Abstract
In Narcissus tazetta, a monocotyledonous bulbous geophyte, floral initiation and differentiation occur within the bulb during the quiescent period in summer, when ambient temperatures are relatively high and the bulb is located underground with no foliage or roots. In many plant species, FLOWERING LOCUS T (FT) and its homologues are considered powerful promoters of flowering. The Narcissus FT gene homologue (NtFT) was isolated, and organ-specific expression patterns of NtFT during the annual cycle and reproductive development under different temperature regimes were analysed using quantitative reverse transcription–PCR (qRT–PCR) and RNA in situ hybridization. During floral induction, NtFT was not expressed in bulb scales, roots, or foliage leaves, but it was detected inside the bulb in the apical meristem and leaf primordia. The expression of another key flowering gene, NLF, the LEAFY homologue in N. tazetta, was also observed only in meristem and leaf primordia within the bulbs; however, its expression did not coincide with that of NtFT during meristem transition to reproductive stage. Under high temperatures (25–30 °C) in the dark, NtFT expression occurred simultaneously with floral induction timing, indicating that floral induction is affected by high temperatures but not by photoperiod or vernalization. Monitoring the apical meristem of Narcissus in February–August of two growing seasons under ambient and controlled storage conditions showed that transition to flowering is temperature dependent and varies between years. Lack of NtFT and NLF expression in foliage leaves suggests that flower initiation control in Narcissus differs from that in common model plants.
Key words: Ambient temperature, flowering control, FLOWERING LOCUS T, LEAFY, Narcissus, NLF, NtFT.
Introduction
Transition of the shoot apical meristem from vegetative to reproductive phase is regulated by a network of signalling pathways responding to both endogenous and environmental cues. The paradigm from model plants, for example Arabidopsis, suggests that these pathways consist of a large group of flowering time genes (Henderson and Dean, 2004; Corbesier and Coupland, 2006; Kanno et al., 2007; Pin and Nilsson, 2012). The signals from the various flowering time pathways are integrated and lead to the activation of a small group of ‘floral integrator’ genes. These include, among others, FLOWERING LOCUS T (FT), LEAFY (LFY), and SUPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1). Activation of these genes triggers the transition to flowering (Boss et al., 2004; Henderson and Dean, 2004; Lee et al., 2006; Tan and Swain, 2006; Kaufmann et al., 2010). At the shoot apices in Arabidopsis, FT and FD probably activate important regulators of floral fate (Xu et al., 2012). LFY appears to play an important role in specifying floral meristem identity (Weigel and Nilsson, 1995), reproductive transition, flower development, and expression of floral organ identity genes (Benlloch et al., 2007).
Perennial plant species differ greatly from annuals in their life cycle and physiological requirements, and therefore might differ in the molecular mechanisms that control flowering (Tan and Swain, 2006; Townsend et al., 2006; Melzer et al., 2008; Jung and Muller, 2009). However, only limited information is available on the molecular aspects of reproductive development in perennial herbaceous plants, geophytes in particular (Kamenetsky et al., 2012).
The monocotyledonous geophyte Narcissus tazetta is one of the most popular ornamentals worldwide. Its annual cycle is naturally adapted to the environmental conditions in Mediterranean regions (Dulberger, 1967; Yahel and Sandler, 1986; Koike et al., 1994; Hanks, 2002). Following quiescence during the hot and dry summer, leaf elongation begins after the first rains in October–November, and flowering occurs in December–January. Foliage leaves remain green until senescence in April–May, and bulbing occurs in May–June. The bulb consists of true scales and leaf bases—the lower parts of the foliage leaves. Flower initiation and differentiation occur within the bulb during the summer, when the underground bulb remains underground with no live roots or foliage leaves. In previous studies, it has been shown that high temperatures (25–30 °C) in late spring (May–June) promote intrabulb florogenesis; temperatures >30 °C delay the subsequent inflorescence differentiation, and low temperatures (12 °C) completely inhibit all stages of florogenesis (Noy-Porat et al., 2009). The N. tazetta LFY homologue, NLF, correlates with intrabulb florogenesis (Noy-Porat et al., 2010). A dramatic increase in NLF expression was observed during floral initiation under ambient summer conditions and at a constant 30 °C, as well as during differentiation of flower primordia. When stored at 12 °C, meristems remained morphologically vegetative, but high NLF expression was observed in these non-differentiated meristems. It was suggested that temperature does not affect NLF expression directly, but might regulate other flower-related genes that are involved in floral transition.
