Abstract
Background
Radioactive iodine lobe ablation (RAI-L-ABL) is a possible alternative to completion thyroidectomy (C-Tx) for follicular thyroid carcinoma (FTC), but no long-term outcome data are available after lobe ablation. We analyzed the long-term outcome of lobe ablation in a series of patients with FTC.
Methods
This was a retrospective study of patients who were treated with lobe ablation between 1983 and 2008. Of 134 patients with FTC, 37 (27.6%) had lobe ablation with 131I (30–32 mCi) (RAI-L-ABL), 68 (50.7%) had C-Tx, and 29 (21.6%) had initial total thyroidectomy (T-Tx). The main outcomes analyzed were 131I uptake after lobe ablation, C-Tx or T-Tx, serum thyroglobulin (Tg), serum thyroid-stimulating hormone (TSH), long-term disease-specific mortality, and disease-free survival.
Results
After lobe ablation, radioiodine uptake was significantly lower for the RAI-L-ABL group (0.6%) than for the C-Tx group (2.0%, p<0.005) or T-Tx group (1.3%, p=0.054). Subsequent remnant ablation was performed in 12 of 37 (32%) patients in the RAI-L-ABL group, in 58 of 68 (85.3%) patients in the C-Tx group, and in 25 of 29 (86.2%) patients in the T-Tx group (p<0.01). With median follow-up of 95 months for the RAI-L-ABL group, 47 months for the C-Tx group, and 53 months for the T-Tx group, there was one death in the RAI-L-ABL group and one death in the T-Tx group. No other RAI-L-ABL patients had detectable disease, whereas patients in the C-Tx group and two patients in the T-Tx group had detectable disease (p=0.18). Long-term stimulated or suppressed Tg of <1 ng/mL were found in 87.5% of the RAI-L-ABL group (n=28), 86.3% of the C-Tx group (n=57), and 77.8% of the T-Tx group (n=21). Tg was detectable in 40.6% of the RAI-L-ABL group compared to 13.8% of C-Tx and 28.6% of T-Tx groups (p<0.05, between groups).
Conclusions
RAI-L-ABL, C-Tx, and T-Tx are equally effective in achieving serum TSH concentrations of >25 mIU/L and preparing patients for conventional 131I treatment and whole body scanning with similar long-term outcomes. However, persistent measurable Tg (range 0.2–2.2 ng/mL) is more common after RAI-L-ABL.
Introduction
As follicular thyroid carcinoma (FTC) can be suspected but not diagnosed by fine needle aspiration (FNA), the FNA diagnosis of “follicular neoplasm” or “suspicious for follicular neoplasm” of thyroid nodules is usually followed by thyroid surgery to determine if the nodules are benign (follicular adenomas) or malignant (FTC or follicular variant of papillary thyroid carcinoma [FVPTC]). Therefore, at surgery all follicular neoplasms are generally removed along with the lobe containing the nodule and the isthmus. In 20% to 30% of such operations the histopathology will reveal FTC or FVPTC (1). If these malignancies are present after hemithyroidectomy, the next step may be completion thyroidectomy (C-Tx) followed by radioiodine (RAI) remnant ablation and whole body scanning (WBS); the latter two procedures cannot be optimally performed if the lobe that remains after hemithyroidectomy is left intact.
When FTC is diagnosed after hemithyroidectomy, the indications for C-Tx are controversial. With larger tumors and/or extensive vascular invasion, the risk of distant hematogenous spread is high. Therefore, RAI remnant ablation/treatment and WBS is generally recommended after C-Tx.
The term “minimally invasive” FTC is variably applied to FTC with capsular invasion alone, (2) or to FTC with capsular invasion and/or vascular invasion of at most a few blood vessels (3). The prognosis for minimally invasive FTC is generally good, but distant spread and death have been reported in patients with minimal vascular invasion or capsular invasion alone (4). Hence, C-Tx followed by remnant ablation and whole body RAI scanning is recommended by some, but not all endocrinologists for minimally invasive FTC. Current guidelines are ambiguous regarding this (5). C-Tx is costly and contributes to additional physical and psychological discomfort. C-Tx is particularly problematic in patients who suffered injury to the recurrent laryngeal nerve during their initial hemithyroidectomy. On the other hand, routine total thyroidectomy (T-Tx) for patients with an FNA diagnosis of follicular neoplasm is also not optimal. T-Tx results in permanent hypothyroidism in most patients and increases the risk of permanent hypoparathyroidism and recurrent laryngeal nerve injury.
We have previously reported our experience with a group of patients with well-differentiated thyroid carcinoma who were treated with RAI lobe ablation (RAI-L-ABL) after hemithyroidectomy as an alternative to C-Tx (6). We demonstrated the efficacy of this procedure in rendering patients markedly hypothyroid for subsequent conventional whole body RAI scanning and treatment. Current guidelines of American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) recommend against RAI ablation after hemithyroidectomy for FTC and FVPTC because it is not known if this approach is as effective as C-Tx followed by radioactive iodine remnant ablation in the long term (5,7).
In this study we performed a retrospective analysis of our experience with lobe ablation in patients with FTC as an alternative to C-Tx. We compared the long-term outcome of a group of patients who had RAI-L-ABL after hemithyroidectomy (RAI-L-ABL group) with that of a group of patients who had C-Tx after hemithyroidectomy and with that of a group of patients who had a T-Tx. As detailed later some of the patients in some of these groups also had radioactive iodine treatment after these initial treatments to ablate residual thyroid tissue.
