Abstract
Background
Guidelines from the National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference recommend a repeat fine-needle aspiration biopsy (FNAB) after 3 months for thyroid nodules with a nondiagnostic (ND) result. Our aims were to assess which factors influenced their clinical management and to determine if the timing of the repeat FNAB affects the diagnostic yield.
Methods
A retrospective institutional review of 298 patients from 1/2006 to 12/2007 with an ND FNAB was performed. The factors influencing the next step in management, including age, gender, history of radiation, presence of Hashimoto's thyroiditis, thyroid-stimulating hormone levels, and ultrasound characteristics, were evaluated. The effect of the time of the repeat FNABs on their diagnostic yield was assessed.
Results
Of the 298 patients in our cohort, 9% were referred directly for surgery, 76% had a repeat FNAB, and 15% were observed. Tumor size was the only independent variable correlated with treatment strategy after a ND FNAB. There was not a significant difference in diagnostic yields between repeat FNABs performed earlier than 3 months compared to those preformed later (p=0.58).
Conclusion
The timing of repeat FNAB for an initial ND FNAB does not affect diagnostic yield of the repeat FNAB.
Introduction
The most accurate and cost-effective tool to evaluate risk of malignancy in thyroid nodules is a fine-needle aspiration biopsy (FNAB) (1,2). However, 2%–20% of FNABs are qualitatively or quantitatively insufficient to make a diagnosis (1). Due to the associated 1%–10% risk of malignancy (3–5), the American Thyroid Association (ATA) and the Bethesda System for Reporting Thyroid Cytopathology recommend performing a repeat FNAB under ultrasound (US) guidance for nondiagnostic (ND) FNABs (2,6). The ideal timing of the repeat FNAB for ND diagnoses (i.e., acellular or inadequate number of follicular cells) has not been objectively established, and waiting for at least 3 months has been suggested to avoid reparative cellular atypia (6,7).
Our aim was to determine whether the recommended 3-month waiting period is necessary before repeating an FNAB for ND specimens and if certain clinical or radiographic characteristics influenced management. We reviewed the management of patients with an initial ND FNAB at our institution to determine (i) if clinical variables or US characteristics influenced the next step in clinical management, and (ii) if timing of repeat biopsy altered the diagnostic yield or the number of biopsies suggestive of malignancy.
Methods
Patients
An IRB-approved search of the Massachusetts General Hospital clinical cytology database was performed from January 2006 through December 2007 to identify patients with an ND FNAB. The interventional radiology database was also surveyed for thyroid core biopsies performed during the same period. An ND FNAB was defined as per the six-tiered Bethesda System for Reporting Thyroid Cytopathology: acellular or an inadequate number of follicular cells (less than six groups of benign follicular cells, each group composed of at least 10 cells) in the specimen, cyst fluid, smear artifact, or obscuring blood (8). All cytology reports were re-reviewed by one cytopathologist (W.C.F.) and found to be concordant with the original reading.
A total of 3594 FNABs were performed at our institution during the study period. Three hundred seventy-five of these biopsies (10%) had an FNAB interpreted as ND. We excluded 51 patients due to lack of documented follow-up. An additional 19 patients were excluded for a concomitant FNAB diagnosed as atypical, suspicious, or malignant. The 298 patients who met the inclusion criteria were divided into three categories based on the next step in management:
• Group I (n=27): Patients who were referred directly for surgery without a repeat FNAB.
-
• Group II (n=226): Patients who had a repeat FNAB at any time after an initial FNAB. To assess the timing of repeat sampling, patients in group II were subdivided:
– Group IIA (n=138): Patients who had early repeat sampling (<3 months)
– Group IIB (n=86): Patients who had late repeat sampling (≥3 months).
• Group III (n=45): Patients in this category were observed and were not subjected to surgery or a repeat FNAB. At least one follow-up after 6 months with an endocrinologist and neck US was required for inclusion.
All patients in the cohort had their electronic medical records and/or charts reviewed for demographic, US, clinical, and pathological variables. Surgical pathological correlation for the index nodule was done for all patients undergoing surgery. Patients in each group were compared based on the following:
1. Demographic and clinical characteristics: age, gender, history of radiation, presence of Hashimoto's thyroiditis, and thyroid-stimulating hormone levels.
