Abstract
This multicenter phase I/II study evaluated the safety, pharmacokinetics, and antitumor effects of repeated doses of NV1020, a genetically engineered oncolytic herpes simplex virus, in patients with advanced metastatic colorectal cancer (mCRC). Patients with liver-dominant mCRC received four fixed NV1020 doses via weekly hepatic artery infusion, followed by two or more cycles of conventional chemotherapy. Phase I included cohorts receiving 3 × 106, 1 × 107, 3 × 107, and 1 × 108 plaque-forming units (PFU)/dose to determine the optimal biological dose (OBD) for phase II. Blind independent computed tomography scan review was based on RECIST (response evaluation criteria in solid tumors) to assess hepatic tumor response. Phase I and II enrolled 13 and 19 patients, respectively. Patients experienced transient mild–moderate febrile reactions after each NV1020 infusion. Grade 3/4 virus-related toxicity was limited to transient lymphopenia in two patients. NV1020 shedding was not detected. Simultaneous cytokine and grade 1 coagulation perturbations were dose-limiting at 1 × 108 PFU/dose, considered the OBD. All 22 OBD patients had previously received 5-fluorouracil; most had received oxaliplatin or irinotecan (50% had both), many with at least one targeted agent. After NV1020 administration, 50% showed stable disease. The best overall tumor control rate after chemotherapy was 68% (1 partial response, 14 stable disease); this did not correlate with baseline variables or chemotherapy. Median time to progression was 6.4 months (95% confidence interval: 2, 8.9); median overall survival was 11.8 months (95% confidence interval: 8.3, 20.7). One-year survival was 47.2%. We conclude that NV1020 stabilizes liver metastases with minimal toxicity in mCRC. It may resensitize metastases to salvage chemotherapy and extend overall survival. A randomized phase II/III trial now appears justified.
Geevarghese et al. report results from a phase I/II multicenter study evaluating the safety, pharmacokinetics, and antitumor effects of repeated doses of NV1020, a genetically engineered oncolytic herpes simplex virus, in patients with advanced metastatic colorectal cancer (mCRC). The authors reveal that NV1020 stabilizes liver metastases with minimal toxicity in mCRC and leads to a one-year survival rate of 47.2%.
Introduction
Among novel treatment strategies for cancer, oncolytic virotherapy has shown encouraging progress and a relatively large number of genetically modified herpes simplex viruses (HSVs) have been evaluated (Nemunaitis, 2002; Kasuya et al., 2007; Vaha-Koskela et al., 2007; Ribacka et al., 2008; Wodarz, 2009). Replication of HSV in transformed cells causes cytolytic cell death and liberation of progeny virions, which infect adjacent tumor cells without adversely affecting untransformed parent cells (Zager et al., 2001; Stanziale et al., 2004; Song et al., 2006; Akiihiro et al., 2008). Additional factors that contribute to tumor-killing activity may include tumor ischemia and induction of cell-mediated immune responses, including local infiltration with natural killer cells (Kucharczuk et al., 1997; Coukos et al., 2003; Prestwich et al., 2008).
NV1020 is a multimutated, replication-competent, highly attenuated derivative of wild-type HSV-1. NV1020 was obtained by plaque purification of a construct designated R7020. R7020 was constructed by Meignier and colleagues (1988), and was evaluated in humans as an attenuated herpesvirus vaccine (Cadoz et al., 1992). R7020 was constructed by the deletion of two regions of the HSV-1 strain F genome, namely (1) the internal repeat (joint region) encompassing the UL56 gene and (2) a region that precludes expression of the UL24 gene (and the gene encoding thymidine kinase [TK]). Deletion of either of the two regions from HSV-1 strains results in viruses that remain replication competent but that are either markedly less pathogenic, or apathogenic, in animals.
Because the endogenous tk gene was inactivated by deleting the UL24/TK region from HSV-1, a functional HSV-1 tk gene was inserted into the NV1020 genome at the site of the deleted internal repeat region. Addition of the functional tk gene ensures that, if necessary, NV1020 infection can be controlled with TK-converted prodrugs, like acylclovir.
It has demonstrated selective antitumor activity in multiple cell lines in vitro and in vivo (Ghosh and Meyers, 1998; Carew et al., 1999; Kooby et al., 1999; Delman et al., 2000; McAuliffe et al., 2000; Cozzi et al., 2001; Shen and Nemunaitis, 2006; Friedman et al., 2009; Markert et al., 2009). Active by multiple routes of administration at relatively low doses, it shows additive effects with other cancer treatment modalities and is genetically stable during manufacture (Meignier et al., 1988; Bennett et al., 2001; Gutermann et al., 2006). In addition to multiple attenuating deletions, insertion of a functional HSV-1 thymidine kinase gene (tk) that expresses TK protein confers sensitivity to antiviral drugs such as acyclovir in the event of uncontrolled replication or toxicity. Fong and colleagues first assessed single intrahepatic artery infusions of NV1020 and reported tolerability, efficacy, and selective tumor infection in metastatic colorectal carcinoma (mCRC) (Kemeny et al., 2006; Fong et al., 2008; Kelly et al., 2008). We have expanded the evaluation of NV1020 to a weekly dosage regimen in extensively pretreated refractory mCRC, using a multicenter phase I/II protocol.
Patients and Methods
Eligibility criteria
Patients were required to be HSV-1 seropositive and to have histologically proven, radiologically progressing, extensively pretreated and refractory (≥2 regimens conventional drug therapy) colorectal adenocarcinoma, with liver-dominant metastases (≤50% involvement). Eligibility criteria included Karnofsky performance score (KPS) ≥70%, survival prognosis ≥4 months, stable comorbidities, and agreement both to use effective contraception and to comply with barrier precautions after virus administration. Laboratory test parameters included white cell count >3 × 103/mm3, absolute neutrophil count >1.5 × 103/mm3, platelet count >100,000/mm3, hemoglobin >9 g/dl, prothrombin time below the institutional upper limit of normal (ULN), serum creatinine ≤2 mg/dl, transaminases, alkaline phosphatase, and total bilirubin <2.5 and 1.5 times the ULN, respectively. Major surgery, chemotherapy, systemic corticosteroids, and other oncological interventions were prohibited less than 4–6 weeks before the first dose of NV1020. Significant intercurrent liver disease, prior hepatic radiotherapy, history of coagulation disorder, HIV infection, prior second malignancies within 5 years, and pregnancy were other specific exclusion criteria.