Patients and Methods
Patients
There were 37 patients with who underwent hemithyroidectomy for FTC and were managed by physicians of the Massachusetts General Hospital Thyroid Unit between 1983 and 2007. These patients were designated the RAI-L-ABL group because they were treated with RAI to ablate the remaining thyroid lobe. Ten (27.0%) of these patients were included in our earlier study (6). In four of these patients the reason for not performing C-Tx was recurrent laryngeal nerve paralysis, and in one patient this was the case because of severe laryngospasm, pulmonary embolism, and aspiration pneumonia at the time of the initial hemithyroidectomy. In the remaining 32 patients, the choice was dictated by patient preference after discussion of the alternative options. The RAI-L-ABL patients received 30–32 mCi of 131I to ablate the remaining thyroid lobe. This dose of RAI was chosen because it was the maximum permitted outpatient dosage of 131I at the time lobe ablation was initiated. The median time between hemithyroidectomy and lobe ablation was 2.5 months (range 1.5–17 months). While thyroid-stimulating hormone (TSH) was not typically measured at the time of lobe ablation, a 24-hour RAI neck uptake was performed before the procedure in all cases. The mean 24-hour RAI neck uptake was 20% (range 7%–28%) in the 26 patients in which this information was available. In the more recent years many of the RAI-L-ABL group patients were instructed to follow a low-iodine diet for 2 weeks before lobe ablation. However, information concerning iodine restriction was not recorded for the majority of patients and, hence, was not analyzed.
There were 68 patients who underwent hemithyroidectomy followed by C-Tx for FTC between 1993 and 2007. They were designated the C-Tx group. The medical records did not indicate if these patients were offered RAI-L-ABL instead of C-Tx.
There were 29 patients who underwent T-Tx as their initial treatment for FTC between 1996 and 2007. They were designated the T-Tx group. The Thyroid Unit computer system does not permit pathological searches before 1993. There were 12 patients (32.4%) in the RAI-L-ABL group who were found to have FTC before 1993, while all patients in C-Tx and T-Tx groups had surgery during or after 1993. For all groups, all available patients with FTC were included if follow-up data were available after initial surgery and WBS procedures. Detailed demographic and clinical data in the three groups are presented in Table 1. For the most important prognostic variables (gender, age, tumor size, and tumor grade), no significant differences were observed among the study groups. The MGH Institutional Review Board approved this study.
Table 1.
Demographic and Clinical Features in the Study Groups
| Group | n | F/Ma | Age at diagnosis, years (SD)a | Tumor size, cm (SD)a | Percent with extensive vascular or capsular invasiona,b |
|---|---|---|---|---|---|
| RAI-L-ABL | 37 | 28/9 | 49.0 (16.9) | 3.0 (1.5) | 16.2 |
| C-Tx | 68 | 49/19 | 47.8 (15.9) | 3.7 (1.9) | 13.6 |
| T-Tx | 29 | 20/9 | 56.1 (18.0) | 3.9 (1.0) | 13.7 |
p=0.95 between groups.
All patients without extensive vascular invasion had minimal capsular or vascular invasion. This information was not available in one patient in the RAI-L-ABL group and one patient in the T-Tx group.
RAI-L-ABL, radioactive iodine lobe ablation; C-Tx, completion thyroidectomy; T-Tx, total thyroidectomy.
WBS and remnant ablation
After RAI-L-ABL, T-Tx, or C-Tx, 131I WBS was performed in some of the patients in the three groups after levothyroxine withdrawal. The median interval between RAI-L-ABL or C-Tx or T-Tx and the WBS was 4.9 months (range 2.8–27.5) in the RAI-L-ABL patients, 3.4 months (range 1.6–22.1) in C-Tx patients, and 2.6 months (range 1.4–11.0) in T-Tx patients. Thirty-four out of 37 (91.9%) RAI-L-ABL patients, 58 out of 68 (74.3%) C-Tx patients, and 25 out of 29 (86.2%) T-Tx patients underwent WBS after thyroid hormone withdrawal. WBS was performed 48 hours after the oral administration of 2 mCi 131I. Serum TSH and thyroglobulin (Tg) were generally measured before the WBS. RAI neck uptake was measured on WBS images. The geometric means of total counts in the region of interest was calculated and normalized to a known 131I source measured in the same camera system. The percent uptake of the administered dose was calculated, after correcting for the radioisotope decay factor.
In some patients, ablation with 30–150 mCi 131I of the surgical remnant or residual thyroid tissue after the initial RAI ablation was performed after 2 weeks on a low-iodine diet according to the protocols and guidelines that were current at the time. This procedure was performed in 12 of 37 (32%) RAI-L-ABL patients, in 58 of 68 (85.3%) C-Tx patients, and in 25 of 29 (86.2%) T-Tx patients (p<0.01). For the 12 RAI-L-ABL patients who received remnant ablation, the mean 131I dose was 95.8±33.0 mCi (mean±SD). This does not include the initial dose of 30 mCi 131I given for RAI-L-ABL. The C-Tx group received 87.9±33.2 mCi for surgical remnant ablation, and the T-Tx group received 92.5±50.9 mCi for surgical remnant ablation. These average doses were not statistically different. On average, patients in the RAI-L-ABL group received a total of 61.1±49.2 mCi, which included 30–32 mCi for the initial RAI-L-ABL.
There were 25 patients in the RAI-L-ABL group who had WBS after their initial RAI-L-ABL. They were deemed not to require additional ablation with RAI at the time of the WBS obtained after thyroid hormone withdrawal. The RAI uptake in their neck at this time was 0.4%±0.7% (mean±SD).
