Abstract
The AP2 domain class of transcription factors is a large family of genes with various roles in plant development and adaptation but with very little functional information in plants other than Arabidopsis. Here, the characterization of an EAR motif-containing transcription factor, SlERF36, from tomato that affects stomatal density, conductance, and photosynthesis is described. Heterologous expression of SlERF36 under the CaMV35S promoter in tobacco leads to a 25–35% reduction in stomatal density but without any effect on stomatal size or sensitivity. Reduction in stomatal density leads to a marked reduction in stomatal conductance (42–56%) as well as transpiration and is associated with reduced CO2 assimilation rates, reduction in growth, early flowering, and senescence. A prominent adaptive response of SlERF36 overexpressors is development of constitutively high non-photochemical quenching (NPQ) that might function as a protective measure to prevent damage from high excitation pressure. The high NPQ leads to markedly reduced light utilization and low electron transport rates even at low light intensities. Taken together, these data suggest that SlERF36 exerts a negative control over stomatal density and modulates photosynthesis and plant development through its direct or indirect effects.
Key words: ERF, flowering, non-photochemical quenching, photosynthesis, repressor, senescence, stomata, stomatal conductance, tobacco, tomato.
Introduction
Plant development is a tightly regulated process that is controlled by both internal and external cues. One of the major factors that govern growth and survival is the ability to respond and adapt to the changing environment during different times of the day and in different seasons of the year. This requires responding rapidly to both biotic and abiotic factors, through changes in gene expression and activating responses that bring about physiological changes. At the genetic level, plants have evolved several gene families that control their development and govern their interactions with the environment (Reichmann and Ratcliffe, 2000). One of these is the plant-specific AP2/ERF domain family of transcription factors that is characterized by the presence of a conserved 58–59 amino acid AP2/ERF domain with different DNA-binding specificities (Sharma et al. 2010). This domain, singly or in combination with other motifs, can activate or repress gene expression in response to developmental or environmental signals, such as heat, drought, cold, and flooding, or in response to pathogenic attack, often by mediating hormonal responses (Mizoi et al., 2012; Gutterson and Reuber, 2004). The AP2/ERF domain family is one of the largest transcription factor families with 122 members in Arabidopsis, 139 in rice, and 146 in tomato (Nakano et al., 2006; Pirrello et al., 2012). Although several members of the family in different plants have been characterized, the function of a large number of AP2/ERF members is still unknown especially outside Arabidopsis. Thus, the functional analysis of AP2/ERF family genes, particularly in target crops like cereals, legumes, and fruits remains a major challenge.
A subclass of the AP2 domain family is the class II type family that functions as dominant repressors of gene expression (Fujimoto et al., 2000; Ohta et al., 2001). These family members possess a C terminal motif L/FDLNL/FxP designated as the ERF-associated Amphiphilic Repression (EAR) motif that has been shown to be involved in repression not only in association with the AP2 domain but also in other transcription factors, such as C2H2 zinc finger proteins and AUX/IAA proteins, that lack the AP2 domain (Kagale et al., 2010; Kagale and Rozwadowski, 2011). In combination with the AP2 domain, there are eight members each of the EAR motif containing genes in Arabidopsis and rice (Nakano et al., 2006) and at least seven in tomato (Sharma et al., 2010). Functional analysis of a few of these genes reveals diverse roles such as in wounding and ethylene response (e.g. AtERF4, Yang et al., 2005), ABA and salt stress signalling (e.g. AtERF7, Song et al., 2005; SlERF3, Pan et al., 2010), ABA-ethylene crosstalk (e.g. AtERF11, Li et al., 2011), cold and drought stresses (e.g. RAP2.1, Dong and Liu, 2010), and herbivory (OsERF3, Lu et al., 2011) Apart from Arabidopsis, the function of this family of genes remains poorly understood. This paper shows that ectopic expression of SlERF36, an EAR motif containing ERF gene, negatively regulates stomatal density and adversely affects several photosynthetic parameters in tobacco, leading to early flowering and senescence.
Materials and methods
Plant material and treatments
All studies were carried out on Solanum lycopersicum var. Ailsa Craig. Plants were grown in soil in glasshouse at 25–27 °C with a 16/8 light/dark photoperiod at a light intensity of about 400 μmol photons m−2 s−1.
For studies on expression in different tissues such as root, stem, leaves, and flowers, these were collected separately from 3-month-old plants, frozen in liquid nitrogen and kept at –70 °C until use for RNA isolation.
RNA isolation, cDNA preparation, and real-time PCR
RNA was isolated from different tissues as described by Asif et al. (2000). RNA was reverse transcribed using REVERTAID MMLV reverse transcriptase (Fermentas) with the 3ʹ-AP primer from Invitrogen (5ʹ-GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT-3ʹ). Two degenerate primers ERF-F1 (5ʹ-GTGGGGDAAATKSGYN KCKGARAT-3ʹ) and ERF-R1 (5ʹ-CTWAAAR SYGCBDYRTCGT ARGC-3ʹ that were designed based on an alignment of the AP2/ERF domain sequences of various ERF genes were used to amplify a fragment of 115 nt. This fragment showed sequence identity with a tomato BAC clone that was similar to the NtERF5. Based on this sequence, forward and reverse primers SlERF36-OF (5ʹ-ATGGATCCTATGAGAAGAGGCAGAGC-3ʹ) and SlERF36-OR (5ʹ-GAAACCAGGATCCTTCAAAGACATAG-3ʹ) were used to amplify the complete open reading frame of 666 nucleotides that encoded a protein of 221 amino acids (SGN-U313854). The fragment was cloned in the T/A cloning vector pTZ57R (Fermentas).
For real-time (RT) PCR analysis, primers SlERF36-FSQ (5ʹ-ACTCATCGTCTTCTGTTGTTGA-3ʹ) and SlERF36-RSQ (5ʹ-ATTAGCATAAACATCATCCATCG-3ʹ) were designed and used in combination with the internal control actin, amplified using the primers ActF1 (5ʹ-ATGACATGGAGAAGATCTGGCATCA-3ʹ) and ActR1 (5ʹ-AGCCTGGATGGCAACATACATAGC-3ʹ). The reactions were run using the Power SyBr Green PCR master mix on a ABI Prism 7000 real-time PCR machine (Applied Biosystems, USA). RNA was isolated from pooled tissue samples collected from several plants at specific stages mentioned above. Reactions were run in triplicates with cycling conditions as follows: 50 °C for 2min, 95 °C for 10min, and 40 cycles of 95 °C for 15 s and 60 °C for 1min. Values were calculated using the comparative Ct (2–ΔΔCt) method. For calculating relative change in expression, the control sample data was averaged and considered as 1 and averaged values of all other samples plotted against the control data.
