Abstract
The objective of this study was to identify barley leaf proteins differentially regulated in response to drought and heat and the combined stresses in context of the morphological and physiological changes that also occur. The Syrian landrace Arta and the Australian cultivar Keel were subjected to drought, high temperature, or a combination of both treatments starting at heading. Changes in the leaf proteome were identified using differential gel electrophoresis and mass spectrometry. The drought treatment caused strong reductions of biomass and yield, while photosynthetic performance and the proteome were not significantly changed. In contrast, the heat treatment and the combination of heat and drought reduced photosynthetic performance and caused changes of the leaf proteome. The proteomic analysis identified 99 protein spots differentially regulated in response to heat treatment, 14 of which were regulated in a genotype-specific manner. Differentially regulated proteins predominantly had functions in photosynthesis, but also in detoxification, energy metabolism, and protein biosynthesis. The analysis indicated that de novo protein biosynthesis, protein quality control mediated by chaperones and proteases, and the use of alternative energy resources, i.e. glycolysis, play important roles in adaptation to heat stress. In addition, genetic variation identified in the proteome, in plant growth and photosynthetic performance in response to drought and heat represent stress adaption mechanisms to be exploited in future crop breeding efforts.
Key words: Abiotic stress, barley, drought, heat, proteomics, Rubisco activase, yield.
Introduction
Drought and heat are among the main abiotic stresses dramatically limiting crop growth and productivity worldwide (Wang et al., 2003). In the field, the co-occurrence of several abiotic stresses, rather than an individual stress condition is most damaging to crop production (Mittler, 2006). For example, the combined effects of heat and drought on yield are more detrimental than the effects of each stress alone, as seen in sorghum (Sorghum bicolor L.; Craufurd et al., 2008), wheat (Prasad et al., 2011), and barley (Savin and Nicolas, 1996). However, most studies to date have addressed the effects of single stresses on plant performance (Ugarte et al., 2007, Harb et al., 2010), and little is known about the molecular mechanisms underlying the acclimation of plants to a combination of different stresses (Mittler, 2006). Recent studies have revealed that the response of plants to a combination of different abiotic stresses is unique and cannot be directly extrapolated from the response of plants to each of the different stresses applied individually (Rizhsky et al., 2004; Ahmed et al., 2013). Breeding of stress-tolerant crops is the most efficient strategy to maintain yield in stress-prone marginal land. It is thus important to identify genetic resources with high stress tolerance and to understand the mechanisms contributing to adaptation to stresses typically co-occurring in the field such as heat and drought.
Barley (Hordeum vulgare L.) is worldwide among the most widely cultivated crops in marginal environments and is often the most common crop in the driest rain-fed farming areas as it is well adapted to abiotic stresses (Baum et al., 2007). Barley has thus been selected or bred for specific adaptation to abiotic stresses in geographically distinct areas of the world. This adaptation of genetically diverse germplasm to similar environmental conditions over a wide geographical range can be exploited for breeding and germplasm exchange. For example, barley germplasm bred by the International Center for Agricultural Research in the Dry Areas, Syria (ICARDA) for the marginal environments of West Asia and Northern Africa (WANA) showed good adaptation to the dry Southern Australian environments (Coventry et al., 2004) and vice versa (Shakhatreh et al., 2001). This germplasm is also interesting in order to study the genetic basis of adaptation to stress in genetically diverse genotypes. Stress adaptation in barley has been attributed to the plasticity of morphological traits such as biomass production, plant growth, tiller number, and peduncle extrusion (von Korff et al., 2008; Shakhatreh et al., 2010). Growth-related responses to stress are typical of stress avoidance strategies which allow the plant to maintain a homeostasis despite changes in the environments. Barley has also been characterized for genetic variation in physiological parameters such as relative water content and chlorophyll fluorescence parameters under stress (Oukarroum et al., 2007; Ahmed et al., 2013). These differences are indicative of differences in stress-tolerance mechanisms which allow the plant to maintain cellular activities under stress (Bartels and Sunkar, 2005). Studies on molecular changes in response to stress in barley have primarily relied on quantification of mRNA changes under stress (Talamè et al., 2007; Guo et al., 2009 von Korff et al., 2009). These studies showed that a large proportion of the transcriptome responded to drought including genes implicated in stress signalling and stress response. However, while mRNA transcript levels can dictate protein abundance, the differential expression of the two macromolecules is not always well correlated (Stylianou et al., 2008; Baerenfaller et al., 2012). Proteomic approaches provide information missing in DNA or mRNA analysis methods in that they focus on the actively translated portion of the genome. Stress resistance is conferred by the proteins, which function in stress signalling, transcription regulation, cellular detoxification, protection of macromolecules, and a panoply of other processes. In recent years, methods for the analysis of the proteome have advanced considerably, and together with emerging sequence information in model crops like barley (The International Barley Sequencing Consortium, 2012), the plant proteome has become increasingly useful for understanding gene function and networks in response to environmental stimuli. Conventional gel-based proteomics has proved useful in barley research to quantify changes in protein abundance in grains during development (Finnie et al., 2006), in roots in response to salt stress (Witzel et al., 2009) and in shoots in response to heat stress (Süle et al., 2004). Proteomic research using fluorescent labels and two-dimensional difference gel electrophoresis (2D-DIGE) has been successfully applied in barley for the identification of proteins associated with malting quality (March et al., 2012) and in Arabidopsis (Shi et al., 2011) and wheat (Gao et al., 2011) to identify proteins responsive to salt. By quantifying changes in protein abundance, one can gain insight into the biochemical processes that underlie the plant’s morphological and physiological acclimations to abiotic stress.
The objectives of this study were to study the molecular basis of abiotic stress responses in two drought-adapted, genetically diverse genotypes, the Syrian landrace selection Arta and the Australian barley cultivar Keel. The specific aims were to: (i) characterize the physiological and morphological responses to drought and heat stress; and (ii) identify leaf proteins differentially regulated in response to drought and heat stress using a proteomics approach based on DIGE and mass spectrometry. The changes in protein abundance are discussed in the context of the physiological and morphological trait plasticity that occurs due to the abiotic stresses.
Materials and methods
Plant material
Two cultivated barley two-row genotypes, Arta and Keel, were grown in a growth chamber to evaluate their performance. Arta is a pure line selection from a Syrian landrace adapted to the driest areas in Syria and is winter barley. These genotypes were selected because they are genetically diverse but adapted to similar drought-prone environments, thus allowing the study of the diversity of molecular and phenotypic changes in response to drought and heat.
Drought and heat treatments
Response to the single or combined effects of drought and heat treatments applied at the generative stage was tested in a duplicated growth chamber experiment. Plants were sown in 96-well trays, stratified at 4 °C for 4 d, and then grown in 8/16 light/dark short-day (SD) conditions for 24 d. Plants were transferred to 16/8 light/dark long-day (LD) conditions for 2 d to acclimate before being potted in 4 l pots containing 1.8kg soil with three plants in each pot. Plants remained in LD for the remainder of the experiment. The SD followed by LD treatment was applied to synchronize flowering. The relative humidity of the chambers was set to 50%, the light intensity was 350 μmol photons m−2 s−1 and the temperature was set to 21/17 °C light/dark. The field capacity (FC) was calculated as the difference in weight between fully hydrated soil and dried soil (Colman, 1947). The soil water content (SWC) of potted plants was adjusted to 50% of the FC. For the drought treatment, the SWC was reduced to 15% FC by controlled withholding of water; pots were weighed daily and watered to match the weight of the heaviest pot. The reduction of the SWC was equal across all drought-treated pots. The SWC of 15% FC for the stressed plants and 50% FC for the control plants was maintained until plant maturity. To control for the added weight of the growing plants, the volumetric water content of random pots was checked weekly using a TDR 100 soil moisture meter (FieldScout) fitted with 12 cm probe rods. When the SWC reached 15% FC in the drought-treated plants, the heat treatment was applied to a subset of the well-watered and drought-treated plants by moving the pots at ZT 3 to an identical chamber set to 21 °C and gradually raising the temperature to 36 °C over 4h. Heat-treated plants remained at 36/32 °C light/dark for 1 week, at which point the temperature was decreased to 21 °C over 4h. Detailed information on phenotyping can be found in Table 1 and in the Supplementary Materials and Methods (available at JXB online).