Follow-up procedures
In this retrospective study, the follow-up protocols varied over time and were therefore not uniform. Patients were followed at variable time intervals and with variable re-staging methods. TSH-stimulated Tg concentrations (either by withdrawal or by administration of recombinant human TSH) were available during follow-up in 11 of 37 (29.7%) of the RAI-L-ABL group, 24 of 68 (35.3%) of the C-Tx group, and in 12 of 33 (36.4%) of the T-Tx group. Follow-up serum Tg concentrations (either stimulated or nonstimulated) were available in 33 (89.2%) RAI-L-ABL patients, 62 (91.2%) C-Tx patients, and 24 (72.7%) T-Tx patients. In each of the three groups, two patients had anti- Tg antibodies preventing Tg measurement. At the last available clinical observation, patients were classified into one of four stages: S0, no clinical evidence of disease and either stimulated or suppressed Tg <1.0 ng/mL; S1, no clinical evidence of disease with detectable stimulated or suppressed Tg (≥1.0 ng/mL); S2, clinically detectable disease; S3, death from thyroid cancer. Patients with no clinical evidence of residual disease with persistent positive Tg antibodies were classified separately (stage Ab+). Owing to the retrospective nature of this study, the group of patients in S2 was heterogeneous with clinically detectable disease being determined by a variety of tests according to the specific clinical situation and clinical judgment at the time of the test. Patient details are provided in the Results section. Table 2 summarizes the study design and procedures.
Table 2.
Study Procedures
| |
RAI-L-ABL group |
C-Tx group |
T-Tx group |
|||
|---|---|---|---|---|---|---|
| Steps | Procedure | na | Procedure | na | Procedure | na |
| Step 1 | Hemithyroidectomy | 37/37 | Hemithyroidectomy | 68/68 | T-Tx | 29/29 |
| Step 2 | Thyroid lobe ablation (30–32 mCi 131I) | 37/37 | C-Tx | 68/73 | — | |
| Step 3 | WBS (2 mCi 131I), Tg, TSH in hypothyroidism | 36/37 | WBS (2 mCi 131I), Tg, TSH in hypothyroidism | 58/68 | WBS (2 mCi 131I), Tg, TSH in hypothyroidism | 25/29 |
| Step 4 | Remnant ablation (30–150 mCi 131I) | 15/37 | Remnant ablation (30–150 mCi 131I) | 58/68 | Remnant ablation (30–150 mCi 131I) | 12/29 |
| Step 5 | Stimulated Tg | 12/37 | Stimulated Tg | 26/68 | Stimulated Tg | 12/29 |
| Step 6 | Unstimulated Tg, clinical restaging at the end of follow-up | 37/37 | Unstimulated Tg, clinical restaging at the end of follow-up | 68/68 | Unstimulated Tg, clinical restaging at the end of follow-up | 29/29 |
Study procedures are described in their temporal sequence. All patients in all the RAI-L-ABL and C-Tx groups underwent Steps 1, 2, and 6. All patients in the T-Tx group underwent Steps 1 and 6.
Number of patients undergoing each step.
WBS, whole body scanning; Tg, thyroglobulin; TSH, thyroid-stimulating hormone.
Statistical analysis
Continuously distributed numerical variables in the three groups were compared with Student's t-test. Noncontinuously distributed numerical variables such as Tg and TSH were compared with the Wilcoxon Sum Rank test. Categorical variables were compared with Chi-square or Fisher's exact test.
All the statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC) and Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA)
Tg assays
Over the years many different Tg assays with progressively increased sensitivity were used at our institution. The Nichols Advantage Tg assay had a lower limit of detection of 1 ng/mL until July 2004, and a lower limit of detection of 0.3 ng/mL from July 2004 to August 2005. Since August 2005 we have used the Immulite assay (Diagnostic Products Corporation, Los Angeles, CA) with a lower detection limit of 0.2 ng/mL. To ensure homogeneity of analysis, Tg results <1 ng/mL were grouped together and considered undetectable regardless of the assay used. We also provide a separate analysis of samples collected after 2003, where the detection limit was considered 0.3 ng/mL.
Results
Efficacy and safety of lobe ablation
Seven patients in the RAI-L-ABL group reported mild to moderate neck tenderness after RAI-L-ABL and one additional patient complained of a cough. The tenderness lasted a few days to a week in most patients and did not require intervention other than the use of over-the-counter pain medications. There were no recorded complications of C-Tx in the C-Tx group.
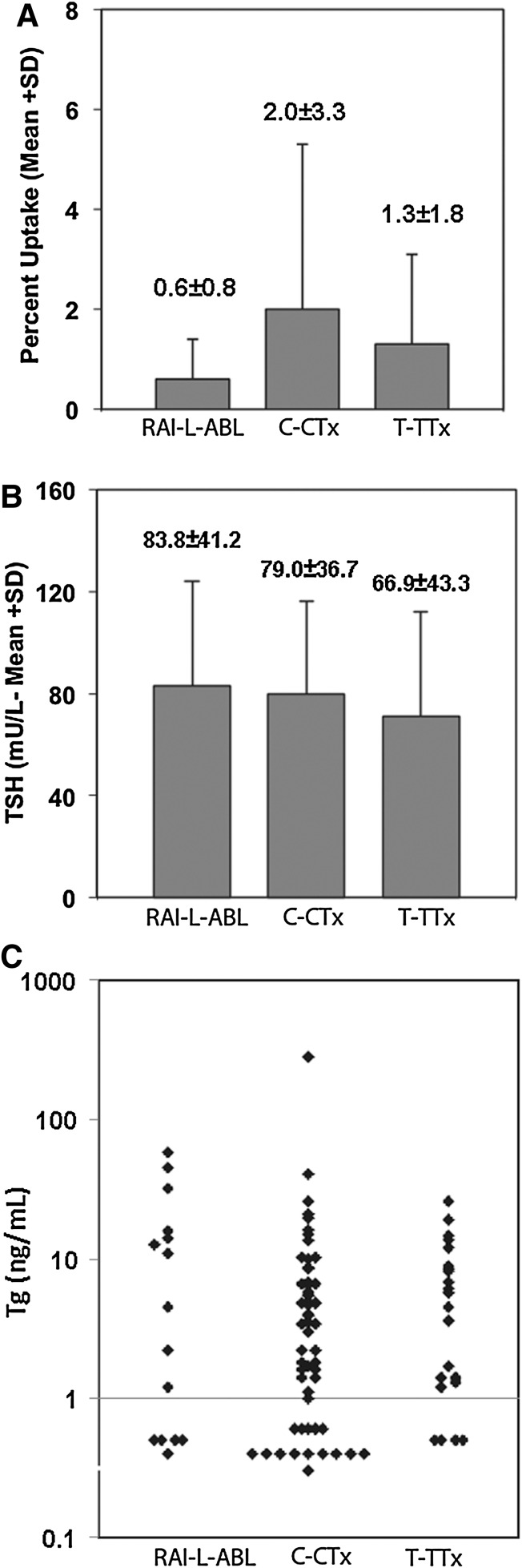
The mean RAI neck uptake was significantly lower after RAI-L-ABL (0.6%±0.8%) than after C-Tx (2.0%±3.3%) or T-Tx (1.3%±1.8%) (p<0.05 for RAI-L-ABL vs. C-Tx and RAI-L-ABL vs. T-Tx; Fig. 1). The serum TSH after levothyroxine withdrawal was similar in the three groups. The serum TSH was 83.1±42.2, 79.0±36.8, and 66.9±43.3 mU/L in the RAI-L-ABL, C-Tx, and T-Tx groups, respectively. All patients had serum TSH values of ≥25 mU/L at the time of WBS (Fig. 1).