Development of transgenic plants
The complete SlERF36 open reading frame was cloned in pBI121 at the BamHI site. Transgenic tobacco plants, (Nicotiana tabacum var. Petit Havana) were generated by Agrobacterium-mediated transformation of leaf disc explants as described by Horsch et al. (1988). Independent transformants, selected on kanamycin on agar, were transferred to soil for hardening and plants grown in a glasshouse maintained at 25 °C. Plants in the T1 generation were selected for phenotypic analysis while homozygous progeny of three independent lines, viz. SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4, in the T2 generation were chosen for comparative physiological analysis.
Physiological studies
For each experiment, homozygous progeny of three independent transgenic lines (five or six plants per line) were grown under natural light in pots as described. Leaf gas exchange and fluorescence were simultaneously measured with a GFS-3000 Portable Gas Exchange Fluorescence System attached with a fluorescence module (PAM-fluorometer 3055-FL, Walz, Germany) on a leaf (generally the fourth leaf from bottom in a 30-day-old plant or fifth leaf from bottom in a 45-day-old plant) enclosed in a temperature-, light-, and humidity-controlled leaf chamber 3010-S (Walz). All comparative measurements were made on plants of the same age (30–45-day-old plants) between 08.00 and 11.00. The leaves were dark adapted at vapour pressure deficit (VPD) ranging between 0.5 and1 kPa, leaf temperature of 25 °C, and CO2 concentration of 400 μmol mol–1, and Fv/Fm measurements were made using a light pulse of 2000 μmol photons m−2 s−1. The leaves were then exposed gradually to increasing photosynthetic photon flux density (PPFD) to 400 μmol photons m−2 s−1 and measurements of steady-state photosynthesis rate (A), stomatal conductance (gs), transpiration (E), internal CO2 concentration (Ci), and quantum yield of PSII in the light (ϕ) were made. Intrinsic water use efficiency was calculated as the A/E ratio.
Light response curves for photosynthesis were constructed using various PPFDs (ranging from 0 to 1200 μmol photons m−2 s−1) incident on the uppermost, fully expanded dark-adapted leaves (generally the fourth and fifth leaves from bottom) as described in Escalona et al. (1999). A/Ci curves were performed over a range of CO2 concentrations between 40 and 2500 μmol mol–1 on 3–5 plants per transgenic line as described in Escalona et al. (1999). VPD response curves for gas exchange parameters A, gs, and E were constructed by exposing the leaves to a range of VPD levels (0.5–3.0 kPa) at 400 μmol photons m−2 s−1 PPFD, leaf temperature of 25 °C and CO2 concentration 400 μmol mol–1. A, gs, and E were recorded simultaneously after steady-state conditions (generally after 10–20min) had been re-established at each VPD level. Light response curves for fluorescence (PSII) and P-700 (PSI) were measured with DUAL-PAM-100 (Walz) as described by Schreiber and Klughammer (2008). Maximal fluorescence and maximal P700 changes were obtained from dark adapted leaves (as described above) and then leaves were exposed to high light (i.e. 1200–1500 μmol photons m−2 s−1) for 30min to obtain a steady state before commencing measurement of several fluorescence parameters every 5min at each PPFD (ranging from 11–2000 μmol photons m−2 s−1). The quantum yield of PSI – Y(I) – is defined by the proportion of overall P700 that is reduced in a given state and not limited by the acceptor side. It is calculated from the complementary PSI quantum yields of non-photochemical energy dissipation due to donor-side limitation and acceptor-side limitation– Y(ND) and Y(NA) (Schreiber and Klughammer, 2008). The quantum yields of PSII, non-photochemical quenching, and non-light-induced non-photochemical fluorescence quenching – Y(II), Y(NPQ), and Y(NO) – were calculated from the measurement of chlorophyll fluorescence, as described by Kramer et al. (2004).
The electron transport rates ETRI and ETRII was calculated as ETR(I or II) = Y(I or II) × PPFD × 0.5 × abs, where Y is the apparent quantum yield, 0.5 is the proportion of absorbed light reaching PSI or PSII, and absI is absorbed irradiance, taken as 0.84 of incident irradiance. NPQ was calculated as (Fm – Fmʹ)/ Fmʹ. Chlorophyll content was measured by isolating chlorophyll from leaf discs and calculated according to Arnon (1949).
Estimation of stomatal density
Leaf epidermis (about 1cm2) from the abaxial surface of fully expanded leaves (fourth leaf from bottom of 45-day-old plants) was peeled off with a pair of forceps and placed immediately in water and later mounted in 10% glycerol and observed under a light microscope (Eclipse TE300 inverted microscope, Nikon). Stomata were counted in an area of 0.25mm2 in three different regions from three independent leaves of the same position from three independent lines.
Statistical analysis
The significance of correlations was tested by using linear regression, with P < 0.05 considered statistically significant. Means were compared by using one-way analysis of variance and post-hoc means comparison (Scheffé Test). All data analysis and plotting were performed with SigmaPlot version 8 0.
Results
The gene identified in these studies encoded a protein of 221 amino acids (Fig. 1) and was designated in a recent study of the tomato ERF family as SlERF36 by Sharma et al. (2010) and as SlERF.F.1 by Pirrello et al. (2012). The gene encoded an ERF-type protein based on the presence of alanine at position 14 and aspartate at position 19 of the AP2/ERF domain (Sakuma et al., 2002). In addition, there was a stretch of seven amino acids (FDLNFPP) at the C-terminal end that has been designated as an EAR motif (ERF-associated amphiphilic repression motif) and shown to be involved in repression of gene expression (Fujimoto et al., 2000; Ohta et al., 2001). As per the classification given by Fujimoto et al. (2000), SlERF36 belongs to group II of ERF transcription factors, members of which have been shown to act as repressors of transcription, while as per the classification of Nakano et al. (2006) it belongs to family VIII B1 (Sharma et al., 2010). BLAST analysis showed 73% amino acid identity with NtERF5 of tobacco while the closest Arabidopsis homologues were AtERF3 (58% amino acid identity) and AtERF7 (50% amino acid identity).
Fig. 1.

Amino acid sequence of the SlERF36 polypeptide. The AP2 domain is shown in bold and underlined. The EAR motif at the C- terminal end has been boxed.
Transcript accumulation during plant growth
As a first step towards the characterization of the function of S1ERF36, its expression pattern in different tissues was investigated using RT-PCR. Highest expression of S1ERF36 was observed in leaves and roots whereas its expression was low in stem and barely detectable in flowers (Supplementary Fig. S1, available at JXB online). Transcript accumulation was low and did not vary much during fruit development and ripening (data not shown and Pirrello et al., 2012).