Table 1.
Summary of traits measured in the genotypes Arta and Keel grown under control, drought, heat, and combination treatments
| Trait | Abbreviation | Unit |
|---|---|---|
| Grain yield | GY | g |
| Total biomass | BM | g |
| Harvest index | HI | g g–1 |
| Plant height | PH | cm |
| Peduncle extrusion | Pedex | cm |
| Spike number | SN | – |
| No. of aborted spikes | AS | – |
| Grains per spike | GS | – |
| Thousand kernel weight | TKW | g |
| Days to maturity | DM | days |
| Total water used per pot | WU | l |
| Water use efficiency of grain yield | WUE | g l–1 |
| Leaf temperature on day 1 | LT_1 | °C |
| Leaf temperature on day 3 | LT_3 | °C |
| Leaf temperature on day 7 | LT_7 | °C |
| Relative water content on day 1 | RWC_1 | % |
| Relative water content on day 3 | RWC_3 | % |
| Relative water content on day 7 | RWC_7 | % |
| Maximum PSII quantum yieldat day 1 | Fv/Fm_1 | Arbitrary |
| Maximum PSII quantum yield at day 3 | Fv/Fm_3 | Arbitrary |
| Maximum PSII quantum yield at day 7 | Fv/Fm_7 | Arbitrary |
| PSII performance index at day 1 | PI_1 | Arbitrary |
| PSII performance index at day 3 | PI_3 | Arbitrary |
| PSII performance index at day 7 | PI_7 | Arbitrary |
Two-dimensional gel electrophoresis
Leaf proteins from three biological replicates were extracted using a trichloric acetic acid/acetone precipitation modified from Cascardo et al. (2001) and fluorescently labelled as detailed in Supplementary Table S1 and Supplementary Materials and Methods. The protein sample was diluted to a total volume of 340 μl in rehydration buffer consisting of 7M urea, 2M thiourea, 2% CHAPS, 20mM DTT, and 0.5% biolyte 3–10 ampholytes (Bio-Rad) and applied to 18 cm immobiline strips pH 3–10 NL (GE Healthcare Life Sciences). Strips for DIGE gels contained a total of 150 μg protein while strips for gels to be post-stained with PageBlue (Fermentas life sciences) contained 500 μg protein. Focusing was accomplished at 20 °C using a Protean isoelectric focusing cell (Bio-Rad) with the following conditions: 14h passive rehydration, 250V for 15min, ramping to 2000V for 1h 45min, and ramping to 10kV for 3h, before maintaining the voltage at 10kV for a total of 30kV/h. The resulting strips were equilibrated in 2D equilibrium buffer (0.1M Tris, 6M urea, 30% glycerol, 2% SDS) containing 2% DTT for 15min and then with 2D equilibrium buffer containing 2.5% iodoacetamide (Sigma) for 15min. Equilibrated strips were placed on 1 mm thick 12% SDS-PAGE gels sized 26×20cm and covered with 0.5% agarose (Bio-Budget). The second dimension was separated using 12 mA/gel for 12h with the EttanDaltSix electrophoresis system (GE Healthcare Life Sciences).
Protein identification
Protein gels were imaged and spots were detected as detailed in Supplementary Materials and Methods. Spots from 2D gels were excised using the Proteineer sp II and tryptically digested and spotted on MTP 600/384 AnchorChip plates using the Proteineer dp system (Bruker Daltonics). Aliquots of the digests were automatically spotted on MTP 600/384 AnchorChip plates for subsequent mass spectrometric analysis according to the thin-layer protocol of Gobom et al. (2001) using the Proteineer dp robot. Peptide mass fingerprints were obtained using the Ultraflex III MALDI ToF/ToF mass spectrometer (Bruker Daltonics). The resulting spectra were processed into peak files with the flexAnalysis version 2.4 software (Bruker Daltonics) by means of the sophisticated numerical annotation procedure algorithm. Peak data were imported into the ProteinScape database system version 3.0 (Bruker), which initiated Mascot version 2.3 (Matrix Science) searches against the UniProt knowledgebase (http://www.uniprot.org/) for H. vulgare (release 2011_06) and the DFCI Barley Gene Index (http://compbio.dfci.harvard.edu/tgi/) version 12 genome database.
Data analysis
Analysis of variance (ANOVA) and correlations were performed using SAS version 9.1 (SAS Institute) and are described in details in Supplementary Materials and Methods. The regulation factor, the log2-transformed fold change in the normalized spot intensity, was calculated between 36 °C and 21 °C treated plants across genotypes and drought treatments as well as between Arta and Keel across both stress treatments. Gene ontology terms for identified proteins were retrieved from AgBase (McCarthy et al., 2006). Singular enrichment analysis was performed with the AgriGO toolkit (Du et al., 2010) as outlined in Supplementary Materials and Methods.
Results
Comparison of effects of drought, heat, and combination treatments on plant performance
Morphological changes caused by the treatments are depicted in the representative photographs of control and stress-treated Arta and Keel plants taken the end of the heat treatment, 9 days after heading initiated (Fig. 1). Drought caused strong reductions in plant height as compared to control in Arta, but not in Keel. In addition, the drought, heat, and combination treatments caused noticeably stronger senescence of lower leaves in Arta than in Keel.
Fig. 1.
Representative Arta and Keel plants after 7 days of control (21 °C) or high (36 °C) temperature and control (50%) or drought (15%) soil water content. Each 4 l pot contains three plants.
Overall, the drought treatment had a stronger effect on morphological traits than on physiological traits. For example, the water regime explained 26% of the variation in grain yield (GY), 57% of the variation for biomass (BM), and 79% of the variation for spike number (SN), while it explained only up to 18% of the variation in relative water content (RWC) and less than 1% of variation in photosynthetic performance index (PI) (Supplementary Table S2). In contrast, the heat treatment had significantly stronger effects on physiological traits than the drought treatment; it explained up to 54% of the variation in water use efficiency (WUE), 34% of the variation in RWC, and 74% of the variation in PI. The heat treatment did not have strong effects on plant growth; it only explained 8% of the variation in BM and 2% for plant height (PH). The strongest phenotypic changes were observed under the combination treatment (Table 2). The combination treatment caused the strongest reduction in GY. GY under the combination treatment was significantly lower in Keel than GY under heat or drought alone, while significant reductions in GY caused by drought and heat were not significantly different from each other (Table 2). While the reductions in GY under drought and heat were similar, drought and heat had different effects on yield component traits. Whereas the drought and combination treatments caused significant reductions in BM and PH, heat did not significantly affect these traits. In addition, the spike number (SN) was significantly reduced by drought and combination treatments as compared to controls, but not significantly affected by heat in both genotypes. In contrast, heat and combination treatments significantly increased the number of aborted spikes (AS) and decreased thousand kernel weight (TKW) in Arta and Keel, while drought did not have significant effects on AS and TKW as compared to control conditions. The harvest index (HI) was significantly reduced under drought, heat, and combination treatments as compared to controls, while the reductions in HI were also significantly greater under heat and combination treatments as compared to the drought treatment in both genotypes. The number of grains per spike (GS) and peduncle extrusion (Pedex) were reduced under drought, heat, and combination treatments, but the differences were not significant. The heat treatment had thus strong effects on generative traits; it explained 32% of the variation of AS, and 72% of variation in TKW. In contrast, drought showed the strongest effects on vegetative traits such as vegetative biomass and plant height.
Table 2.