FIG. 1.
The neck 24 hours RAI uptake (upper panel), TSH (mid panel), and Tg in the three study groups. All studies were performed at the time of thyroid hormone withdrawal after lobe ablation (RAI-L-ABL group) completion thyroidectomy (C-Tx group) or total thyroidectomy (T-Tx group). Numbers over bars in the two upper panels indicate mean and SD. RAI, radioiodine; TSH, thyroid-stimulating hormone; Tg, thyroglobulin.
At the time of WBS, serum Tg was recorded in 15 of 37 RAI-L-ABL patients (40.5%), 54 of 68 C-Tx patients (79.5%), and 22 of 29 (75.9%) T-Tx patients. Absent Tg measurements were due either to the presence of anti-Tg antibodies or to incompleteness of the medical records. Thirteen of the RAI-L-ABL patients (35.1%) either had their lobe ablation before 1994 when Tg measurements were not routinely performed, or their Tg was not reported due to anti-thyroglobulin antibodies. Anti- Tg antibodies were present in 13 of 28 (46.5%) RAI-L-ABL patients, 9 of 68 (13%) C-Tx patients, and 5 of 29 (17%) T-Tx patients (p=0.014, Chi-square). Two patients in the T-Tx group were excluded from Tg calculations because of pre-existing metastatic disease; Tg was markedly elevated in both of these patients. At the time of thyroid hormone withdrawal after lobe ablation, C-Tx, and T-Tx, serum Tg concentrations were not significantly different in the three groups (Fig. 1). Tg concentrations <1 ng/mL were found in 4 of 15 (26.7%) of RAI-L-ABL patients, 13 of 54 (24.1%) C-Tx patients, and in 4 of 29 (14%) T-Tx patients (p=0.37).
Long-term outcome after RAI-L-ABL
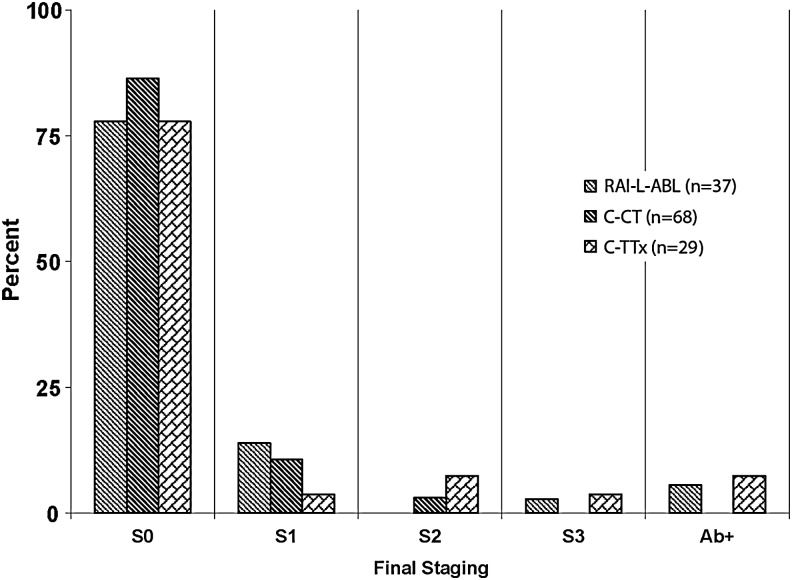
The median follow-up was 95 months in the RAI-L-ABL group, 47 months in the C-Tx group, and 53 months in the T-Tx group (p<0.01, RAI-L-ABL vs. T-Tx and RAI-L-ABL vs. C-Tx). The difference in follow-up duration is a reflection of different sources from which the patients were drawn, as explained in the Patients and Methods section. At the end of the follow-up period, there were two disease-specific deaths, one in the RAI-L-ABL group and one in the T-Tx group. The RAI-L-ABL patient who died had known distant metastatic disease before surgery. He underwent RAI-L-ABL after hemithyroidectomy and debulking of a large extrathyroidal invasive FTC. The T-Tx patient died 10 years after her original diagnosis from RAI refractory pulmonary metastases. Two additional C-Tx patients and two T-Tx patients were alive with distant metastatic disease at the end of follow-up. The distribution of stages S0–S3 (see the Patients and Methods section) is summarized in Figure 2. Chi-square analysis showed no statistically significant differences between the study groups.
FIG. 2.
Distribution of four outcome stages in the study groups, at the end of follow-up. S0 indicates no clinical evidence of disease and either stimulated or suppressed Tg<1.0 ng/mL; S1 indicates no clinical evidence of disease with detectable Tg (≥1.0 ng/mL); S2 indicates clinically detectable disease; S3 indicates death from thyroid cancer. Ab+ indicates patients with no clinical evidence of residual disease, but with persistent positive Tg antibody.