Ethics
The protocol was approved by local institutional review boards and other necessary oversight committees, and the study was conducted in accordance with the 1996 Declaration of Helsinki. An independent Data Monitoring Committee (DMC), with member expertise in virology, oncology, coagulation, and biometrics, was responsible for safety oversight, for approving dose escalations, and for determining OBD. Patients gave their signed informed consent to participate. Data were handled in accordance with the Health Insurance Portability and Accountability Act (HIPAA).
Study design and treatment
This study used an initial phase I dose escalation to determine dose-limiting toxicity (DLT) and to identify an optimal biological dose (OBD) level for evaluation of tumor response, followed by a phase II dose expansion at the OBD.
NV1020 (BioReliance, Rockville, MD) diluted in saline was administered over 10 min under fluoroscopic guidance via transfemoral catheter (or in situ hepatic pump, if present) in four weekly, fixed-dose infusions into the hepatic artery. Hepatic arterial architecture was delineated before the first dose to ensure selective delivery to both lobes of the liver. After infusion, patients were monitored overnight for safety and then discharged for follow-up. Treatment could be delayed (1 week) in the event of persistent virus-related toxicity but no dose reductions were permitted. In phase I, sequential cohorts of three patients received half-log increments of NV1020, starting with four 3 × 106 plaque-forming unit (PFU) infusions. Each patient was observed for toxicity for 7 days after the last infusion before the next patient could be treated; each cohort was monitored for a minimum of 14 days after the last dose, and safety data for the entire cohort at that dose level were reviewed, before dose escalation was approved. Dose levels of 3 × 106, 1 × 107, 3 × 107, and 1 × 108 PFU were evaluated. In phase II, an expanded cohort followed the same procedure but enrollment was concurrent.
Approximately 1 week after the fourth NV1020 infusion, patients started two cycles of additional chemotherapy and/or targeted agents selected by the investigator according to the patient's prior treatment outcomes. Thereafter, either the same chemotherapy or other agents (including experimental drugs) could be continued.
Safety and tumor assessments
Screening assessments consisted of medical history, physical examination including neurological assessment and Mini Mental Status Examination (MMSE) (Folstein et al., 1975), KPS, hematology, coagulation, clinical chemistry, carcinoembryonic antigen (CEA), HSV serostatus, hepatitis and HIV screening, computed tomography (CT) and 2-deoxy-2-[18F]fluoro-d-glucose (18F-FDG) positron emission tomography (PET) scans of abdomen and other sites as clinically indicated for assessing extent of tumor, chest X-ray (CXR), and electrocardiogram (ECG). Physical examination, KPS, hematology, coagulation, chemistry, and CEA were repeated immediately before first dose, together with blood draws for a limited cytokine panel (interleukin [IL]-6, tumor necrosis factor [TNF]-α, and interferon [IFN]-γ), NV1020 and neutralizing antibody titer assay, and various collections for detection of virus shedding. Safety was monitored continuously for up to 24 hr after each infusion and selected tests were repeated on days 3 and 7 each week. Tumor reassessment was performed within 7 days of the final NV1020 dose. Further evaluations were made after two cycles of chemotherapy, and then follow-up was continued at scheduled 12-weekly visits for a total of 12 months. Quarterly telephone contact was maintained indefinitely in order to identify late-onset HSV-related toxicity and to document survival. Permission for autopsy was granted in only three instances, none of which revealed evidence of virally mediated pathology.
Dose-limiting toxicity was determined on the basis of the National Cancer Institute (NCI, Bethesda, MD) Common Toxicity Criteria, version 3 (National Cancer Institute, 2006). Hepatic tumor response was assessed by principal investigators at individual sites, using World Health Organization (WHO, Geneva, Switzerland) criteria (Miller et al., 1981) for CT scans, in conjunction with PET data; patient management was individualized on the basis of these findings. All scans later underwent blinded radiology review by three independent experts. The central CT tumor assessment used modified RECIST (response evaluation criteria in solid tumors) (Therasse et al., 2000) guidelines—the sum of all measurable hepatic lesions was used in these assessments but the 1-month (post-NV1020) scan was not reported as progressive disease when tumor had increased >20% providing that subsequent scans showed stable disease or better (Sze et al., 2003; Reid et al., 2005). The findings of this independent review were used for definitive analyses and reports.
NV1020 pharmacokinetics
Blood was drawn before dosing and 7 and 24 hr after each infusion for NV1020 assay. Before freezing and storage, serum samples were heat-treated to inactivate infectious virus. A BioSprint 15 DNA blood kit (Qiagen, Westburg, The Netherlands) was used for DNA isolation. Extraction efficacy was controlled by NV1020 spiking of each sample in parallel experiments. Quantitative PCR (qPCR) was performed on extracted DNA in order to detect HSV genomes (using an internal spiking control to control PCR conditions). Because HSV-1 and HSV-2 show few differences in the sequence of the probe-binding region, differentiation between both serotypes could be performed by melting point analysis of the amplified DNA fragment. DNA of HSV-1-positive samples was further subjected to a conventional PCR with subsequent gel analysis of the amplified products in order to distinguish between wild-type HSV-1 and NV1020. The limits of detection for the qPCR and conventional PCR were 40 PFU or ∼800 HSV genomes and 160 PFU (corresponding to about 3200 HSV genomes), respectively.
Viral shedding
Before, and for 14 consecutive days after, the first and last NV1020 administrations swabs were collected for detection of wild-type HSV and NV1020 shedding in saliva and from genitalia (penis, scrotum, and perineum or vagina). Swab samples were collected in 1 ml of M4RT transport medium (Remel, Lenexa, KS) and heat-treated before freezing. Virus detection methodology and detection limits were the same as for serum analyses.