In this context, FT and its homologues are considered to be involved in florogenesis, and their function appears to be remarkably conserved in all species tested (Turck et al., 2008). The role of FT and its homologue Hd3a in the photoperiod induction pathway has been extensively studied in Arabidopsis and rice, respectively. In Arabidopsis, FT was found to be regulated directly by CONSTANS (CO) under a long-day photoperiod perceived by the leaves (Abe et al., 2005; Wigge et al., 2005; Corbesier et al., 2007). Tissue-specific overexpression of FT in Arabidopsis caused early flowering when it occurred in the leaf phloem and in the shoot apex (An et al., 2004). CO regulates FT transcription in the leaf phloem; however, it was suggested that the FT protein is translocated from the leaves to the shoot apex and, therefore, FT is the long-sought mobile florigen signal (Kobayashi and Weigel, 2007; Zeevaart, 2007). At the shoot apex, FT interacts directly with the bZIP protein FD which seems to recruit FT to the promoter of AP1 (Abe et al., 2005; Wigge et al., 2005; Kobayashi and Weigel, 2007; Kaufmann et al., 2010). Gene activation by the FT/FD complex is considered the earliest event in the floral transition to occur in the meristem itself (Turck et al., 2008). Recently, Li et al. (2009) showed that a cis-element in FT mRNA allows mobility of this RNA in the plant, suggesting that FT mRNA, along with its protein, may be involved in intraplant spread of the floral stimulus.
Overexpression of FT homologues causes early flowering in tomato and tobacco (Lifschitz and Eshed, 2006), as well as in the monocotyledonous rice (Kojima et al., 2002), wheat, and barley (Yan et al., 2006); it also shortens the juvenile period in Populus (Hsu et al., 2006) and Citrus (Endo et al., 2005; Nishikawa et al., 2010). Ectopic expression of OnFT, an FT homologue from orchid, caused early flowering in Arabidopsis and partially restored the ft-1 mutant phenotype (Hou and Yang, 2009). However, unlike FT in Arabidopsis, OnFT was highly expressed in the buds prior to floral transition, and also showed high expression at the beginning of flower differentiation, which then decreased during flower maturation (Hou and Yang, 2009).
In addition to photoperiod and vernalization pathways, FT has been suggested to be an important component of the ambient temperature signalling pathway in Arabidopsis (Blazquez et al., 2003; Halliday et al., 2003; Balasubramanian et al., 2006; Kumar and Wigge, 2010). However, the involvement of FT in other modes of flowering control has not been studied in detail.
In this report, data are provided, for the first time, on isolation and identification of the FT homologue in the Mediterranean geophyte N. tazetta. Spatial and temporal expression patterns of the FT and LFY homologues were examined in the plant organs during flower induction throughout the summer quiescence period and storage under various conditions, to reveal possible regulation of flower initiation by environmental signals.
Materials and methods
Plant material, growth and storage conditions, and sampling for histological and molecular analyses
Bulbs of N. tazetta cv. Ziva, 13–14cm in circumference, were obtained from commercial producers in Israel. The plants were grown in local soil (Vertisol) in Beer-Tuvia and Bizaron, the southern coastal plain of Israel, and irrigated once every 10 d in October–November and May–June, in addition to natural rainfall (seasonal average of 400mm, falling between October and April).
The effect of soil temperature on floral induction in bulbs was studied during two growing seasons, 2004/2005 and 2007/2008. Soil temperature was recorded at a depth of 10cm at the Negba station, located in close proximity to the commercial fields (Israel Meteorological service http://www.ims.gov.il).
In 2005, bulbs were harvested in April, when the foliage leaves began to dry out. After sorting and cleaning, the bulbs were kept throughout the summer at ambient temperatures of 25–30/16–22 ºC (day/night), or at a constant temperature of 12 °C. To avoid a possible effect of photoperiod, the bulbs were kept in complete darkness. For molecular analyses, 200 bulbs from each treatment were dissected every 3 weeks from April until October 2005, and meristems were collected according to their developmental stage.
For the analysis of the early stages of floral transition, bulbs were harvested from the field once a month from February to July 2008, immediately dissected, and the following organs were collected for molecular analysis: green foliage leaves and their bases, roots, mature scales, young scales, basal plate, leaf primordia, and apical meristem in the central renewal bud (Fig. 2).