Stimulated Tg
Follow-up stimulated Tg levels after levothyroxine withdrawal or after human recombinant TSH administration were available in a limited number of patients. Taking as a denominator the number of patients with measured stimulated Tg, the concentration was <2 ng/mL in 92% (n=12) of the RAI-L-ABL group, 100% (n=25) of the C-Tx group, and 100% of the T-Tx group (n=11). The four patients who died from FTC during follow-up or who had clinical evidence of disease at the last available follow-up point (see above) were excluded from this calculation.
Sensitive Tg levels and Tg antibodies at the end of follow-up
In 104 patients (RAI-L-ABL 26, C-Tx 58, and T-Tx 21) without clinical evidence of disease (S0 or S1), the last available serum Tg measurement (stimulated or unstimulated) was performed after the introduction of a highly sensitive Tg assay in 2005. In this group the lower limit of detection of Tg was 0.2 ng/mL (Table 3). Measurable Tg concentrations (0.2–1.0 ng/mL) were more frequent in the RAI-L-ABL group (34%) than in the C-Tx group (13.8%) or in the T-Tx group (28.6%) (p=0.06). One possible explanation for the higher prevalence of detectable serum Tg is the fact that fewer patients in the RAI-L-ABL group underwent remnant ablation than in the other two groups. However, in the RAI-L-ABL group, Tg was measurable in 4 of 9 (44%) of those who received subsequent remnant ablation and in 5 of 17 (29.4%) of those who did not (Table 3). Therefore, persistent low detectable serum Tg concentrations did not correlate with a history of prior remnant or post-RAI residual tissue ablation. Across groups, persistent measurable Tg concentrations did not correlate with initial tumor size. Of the patients who had positive TgAb at the time of their initial withdrawal WBS, only 1 in 13 in the RAI-L-ABL group and 2 of 9 in the T-Tx group also had positive tests at the end of follow-up.
Table 3.
Patients in the Three Study Groups with Detectable Thyroglobulin at the End of Follow-Up
| |
131I remnant ablation |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
RAI-L-ABL group |
C-Tx group |
T-Tx group |
||||||
| Yes | No | All | Yes | No | All | Yes | No | All | |
| n | 9 | 17 | 26 | 47 | 11 | 58 | 18 | 3 | 21 |
| Tg detectable, n (%) | 4 (44%) | 5 (29%) | 9 (34%) | 8 (17%) | 0 (0%) | 8 (14%) | 5 (28%) | 1 (33%) | 6 (28%) |
Tg detectable at >0.2 ng/mL. Groups are further subdivided according to whether 131I remnant ablation was performed or not.
Discussion
The current management of solitary nonfunctioning thyroid nodules with the cytological diagnosis of follicular neoplasm remains unsatisfactory. Approximately 20%–30% of these nodules prove to be malignant, being either FTC or FVPTC. The current ATA guidelines recommend lobectomy and isthmusectomy as the initial surgical procedure (5), since the majority (70%–80%) of these tumors are benign adenomas on final pathological examination (1). The role of radioactive iodine scanning and therapy after lobectomy and isthmusectomy for FTC remains controversial. When the FTC is large and/or when extensive vascular invasion is present, adjuvant radioactive iodine is generally recommended (5). Smaller, minimally invasive follicular carcinomas with capsular invasion alone have an excellent prognosis with no mortality in one series (8), but a mortality as high as 11% in another (4). FTC with only a few involved blood vessels is thought to have a prognosis between extensively angioinvasive FTC and FTC with capsular invasion alone, but generally closer to the latter.
The role of C-Tx and radioactive iodine for minimally invasive FTC remains uncertain (5). ATA guidelines suggest that “C-Tx should be offered to those patients for whom a near-total or T-Tx would have been recommended had the diagnosis been available before the initial surgery. This includes all patients with thyroid cancer except those with small (<1 cm), unifocal, intrathyroidal, node-negative, low-risk tumors.” However, they do not distinguish between papillary thyroid carcinoma (PTC), which is often bilateral, and FTC, which is rarely bilateral. The 2010 NCCN guidelines note that “C-Tx is also recommended for tumors that, on final histological sections after lobectomy plus isthmusectomy, are minimally invasive follicular carcinomas.” However, they then go on to say that “These tumors may also be simply followed carefully, because minimally invasive follicular carcinomas have an excellent prognosis” (7).
Given the uncertainty of the data as well as the guidelines, many clinicians in the United States and throughout the world recommend C-Tx and radioactive iodine remnant ablation and scanning for almost all patients with FTC. C-Tx is considered a necessary prelude to achieve a sufficiently high serum TSH to permit whole body RAI scanning as well as radioactive iodine remnant ablation and/or therapy of distant metastases (9). Although RAI remnant ablation with recombinant human TSH (rhTSH) is a well-validated procedure (10), the use of rhTSH in a patient with an intact thyroid lobe would likely result in significant thyrotoxicosis and thyroid swelling (11).
Unfortunately, C-Tx is expensive and may be a source of additional physical and psychological discomfort and time away from work. It also carries the additional risk of hypoparathyroidism, and vocal cord paralysis (12). While there have been rare reports of vocal cord paralysis following radioactive iodine treatment for hyperthyroidism (13) and for remnant thyroid tissue ablation (14), this event is extremely rare. For patients with permanent recurrent laryngeal nerve injury after an initial lobectomy and isthmusectomy, a contralateral operation should be avoided unless absolutely necessary. Bilateral recurrent laryngeal nerve injury is a devastating problem. It is estimated that 10,000 completion thyroidectomies are performed annually in the United States (15). We have previously demonstrated that RAI-L-ABL achieves comparably high serum TSH and comparably low neck RAI uptake compared to C-Tx or T-Tx (6). In contrast to our prior study that included patients with both papillary and FTC (6), this study includes only patients with FTC.