NV1020-neutralizing antibodies
Blood was drawn before dosing, 1 month after first NV1020 infusion, and then every 3 months for 1 year. Serum was stored frozen pending an end-point dilution assay. VERO cells were seeded into 96-well plates and infected with HSV at a multiplicity of infection of 1. For sample analysis, virus was preincubated with 2-fold dilutions of patient serum for 2 hr in the presence of guinea pig complement to enhance the detection of neutralizing antibodies. These samples were added to the plated cells. HSV-positive serum was used as reference. After 3 days, surviving cells were stained with methylene blue. The end-point titer of each serum sample was determined (50% reduction in cytopathic effect).
HSV serostatus
Blood was drawn before dosing, and 1 month after the first NV1020 infusion. Patient serum was subjected to Western blot analysis (University of Washington, Seattle, WA) with type-specific antibody specificity of >99%.
Statistical analyses
No formal sample size estimation was used for this early-phase study: 22 fully evaluable patients at the OBD was assumed adequate for the purpose of planning later trials. All patients received at least one dose of NV1020 and thus were included in the safety and efficacy analyses. Descriptive statistical summaries were generated for most data; overall survival and time to progression were determined by Kaplan–Meier analysis.
Results
Patient characteristics
In phase I, 13 patients received NV1020 and were assessable for safety and response. One patient, with rapidly progressing disease on entry, received only two of the four prescribed NV1020 infusions (total exposure, 6 × 107 PFU) and no follow-up chemotherapy before his death from complications of his extensive malignancy. In phase II, 19 enrolled and were fully assessable. Two further patients with rapidly progressing disease on enrollment received only two NV1020 infusions (total exposure, 2 × 108 PFU), and two other patients declined a second cycle of follow-up chemotherapy for personal reasons.
Demographic characteristics for individual cohorts are shown in Table 1. All 22 patients in the OBD group (3 from phase I and 19 from phase II) had 5-fluorouracil (5-FU)-based prior therapy. Most had received oxaliplatin- or irinotecan-containing regimens (50% had received both), 86% had one targeted therapy (24% received ≥2 such biologics), and 29% had prior radiofrequency ablation. After NV1020 administration, 10 (45%) patients received more of the chemotherapy to which they were previously refractory, and 8 (36%) were administered only one new agent.
Table 1.
Patient Characteristics by Dose Levela
| |
NV1020 dose level (PFU) |
|||||
|---|---|---|---|---|---|---|
| |
Phase I |
Phase II |
OBD |
|||
| 3 × 106 | 1 × 107 | 3 × 107 | 1 × 108 | 1 × 108 | 1 × 108 | |
| No. of patients | 3 | 3 | 4 | 3 | 19 | 22 |
| Age at entry, years | ||||||
| Median | 62 | 54 | 62 | 43 | 63 | 60 |
| Range | 57–67 | 50–71 | 57–63 | 40–51 | 33–79 | 33–79 |
| Male | 1 (34%) | 3 (100%) | 1 (25%) | 3 (100%) | 13 (68%) | 16 (73%) |
| White | 2 (67%) | 3 (100%) | 4 (100%) | 3 (100%) | 17 (90%) | 20 (91%) |
| KPS ≥ 90 | 3 (100%) | 3 (100%) | 4 (100%) | 3 (100%) | 18 (95%) | 21 (96%) |
| CEA | ||||||
| Median | 22 | 67 | 8 | 27 | 20 | 24 |
| Range | 12–45 | 2–307 | 4–500 | 5–264 | 2–2808 | 2–2808 |
| Time since primary tumor diagnosis, months | ||||||
| Median | 22 | 19 | 41 | 11 | 21 | 19 |
| Range | 11–22 | 11–26 | 3–79 | 10–27 | 5–51 | 5–51 |
| Pulmonary metastases | 2 (67%) | 1 (33%) | 0 (0%) | 2 (67%) | 6 (32%) | 8 (36%) |
| Number of prior mCRC regimens | ||||||
| Median | 2 | 3 | 3 | 2 | 4 | 4 |
| Range | 2–5 | 3–5 | 3–4 | 1–3 | 1–8 | 1–8 |
Abbreviations: CEA, carcinoembryonic antigen; KPS, Karnofsky performance score; mCRC, metastatic colorectal cancer; OBD, optimal biological dose.
The optimal biological dose level (OBD) comprises 3 patients from phase I and 19 from phase II.
Safety experience
Adverse events reported more commonly during the month of weekly NV1020 infusions (NV1020 phase) are shown in Table 2 by dose level and for all patients receiving the OBD. During the 4–24 hr after each virus infusion, most patients experienced a transient, grade 1 or 2 febrile reaction characterized by various degrees of chills, headache, nausea and vomiting, myalgia, body pains, and fatigue. This was effectively managed with analgesics and antipyretics and did not recur after the NV1020 treatment phase was over. No virus-related, grade 3 or 4 toxicity occurred. No clear dose response for type or frequency of adverse events was apparent. There were no NV1020-related abnormal trends in liver function. Laboratory abnormalities were random, grade 1, and unrelated to virus except for the lymphocyte, neutrophil, and platelet counts, C-reactive protein, D-dimers, and prothrombin times, which showed consistent dose-related perturbations. Transient, asymptomatic grade 3 lymphopenia occurred after the initial infusion (one patient) and after each infusion (one patient) at this dose level. There was also a dose-dependent cytokine trend, with peaks at the 8-hr postdosing sampling point but near normalization by 24 hr (Table 3). All other safety evaluations, and especially the skin/mucosa and neurological/MMSE examinations, showed no within- or between-patient trends. NV1020-related adverse events were not reported during chemotherapy or longer term follow-up.
Table 2.
