. The serum levels of soluble interleukin-2 receptor alpha and lactate dehydrogenase but not of B2-microglobulin correlate with selected clinico-pathological prognostic factors and response to therapy in childhood soft tissue sarcomas. J Cancer Res Clin Oncol. 2010;136:293–305. doi: 10.1007/s00432-009-0661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalocheretis P. Revela I. Spanou E. Drouzas A. Makriniotou I. Iatrou C. Strong correlation of B2-microglobulin (B2-m) with procalcitonin (PCT) in the serum of chronic hemodialysis patients: a role for infections in the dialysis-related amyloidosis? Ren Fail. 2008;30:261–265. doi: 10.1080/08860220701857134. [DOI] [PubMed] [Google Scholar]
- 21.Elefsiniotis IS. Moulakakis A. Pantazis KD. Glynou I. Ketikoglou I. Vezali E. Kada H. Tsianos E. Relationship between serum b2-microglobulin levels and virological breakthrough in HBeAg-negative chronic hepatitis B patients, under long-term treatment schedules including lamivudine. World J Gastroenterol. 2005;11:1922–1928. doi: 10.3748/wjg.v11.i13.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ameri M. Zhou EM. Hsu WH. Western blot immunoassay as a confirmatory test for the presence of anti-Mycoplasma hyopneumoniae antibodies in swine serum. J Vet Diagn Invest. 2006;18:198–201. doi: 10.1177/104063870601800210. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira FP. Starke-Buzetti WA. Detection of antibody to Toxocara vitulorum perieneteric fluid antigens (Pe) in the colostrum and serum of buffalo calves and cows by Western blotting. Vet Parasitol. 2005;129:119–124. doi: 10.1016/j.vetpar.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang YD. Li Y. Xu GB. Dong XY. Yang XA. Feng ZR. Tian C. Chen WF. Detection of antibodies against SARS-CoV in serum from SARS-infected donors with ELISA and Western blot. Clin Immunol. 2004;113:145–150. doi: 10.1016/j.clim.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]