SlERF36 overexpressors show early flowering and early senescence
Because of the large number of ERFs in plants and the possibility of redundancy, a functional analysis of SlERF36 was carried out through ectopic expression of the gene under the CaMV35S promoter in transgenic tobacco. Of the 12 transformants obtained, three designated as SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4 were selected for detailed study. RT-PCR with primers specific to tomato SlERF36 showed the presence of the transcript in all three transgenic lines (Supplementary Fig. S2). Plants in T1 (second) generation were grown on soil in a glasshouse and monitored for various visible growth parameters such as height, time to flowering, leaf shape and size, and leaf senescence throughout the growth period (Fig. 2A). In general, transgenic plants were slow growing and appeared shorter in height with leaves having a lighter shade of green (Supplementary Fig. S3) as compared to control untransformed plants, but there was no change in leaf shape and size between the control and transgenic plants.
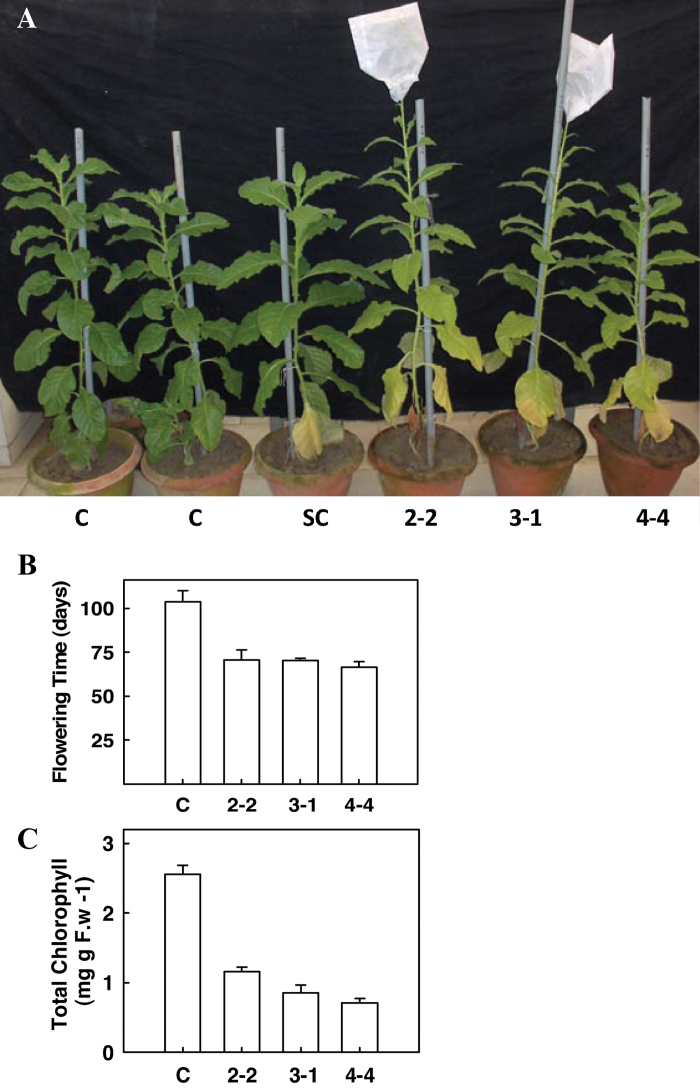
Fig. 2.
(A) The early flowering and early senescence phenotype of transgenic lines expressing SlERF36 under the CaMV35S promoter. Progeny of three independent transgenic lines SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4 in the T1 (second) generation at about 90 days are shown. Plants were grown in the glasshouse at 25–27 °C at 16/8 light-dark cycles. C = Control (Nicotiana tabacum var Petit Havana), SC = segregating control. SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4 are progeny of independent transgenic lines expressing SlERF36. (B) Graphical analysis of flowering time of control and transgenic tobacco plants expressing SlERF36. Values represent the average ± SD of 3–5 plants of each independent transformant. (C) Graphical analysis of total chlorophyll (6th leaf from bottom) of control and transgenic tobacco expressing plants SlERF36. Values represent the average ± SD of 5 leaves of each independent transformant (this figure is available in colour at JXB online).
Transgenic plants also showed early flowering with a difference of about one month between control and transgenic lines (Fig. 2B). While control plants began to flower at 103.25±6.9 days, inflorescence was initiated in transgenic plants in 70.75±5.61 (SlERF36-2-2), 70.25±1.25 (SlERF36-3-1), and 66.5±3.10 (SlERF36-4-4) days. Senescence of individual leaves in transgenic plants occurred earlier with early yellowing and death of the lower leaves as compared to corresponding control leaves (Fig. 2A). Chlorophyll levels of lower leaves (sixth leaf from bottom) were higher in controls (2.55±0.12mg (g FW)–1) as against transgenic leaves where they were 0.71±0.06 (SlERF36-4-4), 0.85±0.10 (SlERF36-3-1), and 1.15±0.06 (SlERF36-2-2) mg (g FW)–1 at the 3-month stage (Fig. 2C).
SlERF36 overexpressors show reduced photosynthesis, conductance, and transpiration
In order to understand the basis of altered growth and other phenotypes in SlERF36 overexpressors, gas exchange and fluorescence parameters were studied in 1-month-old homozygous plants grown under natural light. Interestingly, even at this stage, when visible differences between the control and transgenic plants were not too apparent, marked differences in photosynthetic parameters were observed (Table 1). Control plants showed a photosynthetic rate (A) of 4.97±0.93 μmol CO2 m–2 s–1. In contrast, progeny of transgenic lines SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4 had considerably reduced photosynthetic rates of 2.85±0.28, 1.79±0.19, and 2.34±0.54 μmol CO2 m–2 s–1, which represented a decrease of 43–64% compared to the control. Stomatal conductance (gs) also showed a decrease of 42–56% in the transgenic lines, with 84.2±5.67 mmol water m–2 s–1 in control lines as against 46.76±4.52, 37.45±4.2, and 49.04±9.53 mmol water m–2 s–1 for the progeny of SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4. Transpiration rates were reduced by 34–49% in progeny of the transgenic lines as against control. Water use efficiency (A/E) in the transgenic lines was also reduced to about 70–90% of the control.
Table 1.