Trait means, minimums, and maximums for Arta and Keel under control or drought conditions at either 21°C or 36°C
Definitions of trait abbreviations are given in Table 1. Means that are not significantly different (P < 0.05) share the same superscript letter.
| Trait | Control 21°C Arta | Control 21°C Keel | Control 36°C Arta | Control 36°C Keel | Drought 21°C Arta | Drought 21°C Keel | Drought 36°C Arta | Drought 36°C Keel | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | |
| GY | 11.8 a | 6.5 | 15.1 | 11.7a | 4.2 | 18.2 | 5.6bcd | 2.6 | 11.3 | 6.4b | 4.3 | 8.2 | 6.0bd | 3.1 | 8.5 | 7.7b | 6.0 | 9.0 | 3.2cd | 0.8 | 6.0 | 2.9c | 1.5 | 4.8 |
| BM | 20.7a | 16.2 | 24.3 | 20.2a | 9.5 | 26.1 | 18.7ab | 15.3 | 23.3 | 18.6ab | 13.3 | 23.3 | 13.6c | 11.0 | 17.3 | 15.1bc | 12.8 | 18.3 | 12.3cd | 9.6 | 16.0 | 9.8d | 5.1 | 13.2 |
| HI | 0.56a | 0.40 | 0.73 | 0.57a | 0.44 | 0.79 | 0.29b | 0.14 | 0.52 | 0.35bd | 0.27 | 0.45 | 0.44cd | 0.23 | 0.62 | 0.51ac | 0.45 | 0.58 | 0.25b | 0.08 | 0.47 | 0.30b | 0.22 | 0.43 |
| PH | 49.6ab | 44.0 | 53.8 | 52.5a | 46.5 | 56.4 | 44.9bd | 36.9 | 54.2 | 50.8a | 43.4 | 59.8 | 38.9c | 35.2 | 42.2 | 49.9ab | 45.7 | 54.0 | 42.8cd | 37.7 | 48.7 | 45.3bd | 39.7 | 51.7 |
| Pedex | –3.7a | –4.6 | –2.6 | –5.1ab | –9.7 | –1.9 | –3.7a | –8.8 | 2.9 | –5.9ab | –9.0 | –2.9 | –6.1ab | –9.1 | –2.5 | –5.8ab | –11.3 | –3.4 | –5.5ab | –7.8 | –1.2 | –6.8b | –8.7 | –4.5 |
| SN | 22.6ac | 16.0 | 30.0 | 22.2ac | 10.7 | 33.0 | 25.9a | 16.6 | 35.3 | 26.7a | 20 | 36.3 | 14.9b | 13 | 16.7 | 17.6bc | 12.7 | 25.0 | 18.5bc | 11.7 | 25.7 | 14.9b | 5.7 | 21.3 |
| AS | 3.5ac | 0.3 | 10.7 | 0.4a | 0.0 | 2.7 | 11.1b | 4.0 | 22.0 | 6.1cd | 0.3 | 16.0 | 3.2ac | 1.7 | 5.3 | 2.1a | 0.0 | 8.0 | 8.8bd | 4.7 | 13.7 | 3.3ac | 0.7 | 7.7 |
| GS | 12.2a | 10.9 | 14.2 | 12.1a | 9.3 | 17.2 | 10.7a | 6.8 | 15.1 | 10.4a | 8.2 | 14.7 | 11.2a | 5.7 | 13.6 | 11.3a | 9.6 | 13.0 | 10.8a | 6.2 | 14.4 | 10.0a | 7.8 | 12.6 |
| TKW | 50.2a | 40.6 | 63.8 | 44.1b | 33.1 | 55.0 | 35.5c | 32.1 | 41.2 | 30.4cd | 27.0 | 33.9 | 46.0ab | 36.7 | 51.3 | 44.5ab | 41.2 | 48.4 | 29.9cd | 24.3 | 37.1 | 26.3d | 23.4 | 29.6 |
| DM | 90a | 68 | 107 | 88a | 63 | 109 | 97a | 82 | 105 | 93a | 68 | 107 | 90a | 74 | 105 | 88a | 68 | 98 | 96a | 88 | 102 | 90a | 63 | 100 |
| WU | 7.54a | 5.89 | 9.27 | 6.70a | 4.71 | 7.96 | 7.65a | 6.27 | 9.94 | 6.65a | 4.81 | 8.06 | 3.45b | 1.63 | 4.26 | 3.42b | 2.91 | 3.95 | 3.61b | 1.66 | 5.60 | 3.21b | 2.62 | 3.98 |
| WUE | 1.6ad | 0.9 | 2.1 | 1.7ab | 0.8 | 2.7 | 0.7c | 0.4 | 1.4 | 1.0cd | 0.6 | 1.3 | 1.8ab | 1.3 | 2.8 | 2.3b | 1.8 | 3.1 | 0.9c | 0.2 | 1.8 | 0.9c | 0.4 | 1.7 |
| LT_1 | 20.3a | 17.1 | 24.5 | 21.1ab | 18.7 | 25.0 | 35.0c | 32.8 | 36.2 | 33.2d | 31.8 | 35.3 | 22.8e | 19.5 | 25.4 | 22.0be | 19.5 | 25.1 | 36.5cf | 33.8 | 39.0 | 37.2f | 35.7 | 38.4 |
| LT_3 | 21.7a | 18.1 | 24.4 | 21.9a | 20.0 | 24.3 | 37.0b | 35.7 | 39.3 | 35.9b | 32.9 | 37.9 | 22.5a | 19.1 | 25.8 | 22.6a | 19.7 | 25.3 | 37.1b | 35.5 | 38.6 | 37.0b | 35.5 | 38.9 |
| LT_7 | 23.0a | 17.6 | 24.4 | 21.4a | 18.3 | 23.4 | 33.2b | 27.6 | 38.9 | 34.6bc | 32.2 | 37.5 | 22.3a | 18.8 | 25.1 | 21.8a | 18.4 | 24.8 | 36.4cd | 32.9 | 39.7 | 37.2d | 33.5 | 39.4 |
| RWC_1 | 90.3a | 87.2 | 93.3 | 83.2abc | 65.8 | 96.4 | 81.6abc | 55.3 | 91.2 | 78.3bc | 67.4 | 100.0 | 87.1ab | 75.7 | 93.6 | 85.1ab | 70.2 | 96.2 | 74.2c | 55.4 | 92.2 | 79.2bc | 53.8 | 94.7 |
| RWC_3 | 88.5a | 76.1 | 93.6 | 87.5ab | 75.1 | 97.3 | 76.2bcd | 49.9 | 88.4 | 80.5abc | 70.5 | 87.3 | 79.8abc | 63.1 | 90.8 | 78.3abcd | 65.5 | 93.9 | 67.3d | 54.8 | 83.1 | 72.9cd | 61.0 | 82.2 |
| RWC_7 | 82.9a | 77.7 | 90.2 | 79.4ab | 69.9 | 90.4 | 70.4bd | 63.2 | 77.4 | 74.4ab | 63.8 | 80.2 | 76.7ab | 58.7 | 85.2 | 75.6ab | 58.0 | 87.7 | 60.8cd | 43.1 | 70.5 | 59.4c | 50.8 | 69.3 |
| Fv/Fm_1 | 0.836a | 0.813 | 0.863 | 0.835a | 0.814 | 0.866 | 0.795b | 0.788 | 0.801 | 0.784bc | 0.771 | 0.792 | 0.835a | 0.812 | 0.862 | 0.826a | 0.806 | 0.845 | 0.785bc | 0.751 | 0.804 | 0.775c | 0.767 | 0.785 |
| Fv/Fm_3 | 0.820a | 0.760 | 0.838 | 0.816ab | 0.799 | 0.821 | 0.789bc | 0.720 | 0.829 | 0.770c | 0.747 | 0.785 | 0.829a | 0.806 | 0.839 | 0.811ab | 0.795 | 0.822 | 0.791bc | 0.724 | 0.834 | 0.732d | 0.676 | 0.765 |
| Fv/Fm_7 | 0.829bc | 0.818 | 0.846 | 0.803bc | 0.765 | 0.820 | 0.782abc | 0.716 | 0.809 | 0.728a | 0.566 | 0.782 | 0.834c | 0.823 | 0.863 | 0.815bc | 0.806 | 0.832 | 0.761ab | 0.617 | 0.810 | 0.564d | 0.322 | 0.722 |
| PI_1 | 3.3a | 2.1 | 5.2 | 3.5a | 2.7 | 4.8 | 1.9b | 1.5 | 2.3 | 2.3bc | 1.9 | 2.7 | 3.0ac | 1.0 | 4.4 | 3.4a | 2.6 | 4.2 | 2.0b | 1.5 | 2.9 | 2.1b | 1.7 | 2.6 |
| PI_3 | 2.5a | 1.9 | 2.9 | 3.1a | 2.6 | 3.5 | 1.8b | 0.6 | 3.3 | 1.8b | 1.2 | 2.3 | 2.9a | 2.1 | 3.7 | 3.0a | 2.2 | 3.8 | 1.8b | 0.7 | 3.5 | 1.0c | 0.5 | 1.4 |
| PI_7 | 3.0a | 2.2 | 3.7 | 2.8a | 2.2 | 3.4 | 1.6b | 0.6 | 2.4 | 1.4b | 0.3 | 1.9 | 3.6c | 2.8 | 4.8 | 3.2ac | 2.9 | 3.8 | 1.1b | 0.1 | 1.8 | 0.3d | 0.0 | 0.8 |
Under drought, GY was positively correlated to BM (0.70) and PH (0.56), while under heat and combination treatments GY correlated with BM (0.57 and 0.46, respectively), but not with PH (Supplementary Table S3). Under heat alone, GY correlated negatively with AS (-0.54) and positively with TKW (0.58). Different yield components were thus correlated to GY under the different treatments.