Adverse Events Occurring in ≥10% of Patients During 1-Month NV1020 Administration Phase by Dose Levela
| |
NV1020 dose level (PFU) |
|||||
|---|---|---|---|---|---|---|
| |
Phase I |
Phase II |
OBD |
|||
| 3 × 106(n = 3) | 1 × 107(n = 3) | 3 × 107(n = 4) | 1 × 108(n = 3) | 1 × 108(n = 19) | 1 × 108(n = 22) | |
| Pyrexia | 3 (100%) | 2 (67%) | 4 (100%) | 100% | 18 (95%) | 21 (95%) |
| Chills | 3 (100%) | 0 (0%) | 2 (50%) | 2 (67%) | 11 (58%) | 13 (59%) |
| Nausea | 3 (100%) | 0 (0%) | 2 (50%) | 0 (0%) | 12 (63%) | 12 (55%) |
| Myalgia, pain at other sites | 1 (33%) | 1 (33%) | 1 (25%) | 0 (0%) | 11 (58%) | 11 (50%) |
| Headache | 0 (0%) | 1 (33%) | 2 (50%) | 1 (33%) | 10 (47%) | 11 (50%) |
| Fatigue | 0 (0%) | 1 (33%) | 1 (25%) | 1 (33%) | 7 (37%) | 8 (36%) |
| Vomiting | 1 (33%) | 0 (0%) | 2 (50%) | 1 (33%) | 7 (37%) | 8 (36%) |
| Abdominal pain | 0 (0%) | 1 (33%) | 1 (25%) | 0 (0%) | 5 (26%) | 5 (23%) |
| Diarrhea | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 4 (21%) | 4 (18%) |
| Abdominal distention | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (16%) | 3 (14%) |
| Constipation | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (16%) | 3 (14%) |
| Hypotension | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (16%) | 3 (14%) |
The optimal biological dose (OBD) level comprises 3 patients from phase I and 19 from phase II.
Table 3.
| Laboratory variable | Mean baseline (range) | Mean maximum (range) | Time to peak/nadir (hr) |
|---|---|---|---|
| Interferon-γ (pg/ml) | 0.7 (0.6–1.2) | 102 (20–434) | 7 |
| Interleukin-6 (pg/ml) | 10 (6–16) | 200 (29–1000) | 7 |
| Tumor necrosis factor (pg/ml) | 1 (0.6–2.1) | 7.75 (4.2–18) | 7 |
| ANC (× 103/μl) | 4.6 (3.0–6.7) | 3.5 (3.0–4.4) | 24 |
| Lymphocyte count (%) | 23 (19–28) | 16 (4–26) | 24 |
| Platelet count (× 103/μl) | 213 (148–330) | 153 (101–209) | 24 |
| Prothrombin time (sec) | 11 (9–15) | 12 (10–16) | 24 |
| C-reactive protein (mg/dl) | 4.3 (1.0–13.6) | 21.4 (8.6–52.3) | 24 |
Abbreviation: ANC, absolute neutrophil count.
1 × 108 PFU (optimal biological dose, n = 22).
Note: Blood samples were drawn before NV1020 administration and 7 ± 1 hr, 24 ± 1 hr, 72 ± 1 hr, and 7 ± 1 day postinfusion.
Virology
During the initial month of virus administration, NV1020 was never detected in any serum, saliva, or genital swab samples. However, wild-type HSV-1 DNA was detected in single-day serum samples at random times from each of four patients and on multiple days from one patient. Two of these patients also shed HSV-1 from their skin or buccal mucosa at other time points but none reported symptoms. Sporadically, saliva or skin swabs from 10 others tested positive for HSV-1 during the study; 3 reported one episode each of oral lesions typical in location, frequency, and severity for them of herpes labialis.
Pretreatment NV1020-neutralizing antibody titer in all patients (mean: 499; range: 12, 3072) rose markedly after the NV1020 infusions (mean: 3689; range: 384, 8689) independent of dose. By 12 months, titers were close to baseline (mean: 653; range: 271, 1086). Pretreatment, 26 patients (81%) tested seronegative for HSV-2 but 9 of these (35%) converted to seropositivity after the NV1020 infusions.
Antitumor response
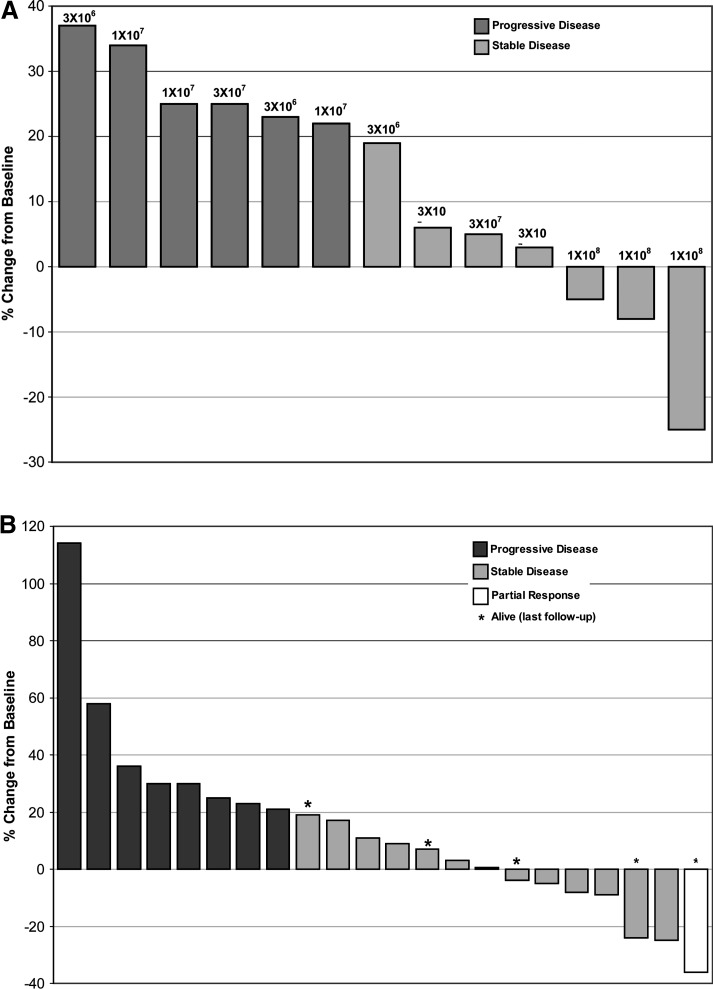
After NV1020 infusions at the lowest dose level (3 × 106) hepatic metastases in all three patients showed steady progression. With 1 × 107 PFU, one of three patients showed marginal stabilization. After the 3 × 107 and 1 × 108 PFU dose levels, CT scans for three of four and three of three patients showed disease stabilization (SD), respectively. Tumors progressed in two patients in the lower dose level at month 3, whereas more durable responses were observed for all three patients administered the highest dose level. Maximal changes in tumor diameter after NV1020 are shown in Fig. 1A. Median time to progression (TTP) for all 13 phase I patients was 3.5 months (95% confidence interval [CI]: 2.8, 6.9) and median survival was 12.4 months (95% CI: 9.6, 15.0).