2. US characteristics: size, echogenicity, margin, microcalcifications, and vascular flow of the index nodule.
Biopsy procedures
All ND FNABs were performed under US guidance by an endocrinologist (n=179, 65%), a surgeon (n=22, 8%), or a radiologist (n=75, 27%). Three to four passes of the index nodule was performed using a 10-mL syringe and 25–27-gauge needles. Six to eight alcohol-fixed smears were prepared from the aspirate for Papanicolaou staining, while the remainder was prepared for ThinPrep by rinsing the needle hub in Cytolyte. Repeat FNABs were performed in the same manner. Confirmation of specimen adequacy by a dedicated cytopathologist is not available at our institution.
A subset of the patients in group II who underwent repeat FNAB also had core biopsy performed immediately afterward (n=62) by an interventional radiologist (A.E.S.). Core biopsies were performed under US guidance using 20-gauge Temno Evolution-cutting core biopsy needles (Cardinal Healthcare, Dublin, OH) with a 10- or 20-mm adjustable needle throw as previously described (9). These 62 patients were participants in the study by Samir et al. in which we reported that the combination of FNAB and core biopsy after an ND FNAB resulted in a higher diagnostic yield compared to FNA alone (9).
Statistical analysis
Univariate analysis was done using the Fisher exact test for categorical variables and a Student's t-test for continuous normal data. Timing of repeat biopsy was analyzed as both a categorical (< or ≥3 months) as well as continuous variable. Multinomial logistic regression was performed for multivariate analysis of the three primary treatment strategies. The McNemar's test was used to test concordance between the core biopsy and repeat FNAB.
Results
Nodule size was significantly larger in patients referred directly for surgery following an initial ND FNAB
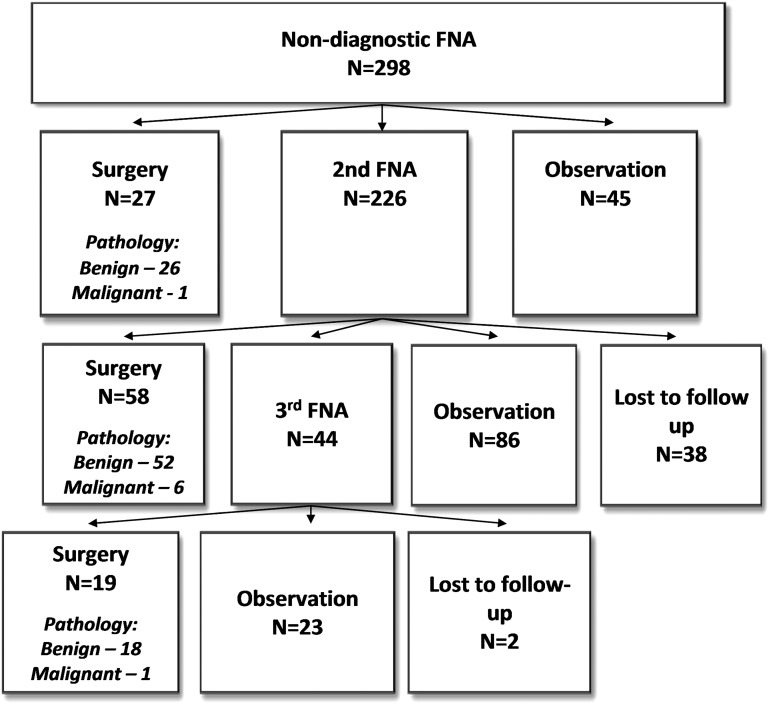
The 298 patients in our cohort consisted of 226 (76%) female patients and 72 (24%) male patients, with a mean age of 62 (±15). Over the 2-year period, 27/298 (9%) patients were referred directly for surgery (group I), 226/298 (76%) had a repeat FNAB (group II), and 45/298 (15%) patients were observed (group III) (Fig. 1). On the univariate analysis, there was a significant difference in age among the groups, but no other differences in the demographic variables (Table 1). Furthermore, there was no difference in the proportion of patients with a prior or concomitant benign FNAB or an additional ND FNAB between the three treatment groups (p=0.29). Patients in group I (surgery) had larger nodules than in group II (repeat FNA, p=0.002) and group III (observation, p<0.001). On the adjusted analysis, increasing tumor size correlated with the odds of going directly to surgery over repeat FNA (odds ratio 4.5, confidence interval 1.9–10.5). Other worrisome US characteristics, including echogenicity, margin, microcalcifications, and vascular flow, did not differ between the three groups.
FIG. 1.
Clinical strategy and surgical outcomes. FNA, fine-needle aspiration.