Analysis of various gas exchange parameters in 1-month-old control and transgenic SlERF36 expressing tobacco plants grown under natural light
Fluorescence data was obtained simultaneously with gas exchange using the GFS-3000 system attached with fluorescence measurement head (3055-FL) which serves as light source for gas exchange and fluorescence measurements as well as quantum yield measurements. Electron transport rate and NPQ were calculated from Fm and apparent quantum yield (Y) measurements made on dark and light adapted leaves, respectively, as ETR = Y × PPFD × 0.5×0.84, where Y = apparent quantum yield, and NPQ = (Fm – Fmʹ)/Fmʹ. Values represent the average of six homozygous progeny plants of each independent line (2-2, 3-1, and 4-4). Parameters in bold are significantly different in at least two transgenic lines (P < 0.001). *P < 0.05, **P < 0.01**, ***P < 0.001.
| Parameter | Control | SIERF36-2-2 | SIERF36-3-1 | SIERF36-4-4 |
|---|---|---|---|---|
| Photosynthetic efficiency, A (μmol CO2 m–2 s–1) | 4.97±0.93 | 2.85±0.28*** | 1.79±0.19*** | 2.34±0.54*** |
| Stomatal conductance, gs (mmol H2O m–2 s–1) | 84.20±5.67 | 46.76±4.52*** | 37.45±4.2*** | 49.04±9.53*** |
| Transpiration rate, E (mmol H2O m–2 s–1) | 0.93±0.18 | 0.59±0.05*** | 0.48±0.04*** | 0.62±0.11*** |
| Water use efficiency (A/E) | 5.34±0.29 | 4.80±0.51* | 3.73±0.50*** | 3.78±0.26*** |
| Apparent quantum yield | 0.60±0.01 | 0.50±0.04 | 0.46±0.04* | 0.48±0.06* |
| Electron transport rate, ETR (μmol m–2 s–1) | 96.35±3.47 | 84.78±7.76 | 76.96±7.25* | 81.39±11.33* |
| Non-photochemical quenching, NPQ | 0.69±0.16 | 0.95±0.22 | 1.22±0.32*** | 1.34±0.15*** |
| Maximum quantum yield (Fv/Fm) | 0.835±0.001 | 0.82±0.01 | 0.84±0.004 | 0.84±0.05 |
In order to obtain an insight into the reasons for the reduced photosynthesis and transpiration, fluorescence kinetics and total yield were checked using the GFS system. No change was observed in the Fv/Fm ratios between the control and transgenic lines with Fv/Fm ratios of around 0.83–0.84 in all lines, suggesting that the function of reaction centres was not altered in transgenic plants relative to the control. However, the apparent quantum yield and electron transport rate of PSII was reduced in transgenic lines (Table 1). Interestingly, NPQ was substantially higher in progeny of all transgenic lines as compared to control plants.
SlERF36 overexpressors show defects in light utilization and CO2 assimilation capacities
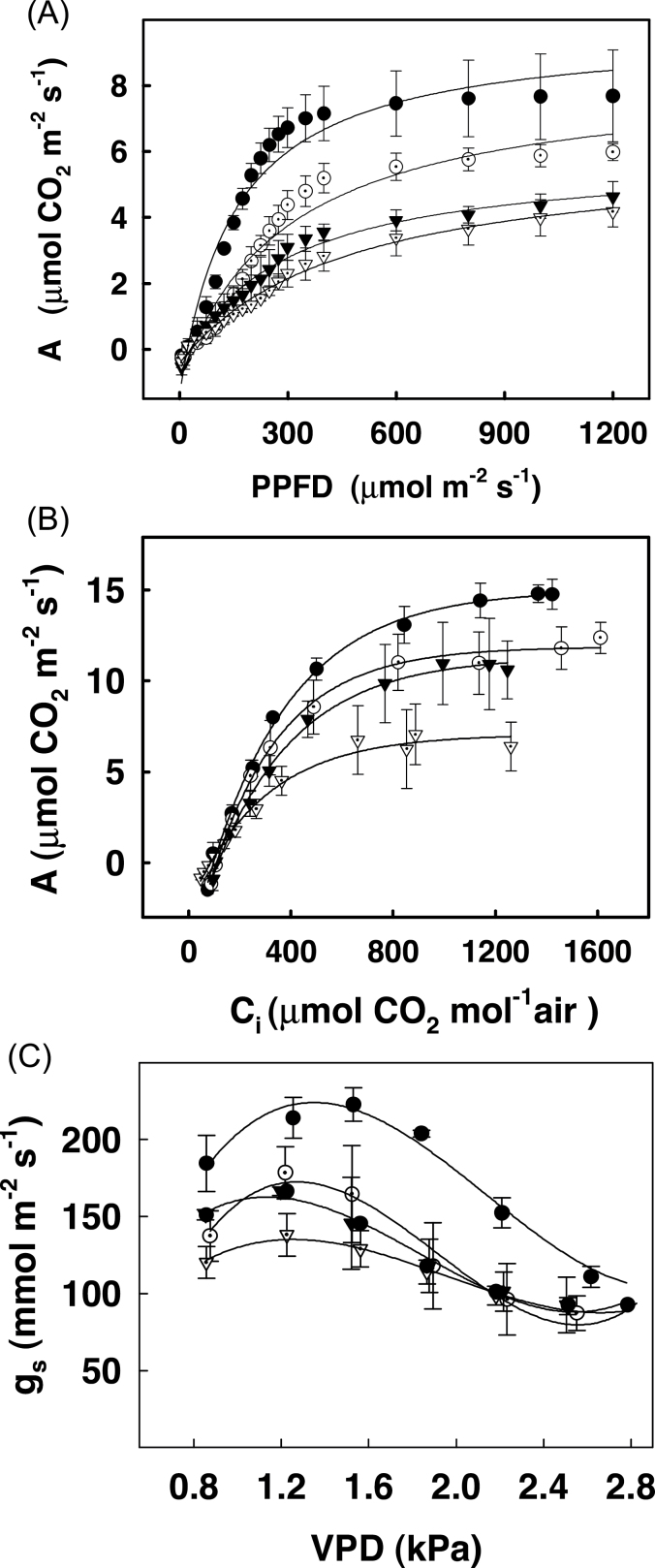
In order to test whether the ability of the transgenic plants to utilize light was selectively disturbed, measurements of PPFD response curves were carried in the transgenic plants. The photosynthetic rates at various light intensities (at 400 μmol CO2 mol–1) in transgenic lines showed considerable decrease from those of control plants right from 50 μmol m–2 s–1 onwards (Fig. 3A). As light intensity increased, there was an increase in photosynthetic rates in both the control and the transgenic lines, although rates of transgenic lines continued to remain much lower than those of control plants. Interestingly, the decrease in photosynthetic rates was more prominent at lower light intensities than at higher light intensities in transgenic lines compared to control plants. This indicated that the ability to utilize light optimally was markedly affected in the transgenic lines. There was, however, no change in the point at which light saturation occurred. In control as well as transgenic lines, saturation of photosynthesis occurred at a PPFD of about 450 μmol m–2 s–1. The photosynthesis rates were also calculated as a function of changing gs obtained from light response curves. As shown in Supplementary Fig. S4, there was a linear relationship between A and gs, although the rates of control plants were higher than in transgenic plants.
Fig. 3.