WUE, RWC, maximum quantum efficiency of photosystem II (Fv/Fm), PI, and leaf temperature (LT) were significantly affected by heat and combination treatments, but not by drought (Table 2). WUE dropped from 1.6 and 1.7 under control to 0.7 and 1.0 under heat and 0.9 and 0.9 under combination treatments in Arta and Keel, respectively. In addition, at 3 and 7 days after start of the treatment, Arta and Keel plants had significantly lower RWC due to the combination treatment (Table 2). The integrated chlorophyll fluorescence measurement PI was significantly reduced under heat and combination treatments in both genotypes at all analysed time points. In contrast, Fv/Fm was only significantly reduced in Keel under heat and combination treatments at all three time points analysed. LT (as opposed to the ambient temperature) was significantly increased by drought 1 day after treatment start in Arta plants (22.8 °C) compared to well-watered controls (20.3 °C), but no significant differences in LT were detected between drought-treated and well-watered Keel plants (Table 2). LT was significantly higher in Arta and Keel plants grown under heat than plants grown under control temperatures, with mean values ranging from 33.2 to 37.0 °C and from 20.3 to 23.0 °C, respectively. After 7 days of heat treatment, both Arta and Keel plants experienced higher LT when additionally treated with drought (36.4 and 37.2 °C, respectively) as compared to the heat treatment alone (33.2 and 34.6 °C, respectively).
The experiment revealed the strongest genetic variation for the traits PH (24%), AS (17%), and Fv/Fm (15%) (Supplementary Table S2). Arta showed a significantly stronger reduction in PH under drought than Keel. In addition, heat resulted in a significantly higher amount of AS in Arta than in Keel (Table 2). Finally, in Keel, the combination treatment resulted in a significantly lower Fv/Fm and PI as compared to the heat treatment alone and as compared to the Fv/Fm and PI under the combination treatment in Arta. The two genotypes thus showed different physiological responses to the heat and combination treatments.
Characterization and quantification of the barley leaf proteome
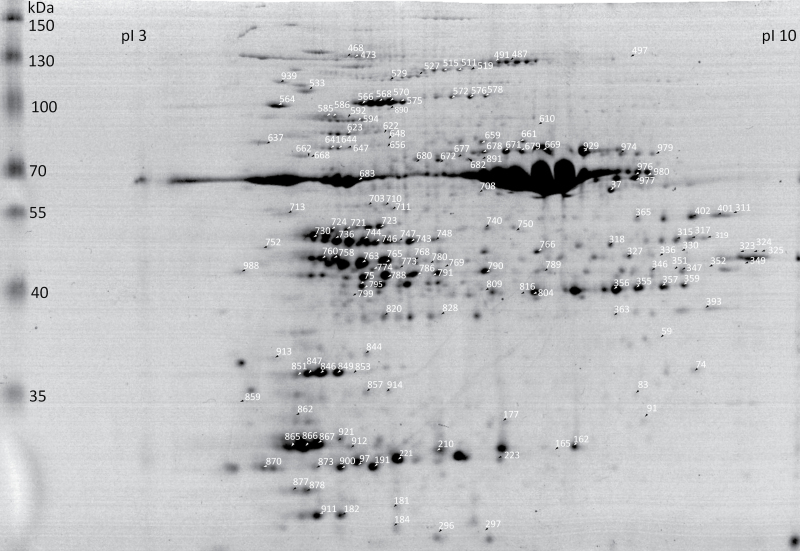
Spot detection on Coomassie-stained gels (Fig. 2) revealed a total of 525 distinct spots that were submitted for identification by peptide mass finger printing and subsequent peptide fragmentation analysis. Mascot search against the Uniprot database for H. vulgare or the DFCI Barley Gene Index database revealed the identity of 296 proteins from a total of 525 spots. The 296 identified spots were matched to a total of 145 unique accessions. Spots identified with the same protein accession were considered to be isoforms. Sixty-two of the proteins identified in the barley leaf proteome had isoforms present.
Fig. 2.
Representative Coomassie-stained 12% SDS-PAGE containing 400 μg total leaf protein. Numbered arrows indicate spots that were identified by MS and significantly regulated between conditions or between genotypes (Supplementary Table S4).
Using a singular enrichment analysis with gene ontology terms and the UniProt database, over half of the identified proteins were present in intracellular compartments (59%) and the cytoplasm (52%), as well as in organelles (51%) and were significantly enriched compared to the background (22, 9, and 15%, respectively; Supplementary Fig. S1). The largest fold enrichment was for the term ‘plastid’, which was present in 45% of leaf proteins and in less than 2% of background proteins.
To quantify differences in protein accumulation due to heat and drought treatments, the barley leaf proteome of samples harvested three days after treatment start was visualized using DIGE, as seen in a representative image in Supplementary Fig. S2. Samples from this time point were chosen based on the RWC and PI measurements; 3 days after treatment start was the first time point where the combination treatment resulted in significant changes in RWC and PI in both genotypes compared to controls. Spot detection on a composite fluorescent image comprising all superimposed gel images resolved 1005 distinct spots. Based on DIGE, this study identified 305 spots significantly differentially regulated by the heat treatment, 473 spots different between genotypes, and 35 spots different due to the interaction between temperature and genotype. However, no spots were found to be significantly regulated by the drought treatment. Of the 305 spots found to be differentially regulated by heat, 99 were identified via mass spectrometry (32 downregulated, 67 upregulated). Of the 473 spots found to be differentially regulated between genotypes, 125 were identified (90 downregulated in Keel, 35 downregulated in Arta). Additionally, mass spectrometry helped identify 14 out of the 35 proteins controlled by a significant interaction effect of the genotype and heat treatment (Supplementary Figs. S3 and S4).
The biological functions of differentially regulated proteins included roles in metabolism, photosynthesis, transport, response to abiotic stimulus, and response to stress (Table 3 and Supplementary Table S4). The proteomic analysis revealed the upregulation of structural components of the photosystem, i.e. the chlorophyll a-b binding protein of LHCII type III (Lhcb3) (spot 870), chloroplast oxygen-evolving enhancer protein 1 (PsbO) (spots 846, 847, and 851) and oxygen-evolving enhancer protein 2 (PsbP) (spots 97 and 221). While the overall trend of genotype-specific protein regulation was to be downregulation in Keel, photosynthesis-associated proteins had the opposite trend; 14 out of the 20 photosynthetic proteins were detected to be upregulated in Keel. In addition, proteins involved in detoxification, energy metabolism, and protein biosynthesis were differentially regulated by heat and between genotypes. These involved, for example, the upregulation of glycolytic proteins under heat, i.e. fructose-bisphosphate aldolase (spot 788) and cytosolic glyceraldehyde-3-phosphate dehydrogenase (spots 327 and 330). In addition, chaperones (spots 564 and 939), proteases (spots 586 and 527), elongation factors (spots 533 and 723), and initiation factor 4A (spot 703 and 710) were upregulated under heat. A large number of proteins differentially regulated between genotypes had functions in photorespiration and these were primarily upregulated in Arta as compared to Keel irrespective of the treatment. The proteomic data thus revealed a large number of proteins differentially regulated between genotypes and between heat and control conditions and the majority of these proteins had functions in photosynthesis, energy metabolism, and detoxification.