Other groups have also investigated RAI-L-ABL in preparation for later ablation of residual thyroid tissue. Arad et al. (16) attempted lobe ablation with doses of 131I up to 150 mCi. They reported a very high failure rate, but the scanty details reported do not permit analysis of their study. Bal et al. used 131I (15–60 mCi; mean 31.8±11.7 mCi) to ablate the remaining thyroid lobe after thyroid lobectomy (17). They achieved complete ablation in 57% of patients, whereas the mean neck uptake was 3.1%±2.4% in the remaining patients. Complete ablation was achieved in 92% of cases after two doses of 131I (17). Hoyes et al. (18) used higher doses of 131I (100 mCi) in a group of 60 patients after lobectomy. Ninety percent of patients with an intact lobe achieved <1% uptake in this study. In both studies 131I lobe ablation resulted in a sufficiently high serum TSH concentration to permit whole body RAI scanning and was comparable to C-Tx. More recently, Santra et al. reported follow-up data on a series of patients with both FTC and PTC (19). In this large group of 360 patients, the relapse rate was the same in patients undergoing lobe ablation or C-Tx. However, the protocol in this study included repeated 131I doses until a neck uptake <0.2% was obtained. In contrast, our study analyzed whether single-dose lobe ablation is comparable to C-Tx or T-Tx in preparing patients with FTC for WBS and in long-term outcome, without specific neck uptake goals.
It is important to understand the goal of RAI-L-ABL in FTC. FTC is almost invariably a unilateral tumor. Therefore, C-Tx is performed to permit radioactive iodine scanning/therapy, not to remove residual cancer. Similarly, our view of the goal of lobe ablation is to permit subsequent RAI scanning/therapy. The first question is whether lobe ablation achieves the goal of an elevated serum TSH>25 mU/L and minimal residual radioactive iodine uptake. Based on the data in this study and our prior study (6), the answer is clearly yes. The mean serum TSH after lobe ablation was 83 μU/mL and all patients had serum TSH>25 mU/L. Furthermore, the mean 24-hour RAI uptake after RAI-L-ABL was 0.6%, and more patients in the RAI-L-ABL group had very low (<1%) RAI neck uptake and serum Tg concentrations at the time of thyroid hormone withdrawal compared to the C-Tx group and the T-Tx group. As a consequence, the RAI-L-ABL group was less likely to receive remnant ablation than the C-Tx and T-Tx groups and therefore received a lower mean dose of 131I. The significance of this observation is limited by the retrospective nature of this study, but does suggest a potential additional benefit for lobe ablation.
The 2010 NCCN guidelines note that RAI-L-ABL “is not recommended for patients who have undergone lobectomy or lobectomy plus isthmusectomy as initial surgery” (7). The recent ATA guidelines also specifically recommend against lobe ablation (recommendation no. 29), but do not distinguish lobe ablation for PTC from FTC (5). They comment: “It is unknown whether this approach results in similar long-term outcomes. Consequently, routine RAI ablation in lieu of C-Tx is not recommended.” They do not mention that the long-term benefits of C-Tx and remnant ablation, for minimally invasive FTC compared with observation alone, are also unknown.
The second question concerning RAI-L-ABL must be: What is the long-term efficacy of lobe ablation after lobectomy and isthmusectomy for FTC? Our study provides some preliminary data to address that question. Leblanc et al. reported follow-up data on 98 patients, primarily with PTC who received 100 mCi 131I after thyroid lobectomy (20). The 6-year recurrence rate of 6% in the lobe ablation group was not statistically different from that of the control group who received 100 mCi 131I after T-Tx (20). Our current retrospective study provides long-term follow-up on a large cohort of patients with FTC. Survival and disease free survival were indistinguishable between the three groups (RAI-L-ABL, C-Tx, and T-Tx). The number of patients with FTC studied (n=141) and the length of follow-up is comparable to that reported in other series commenting on the prognosis of this relatively uncommon tumor (8, 21).
During long-term follow-up there were differences in serum Tg concentrations between the three study groups. Low but measurable serum Tg was more often found in the RAI-L-ABL group than in the C-Tx and T-Tx groups. Detectable Tg appeared to be independent of whether or not the patient received subsequent remnant ablation after lobe ablation. These persistent detectable Tg concentrations did not correlate with clinically relevant persistent or recurrent disease after a median follow-up of 7.25 years. Persistent detectable serum Tg concentrations also occur after T-Tx and remnant ablation in patients with PTC (22). In PTC patients, low, persistent, but stable serum Tg concentrations are not associated with adverse outcomes (22–24). In PTC persistent low Tg concentrations are often assumed to be due to undetectable small lymph-node metastases. (25). Nodal disease is uncommon in FTC with the exception of the Hurthle cell variant (26). Therefore, we do not believe that lymph node metastases can explain our findings. Rather, it seems likely that small persistent remnants of normal thyroid tissue or benign microscopic nodules are responsible for this observation. It has been estimated that 100 mCi of 131I would be required to destroy a 3-mm functionally cold thyroid nodule, when relying on the irradiation from the surrounding normal thyroid tissue (27). It is possible that small, functionally cold area of thyroid tissue survive RAI-L-ABL while they would be removed by C-Tx. This is a speculative explanation for the persistent low Tg concentrations, which occurred more frequently in the RAI-L-ABL than in the control groups. It is also possible that surgical removal achieves a more complete long-term removal of thyroid tissue by virtue of changes in the vasculature of the thyroid bed. Regardless of the explanation, based on our clinical experience we suggest that low stable serum Tg concentrations after lobe ablation for FTC have little clinical significance.
Our data also suggest that RAI-L-ABL may be associated with the transient appearance of anti-Tg antibodies, a phenomenon that is reminiscent of the effect of RAI on TSH receptor antibodies in patients with Graves' disease (28) However, our understanding of this phenomenon is limited by the lack of baseline Tg antibody levels.