FIG. 1.
Maximal change in hepatic tumor diameter after NV1020 administration. Progression is defined as a ≥20% increase in the size of all measurable hepatic lesions, partial response is ≥30%, and stable disease is intermediate change. (A) Phase I dose ranging: NV1020, 3 × 106–1 × 108 PFU; n = 13. (B) Optimal biological dose: NV1020, 1 × 108 PFU; n = 22.
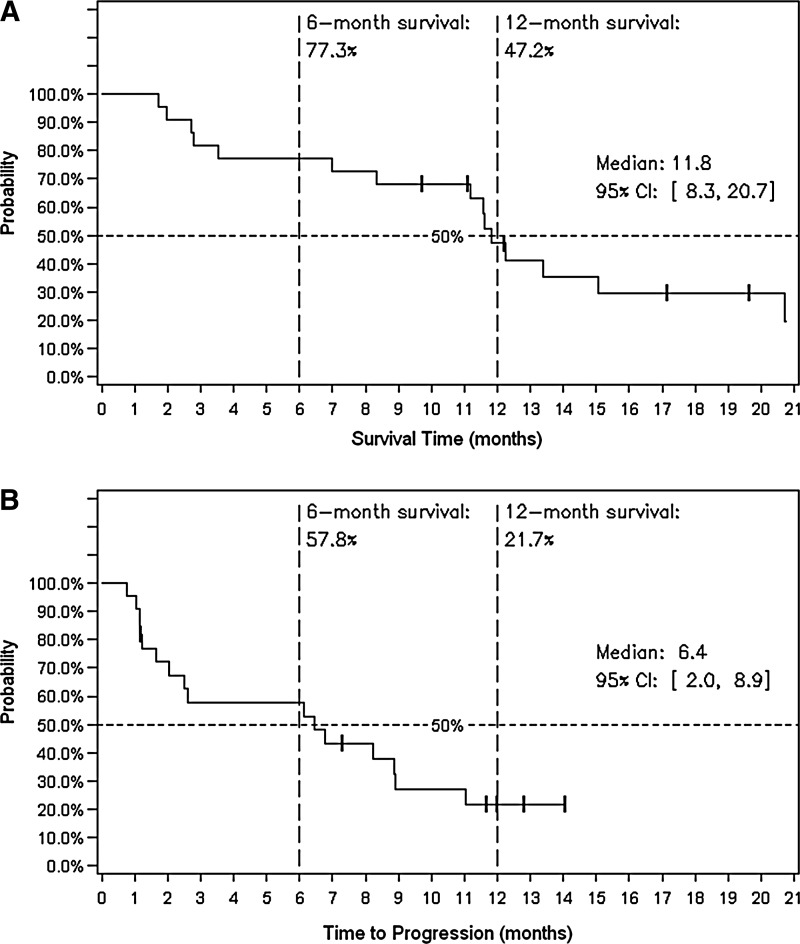
After NV1020 treatment at the OBD (n = 22), 11 patients (50%) initially showed stable disease (SD). Subsequent to chemotherapy, one patient experienced a partial response (PR) lasting more than 6 months (i.e., end of formal tumor follow-up) and was still alive when last contacted 30 months after enrollment. Best response tumor control rate during the study was 68% (1 PR, 14 SD), shown in Fig. 1B. Response showed no clear correlation with initial tumor size, SUV, viral antibody titer, or CEA level, nor with time since primary tumor resection, nor with pre- or post-NV1020 chemotherapy regimen. Median TTP for this group was 6.4 months (95% CI: 2, 8.9) and median overall survival (OS) was 11.8 months (95% CI: 8.3, 20.7). The 12-month survival rate was 47.2% and 5 (22.7%) were alive at the most recent survival follow-up (range: 9.7, 19.6 months). Kaplan–Meier probability curves for TTP and OS are shown in Fig. 2.
FIG. 2.
Tumor progression and overall survival (in months) after NV1020 administration at the optimal biological dose, n = 22. (A) Kaplan–Meier graph for overall survival. (B) Kaplan–Meier graph for time to progression.
Discussion
This study demonstrated that repeated hepatic arterial infusions of NV1020 at dose levels up to 1 × 108 PFU were remarkably well tolerated and corroborated the findings reported using the same dose range for single infusions (Kemeny et al., 2006). Locoregional infusion, which minimizes whole body systemic virus exposure and circumvents potential host immune neutralization, was well accepted by physicians and patients. No dermal or neural toxicity was observed and the only consistent side effect was a self-limiting viral syndrome during the first 24 hr after NV1020 administration, mitigated effectively with symptomatic treatment. In no instances did toxicity delay discharge home or further NV1020 infusions. Clinical adverse events did not appear to be dose-related. Both the scope and frequency of adverse events during chemotherapy that followed NV1020 administration were unremarkable and nothing suggested possible drug interactions, viral reactivation, or systemic dissemination during the patients' eventual clinical decline.
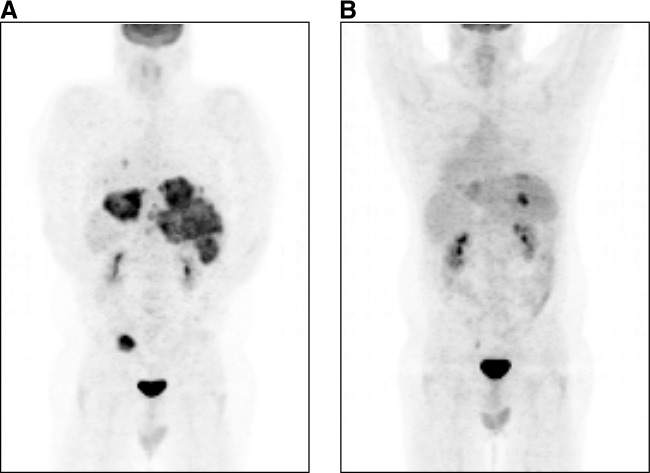
No clinical DLT was defined and thus a maximal tolerated dose (MTD) could not be identified. However, individual infusions of NV1020 induced a dose-related transient increase in all three cytokines (IL-6, TNF-α, and IFN-γ). At 1 × 108 PFU, this was associated with various subclinical hematological correlates; although short-lived, the consistency of the prothrombin time prolongation (approximately 1 sec for <24 hr) prompted discussion within the DMC about the risk of significant coagulopathy and potentially serious clinical outcomes in the event of further dose escalation. All three patients at that dose level had shown antitumor effects (SD, one patient also had complete remission of both local pelvic recurrence and pulmonary metastases, shown in Fig. 3). Consequently, further dose escalation was not considered justified and 1 × 108 PFU was designated the OBD for cohort expansion.