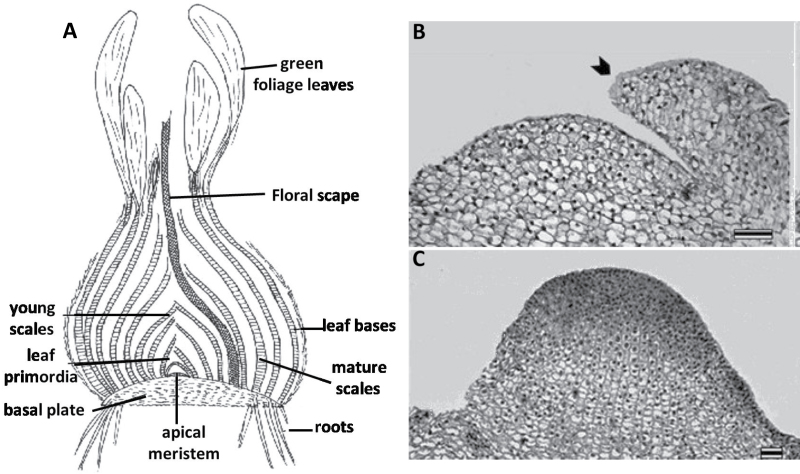
Fig. 2.
Morphological analysis of N. tazetta. (A) Schematic representation of bulb structure in April–May. Note leaf bases of the foliage leaves, functioning as storage scales after leaf blade senescence. Mature scales were differentiated directly as storage organs within the bulb. The indicated organs were collected for the molecular analysis. (B) Vegetative meristem in April. Bar=100 μm. Arrowhead indicates new leaf primordium. (C) Reproductive meristem in June. Bar=100 μm.
Morphological changes in the apical meristem were monitored from February to August with a stereoscope (Stemi 2000-C, Zeiss) and light microscope (Leica DM LB). Histological sections were prepared as described previously (Noy-Porat et al., 2010). The meristem dimensions were measured on histological sections from the centre of the meristem, under the microscope.
For molecular analyses, plant tissues were collected in liquid nitrogen, sorted by developmental stage according to morphological characteristics (Noy-Porat et al., 2009), and stored at –80 °C. A total of ~2000 bulbs were dissected during the whole period and their organs collected.
Nucleic acid isolation
RNA from all collected plant parts was isolated according to Jaakola et al. (2001). DNA was isolated from green foliage leaves by a CTAB (cetyltrimethyl ammonium bromide)-based method (Noy-Porat et al., 2009). For vegetative and reproductive meristems and leaf primordia, each sample consisted of RNA isolated from ~100 bulbs. For all other plant parts (Fig. 2), each sample consisted of RNA isolated from 10 bulbs.
Gene identification
The Narcissus homologue of FT (NtFT) was amplified using degenerate primers designed according to FT homologues from various plant species. The following primers were used: forward, 5′-ATGGTRGAYCCDGATGYWCCRAG-3′ and reverse, 5′-RTTRAARTTYTGNCGCCANC-3′.
Alignment of the partial amino acid sequence of the Narcissus (NtFT) cDNA with those of FT homologues from various species was performed using ClustalW. The accession numbers of the homologues were as follows: Arabidopsis NP_176726 (FT), NP_193770 (TSF), NP_196004 (TFL1), NP_201010 (BFT), NP_173250 (MFT), NP_180324 (ATC); BAG12904 (Populus nigra), BAF96645 (Citrus unshiu), AAW23034 (Triticum aestivum), NP_001056860 (Oryza sativa), and ACC59806 (Oncidium ‘Gower Ramsey’).
The Narcissus homologue of LFY (NLF) was amplified by standard reverse transcription–PCR (RT–PCR) using the following primers: forward, 5′- TTGGGCTTGTTGATGTAGCTT-3′ and reverse, 5′-GAGCTCGACGACATGATG-3′. The PCR products were analysed on an agarose gel and cloned into a pGEM-T easy vector (Promega, Madison, WI, USA) for sequencing (Noy-Porat et al., 2010).
Quantitative RT–PCR (qRT–PCR)
Spatial and temporal analyses of the expression of genes during florogenesis were performed using qRT–PCR of all sampled plant tissues (Fig. 2). For reverse transcription, 1 μg of total RNA from each sample was digested with RQ1-DNase (Promega). cDNA first-strand synthesis was performed using the Verso cDNA kit (ABgene, Surrey, UK). For NtFT, qRT–PCR was carried out in a 20 μl reaction volume using the AbsoluteBlue QPCR mix (ABgene) and the PerfectProbe gene detection mix (PrimerDesign, Southampton, UK) containing the probe and primers at a final concentration of 0.2 μM. The cDNA was diluted 1:5, and 5 μl were added to the reaction. For NLF, quantitative real-time PCR was carried out in a 20 μl reaction volume using Absolute QPCR SYBR Green mix (ABgene). The reaction mixture included a final primer concentration of 0.3 μM, and 2 μl of cDNA. Actin was used as an internal control (using SYBR Green chemistry).