Response of net photosynthesis, A, to PPFD (3A) and to sub-stomatal CO2 concentrations Ci, (3B) and that of stomatal conductance gs to VPD (3C) in leaves of control (●), SIERF36–2-2 (O), SIERF36–3-1 (▼) and SIERF36–4-4 (▽) lines of tobacco. Measurements were carried out as described in material and methods. Values are average ± SEs of three to five replicates. Curves were adjusted to a hyperbolic function y=y o + a*x/(b + x) for 3A and 3B and to a non-linear regression for 3C using the equation gs=aVPD3+ bVPD2+cVPD+ d (r2 values ranging from 0.8–0.98).
Measurements of A/Ci response curves were also carried out in control and transgenic plants at 400 μmol light to test whether the ability to utilize CO2 was affected (Fig. 3B). Surprisingly, although increasing Ci in transgenic lines led to increased photosynthetic rates, these were considerably lower than those in control plants at any given point by 30–50%. The initial slope of A/Ci curve was similar in control and transgenic plants but the rates in transgenic plants were reduced at higher Ci (>200 μmol mol–1).
The sensitivity of stomata was also tested by measuring stomatal conductance at different vapour pressure deficits. As shown in Fig. 3C, an increase in VPD led to an increase in stomatal conductance up to 1.2 kPa in both control and transgenic lines followed by a decrease thereafter, indicating that the stomata in these lines retained their sensitivity to changes in VPD. The absolute values of gs were expectedly much lower in transgenic lines as compared to control.
SlERF36 overexpressors have reduced electron transport rate and high NPQ
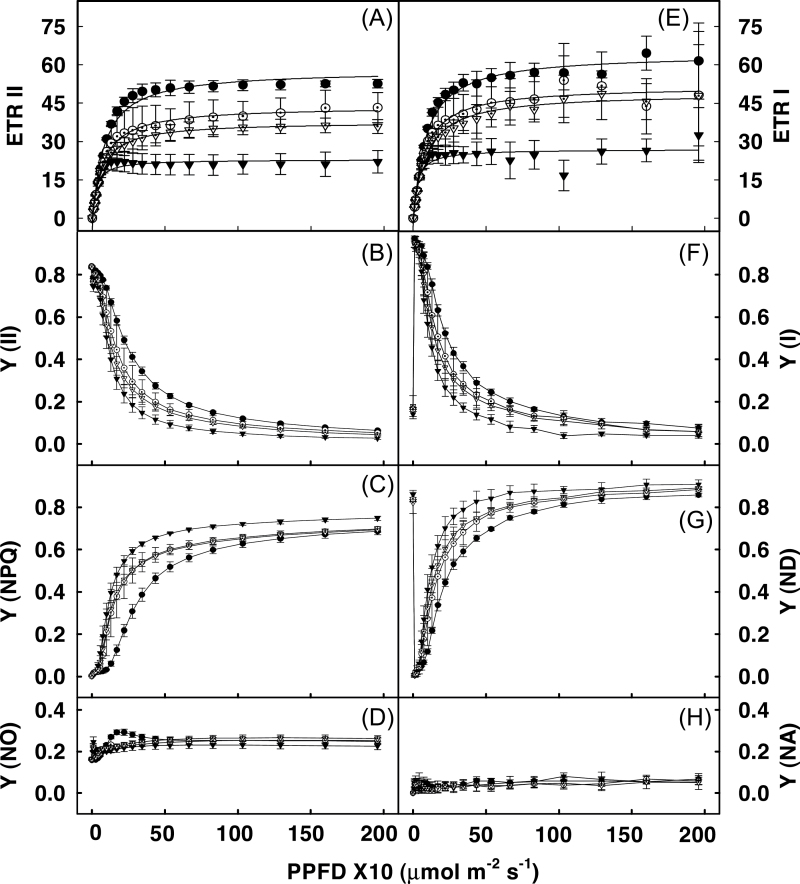
In order to obtain a more detailed insight into the processes affecting light and CO2 utilization in the transgenic lines, the limitation parameters of photosystems I and II were measured. Both ETR II (Fig. 4A) and ETR I (Fig. 4E) were considerably reduced in transgenic lines, showing a decrease of 30–50% over those in control lines. A further analysis of the decrease in the ETRs was performed by measuring three complementary quantum yields of energy conversion, namely Y(II), Y(NPQ), and Y(NO) for PSII and Y(I), Y(ND), and Y(NA) for PSI. As shown in Fig. 4B, Y(II) in all transgenic plants showed a considerable decrease particularly at light intensities less than 200 μmol m–2 s–1. This decrease in Y(II) appeared to be primarily due to an increase in NPQ (Fig. 4C) and not Y(NO) since latter remained almost constant in control and transgenic plants (Fig. 4D). Like Y(II), Y(I) was also decreased in transgenic plants (Fig. 4F). This decrease seemed to be due to marked donor side limitation of PSI (due to lower PSII ETR) as reflected by the elevated Y(ND), which was 30% higher in transgenic lines than in control leaves (Fig. 4G) while the corresponding Y(NA) was similar in both the control and the transgenics (Fig. 4H).
Fig. 4.
Light response curve for electron transport rate of PSII (ETRII) and PSI (ETRI) and their respective quantum yields for leaves of control (●), SIERF36–2-2 (O), SIERF36–3-1 (▼) and SIERF36–4-4 (▽) lines of tobacco. (A) electron transport rate for PSII, ETRII; (B) photochemical quantum yield for PSII, Y(II); (C) quantum yield of light-induced non-photochemical fluorescence quenching for PSII, Y(NPQ); (D) quantum yield of non-light-induced non-photochemical fluorescence quenching for PSII, Y(NO); (E) electron transport rate for PSI, ETRI; (F) photochemical quantum yield for PSI, Y(I); (G) Quantum yield of non-photochemical energy dissipation in PSI due to donor side limitation, Y(ND); (H) Quantum yield of non-photochemical energy dissipation in PSI due to acceptor side limitation Y(NA). Measurements were carried out as described in material and methods at light intensities ranging up to 2000. Values are average ± SEs of three to five replicates. Curves were adjusted to a hyperbolic function y=y o + a*x/(b + x) for A and E.
SlERF36 expression leads to reduced stomatal density in transgenic lines.