FIG. 3.
Patient 401. Posterior views of distant antitumor response in a 40-year-old white male after NV1020 hepatic artery infusion. (A) PET scan after failing FOLFOX (5-fluorouracil, folinic acid, oxaliplatin) shows extensive hepatic with pulmonary metastases, with local recurrence of sigmoid colon adenocarcinoma. (B) PET scan 5 months after four 1 × 108 PFU infusions of NV1020 followed by repeat FOLFOX shows marked regression of hepatic metastases, together with complete clearance of pulmonary and pelvic tumor.
Rigorous swabbing and salivary collections for up to 14 days after the last infusion confirmed no shedding of NV1020. However, wild-type HSV-1 was shed at sporadic, usually solitary, time points by 38% of the patients and was detected in the serum of an additional three. Three patients experienced what was described as “typical” single recurrences of their intermittent herpes lip lesions. This frequency reflects the literature for a population of HSV-1-seropositive patients (Whitley and Gnann, 1993; Stanberry and Jorgensen, 1997). On the basis of the absence of NV1020 shedding and the satisfactory safety profile of NV1020 reviewed in phase I, protocolized barrier precautions were relaxed in phase II. The immunocompetence of NV1020 was confirmed by the universal and dramatic rise in NV1020-neutralizing antibodies within 1 month of starting infusions. Insertion of a 5.2-kb fragment of HSV-2 DNA (including HSV-2 glycoprotein G) in order to improve immunogenicity and to attenuate pathogenicity of an NV1020 vaccine was responsible for the HSV-2 seroconversion observed in some patients. One HSV-1-seronegative patient was inadvertently enrolled: her postinfusion viral syndrome was mild and no noteworthy other adverse events were observed. Others have treated small numbers of HSV-1-seronegative patients with a similar HSV construct without untoward toxicity (Markert et al., 2009), suggesting that this eligibility constraint might be lifted in future studies.
Although this was not a randomized, controlled study the biological activity and antitumor findings were noteworthy. Our sample of mCRC patients was highly pretreated and had documented relapse immediately before study entry after extensive therapy. Yet 50% showed stabilization of hepatic metastases at the first radiological assessment after just four infusions of NV1020 at the OBD. Subsequent additional conventional systemic chemotherapy yielded an overall clinical control rate of 68% and median TTP of 6.4 months. These outcomes were unexpected considering that 45% of patients were given (as third- or fourth-line treatment) only drugs to which they were previously refractory, and another 36% received just one new agent. Furthermore, overall median survival was also longer than historical data would predict after salvage therapy (Cunningham et al., 2004; Gholam et al., 2006; Saletti and Cavalli, 2006; Van Cutsem et al., 2007; Capdevila et al., 2008).
Thus, it is reasonable to conclude that NV1020 stabilizes liver metastases in highly advanced, refractory mCRC and may extend survival by resensitizing tumors to chemotherapy through specific, systemic oncolytic immune response mechanisms (Toda et al., 1999; Muruve, 2004; Diaz et al., 2007). Coupled with the absence of significant virus-related toxicity under the conditions in this study, NV1020 warrants further evaluation because the majority of patients with mCRC eventually succumb to progressive hepatic tumor burden and the goal of newer multimodality approaches is to extend survival regardless of radiographic regression (Biasco et al., 2005; Saltz, 2005; Saunders and Iveson, 2006; Goldberg et al., 2007). A larger, randomized phase II/III trial studying combination therapy with cytotoxic and targeted agents is now justified. Evaluation for other indications (e.g., hepatocellular carcinoma) or for other vascular beds (e.g., mesenteric), or combining NV1020 with other cytoreductive treatment modalities, may open a wide new array of possibilities in view of its unique mode of action.
Acknowledgments
This study would not have been possible without the good will of our patients and families. Special thanks are due to the research staff at each investigational center, including subinvestigators, nurses, coordinators, research pharmacists, and support staff in administration, legal, finance, and other departments that are also essential for trials of this kind. Sam Gambhir, M.D., and Andrei Iagaru, M.D., provided independent PET oversight; Alice Chen and her staff at MediGene, Inc., provided operational oversight. Matthias Karrasch was medical backup, Dominika Weinelt reviewed safety reports, and Eunice Braz provided QC support; these scientists were all at MediGene AG. Pam Larsen at MediGene, Inc., provided regulatory support, Synteract (Carlsbad, CA) provided data management support, and Mayo Labs (Rochester, MN) provided centralized laboratory services. The DMC comprised Roger Flora, Ph.D., Dan Hoth, M.D., Howard Liebman, M.D., Jack Macdonald, M.D., Kenneth Tyler, Ph.D., and Anna Wald, M.D. This trial was funded by MediGene, Inc. (San Diego, CA).
Author Disclosure Statement
For S.K.G., no competing financial interests exist (Vanderbilt University received research funding for this study).
For D.A.G, no competing financial interests exist (the University of Pittsburgh received research funding for this study). H.A.d.H. received consulting fees from MediGene, Inc. M.H. is a paid employee of MediGene AG. A.E.K. is a paid employee of MediGene AG. A.M. is a paid employee of MediGene AG and holds stock in the company. For J.N., no competing financial interests exist (the Mary Crowley Medical Research Center received research funding for this study). For T.R.R, no competing financial interests exist (UCSD received research funding for this study). D.Y.S. received consulting fees from MediGene, Inc. For K.K.T., no competing financial interests exist (Harvard Medical School received research funding for this study). H.T. is a paid employee of MediGene AG.