Table 3.
Proteins discussed in the text which were differentially regulated by temperature (T), genotype (G), or an interaction effect (G×T)
Proteins quantified by DIGE, identified via mass spectrometry and grouped according to their biological function. Spot number (No.) in Fig. 2 is given in addition to the Uniprot protein name and accession number. Predicted molecular weight (MW), isoelectric point (pI), Mascot score (Score), and percentage sequence coverage (SC%) are based on Mascot searches. The regulation factors, the log2 fold change in protein expression, are given for plants grown at 36 °C over plants grown at 21 °C (36/21) across both genotypes, for Keel plants over Arta plants (K/A) across all treatments, for heat-treated Keel plants over heat-treated Arta plants (K36/A36) and for control Keel plants over control Arta plants (K21/A21). Regulation factors corresponding to significant (P < 0.05) changes in expression are underlined. The complete list of differentially regulated proteins can be found in Supplementary Table S3.
No. 
|
Protein name | UniRef100 | MW | pI | Score | SC% | SE | 36/21 | K/A | K36/A36 | K21/A21 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbohydrate metabolic process | |||||||||||
| 327 | Glyceraldehyde-3-phosphate dehydrogenase, cytosolic | P26517 | 122.3 | 7.0 | 154.6 | 2.4 | T | 1.29 | 1.02 | –1.18 | 1.30 |
| 330 | Glyceraldehyde-3-phosphate dehydrogenase, cytosolic | P26517 | 48.3 | 7.0 | 49.0 | 2.6 | T/G/G×T | 1.68 | –1.97 | –2.36 | –1.49 |
| 788 | Fructose- bisphosphatealdolase | F2ELD1 | 41.9 | 7.5 | 304.6 | 10.8 | T | 1.22 | 1.09 | 1.07 | 1.10 |
| Photosynthesis | |||||||||||
| 97 | Oxygen-evolving enhancer protein 2, chloroplastic | Q00434 | 96.0 | 6.9 | 44.9 | 0.9 | T/G | 1.46 | 2.14 | 2.10 | 2.21 |
| 221 | Oxygen-evolving enhancer protein 2, chloroplastic | Q00434 | 96.0 | 6.9 | 91.5 | 7.0 | T/G | 1.29 | 1.86 | 1.84 | 1.88 |
| 846 | Predicted protein | F2CRK1 | 34.4 | 5.6 | 306.6 | 11.6 | T/G | 1.29 | 1.80 | 1.82 | 1.78 |
| 847 | Chloroplast oxygen-evolving enhancer protein 1 | A5JV93 | 68.7 | 7.0 | 198.6 | 3.7 | T/G | 1.55 | 2.53 | 2.52 | 2.55 |
| 851 | Predicted protein | F2CRK1 | 34.4 | 5.6 | 199.0 | 11.6 | T/G | 1.63 | 2.39 | 2.28 | 2.58 |
| 870 | Chlorophyll a-b binding protein of LHCII type III, chloroplastic | P27523 | 91.6 | 7.0 | 108.6 | 1.2 | T/G | 1.75 | 1.98 | 2.05 | 1.86 |
| Response to abiotic stimulus | |||||||||||
| 586 | ATP-dependent zinc metalloprotease FTSH 1, chloroplastic | Q5Z974 | 80.0 | 6.9 | 74.2 | 9.2 | T | 2.69 | –1.29 | –1.37 | –1.12 |
| 527 | ATP-dependent Clp protease ATP-binding subunit clpA homologue CD4B, chloroplastic | P31542 | 173.3 | 6.9 | 124.0 | 7.9 | T/G | –2.07 | –1.76 | –2.35 | –1.55 |
| 564 | Chloroplast heat shock protein 70 | A4ZYQ0 | 134.0 | 7.0 | 170.2 | 1.8 | T | 1.85 | –1.10 | –1.21 | 1.08 |
| 939 | Heat-shock protein | Q43638 | 103.9 | 7.0 | 122.8 | 2.2 | T/G | 2.90 | –1.43 | –1.54 | –1.16 |
| Translation | |||||||||||
| 533 | Elongation factor EF-G | Q9SI75 | 61.3 | 6.9 | 64.2 | 16.0 | T/G/G×T | 2.41 | –1.45 | –1.52 | –1.29 |
| 703 | Eukaryotic initiation factor 4A | P41378 | 143.9 | 6.9 | 121.0 | 4.8 | T/G/G×T | 1.73 | –1.65 | –1.96 | –1.24 |
| 710 | Eukaryotic initiation factor 4A | P41378 | 68.3 | 7.0 | 74.4 | 2.0 | G | –1.11 | –1.56 | –1.86 | –1.34 |
| 723 | Elongation factor Tu | Q8W2C3 | 144.5 | 6.9 | 298.2 | 2.4 | G | –1.08 | –1.34 | –1.40 | –1.28 |
Discussion
Drought affected plant growth but not photosynthesis
The reductions in grain yield due to the drought and heat treatments, while of similar magnitude, were due to different changes in yield component traits. Drought had major effects on plant growth, notably biomass and spike number, while the heat stress primarily affected the generative organs of the plant, the number of aborted spikes, and kernel weight. In addition, while drought did not significantly impact photosynthetic efficiency, photosynthesis was significantly compromised under heat and under the combination treatment. Consequently, drought affected plant growth but not photosynthesis. The strong reduction in growth and simultaneous maintenance of photosynthesis under drought as seen in the present study confirm recent studies in Arabidopsis, which demonstrated that mild drought primarily affected plant growth, but had minor effects on the photosynthesis rate (Muller et al., 2011; Skirycz et al., 2011; Verelst et al., 2012). The results are thus consistent with the hypothesis that plants reduce their growth as a primary adaptation response to stress rather than as a secondary consequence of resource limitations (Muller et al., 2011). In particular, under drought, biomass was positively correlated with yield. Thus, under non-lethal stress, limiting growth reduction might provide a strategy to increase productivity under stress. In the present study, drought also reduced the number of spikes presumably through reducing tiller number. The reduction in spike number due to the inhibition of tillering is a known reaction to drought (El Soda et al., 2010), but its molecular basis is not established. However, drought-induced inhibition of tillering may share similar pathways to the inflorescence-induced inhibition of tillering. The repression of tillering that occurs during anthesis is thought to be due to a combination of auxin signalling and resource competition between the apical buds, which form new tillers, and the stem apex (Jewiss, 1972).
Analysis of leaf proteomes under drought did not reveal significant changes of protein abundance as compared to control conditions. This is in contrast to the large number of transcripts differentially regulated in barley plants subjected to drought at the generative stage as seen by Guo et al. (2009). Homeostasis of the proteome could imply that the plants had acclimated to the drought stress (Harb et al., 2010). Acclimation to drought on the physiological level in the present study was evident in the maintenance of photosynthesis and water status under drought. Differences in stress symptoms might also be due to differences in the severity and duration of the stress application as compared to other studies (Talamè et al., 2007). However, the present data may also indicate a higher stability of the proteome as compared to the transcriptome under drought stress. Interestingly, a recent study has shown that while 1222 transcripts were differentially regulated in Arabidopsis subjected to mild drought, 34 out of 2081 proteins were significantly changed with an average regulation factor of 1.5 (Baerenfaller et al., 2012). This suggests a higher stability of the proteome as compared to the transcriptome in response to environmental perturbation. These data suggest that barley adapted to non-lethal drought by avoidance mechanisms, in particular through the reduction of growth. The resistance strategy ensured homeostasis of the cell which was reflected in the maintenance of photosynthesis and stability of the proteome under drought.