The majority of patients with FTC reported in this study had minimally invasive FTC characterized by capsular invasion alone and or minimal vascular invasion. One could argue that the excellent outcome in the three groups might have been achieved without any additional therapy (29). However, given the uncertainties in current guidelines, most patients with FTC appear to be treated with C-Tx and radioactive iodine remnant ablation/therapy. In this article we do not address the need for additional therapy in minimally invasive FTC treated initially with lobectomy and isthmusectomy. We merely point out that RAI-L-ABL appears to achieve a similar outcome when compared to C-Tx and T-Tx.
We suggest, therefore, that lobe ablation with RAI is an acceptable alternative to C-Tx in patients with minimally invasive FTC, when radioactive iodine WBS and remnant ablation are considered appropriate. For more aggressive FTC with extensive vascular invasion, it is possible that the timing of treatment with radioactive iodine is of importance. Radioactive iodine remnant ablation/therapy is usually delayed for ∼3 or more months after RAI-L-ABL; hence, when rapid therapy is considered essential, C-Tx should be considered. In such aggressive cases, RAI-L-ABL may be limited to patients in whom C-Tx carries significant risk, especially those with recurrent laryngeal nerve paralysis (27). While some literature (19) suggests excellent outcomes of lobe ablation in patients with PTC as well, we very rarely use RAI-L-ABL in our PTC patients. The multifocality often observed in PTC raises the concern that small tumor foci in the remnant lobe may not be adequately treated with this procedure. Another controversial entity not addressed here is the encapsulated FVPTC. It is uncertain whether additional therapy is necessary after lobectomy and isthmusectomy for these patients (30). However, if additional therapy is contemplated, then RAI-L-ABL could be considered as an alternative to C-Tx if contralateral nodules are absent.
There are some limitations to the present study. While all patients were classified as having FTC, the criteria for this diagnosis, and in particular its distinction from the follicular variant of PTC, have changed over time. It is therefore possible that our series includes some patients with the latter entity. Because of the long time period since surgery in most cases, we were not able to independently re-review the pathological specimen. This is a retrospective study and is therefore susceptible to possible selection bias. It is possible that, in our series, RAI-L-ABL was offered to patients thought to have the best potential outcome. In recognition of this limitation, we propose that RAI-L-ABL might be offered precisely and primarily to this group of patients. Given the long natural history of well-differentiated thyroid carcinoma, most outcome studies in the field are retrospective. As a consequence of the retrospective nature of this study, monitoring protocols were heterogeneous. Many of our patients did not have stimulated Tg testing. However, Tg is a surrogate marker of thyroid cancer. Important hard end-points such as death and metastatic disease did not differ between our study groups.
Author Disclosure Statement
G.B., M.G., S.P., and J.Y. have nothing to disclose. D.S.R. reports research funds via M.D. Anderson, from Genzyme, Inc.; G.H.D. is a consultant to Genzyme Corporation.
References
- 1.Baloch ZW. LiVolsi VA. Asa SL. Rosai J. Merino MJ. Randolph G. Vielh P. DeMay RM. Sidawy MK. Frable WJ. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–437. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 2.Baloch Z. Li Volsi VA. Pathology of the thyroid gland. In: Li Volsi VA, editor; Asa SL, editor. Endocrine Pathology. Churchill Livingstone; New York: 2002. pp. 61–88. [Google Scholar]
- 3.Thompson LDR. Malignant neoplasms of the thyroid gland. In: Thompson LDR, editor. Endocrine Pathology. Churchill Livingstone Elsevier; Philadelphia, PA: 2006. pp. 61–88. [Google Scholar]
- 4.D'Avanzo A. Treseler P. Ituarte PH. Wong M. Streja L. Greenspan FS. Siperstein AE. Duh QY. Clark OH. Follicular thyroid carcinoma: histology and prognosis. Cancer. 2004;100:1123–1129. doi: 10.1002/cncr.20081. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 6.Randolph GW. Daniels GH. Radioactive iodine lobe ablation as an alternative to completion thyroidectomy for follicular carcinoma of the thyroid. Thyroid. 2002;12:989–996. doi: 10.1089/105072502320908321. [DOI] [PubMed] [Google Scholar]
- 7.Tuttle RM. Ball DW. Byrd D. Dilawari RA. Doherty GM. Quan-Yang D. Hormoz E. Farrar W. Haddad RI. Hunt JP. Kandeel F. Kloos RT. Kopp P. Lamonica DM. Loree TR. Lydiatt WM. Mccaffrey J. Moley JF. Olson JA. Parks L. Ridge JA. Shah JP. Sherman SI. Sturgeon C. Waguespack SG. Wang TN. Wirth LJ. NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma. 2010. www.nccn.org. www.nccn.org NCCN. [DOI] [PubMed]
- 8.van Heerden JA. Hay ID. Goellner JR. Salomao D. Ebersold JR. Bergstralh EJ. Grant CS. Follicular thyroid carcinoma with capsular invasion alone: a nonthreatening malignancy. Surgery. 1992;112:1130–1136. discussion 1136–1138. [PubMed] [Google Scholar]
- 9.De Carlucci D., Jr. Tavares MR. Obara MT. Martins LA. Hojaij FC. Cernea CR. Thyroid function after unilateral total lobectomy: risk factors for postoperative hypothyroidism. Arch Otolaryngol Head Neck Surg. 2008;134:1076–1079. doi: 10.1001/archotol.134.10.1076. [DOI] [PubMed] [Google Scholar]