References
- Akiihiro N. Chenhong L. Azhang L. Ushjima Y. Ishida D. Kamakura M. Fujimoto Y.F. Goshima F. Kikkawa F. Nishiyama Y. Non-engineered, naturally oncolytic herpes simplex virus HSV1 HF-10: Applications for gene therapy. Curr. Gene Ther. 2008;8:208–221. doi: 10.2174/156652308784746422. [DOI] [PubMed] [Google Scholar]
- Bennett J.J. Malhotra S. Wong R.J. Delman K. Zager J. St-Louis M. Johnson P. Fong Y. Interleukin 12 secretion enhances antitumor efficacy of oncolytic herpes simplex viral therapy for colorectal cancer. Ann. Surg. 2001;233:819–826. doi: 10.1097/00000658-200106000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasco G. Derenzini E. Grazi G.L. Ercolani G. Ravaioli M. Pantaleo M.A. Brandi G. Treatment of hepatic metastases from colorectal cancer: Many doubts, some certainties. Cancer Treatment Rev. 2005;32:214–228. doi: 10.1016/j.ctrv.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Cadoz M. Micoud M. Seigneurin J.M. Mallaret M.R. Baccard C. Morand P. Phase 1 trial of R7020: A live attenuated recombinant herpes simplex (HSV) candidate vaccine. Paper presented at the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy; Anaheim, CA: 1992. Oct 11–14, 1992. [Google Scholar]
- Capdevila J. Ramos F. Macarulla T. Elez E. Tabernero J. The role of salvage treatment in advanced colorectal cancer. Crit. Rev. Oncol. Hematol. 2008;71:53–61. doi: 10.1016/j.critrevonc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Carew J.F. Kooby D.A. Halterman M.W. Federoff H.J. Fong Y. Selective infection and cytolysis of human head and neck squamous cell carcinoma with sparing of normal mucosa by a cytotoxic herpes simplex virus type 1 (G207) Hum. Gene Ther. 1999;10:1599–1606. doi: 10.1089/10430349950017608. [DOI] [PubMed] [Google Scholar]
- Coukos G. Courreges M.C. Benencia F. Intraperitoneal oncolytic and tumor vaccination therapy with replication-competent recombinant virus: The herpes paradigm. Curr. Gene Ther. 2003;3:113–125. doi: 10.2174/1566523034578401. [DOI] [PubMed] [Google Scholar]
- Cozzi P. Malhotra S. McAuliffe P. Kooby D. Federoff H.J. Huryk B. Johnson P. Scardino P.T. Heston W.D.W. Fong Y. Intravesical oncolytic viral therapy using attenuated, replication-competent herpes simplex viruses G207 and NV1020 is effective in the treatment of bladder cancer in an orthotopic syngeneic model. FASEB J. 2001;15:1306–1308. doi: 10.1096/fj.00-0533fje. [DOI] [PubMed] [Google Scholar]
- Cunningham D. Humblet Y. Siena S. Khayat D. Blieberg H. Santoro A. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- Delman K.A. Bennett J.J. Zager J.S. Burt B.M. McAuliffe P.F. Petrowsky H. Kooby D.A. Hawkins W.G. Horsburgh B.C. Johnson P. Fong Y. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum. Gene Ther. 2000;11:2465–2472. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- Diaz R.M. Galivo F. Kottke T. Wongthida P. Qiao J. Thompson J. Valdes M. Barber G. Vile R.G. Oncolytic immunotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Folstein M.F. Folstein S.E. McHugh P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fong Y. Kim T. Bhargava A. Schwartz L.H. Brown K. Bordy L. Covey A. Karrasch M. Getrajdman G. Mescheder A. Jarnagin W. Kemeny N. A herpes oncolytic virus can be delivered via the vasculature to produce biologic changes in human colorectal cancer. Mol. Ther. 2008;17:389–394. doi: 10.1038/mt.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman G.K. Pressey J.G. Reddy A.T. Markert J.M. Gillespie G.Y. Herpes simplex virus oncolytic therapy for pediatric malignancies. Mol. Ther. 2009;17:1125–1135. doi: 10.1038/mt.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholam D. Giacchetti S. Brezault-Bonnet C. Bouchahda M. Hauteville D. Adam R. Ducot B. Ghemard O. Kustlinger F. Jasmin C. Levi F. Chronomodulated irinotecan, oxaliplatin, and leucovorin-modulated 5-fluorouracil as ambulatory salvage therapy in patients with irinotecan- and oxaliplatin-resistant metastatic colorectal cancer. Oncologist. 2006;11:1072–1080. doi: 10.1634/theoncologist.11-10-1072. [DOI] [PubMed] [Google Scholar]
- Ghosh J. Myers C.E. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R.M. Rothenberg M.L. Van Cutsem E. Benson A.B. Blanke C.D. Diasio R.B. Grothey A. Lenz H.J. Meropol J. Ranamathan R.K. Becerra C.H.R. Wickham R. Armstrong D. Viele C. The continuum of care: A paradigm for the management of metastatic colorectal cancer. Oncologist. 2007;12:38–50. doi: 10.1634/theoncologist.12-1-38. [DOI] [PubMed] [Google Scholar]
- Gutermann A. Mayer E. von Dehn-Rothfelser K. Breidenstein C. Weber M. Muench M. Gungor D. Suehnel J. Moebius U. Lechmann M. Efficacy of oncolytic herpesvirus NV1020 can be enhanced by combination with chemotherapeutics in colon carcinoma cells. Hum. Gene Ther. 2006;17:1241–1253. doi: 10.1089/hum.2006.17.1241. [DOI] [PubMed] [Google Scholar]
- Kasuya H. Takeda S. Shimoyama S. Shikano T. Nomura N. Kanazumi N. Nomoto S. Sugimoto H. Nakao A. Oncolytic virus therapy: Forward. Curr. Cancer Drug Targets. 2007;7:123–125. doi: 10.2174/156800907780058826. [DOI] [PubMed] [Google Scholar]