Table 1.
Patient and Ultrasound Variables
| Variable | Total (n=298) | Group I—surgery (n=27) | Group II—repeat FNA (n=226) | Group III—observation (n=45) | p-value |
|---|---|---|---|---|---|
| Age (mean±SD) | 62±14.6 | 54±16.8 | 63±14.2 | 61±14.2 | 0.01 |
| Female (%) | 226 (76%) | 22 (81%) | 167 (75%) | 36 (80%) | 0.61 |
| History of neck radiation [n (%)] | 16/276 (6%) | 2/26 (8%) | 11/205 (5%) | 3/45 (7%) | 0.74 |
| Hashimoto's thyroiditis [n (%)] | 10/275 (4%) | 0/26 (0%) | 8/203 (4%) | 2/45(4%) | 0.75 |
| TSH median (n [IQR]) | 1.3 [1.3] | 1.0 [1.0] | 1.3 [1.25] | 1.23 [1.6] | 0.76 |
| Index nodule sizea | |||||
| <2 cm [n (%)] | 130 (57%) | 2 (14%) | 101 (56%) | 27 (79%) | <0.01 |
| 2–4 cm [n (%)] | 78 (34%) | 7 (50%) | 65 (36%) | 6 (18%) | |
| >4 cm [n (%)] | 19 (8%) | 5 (36%) | 13 (7%) | 1 (3%) | |
Based on the index nodule size reported on 227 ultrasounds.
SD, standard deviation; TSH, thyroid-stimulating hormone; IQR, interquartile range.
Boldface p-values indicate statistical significance.
Eighty-five percent of patients were treated per guideline recommendations following an initial ND FNAB
Group I: Of the 27/298 patients referred directly for surgery without repeat sampling, the final pathological diagnosis included the following: 14 follicular adenomas (52%), 12 benign adenomatous nodules (44%), and 1 follicular carcinoma (4%).
Group II: Table 2 shows the results of the 226 repeat FNABs and the final surgical pathological diagnosis of the 58 patients in group II who went to surgery after a second FNAB. Six of these 58 (10%) patients had cancers diagnosed at surgery. The one benign FNAB that had a classical papillary thyroid carcinoma (PTC) found on final pathology was diagnosed accurately by a core biopsy performed at the same time. Additionally, there were two repeat ND FNABs that had follicular carcinoma and medullary thyroid carcinoma diagnosed on final surgical pathology and two nodules with atypia that were diagnosed at classical PTC and follicular variant PTC.
Table 2.
Cytological Diagnosis and Surgical Pathology for Group II Patients
| |
|
Surgical pathology (n=58) |
|
|---|---|---|---|
| Cytological diagnosis on second FNAB | n=226 | Malignant | Benign |
| Atypia of undetermined significance | 24 (11%) | 2 | 11 |
| Nondiagnostic | 93 (41%) | 2 | 26 |
| Benign | 101 (45%) | 1 | 9 |
| Suspicious for malignancy | 2 (0.9%) | 0 | 1 |
| Suspicious for a follicular neoplasm | 5 (2.2%) | 0 | 5 |
| Malignant | 1 (0.4%) | 1 | 0 |
FNAB, fine-needle aspiration biopsy.
A third FNAB was performed in 44 patients following a second FNAB. Surgery was done in 19 (44%) of these patients following a third FNAB. The indication for surgery in these 19 patients was a third ND result or to differentiate between cellular hyperplastic nodule, adenoma, and follicular carcinoma. On final pathology, 18 nodules were benign, and one patient had a follicular variant of PTC.
Overall, of the 298 patients in our cohort, 104 (35%) eventually underwent surgery, of which 8/104 (8%) had a malignancy. Surgical pathological correlation is reported on the index nodule that was initially ND FNA in all cases. The proportion of patients undergoing surgery with malignant pathology was not significantly different in patients undergoing repeat FNAB (p=0.60).