- 10.Pacini F. Ladenson PW. Schlumberger M. Driedger A. Luster M. Kloos RT. Sherman S. Haugen B. Corone C. Molinaro E. Elisei R. Ceccarelli C. Pinchera A. Wahl RL. Leboulleux S. Ricard M. Yoo J. Busaidy NL. Delpassand E. Hanscheid H. Felbinger R. Lassmann M. Reiners C. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006;91:926–932. doi: 10.1210/jc.2005-1651. [DOI] [PubMed] [Google Scholar]
- 11.Fast S. Nielsen VE. Bonnema SJ. Hegedus L. Dose-dependent acute effects of recombinant human TSH (rhTSH) on thyroid size and function: comparison of 0.1, 0.3 and 0.9 mg of rhTSH. Clin Endocrinol (Oxf) 2009;72:411–416. doi: 10.1111/j.1365-2265.2009.03650.x. [DOI] [PubMed] [Google Scholar]
- 12.Calabro S. Auguste LJ. Attie JN. Morbidity of completion thyroidectomy for initially misdiagnosed thyroid carcinoma. Head Neck Surg. 1988;10:235–238. doi: 10.1002/j.1930-2398.1988.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 13.Cambil T. Gil E. Ponce C. Ruiz JV. Castro J. [Bilateral vocal cords paresis following iodine therapy] Rev Esp Med Nucl. 2003;22:97–99. doi: 10.1016/s0212-6982(03)72152-1. [DOI] [PubMed] [Google Scholar]
- 14.Lee TC. Harbert JC. Dejter SW. Mariner DR. VanDam J. Vocal cord paralysis following I-131 ablation of a postthyroidectomy remnant. J Nucl Med. 1985;26:49–50. [PubMed] [Google Scholar]
- 15.Bryson PC. Shores CG. Hart C. Thorne L. Patel MR. Richey L. Farag A. Zanation AM. Immunohistochemical distinction of follicular thyroid adenomas and follicular carcinomas. Arch Otolaryngol Head Neck Surg. 2008;134:581–586. doi: 10.1001/archotol.134.6.581. [DOI] [PubMed] [Google Scholar]
- 16.Arad E. O'Mara RE. Wilson GA. Ablation of remaining functioning thyroid lobe with radioiodine after hemithyroidectomy for carcinoma. Clin Nucl Med. 1993;18:662–663. doi: 10.1097/00003072-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Bal CS. Kumar A. Pant GS. Radioiodine lobar ablation as an alternative to completion thyroidectomy in patients with differentiated thyroid cancer. Nucl Med Commun. 2003;24:203–208. doi: 10.1097/00006231-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Hoyes KP. Owens SE. Millns MM. Allan E. Differentiated thyroid cancer: radioiodine following lobectomy—a clinical feasibility study. Nucl Med Commun. 2004;25:245–251. doi: 10.1097/00006231-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Santra A. Bal S. Mahargan S. Bal C. Long-term outcome of lobar ablation versus completion thyroidectomy in differentiated thyroid cancer. Nucl Med Commun. 2011;32:52–58. doi: 10.1097/MNM.0b013e328340e74c. [DOI] [PubMed] [Google Scholar]
- 20.Leblanc G. Tabah R. Liberman M. Sampalis J. Younan R. How J. Large remnant 131I ablation as an alternative to completion/total thyroidectomy in the treatment of well-differentiated thyroid cancer. Surgery. 2004;136:1275–1280. doi: 10.1016/j.surg.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 21.Brennan MD. Bergstralh EJ. van Heerden JA. McConahey WM. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991;66:11–22. doi: 10.1016/s0025-6196(12)61170-7. [DOI] [PubMed] [Google Scholar]
- 22.Alzahrani AS. Mohamed G. Al Shammary A. Aldasouqi S. Abdal Salam S. Shoukri M. Long-term course and predictive factors of elevated serum thyroglobulin and negative diagnostic radioiodine whole body scan in differentiated thyroid cancer. J Endocrinol Invest. 2005;28:540–546. doi: 10.1007/BF03347243. [DOI] [PubMed] [Google Scholar]
- 23.Chao M. Management of differentiated thyroid cancer with rising thyroglobulin and negative diagnostic radioiodine whole body scan. Clin Oncol (R Coll Radiol) 2010;22:438–447. doi: 10.1016/j.clon.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Pacini F. Agate L. Elisei R. Capezzone M. Ceccarelli C. Lippi F. Molinaro E. Pinchera A. Outcome of differentiated thyroid cancer with detectable serum Tg and negative diagnostic (131)I whole body scan: comparison of patients treated with high (131)I activities versus untreated patients. J Clin Endocrinol Metab. 2001;86:4092–4097. doi: 10.1210/jcem.86.9.7831. [DOI] [PubMed] [Google Scholar]
- 25.Kloos RT. Approach to the patient with a positive serum thyroglobulin and a negative radioiodine scan after initial therapy for differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:1519–1525. doi: 10.1210/jc.2007-2357. [DOI] [PubMed] [Google Scholar]
- 26.Shaha AR. Shah JP. Loree TR. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg. 1996;172:692–694. doi: 10.1016/s0002-9610(96)00310-8. [DOI] [PubMed] [Google Scholar]
- 27.Allan E. Owens SE. Waller ML. Differentiated thyroid cancer: lobectomy and radioiodine, a treatment suitable for all cases? Nucl Med Commun. 1999;20:983–989. [PubMed] [Google Scholar]
- 28.Laurberg P. Wallin G. Tallstedt L. Abraham-Nordling M. Lundell G. Torring O. TSH-receptor autoimmunity in Graves' disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008;158:69–75. doi: 10.1530/EJE-07-0450. [DOI] [PubMed] [Google Scholar]
- 29.Thompson LD. Wieneke JA. Paal E. Frommelt RA. Adair CF. Heffess CS. 2001 A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer. 91:505–524. doi: 10.1002/1097-0142(20010201)91:3<505::aid-cncr1029>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Liu J. Singh B. Tallini G. Carlson DL. Katabi N. Shaha A. Tuttle RM. Ghossein RA. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107:1255–1264. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]