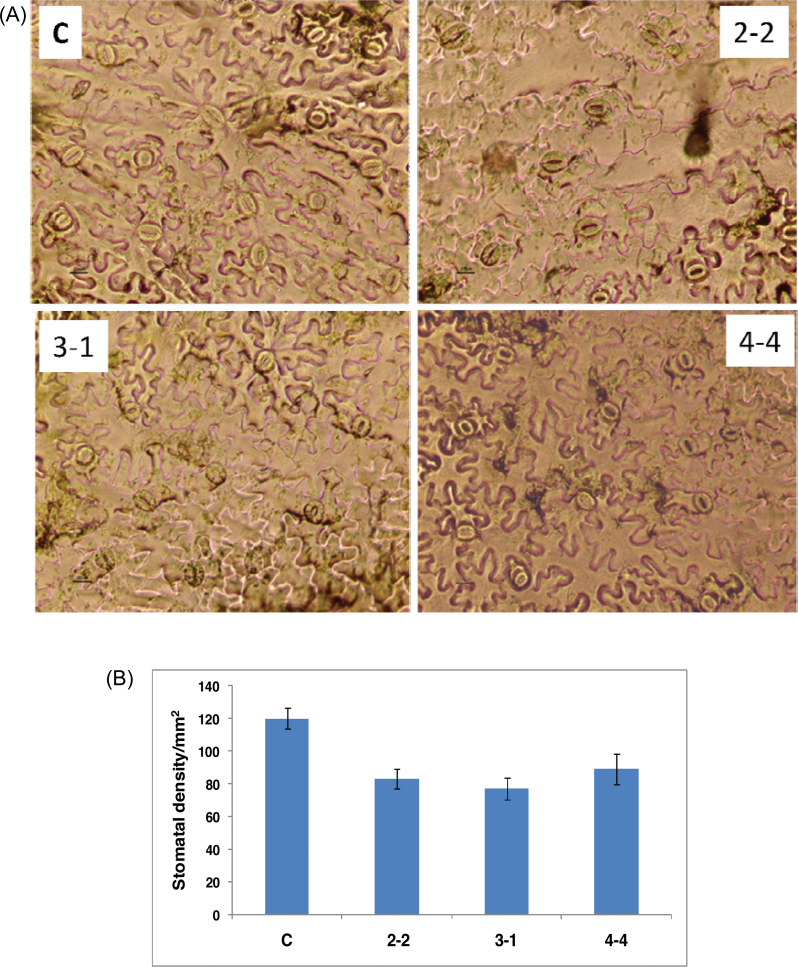
Since several photosynthetic parameters including stomatal conductance and light and CO2 utilization were grossly affected in transgenic lines, this study investigated whether there was a change in the stomatal density between the control and the transgenic lines by measuring the stomata in several leaves of the control and transgenic lines (three leaves/line at three different locations in all three transgenic lines). A considerable decrease in the stomatal density was observed in all the transgenic lines (Fig. 5A). While control leaves had about 120±6.5 stomata mm–2, the transgenic lines SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4 showed 83±6, 77±6.8 and 89±9.5 stomata mm–2 (Fig. 5B). This represented a decrease of 25–35% in the total abaxial stomatal density as compared to control plants. There was, however, no change in the stomatal size from that in the control plants.
Fig. 5.
(A) Leaf abaxial surface showing stomatal density in control (C) and transgenic SlERF36 expressing tobacco plants of three independent lines, SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4. Stomatal density from leaf epidermal peels was estimated in the leaf sections at three different regions of three different leaves (4th leaf from bottom) under a light microscope (Nikon Eclipse TE300 Inverted microscope). The small black bar at the base of each picture on the left hand side represents a length of 20 μm. (B) Graphical estimation of the stomatal density of the lower leaf epidermis of control (C) and transgenic SlERF36 expressing tobacco leaves of lines SlERF36-2-2, SlERF36-3-1, and SlERF36-4-4 shown in figure 5A. Values represent an average stomatal density ± SD in an area of 1mm2 of three independent leaves (from the same position) (this figure is available in colour at JXB online).
Discussion
The growth of plants and their adaptation to the environment is fine-tuned by the controlled expression of various transcriptional regulators that include transcription factors, co-activators, repressors, and other accessory proteins. SlERF36/SlERF.F.1 is a C-terminal EAR-motif-containing AP2/ERF domain repressor protein that seems to control plant development and photosynthesis either by negatively regulating stomatal density and affecting stomatal conductance or through an independent effect on density.
One of the major effects of SlERF36 expression in tobacco is a reduction of 25–35% in stomatal density (but without a change in size or sensitivity) in progeny of several independent transgenic lines. This is accompanied with a drastic effect on specific photosynthesis parameters that include a 42–56% decrease in stomatal conductance and transpiration, a lower rate of photosynthesis, decreased water use efficiency, lower rates of electron transport, and decreased quantum yield, leading finally to the onset of early flowering and senescence. While the decrease in density and conductance/transpiration are related and could in principle affect ETR and photosynthesis, they could also in turn be affected by the decrease in photosynthesis or be entirely independent effects from a direct or indirect action of SlERF36. Stomatal density is under the control of endogenous developmental cues but is also affected by changes in light intensity, CO2 concentration, humidity, and water availability. Plants respond to changing CO2 levels with a change in stomatal aperture as a short-term adaptation and with a change in both stomatal density and size over a longer timescale (Woodward, 1987; Woodward and Kelly, 1995). This adaptation mainly consists of a reduction in stomatal density in response to higher CO2 levels (Woodward et al., 2002; Hetherington and Woodward, 2003). Fossil data have also revealed that lower atmospheric CO2 was associated with an increase in stomatal density and conductance and a reduction in size while higher atmospheric CO2 was associated with decrease in stomata (Royer, 2001; Franks and Beerling, 2009). In fact, CO2 doubling leads to an average decrease of almost 22% in stomatal density in 48 Arabidopsis accessions (Woodward et al., 2002) and up to 29% in 110 other species (Woodward and Kelly, 1995). Stomatal conductance is also known to follow an inverse relationship with ambient CO2 in angiospermic plants, decreasing at high CO2 and increasing at low CO2 levels (Brodribb et al., 2009). This decrease in stomatal density and conductance most likely allows plants to fine tune their responses to the environment and control CO2 assimilation and water utilization better (Franks and Beerling, 2009; Brodribb et al., 2009). Given the approximately 22–30% decrease in stomata in response to CO2 concentrations as high as twice the normal levels, it may be expected that an almost 25–35% decrease in stomatal density (and a 42–56% decrease in conductance) in transgenic SlERF36 plants but without a corresponding increase in CO2 concentration could severely restrict the uptake/availability of CO2 to the plant and have a cascading effect on the other photosynthetic parameters.
One of the parameters that undergoes a drastic reduction along with the reduced stomatal density is the stomatal conductance which in turn could affect CO2 uptake. This in turn severely limits the photosynthetic rate as evident from the 30–50% decrease in CO2 assimilation in transgenic plants. Such an effect of reduced stomatal density and conductance on CO2 uptake, photosynthesis, and productivity has also been reported in several previous studies in various plants (Freeland, 1948; Heichel, 1971; Fu et al., 2010). Conversely, stomatal density is markedly affected by the environment and hence an effect of reduced photosynthesis on stomatal density reduction cannot be ruled out. However, studies by Miyazawa et al. (2006) on Populus show that stomatal density of upper leaves was affected by conductance of lower leaves but was independent of photosynthesis. Similarly, Beerling and Woodward (1995) observed that the effect of increased CO2 on stomatal density reduction was also seen in variegated leaves indicating that it was an effect independent of photosynthesis.