Timing of the repeat FNAB does not affect diagnostic yield
Patients in group II (repeat FNA, n=226) were analyzed based on whether they had their repeat FNAB early (<3 months, group IIA) or late (≥3 months, group IIB). Timing of the repeat biopsy was not available for two patients; therefore they were excluded from the analyses relating to timing of the repeat FNAB. Early repeat FNAB was done in 138 (63%) patients versus 86 (27%) in the late group. Patients in the early group (group IIA) had their repeat FNAB, a median of 37 days from the initial ND FNAB compared to 184 days in the late subgroup (group IIB). Demographic and clinical factors did not differ between groups IIA and IIB (Table 3). Moreover, US characteristics did not differ between early and late repeat biopsy groups: proportion of cystic nodules (p=0.83), degree of echogenicity (p=0.43), border irregularity (p=0.08), increased vascularity (p=0.66), or presence of microcalcifications (p=0.51). The timing of the repeat FNAB did not significantly alter the diagnostic yield on the repeat FNAB (p=0.58): 57/138 (41.3%) in group IIA versus 35/86 (40.7%) of patients in group IIB. Timing remained not correlated with diagnostic yield with the regression analysis using time between FNABs as a continuous variable (p=0.45). Furthermore, there was no significant difference in the cytological diagnoses between the early and late subgroups (p=0.66) (Table 4).
Table 3.
Comparison of Clinical and Ultrasound Variables Between Those Undergoing Repeat Fine-Needle Aspiration Biopsy Before and After 3 Months (Group IIA Vs. IIB, n=224)
| Variable | Group IIA (<3 months) (n=138) | Group IIB (≥3 months) (n=86) | p-Value |
|---|---|---|---|
| Age (mean±SD) | 64.6±13.6 | 60.9±15.1 | 0.06 |
| Female [n (%)] | 101 (73%) | 66 (77%) | 0.63 |
| Hashimoto's thyroiditis [n (%)] | 6 (5%) | 2 (2%) | 0.47 |
| TSH median (n [IQR]) | 1.8 [1.6] | 1.2 [0.9] | 0.11 |
| Index nodule sizea | |||
| <2 cm [n (%)] | 59 (54%) | 42 (60%) | |
| 2–4 cm [n (%)] | 39 (36%) | 26 (37%) | |
| >4 cm [n (%)] | 11 (10%) | 2 (3%) | 0.18 |
Table 4.
Diagnostic Yield of Repeat Fine-Needle Aspiration Biopsys Based on Time Period Between Fine-Needle Aspiration Biopsy
| Diagnosis on repeat FNAB | Group IIA (<3 months) n=138 | Group IIB (≥3 months) n=86 |
|---|---|---|
| Atypia/follicular lesion of undetermined significance [n (%)] | 14 (10.1%) | 9 (10.5%) |
| Nondiagnostic [n (%)] | 57 (41.3%) | 35 (40.7%) |
| Benign [n (%)] | 63 (45.7%) | 38 (44.2%) |
| Suspicious for follicular neoplasm [n (%)] | 0 | 2 (2.3%) |
| Suspicious for malignancy [n (%)] | 3 (2.2%) | 2 (2.3%) |
| Malignant [n (%)] | 1 (0.7%) | 0 |
Discussion
In this study, we report the clinical management of 298 patients at our institution with an ND FNAB, defined as an acellular specimen or inadequate number of follicular cells, over a 2-year period. Particular attention was paid to the analysis of pathological results in patients with repeat biopsies performed after the recommended 3-month waiting period. While most series report a 5%–20% rate of ND FNABs, the Bethesda system for reporting thyroid cytopathology states that the rate of ND FNABs should ideally not exceed 10% (1). Our ND FNAB rate over the 2-year study period was 10%.
The Bethesda system reports a risk of malignancy associated with an ND FNAB between 1% and 4% (1), with other series citing a range of 2%–51% (3–4,7,10). Our data show the overall rate of malignancy in patients who underwent surgery to be 8%, consistent with the current literature. Moreover, at our institution, most patients were treated according to the National Cancer Institute/ATA guidelines with either surgery or repeat sampling (85%). Other than the size of the nodule, none of the clinical or imaging variables assessed in this study correlated with the treatment group. Although speculative, based on the retrospective nature of this study, the decision to proceed directly to surgery was likely multifactorial and based on tumor size, clinical suspicion, and patient preference. Moreover, patients who proceeded directly to surgery had larger nodules, but no statistically significant difference in the malignancy rate compared to those who underwent surgery after a repeat biopsy.
Benefits of repeat biopsy were noted in our study; repeating the FNAB yielded a benign diagnosis in almost half of the repeat FNAB nodules, avoiding unnecessary surgery. This underscores the usefulness of a repeat FNAB in the stratification and management of patients with an initial ND FNAB. Moreover, the addition of core biopsy in combination to repeat FNAB where available may improve diagnostic yield (9). A synopsis of the NCI State of Science Conference as well as the Bethesda system recommends that surgery be strongly considered in patients with two or more consecutive ND FNABs (1,6). In our cohort, approximately one-third of the patients with consecutive ND FNABs were referred for surgery. The risk of malignancy in patients who referred for surgery following two ND FNABs is reported to be ∼10% (1,4), similar to the 10.1% rate of malignancy following two and 5.3% following three ND FNABs in our study.