Heat affected photosynthesis rates
The heat treatment caused significant changes in reproductive organs and affected TKW and spike abortion rates, while the vegetative traits biomass and plant height remained relatively unaffected. At the same time, heat caused a significant reduction of RWC and photosynthetic efficiency. The RWC was likely reduced by an increase in transpiration as seen in the reduced leaf temperature under heat as compared to the ambient temperature. As ambient temperature increases, plants attempt to cool themselves by opening stomata and increasing transpiration (Schulze et al., 1973). However, the RWC under heat was not significantly different from the RWC under drought conditions, while photosynthesis rates were significantly different between these two stresses. This suggested that photosynthesis was significantly reduced under heat not because of suboptimal water content in the cells but rather by a direct detrimental effect of heat on the photosynthetic apparatus. These findings are in line with previous observations that the optimum temperature for photosynthesis in barley is 20 °C and photosynthesis rates are decreased by more than 50% at 35 °C (Todd, 1982). The detrimental effect of heat on the photosynthetic apparatus was also indicated by the proteome data, which demonstrated an increase in the turnover of photosynthesis-related proteins under heat.
A reduction in Fv/Fm and PI in the leaves and senescence of the lower leaves (Fig. 1) indicated that the ability to photosynthesize was permanently decreased under heat. In addition, the PI was significantly reduced under heat which suggests that both the light-dependent and light-independent mechanisms, e.g. carbon fixation, were damaged or inhibited due to high temperature. Thus, the heat treatment had a detectable effect on photoinhibition and the availability of carbon dioxide was probably limited by stomata closure. Evidence of a reduction of the carbohydrate pool in heat-treated plants was also provided by the reduction in kernel weight and by the upregulation of glycolysis enzymes observed in the proteomic data.
Heat also reduced total grain yield by increasing the abortion rate of spikes. Floret fertility and grain setting, as measured by the number of aborted spikes, was reduced under the heat treatment. Reproductive growth is known to be more sensitive to heat stress than vegetative growth in barley. In particular, anthers are prone to growth inhibition and lose the ability to produce pollen when heat stressed (Oshino et al., 2007). Heat thus caused yield reductions primarily by affecting photosynthesis, carbon fixation, and floret fertility. Strong effects of the short-term heat treatment on physiology indicated that the homeostasis of the cells was disturbed.
As in stress-prone environments drought and heat often co-occur, this study compared the combined effects of drought and heat with that of the single stresses on plant performance. The changes in plant performance were greater under the combination treatment than under heat or drought alone (Table 2). The strongest effect of the combination treatment was observed for PI suggesting that photosynthesis was most sensitive to the simultaneous application of drought and heat, presumably because leaf cooling through transpiration was severely reduced by the limited water supply. Reduced transpiration and leaf cooling in the combination treatment was also suggested by the difference in leaf temperature between the heat (33–34 °C) and the combination treatment (36–37 °C) (Table 2). In addition, grain yield was significantly more reduced by the combination treatment than by the single stresses, presumably because drought and heat affected unique yield component traits such as spike number and spike fertility, which resulted in an overall reduced yield in the combination treatment.
Proteomic basis of morphological plasticity and physiological responses to heat stress
The proteome analysis identified significant differences in protein abundance only under heat, and not under drought. This reflects the strong effects of heat on plant physiology in contrast to the physiological homeostasis seen under drought. Under heat, a large number, 99 out of 296 detected proteins were differentially regulated, and these had predominantly functions in photosynthesis, detoxification, energy metabolism, and protein biosynthesis (Table 3 and Supplementary Table S4). In many cases, several spots were detected per protein. These may represent close homologues, which could not be resolved based on the mass spectrometric data, but may also be isoforms due to differential post-translational modifications (Röhrig et al., 2006). The high frequency of differentially regulated proteins with functions in photosynthesis may be explained by the enrichment of plastid proteins in the present leaf proteome analysis (Supplementary Fig. S2). The proteomic analysis revealed structural components of the light-harvesting complex (Lhcb3) and the oxygen-evolving complexes (PsbO and PsbP) as being significantly upregulated under the heat treatment. It is tempting to speculate that the upregulation of Lhcb3, PsbO, and PsbP might be due to the de novo synthesis of peptides that are en route to replace them and the lag in degradation of damaged proteins removed from the photosystem. The upregulation of Lhcb3, PsbO, and PsbP proteins was therefore likely due to their increased replacement after damage by heat as indicated by the chlorophyll fluorescence data. The reduction of the PI due to the heat treatment was indicative of inhibition of the light-independent reactions of photosynthesis. Rubisco, the enzyme catalysing the rate-limiting step of carbon fixation, is inhibited by side-products of photorespiration which stabilize the active site of Rubisco in a closed conformation (Pearce and Andrews, 2003). Interestingly, Rubisco activase B showed the highest upregulation under heat, while Rubisco activase A was downregulated under heat (Table 3). Rubisco activase frees the catalytic site of Rubisco from inhibitory sugar phosphates by forcing the active site into an open conformation (Portis et al., 2007). Rubisco activases have been characterized on the genomic level in barley (Rundle and Zielinski, 1991), wheat (Law and Crafts-Brandner, 2001), rice (Orysa sativa L.) (To et al., 1999), cotton (Gossypium hirsutum L.) (Salvucci et al., 2003), maize (Zea mays L.) (Ayala-Ochoa et al., 2004), and Arabidopsis (Werneke et al., 1989). Rubisco activase A with two different splice variants is present in all mentioned species and is known to be heat inactivated starting at 35 °C (Crafts-Brandner et al., 1997). In contrast, Rubisco activase B has only been detected in barley, wheat, maize, and cotton but not in rice or Arabidopsis. It has already been shown that Rubisco activase B is induced by heat on the transcript level in wheat (Wang et al., 2011) and on the protein level in cotton (Law et al., 2001). However, the thermostability of Rubisco activase B is currently untested. The upregulation of Rubisco activase B under heat treatment suggested a specific role for Rubisco activase B in maintaining the activity of Rubisco under high temperature conditions, possibly by being more thermostable than Rubisco activase A.
Interestingly, the proteomic data showed that several glycolytic proteins were upregulated under the heat treatment. The prime functions of glycolysis are to generate carbon skeletons, reductants, and ATP, which can confer a bioenergetic advantage that can extend the survival time of plant cells that have become ATP-depleted due to environmental stresses. Increased levels of GAPDH transcripts have been observed under environmental stress conditions, such as dehydration in Craterostigma plantagineum (Velasco et al., 1994), during heat shock in Arabidopsis plants (Yang et al., 1993), and during anaerobic stress in maize (Chang et al., 2000). Thus, there is supporting evidence for the activation of ATP-generating pathways under different stresses, presumably to cope with a higher demand for ATP to maintain homeostasis under stress conditions. A higher energy demand was also suggested by the upregulation of ATP synthase subunit alpha in the mitochondria and plastids upon heat stress (Table 3). For example, higher transcript levels of ATP synthase were observed in rice upon salt and osmotic stress (Zhang et al., 2006). A higher energy was probably required for a higher rate of protein degradation and biosynthesis as suggested by the upregulation of chaperones, proteases, elongation factors, and initiation factor 4A. Analysis of the proteome of heat-stressed barley plants thus suggested thermolability and a more rapid protein turnover of photosynthesis-related proteins, while drought did not affect cellular homeostasis.