- Kelly K.J. Wong J. Fong Y. Herpes simplex virus NV1020 as a novel and promising therapy for hepatic malignancy. Expert Opin. Investig. Drugs. 2008;17:1105–1113. doi: 10.1517/13543784.17.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny N. Brown K. Covey A. Kim T. Bhargava A. Brody L. Guilfoyle N.P. Haag N.P. Karrasch M. Glasschroeder B. Knoll A. Getrajdman G. Kowal K.J. Jarnagin W.R. Fong Y. Phase I, open-label, dose escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum. Gene Ther. 2006;17:1–11. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- Kooby D.A. Carew J.F. Halterman M. Mack J. Bertino J. Blumgart L.H. Federoff H.J. Fong Y. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207) FASEB J. 1999;13:1325–1334. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- Kucharczuk B. Randazzo J.C. Change M.Y. Amin K.M. Elshami A.A. Sterman D.H. Rizk N.P. Molnar-Kimber K.L. Brown S.M. MacLean A.R. Litzky L.A. Fraswer N.W. Albelda S.M. Kaiser L.R. Use of a replication-restricted herpes virus to treat experimental human malignant mesothelioma. Cancer Res. 1997;57:466–471. [PubMed] [Google Scholar]
- Markert J. Liechty P. Wang W. Faston S. Braz E. Karrasch M. Nabors L. Markiewicz M. Lakeman A. Palmer C. Parker J. Whitley R. Gillespie G. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe P. Jarnagin W. Johnson P. Delman K.A. Federoff H. Fong Y. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. J. Gastrointest. Surg. 2000;4:580–588. doi: 10.1016/s1091-255x(00)80106-7. [DOI] [PubMed] [Google Scholar]
- Meignier B. Longnecker R. Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J. Infect. Dis. 1988;158:602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- Miller A.B. Hoogstraten B. Staquet M. Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Muruve D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute, Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) v3.0. 2006. http://ctep.cancer.gov/reporting/ctc.html. [Jul;2010 ]. http://ctep.cancer.gov/reporting/ctc.html
- Nemunaitis J. Live viruses in cancer treatment. Oncology. 2002;16:1483–1492. [PubMed] [Google Scholar]
- Prestwich R.J. Harrington K.J. Vile R.G. Melcher A.A. Immunotherapeutic potential of oncolytic virotherapy. Lancet Oncol. 2008;9:610–611. doi: 10.1016/S1470-2045(08)70163-3. [DOI] [PubMed] [Google Scholar]
- Reid T.R. Freeman S. Post L. McCormick F. Sze D.Y. Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Ther. 2005;12:673–681. doi: 10.1038/sj.cgt.7700819. [DOI] [PubMed] [Google Scholar]
- Ribacka C. Pesonen S. Hemminki A. Cancer, stem cells, and oncolytic viruses. Ann. Med. 2008;40:496–505. doi: 10.1080/07853890802021342. [DOI] [PubMed] [Google Scholar]
- Saletti P. Cavalli F. Metastatic colorectal cancer. Cancer Treatment Rev. 2006;32:557–571. doi: 10.1016/j.ctrv.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Saltz L. Metastatic colorectal cancer: Is there one standard approach? Oncology. 2005;19:1147–1148. [PubMed] [Google Scholar]
- Saunders M. Iveson T. Management of advanced colorectal cancer: State of the art. Br. J. Cancer. 2006;95:131–138. doi: 10.1038/sj.bjc.6603233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. Nemunaitis J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006;13:975–992. doi: 10.1038/sj.cgt.7700946. [DOI] [PubMed] [Google Scholar]
- Song T.-J. Eisenberg D.P. Adusumilli P.S. Hezel M. Fong Y. Oncolytic herpes viral therapy is effective in the treatment of hepatocellular carcinoma cell lines. J. Gastrointest. Surg. 2006;10:532–542. doi: 10.1016/j.gassur.2005.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry L. Jorgensen D. Herpes simplex viruses 1 and 2. In: Evans A., editor; Kaslo R., editor. Viral Infections of Humans: Epidemiology and Control. Plenum Medical Book; London: 1997. pp. 419–454. [Google Scholar]
- Stanziale S.F. Stiles B.M. Bhargava A. Kerns S.A. Kalakonda N. Fong Y. Oncolytic herpes simplex virus-1 mutant expressing green fluorescent protein can detect and treat peritoneal cancer. Hum. Gene Ther. 2004;15:609–618. doi: 10.1089/104303404323142051. [DOI] [PubMed] [Google Scholar]
- Sze D. Freeman S. Slonim S.M. Reid T.R. Dr. Gary J. Becker Young Investigator Award: Intraarterial adenovirus for metastatic gastrointestinal cancer: Activity, radiographic response, and survival. J. Vasc. Intervent. Radiol. 2003;14:279–290. doi: 10.1097/01.rvi.0000058422.01661.1e. [DOI] [PubMed] [Google Scholar]
- Therasse P.T. Arbuck S.G. Eisenhauer E.A. Wanders J. Kaplan R.S. Rubinstein L. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Toda M. Rabkin S.D. Kojima H. Martuza R.L. Herpes simplex virus as an in situ cancer vaccine for the induction of specific antitumor immunity. Hum. Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- Vaha-Koskela M. Heikkila J. Kinkkanen A.E. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E. Peeters M. Siena S. Humblet Y. Hendlisz A. Heyns B. Canon J.-L. Van Laethen J.-L. Maurel J. Richardson G. Wolf M. Amado R.G. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- Whitley R. Gnann J. The epidemiology and clinical manifestations of herpes simplex virus infections. In: Roizman B., editor; Whitley R., editor; Lopez C., editor. The Human Herpes Viruses. Raven Press; New York: 1993. pp. 69–105. [Google Scholar]
- Wodarz D. Use of oncolytic viruses for the eradication of drug-resistant cancer cells. J. R. Soc. Interface. 2009;6:179–186. doi: 10.1098/rsif.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager J.S. Delman K.A. Malhotra S. Ebright M.I. Bennett J.J. Kates T. Halterman M. Federoff H. Fong Y. Combination vascular delivery of herpes simplex oncolytic viruses and amplicon mediated cytokine gene transfer is effective therapy for experimental liver cancer. Mol. Med. 2001;7:561–568. [PMC free article] [PubMed] [Google Scholar]