Interestingly, while a reduction in stomatal density and conductance could restrict CO2 uptake and assimilation, increasing internal CO2 does not restore the photosynthetic rates of the affected transgenic plants to those of the control. They continue to remain lower than control at any given Ci concentration (Fig. 3B). This shows that CO2 is not the only limiting factor that is responsible for the photosynthetic defects in the transgenic plants. Rather, the plants appear to have undergone a reconditioning to an environment of reduced internal CO2 that prevents them from making optimum utilization of light. This is evident from the fact that electron transport rates as well as the quantum yields of PSII and PSI are lower in transformants. The defect actually lies on the PSII side, leading to a reduction in the flow of electrons from PSII to PSI. The low PSII ETRs seem to result from considerably high levels of NPQ (Fig. 4C) as a protective mechanism against an environment of high excitation pressure due to low stomatal conductance and a limitation in CO2 availability.
The increase in levels of NPQ is a response to avoid damage to the photosynthetic apparatus from high excitation pressure usually in response to high light conditions (Demmig-Adams and Adams, 1996; Oquist and Huner, 2003). But they may also result from a reduction in conductance beyond a certain critical value, as shown previously in Phaseolus vulgaris by Omasa and Takayama (2003). By simultaneous measurement of NPQ and stomatal conductance after treating leaves with ABA, they observed that at conductance values greater than 80 mmol H2O m–2 s–1, the NPQ remained between 0.16 to 0.69. However, a reduction in stomatal conductance to less than 60 mmol m–2 s–1 resulted in drastic increase in NPQ with values reaching beyond 1.5. This is very similar to values observed in the current study wherein the SlERF36-expressing transgenic lines had stomatal conductances of lower than 50 mmol H2O m–2 s–1 and high NPQ values ranging between 1.34 and 0.95 as against a conductance of 84 mmol H2O m–2 s–1 and NPQ of only 0.69 in control plants. That this adaptation helps in protection of the reaction centres of the photosynthetic machinery in transgenic SlERF36 plants is obvious from the fact that the openness of the reaction centres (as seen from an unchanged Fv/Fm ratio) is not affected in transgenic lines. What is surprising, however, is that, unlike most plants where high NPQ is a response to high light intensity, the transgenic SlERF36 plants seem to have constitutively high NPQ even at light intensities as low as 50 μE (where NPQ is almost 4-times higher in transgenic plants) (Fig. 4C). A consequence of the high NPQ is that only a fraction of the available light (even at low light intensities) is used for photosynthesis in the transgenic lines. This difference in light utilization between the control and the transgenic lines in addition to the reduced conductance and possibly reduced CO2 availability leads to markedly reduced photosynthetic rates and lower quantum yield. The ensuing stress probably leads to early flowering and senescence.
These observations on the adverse effects of a 25–35% reduction in stomatal density on stomatal conductance, photosynthesis, and water use efficiency in tobacco are in contrast to several reports in Arabidopsis where a similar reduction in stomatal density in mutants like gtl1 (Yoo et al., 2010), edt1 (Yu et al., 2008), and gpa1 (Nilson and Assman, 2010) actually leads to higher water use efficiency. However, in these mutants, although transpiration was considerably reduced, stomatal conductance was still quite high, as a result of which CO2 assimilation rates only underwent a negligible change. These changes in conductances and CO2 assimilation rates between Arabidopsis and tobacco for the same reduction in stomata may reflect inherent adaptive response strategies between Arabidopsis and tobacco. Understanding the effects of changes in stomatal density and conductance in the same genetic background and under the same growth conditions have so far been difficult and were only recently made through comparison of different stomatal density mutants in Arabidopsis (Doheny-Adams et al., 2012). The SlERF36-expressing lines provide interesting study material for such a study in a different plant, tobacco.
How might SlERF36 exert its effects? SlERF36 could either have a direct effect on stomatal density that affects other components or a direct effect on photosynthesis that affects density or multiple independent effects. SlERF36 contains a C-terminal EAR motif and has recently been shown to actively repress the ethylene responsive GCC box in vitro (Pirrello et al., 2012). Other studies have also reported the EAR motif to act as an active repressor. The mechanism of repression is believed to be through the interaction with histone deacetylases that mediate silencing of the target genes (Song et al., 2005; Kagale and Rozwadowski, 2011). SlERF36 could target some component of the RuBP regeneration pathway leading to a cascading effect on ETRs and NPQ and possibly density and conductance. Conversely, SIERF36 could target a component of the stomatal development pathway. This pathway incidentally consists of several negative regulators such as TOO MANY MOUTHS, ERECTA, ERL1, ERL2, epidermal growth factors such as EPF1, EPF2, CHALLAH, and other negative regulators such as SDD1 and the MAPK kinase kinase, YODA (Shimada et al., 2011). These control stomatal density through control of asymmetric cell division of the meristemoid mother cells such that at least one intervening cell separates adjacent stomata. Given the greater spacing in adjacent stomata amongst plants expressing SlERF36, SlERF36 might exert a control over the levels of some regulator that is possibly involved in the decision about spacing. The fact that SlERF36 suppresses the GCC box (Pirrello et al., 2012) also raises the intriguing possibility of the involvement of ethylene in stomatal development and/or photosynthesis. Identifying the precise targets of SlERF36, the role of the EAR motif in their regulation and the direct or indirect action of SlERF36 targets on photosynthesis, NPQ, stomatal density and conductance would thus be the subject of more detailed studies.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Transcript accumulation of SlERF36 in different tissues.
Supplementary Fig. S2. Detection of SlERF36 transcripts in transgenic plants by reverse-transcription PCR.
Supplementary Fig. S3. Leaf colour of transgenic SlERF36-expressing plants.
Supplementary Fig. S4. Response of photosynthesis to changing stomatal conductance at different light intensities.
Acknowledgements
The authors are grateful to Dr PV Sane, ex-Director, CSIR-National Botanical Research Institute, for helpful discussions and interpretations with the defects in the photosynthetic machinery. They thank Mr Ram Awadh for taking care of the transgenic plants. The work was supported with financial grants from the Department of Biotechnology, India. A SRF Fellowship to R.K.U. from DBT and CSIR and a CSIR-funded SIP grant to D.K.S. and R.S. are gratefully acknowledged.