Barley genotypes Arta and Keel responded uniquely to heat and drought treatments
The identification of genetic differences in stress responses is an important basis for improving plant performance under stress. The differences in responses to drought and heat, in particular in growth, spike fertility, and chlorophyll fluorescence seen between Arta and Keel, suggested that the two genotypes have unique mechanisms for coping with environmental stresses. Arta was characterized by a stronger growth reduction under drought than Keel. Under heat, Arta showed a higher spike abortion rate as compared to Keel. Finally, under the combination treatment Arta had a higher Fv/Fm than Keel. Genetic variation in the efficiency of photosynthesis under stress has already been detected in diverse barley genotypes (Oukarroum et al., 2007; Ahmed et al., 2013). Interestingly, Keel tended to show higher yield under drought and heat alone, while Arta tended to show a higher yield under the combination treatment. Differences in growth and yield under the different stress regimes suggested that Keel is better adapted to mild stress as it maintains growth and yield. In contrast, under more severe stress as represented by the combination treatment Arta performed better presumably through a stronger morphological adaptation and maintenance of photosynthesis. The genetic difference was supported by the high number of proteins differentially regulated between the genotypes. Interestingly, a large number of these differential proteins had roles in photorespiration, and these were primarily downregulated in Keel. Especially under stress conditions that lead to reduced rates of photosynthetic CO2 assimilation, photorespiration serves as energy sink preventing the over-reduction of the photosynthetic electron transport chain and photoinhibition. Differences in the efficiency of photorespiration between Arta and Keel may explain the difference in photosynthetic performance under the combination stress. This study also observed 14 protein spots regulated by interaction effects between genotype and environment which are thus potential outputs of unique adaptations to heat stress that have evolved between Arta and Keel (Supplementary Figs. S3 and S4). Arta and Keel are parents of a recombinant inbred population, which shows transgressive segregation for agronomic performance under drought in Mediterranean environments (J. Rollins, B. Drosse M. A. Mulki, S. Grando, M. Baum, M. Singh, S. Ceccarelli, M. von Korff, in revision) unpublished results). Identification of the unique stress adaptation strategies in Arta and Keel will allow further dissection of the genetic basis of this transgressive performance in the offspring.
Conclusion
The data presented in this study suggested that barley has adapted to non-lethal drought by avoidance mechanisms, such as the reduction of growth which allowed the plants to maintain a cellular homeostasis as seen in the stability of photosynthesis and of the proteome under drought. In contrast, heat affected RWC, photosynthesis, and floret fertility and caused a rapid turnover of photosynthesis-related proteins. Based on the protein changes observed, it is proposed that important heat-tolerance mechanisms include protein quality control and de novo synthesis, which cause a higher energy demand as seen in the upregulation of ATP generating pathways. Reduced CO2 availability due to stomatal closure under heat was counter-balanced by the activation of Rubisco by Rubisco activase B. Inhibition of the light-independent reactions of photosynthesis likely caused the production of radical oxygen species as seen in the increase of scavenging and detoxifying enzymes. Genetic variation in stress responses between Arta and Keel, in particular in growth, spike fertility, and photosynthesis can be exploited in future crop breeding efforts.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Materials and Methods
Supplementary Table S1. Dye swap setup of the DIGE experiment using Cy2, Cy3, and Cy5.
Supplementary Table S2. Summary of the four-way analysis of variance for the traits measured in Arta and Keel genotypes under the two soil water content treatments at 21 or 36 °C.
Supplementary Table S3. Spearman’s rank correlation coefficients for phenotypic traits measured in both genotypes under control, drought conditions, under heat, and combination conditions.
Supplementary Table S4. All proteins differentially regulated due to temperature, genotype, or an interaction effect of the two as quantified by DIGE and identified via mass spectrometry
Supplementary Fig. S1. Singular enrichment analysis of cellular component gene ontology terms present in the barley leaf proteome as identified by mass spectrometry compared to all terms present in Uniprot entries for Hordeum vulgare.
Supplementary Fig. S2. Representative fluorescent image of a 2D-DIGE gel containing leaf total protein labelled with Cy3.
Supplementary Fig. S3. Spot intensities of proteins with genotype by temperature interaction effects where the spot intensity is not significantly different between genotypes under control conditions.
Supplementary Fig. S4. Spot intensities of proteins with genotype by temperature interaction effects.
Acknowledgements
This work was supported by the Max Planck Society and by a grant from the German Plant Genome Research Initiative of the Federal Ministry of Education and Research to MvK and SET.
References
- Ahmed IM, Dai H, Zheng W, Cao F, Zhang G, Sun D, Wu F. 2013. Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiology and Biochemistry 63, 49–60 [DOI] [PubMed] [Google Scholar]
- Ayala-Ochoa A, Vargas-Suarez M, Loza-Tavera H, Leon P, Jimenez-Garcia LF, Sanchez-de-Jimenez E. 2004. In maize, two distinct ribulose 1,5-bisphosphate carboxylase/oxygenase activase transcripts have different day/night patterns of expression. Biochimie 86, 439–449 [DOI] [PubMed] [Google Scholar]
- Baerenfaller K, Massonnet C, Walsh S, et al. 2012. Systems-based analysis of Arabidopsis leaf growth reveals adaptation to water deficit. Molecular Systems Biology 8, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D, Sunkar R. 2005. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences 24, 23–58 [Google Scholar]
- Baum M, von Korff M, Guo P, Lakew B, Udupa SM, Sayed H, Choumane W, Grando S, Ceccarelli S. 2007. Molecular approaches and breeding strategies for drought tolerance in barley. In: Varshney R, Tuberosa r, eds. Genomic assisted crop improvement: vol. 2: genomics applications in crops. The Netherlands: Springer, 51–79 [Google Scholar]
- Cascardo JCM, Buzeli RA, Almeida RS, Otoni WC, Fontes EP. 2001. Differential expression of the soybean BiP gene family. Plant Science 160, 273–281 [DOI] [PubMed] [Google Scholar]
- Chang WWP, Huang L, Shen M, Webster C, Burlingame AL, Roberts JKM. 2000. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiology 122, 295–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman EA. 1947. A laboratory procedure for determining the field capacity of soils. Soil Science 63, 227–284 [Google Scholar]
- Coventry SJ, Baum M, Sayed H, Grando S, Ceccarelli S, Barr AR, Eglinton JK. 2004. The genetic basis of adaptation to low rainfall environments in Australia. In: Proceedings of the 9th International Barley Genetics Symposium, Brno, Czech Republic, 920–926 [Google Scholar]
- Crafts-Brandner SJ, van de Loo FJ, Salvucci ME. 1997. The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiology 114, 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craufurd PQ, Flower DJ, Peacock JM. 2008. Effect of heat and drought stress on sorghum (Sorghum bicolor). I. Panicle development and leaf appearance. Experimental Agriculture 29, 61 [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z. 2010. AgriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Research 38, W64–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Soda M, Nadakuduti SS, Pillen K, Uptmoor R. 2010. Stability parameter and genotype mean estimates for drought stress effects on root and shoot growth of wild barley pre-introgression lines. Molecular Breeding 26, 583–593 [Google Scholar]
- Finnie C, Bak-Jensen KS, Laugesen S, Roepstorff P, Svensson B. 2006. Differential appearance of isoforms and cultivar variation in protein temporal profiles revealed in the maturing barley grain proteome. Plant Science 170, 808–821 [Google Scholar]
- Gao L, Yan X, Li X, Guo G, Hu Y, Ma W, Yan Y. 2011. Proteome analysis of wheat leaf under salt stress by two-dimensional difference gel electrophoresis (2D-DIGE). Phytochemistry 72, 1180–1191 [DOI] [PubMed] [Google Scholar]
- Gobom J, Schuerenberg M, Mueller M, Theiss D, Lehrach H, Nordhoff E. 2001. Alpha-cyano-4-hydroxycinnamic acid affinity sample preparation. A protocol for MALDI-MS peptide analysis in proteomics. Analytical Chemistry 73, 434–438 [DOI] [PubMed] [Google Scholar]