Although the guidelines for repeating an ND FNAB are well established, the time interval between the initial and repeat FNAB is not. A synopsis of the State of Science Conference recommends waiting for at least 3 months before performing a repeat FNAB to avoid post-FNAB reparative cellular atypia (7). In our study, 63% of patients had a biopsy before 3 months, and we found no significant differences in the diagnostic yield or pathologic results on a repeat FNAB based on timing of the repeat FNAB. Therefore, we feel that the timing of repeat FNAB can be based on clinician and patient preference, as we saw no obvious downside to an early biopsy.
Being retrospective, our study has several limitations. We excluded 14% of the patients who had ND FNABs during our study period due to lack of follow-up, lending to potential selection bias. Our study population was also subject to missing data by virtue of its retrospective nature. Moreover, some of the subanalyses, such as radiation exposure, may be underpowered to detect significant associations with clinical practice. In addition, the decision to refer a patient for surgery, perform repeat sampling, or observe is subject to clinician bias apart from the factors captured in the design. The timing of repeat FNAB and the decision to include core biopsy after an initial ND FNAB was subject to scheduling or clinical bias; we had no way to determine what these biases were, as they were not always recorded. Lastly, we were unable to know in which cases the FNAB and/or core biopsy reports were accessed by the pathologist or cytopathologist when making cytological or pathological diagnoses.
In conclusion, patients with an initial ND FNAB undergoing surgery have an 8% overall chance of malignancy in the index nodule on final pathology. Repeat FNAB can be performed after an initial ND FNAB without waiting 3 months, without compromising diagnostic yield of the sample. Core biopsy may be a useful adjunct to repeat biopsy in some cases. Finally, we found an 8% incidence of cancer in patients who ultimately underwent surgery. While this is the select group of patients with, along with larger nodule size, other unmeasured clinical factors, this rate is not insignificant. Therefore, other known factors associated with malignancy (11), such as the size of the nodule, should continue to guide the clinical strategy in these patients.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Cibas ES. Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Alexander EK. Heering JP. Benson CB. Frates MC. Doubilet PM. Cibas ES. Marqusee E. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab. 2002;87:4924–4927. doi: 10.1210/jc.2002-020865. [DOI] [PubMed] [Google Scholar]
- 4.McHenry CR. Walfish PG. Rosen IB. Non-diagnostic fine needle aspiration biopsy: a dilemma in management of nodular thyroid disease. Am Surg. 1993;59:415–419. [PubMed] [Google Scholar]
- 5.Yang J. Schnadig V. Logrono R. Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 6.Layfield LJ. Abrams J. Cochand-Priollet B. Evans D. Gharib H. Greenspan F. Henry M. LiVolsi V. Merino M. Michael CW. Wang H. Wells SA. Post-thyroid FNA testing and treatment options: a synopsis of the National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:442–448. doi: 10.1002/dc.20832. [DOI] [PubMed] [Google Scholar]
- 7.Baloch Z. LiVolsi VA. Jain P. Jain R. Aljada I. Mandel S. Langer JE. Gupta PK. Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol. 2003;29:203–206. doi: 10.1002/dc.10361. [DOI] [PubMed] [Google Scholar]
- 8.Baloch ZW. Cibas ES. Clark DP. Layfield LJ. Ljung BM. Pitman MB. Abati A. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008;5:6. doi: 10.1186/1742-6413-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samir AE. Vij A. Seale MK. Desai G. Halpern E. Faquin WC. Parangi S. Hahn PF. Daniels GH. Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior nondiagnostic fine-needle aspirate. Thyroid. 2012;22:461–467. doi: 10.1089/thy.2011.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow LS. Gharib H. Goellner JR. van Heerden JA. Nondiagnostic thyroid fine-needle aspiration cytology: management dilemmas. Thyroid. 2001;11:1147–1151. doi: 10.1089/10507250152740993. [DOI] [PubMed] [Google Scholar]
- 11.Tuttle RM. Lemar H. Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998;8:377–383. doi: 10.1089/thy.1998.8.377. [DOI] [PubMed] [Google Scholar]