References
- Arnon DI. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris . Plant Physiology 24, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif MH, Dhawan P, Nath P. 2000. A simple procedure for the isolation of high quality RNA from ripening banana fruit. Plant Molecular Biology Reports 18, 109–115 [Google Scholar]
- Beerling DJ, Woodward FI. 1995. Stomatal responses of variegated leaves to CO2 enrichment. Annals of Botany 75, 507–511 [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Field TS. 2009. Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytologist 183, 839–847 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III 1996. Xanthophyll cycle and light stress in nature: uniform response to excess direct sunlight among higher plant species. Planta 198, 460–470 [Google Scholar]
- Doheny-Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE. 2012. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philosophical Transactions of the Royal Society B 367, 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C-J, Liu J-Y. 2010. The Arabidopsis EAR-motif-containing protein RAP2.1 functions as an active transcriptional repressor to keep stress responses under tight control. BMC Plant Biology 10, 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalona JM, Flexas J, Medrano H. 1999. Stomatal and non-stomatal limitations of photosynthesis under water stress in field grown grapevines. Australian Journal of Plant Physiology 26, 421–433 [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences, USA 106, 10343–10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeland RO. 1948. Photosynthesis in relation to stomatal frequency and distribution. Plant Physiology 23, 595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QS, Zhao B, Wang YJ, Ren S, Guo YD. 2010. Stomatal development and associated photosynthetic performance of capsicum in response to differential light availabilities. Photosynthetica 48, 189–198 [Google Scholar]
- Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. 2000. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. The Plant Cell 12, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterson N, Reuber TL. 2004. Regulation of disease resistance pathways by AP2/ERF transcription factors. Current Opinion in Plant Biology 7, 465–471 [DOI] [PubMed] [Google Scholar]
- Heichel GH. 1971. Genetic control of epidermal cell and stomatal frequency in maize. Crop Science 11, 830–832 [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry J, Hoffmann N, Neidermeyer J, Rogers SG, Fraley RT. 1988. Leaf disc transformation. In Gelvin SB, Schilperoort RA, editors, Plant molecular biology manual. Dordrecht, The Netherlands: Kluwer Academic Publishers, A5: 1–9 [Google Scholar]
- Kagale S, Links MG, Rozwadowski K. 2010. Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis . Plant Physiology 15, 1109–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S, Rozwadowski K. 2011. EAR motif mediated transcriptional repression in plants. Epigenetics 6, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DM, Johnson G, Kiirats O, Edwards GE. 2004. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynthesis Research 79, 209–218 [DOI] [PubMed] [Google Scholar]
- Lake JA, Woodward FI. 2008. Response of stomatal numbers to CO2 and humidity: control by transpiration rate and abscisic acid. New Phytologist 179, 397–404 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang L, Yu Y, Quan R, Zhang Z, Zhang H, Huang R. 2011. The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis . The Plant Journal 68, 88–99 [DOI] [PubMed] [Google Scholar]
- Lu J, Ju H, Zhou G, Zhu C, Erb M, Wang X, Wang P, Lou Y. 2011. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. The Plant Journal 68, 583–596 [DOI] [PubMed] [Google Scholar]
- Miyazawa S-I, Livingston NJ, Turpin DH. 2006. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa × P. deltoides). Journal of Experimental Botany 57, 373–380 [DOI] [PubMed] [Google Scholar]
- Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. 2012. AP2/ERF family transcription factors in plant abiotic stress responses. Biochimica et Biophysica Acta 1819, 86–96 [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. 2006. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology 140, 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson SE, Assmann SM. 2010. The α-subunit of the Arabidopsis heterotrimeric G protein, GPA1, is a regulator of transpiration efficiency. Plant Physiology 152, 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. 2001. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. The Plant Cell 13, 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omasa K, Takayama K. 2003. Simultaneous measurement of stomatal conductance, non-photochemical quenching, and photochemical yield of photosystem II in intact leaves by thermal and chlorophyll fluorescence imaging. Plant Cell Physiology 44, 1290–1300 [DOI] [PubMed] [Google Scholar]
- Oquist G, Huner N. 2003. Photosynthesis of overwintering evergreen plants. Annual Review of Plant Biology 54, 329–355 [DOI] [PubMed] [Google Scholar]
- Pan I-C, Li C-W, Su R-C, Cheng C-P, Lin C-S, Chan M-T. 2010. Ectopic expression of an EAR motif deletion mutant of SlERF3 enhances tolerance to salt stress and Ralstonia solanacearum in tomato. Planta 232, 1075–1086 [DOI] [PubMed] [Google Scholar]
- Pirrello J, Prasad BCN, Zhang W, et al. 2012. Functional analysis and binding affinity of tomato ethylene response factors provide insight on the molecular bases of plant differential responses to ethylene. BMC Plant Biology 12, 190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann JL, Ratcliffe OJ. 2000. A genomic perspective on plant transcription factors. Current Opinion in Plant Biology 3, 423–434 [DOI] [PubMed] [Google Scholar]
- Royer DL. 2001. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Review of Palaeobotany and Palynology 114, 1–28 [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K. 2002. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration and cold-inducible gene expression. Biochemical and Biophysical Research Communications 290, 998–1009 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Klughammer C. 2008. Saturation pulse method for assessment of energy conversion in PSI. PAM Application Notes 1, 11–14 [Google Scholar]
- Sharma MK, Kumar R, Solanke AU, Sharma R, Tyagi AK, Sharma AK. 2010. Identification, phylogeny, and transcript profiling of ERF family genes during development and abiotic stress treatments in tomato. Molecular Genetics and Genomics 284, 455–475 [DOI] [PubMed] [Google Scholar]
- Shimada T, Sugano SS, Hara-Nishimura I. 2011. Positive and negative peptide signals control stomatal density. Cellular and Molecular Life Sciences 68, 2081–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CP, Agarwal M, Ohta M, Guo Y, Halfter U, Wang P, Zhu JK. 2005. Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. The Plant Cell 17, 2384–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward FI, Kelly CK. 1995. The influence of CO2 concentration on stomatal density. New Phytologist 131, 311–327 [Google Scholar]
- Woodward FI, Lake JA, Quick WP. 2002. Stomatal development and CO2: ecological consequences. New Phytologist 153, 477–484 [DOI] [PubMed] [Google Scholar]
- Woodward FI. 1987. Stomatal numbers are sensitive to increases in CO2 from preindustrial levels. Nature 327, 617–618 [Google Scholar]
- Yang Z, Tian L, Latoszek-Green M, Brown D, Wu K. 2005. Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Molecular Biology 58, 585–596 [DOI] [PubMed] [Google Scholar]
- Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV. 2010. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. The Plant Cell 22, 4128–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Chen X, Hong YY, Wang Y, Xu P, Ke SD, Liu HY, Zhu JK, Oliver DJ, Xiang CB. 2008. Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. The Plant Cell 20, 1134–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.