- Guo P, Baum M, Grando S, Ceccarelli S, Bai G, Li R, von Korff M, Varshney RK, Graner A, Valkoun V. 2009. Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. Journal of Experimental Botany 60, 3531–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MMR, Pereira A. 2010. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiology 154, 1254–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewiss OR. 1972. Tillering in grasses – its significance and control. Grass and Forage Science 27, 65–82 [Google Scholar]
- Law RD, Crafts-Brandner SJ. 2001. High temperature stress increases the expression of wheat leaf ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein. Archives of Biochemistry and Biophysics 386, 261–267 [DOI] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ, Salvucci ME. 2001. Heat stress induces the synthesis of a new form of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in cotton leaves. Planta 214, 117–125 [DOI] [PubMed] [Google Scholar]
- March TJ, Richter D, Colby T, Harzen A, Schmidt J, Pillen K. 2012. Identification of proteins associated with malting quality in a subset of wild barley introgression lines. Proteomics 12, 2843–2851 [DOI] [PubMed] [Google Scholar]
- McCarthy F, Wang N, Magee GB, et al. 2006. AgBase: a functional genomics resource for agriculture. BMC Genomics 7, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. 2006. Abiotic stress, the field environment and stress combination. Trends in Plant Science 11, 15–19 [DOI] [PubMed] [Google Scholar]
- Muller B, Pantin F, Génard M, Turc O, Freixes S, Piques MC, Gibon Y. 2011. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. Journal of Experimental Botany 62, 1715–1729 [DOI] [PubMed] [Google Scholar]
- Oshino T, Abiko M, Saito R, Ichiishi E, Endo M, Kawagishi-Kobayashi M, Higashitani A. 2007. Premature progression of anther early developmental programs accompanied by comprehensive alterations in transcription during high-temperature injury in barley plants. Molecular Genetics and Genomics 278, 31–42 [DOI] [PubMed] [Google Scholar]
- Oukarroum A, Madidi SE, Schansker G, Strasser RJ. 2007. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environmental and Experimental Botany 60, 438–446 [Google Scholar]
- Pearce FG, Andrews TJ. 2003. The relationship between side reactions and slow inhibition of ribulose-bisphosphate carboxylase revealed by a loop 6 mutant of the tobacco enzyme. Journal of Biological Chemistry 278, 32526–32536 [DOI] [PubMed] [Google Scholar]
- Portis AR, Li C, Wang D, Salvucci ME. 2007. Regulation of Rubisco activase and its interaction with Rubisco. Journal of Experimental Botany 59, 1597–1604 [DOI] [PubMed] [Google Scholar]
- Prasad PVV, Pisipati SR, Momcilovic I, Ristic Z. 2011. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. Journal of Agronomy and Crop Science 197, 430–441 [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. 2004. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology 134, 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrig H, Schmidt J, Colby T, Bräutigam A, Hufnagel P, Böhm N, Bartels D. 2006. Desiccation of the resurrection plant Craterostigma plantagineum induces dynamic changes in protein phosphorylation. Plant, Cell and Environment 29, 1606–1615 [DOI] [PubMed] [Google Scholar]
- Rundle SJ, Zielinski RE. 1991. Organization and expression of two tandemly oriented genes encoding Ribulose bisphosphate carboxylase/oxygenase activase in barley. Journal of Biological Chemistry 266, 4677–4685 [PubMed] [Google Scholar]
- Salvucci ME, van de Loo FJ, Stecher D. 2003. Two isoforms of Rubisco activase in cotton, the products of separate genes not alternative splicing. Planta 216, 736–744 [DOI] [PubMed] [Google Scholar]
- Savin R, Nicolas M. 1996. Effects of short periods of drought and high temperature on grain growth and starch accumulation of two malting barley cultivars. Australian Journal of Plant Physiology 23, 201 [Google Scholar]
- Schulze ED, Lange OL, Kappen L, Buschbom U, Evenari M. 1973. Stomatal responses to changes in temperature at increasing water stress. Planta 110, 29–42 [DOI] [PubMed] [Google Scholar]
- Skirycz A, Vandenbroucke K, Clauw P, et al. 2011. Survival and growth of Arabidopsis plants given limited water are not equal. Nature Biotechnology 29, 212–214 [DOI] [PubMed] [Google Scholar]
- Shakhatreh Y, Haddad N, Alrababah M, Grando S, Ceccarelli S. 2010. Phenotypic diversity in wild barley(Hordeum vulgare L. ssp. spontaneum(C. Koch) Thell.) accessions collected in Jordan. Genetic Resources and Crop Evolution 57 (1), 131–146 [Google Scholar]
- Shakhatreh Y, Kafawin O, Ceccarelli S, Saoub H. 2001. Selection of barley lines for drought tolerance in low rainfall areas. Journal of Agronomy and Crop Science 186, 131–146 [Google Scholar]
- Shi S, Chen W, Sun W. 2011. Comparative proteomic analysis of the Arabidopsis cbl1 mutant in response to salt stress. Proteomics 11, 4712–4725 [DOI] [PubMed] [Google Scholar]
- Stylianou IM, Affourtit JP, Shockley KR, Wilpan RY, Abdi FA, Bhardwaj S, Rollins J, Churchill GA, Paigen B. 2008. Applying gene expression, proteomics and single-nucleotide polymorphism analysis for complex trait gene identification. Genetics 178, 1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süle A, Vanrobaeys F, Hajós GY, Van Beeumen J, Devreese B. 2004. Proteomic analysis of small heat shock protein isoforms in barley shoots. Phytochemistry 65, 1853–1863 [DOI] [PubMed] [Google Scholar]
- Talamè V, Ozturk NZ, Bohnert HJ, Tuberosa R. 2007. Barley transcript profiles under dehydration shock and drought stress treatments: a comparative analysis. Journal of Experimental Botany 58, 229–240 [DOI] [PubMed] [Google Scholar]
- The International Barley Sequencing Consortium 2012. A physical, genetic and functional sequence assembly of the barley genome. Nature 491, 711–716 [DOI] [PubMed] [Google Scholar]
- To KY, Suen DF, Chen SC. 1999. Molecular characterization of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in rice leaves. Planta 209, 66–76 [DOI] [PubMed] [Google Scholar]
- Todd GW. 1982. Photosynthesis and respiration of vegetative and reproductive parts of wheat and barley plants in response to increasing temperature. Proceedings of the Oklahoma Academy of Science 62, 57–62 [Google Scholar]
- Ugarte C, Calderini DF, Slafer GA. 2007. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crops Research 100, 240–248 [Google Scholar]
- Velasco R, Salamini F, Bartels D. 1994. Dehydration and ABA increase mRNA levels and enzyme activity of cytosolic GAPDH in the resurrection plant Craterostigma plantagineum . Plant Molecular Biology 26, 541–546 [DOI] [PubMed] [Google Scholar]
- Verelst W, Bertolini E, De Bodt S, Vandepoele K, Demeulenaere M, Pè ME, Inzé D. 2012. Molecular and physiological analysis of growth-limiting drought stress in Brachypodium distachyon leaves. Molecular Plant 6, 311–322 [DOI] [PubMed] [Google Scholar]
- von Korff M, Grando S, This D, Baum M, Ceccarelli S. 2008. Quantitative trait loci (QTL) associated with agronomic performance of barley under drought. Theoretical and Applied Genetics 117, 653–669 [DOI] [PubMed] [Google Scholar]
- von Korff M, Radovic S, Choumane W, Stamati K, Udupa S M, Grando S, Ceccarelli S, Mackay I, Powell W, Baum M, Morgante M. 2009. Asymmetric allele-specific expression in relation to developmental variation and drought stress in barley hybrids. Plant Journal 59, 14–26 [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14 [DOI] [PubMed] [Google Scholar]
- Wang X, Cai J, Jiang D, Liu F, Dai T, Cao W. 2011. Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. Journal of Plant Physiology 168, 585–593 [DOI] [PubMed] [Google Scholar]
- Werneke JM, Chatfield JM, Ogren WL. 1989. Alternative mRNA splicing generates the two ribulosebisphosphate carboxylase/oxygenase activase polypeptides in spinach and Arabidopsis . The Plant Cell 1, 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel K, Weidner A, Surabhi GK, Börner A, Mock HP. 2009. Salt stress-induced alterations in the root proteome of barley genotypes with contrasting response towards salinity. Journal of Experimental Botany 60, 3545–3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kwon HB, Peng HP, Shih MC. 1993. Stress responses and metabolic regulation of glyceraldehyde-3-phosphate dehydrogenase genes in Arabidopsis . Plant Physiology 101, 209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Takano T, Liu S. 2006. Identification of a mitochondrial ATP synthase small subunit gene (RMtATP6) expressed in response to salts and osmotic stresses in rice (Oryza sativa L.). Journal of Experimental Botany 57, 193–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.