Primers and probe used for NtFT were: 5′-AGAGATAGTGTG TTATGAAAGTCC-3′ (forward), 5′-TGCCTACCCAATTAGCGA AA-3′ (reverse), and 5′-FAM-CCACCAAAACAAAGCGATGAAT CCCCTTGGTGG-3′ (probe); for NLF: 5′-GACGCTTCGAG TCCCTTAACAA-3′ and 5′-TTCGCCTCCGCTTTCATG-3′; for ACTIN: 5′-ATCAAGGAGAAACTGGCTTATGTTG-3′(forward) and 5′-CCATCAGGAAGTTCGTAGCTCTTC-3′ (reverse).
The qRT–PCR was performed in a Rotor-Gene 6000 apparatus (Corbett Life Science, Germantown, MD, USA). Each result is the mean of three biological replicates, each with two technical repeats.
In situ hybridization
Tissues were fixed in FAA (formaldehyde:acetic acid:alcohol, 5:5:90, v/v/v) for at least 2 d and then embedded in ParaPlast. Tissue sections (10 μm) were mounted on SuperFrost Plus slides (Menzel-Glaser, Braunschweig, Germany) and left for 2h on a 40 °C hot plate. A probe was designed based on areas of the gene that are unique to NtFT and are much less conserved in other genes of the family. The 240bp segment of NtFT was cloned into a StrataClone vector (Stratagene, La Jolla, CA, USA), with a T7 promoter sequence attached to the 3′ end of the gene and a T3 promoter sequence attached to its 5′ end. Digoxigenin (DIG)-labelled RNA sense and antisense probes were then generated using the MEGAscript kit (Ambion, Austin, TX, USA) and DIG RNA labelling mix (Roche Applied Science, Indianapolis, IN, USA). The probes were later purified using the MEGAclear kit (Ambion), and quantified by running 1 μl on an agarose gel and measuring the concentration in a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE, USA). The specificity of the probe was tested on a sense control as well as on tissues that do not express NtFT. In situ hybridization was performed as described previously (Noy-Porat et al., 2009).
Results
Isolation of the FT homologue in N. tazetta
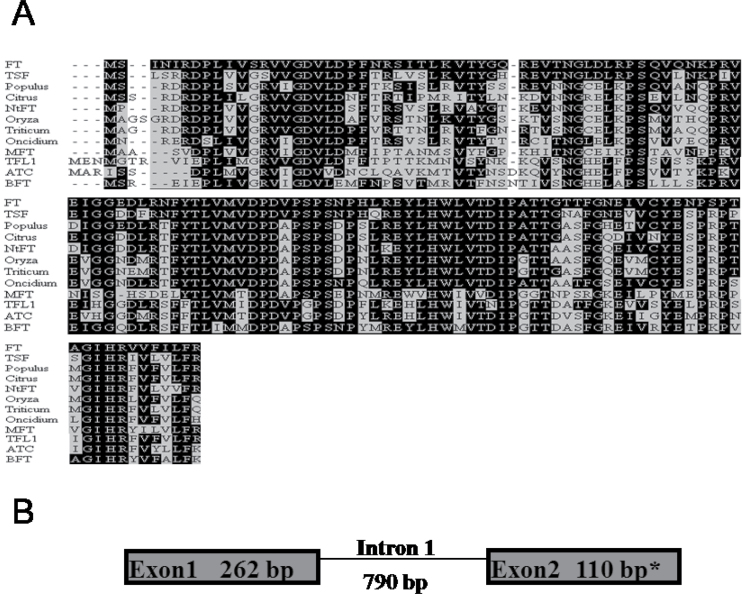
RT–PCR with primers designed according to conserved domains of FT homologues from different species amplified a cDNA fragment of 372bp in N. tazetta. Sequence analysis of the partial translation product revealed 75% similarity to FT and TSF from Arabidopsis, and 80–84% similarity to FT homologues from rice, wheat, Populus, Citrus, and orchid (Fig. 1A). The Narcissus gene was less similar to other genes of the FT family, showing 53–58% similarity to TFL1, MFT, BFT, and ATC from Arabidopsis (Fig. 1A). It was therefore classified as an FT homologue, named NtFT, and deposited in GenBank under accession no. HM537233. Using the identified NtFT sequence, a 1350bp fragment was isolated from N. tazetta DNA by PCR. It contained a large intron with location and size similar to those of introns found in other FT homologues (Fig. 1B).
Fig. 1.
NtFT gene structure. (A) Multiple alignment of the NtFT protein deduced from the cDNA sequence with FT homologues from different plant species. Accession numbers are listed in the Materials and methods. Similar amino acids were identified using BoxShade version 3.2. Black shading, identical residues; grey shading, similar residues. (B) Exon/intron arrangements and sizes (in bp) of NtFT. *Only part of exon 2 was identified and therefore its size is not complete.
Anatomical and morphological observations of floral transition
During the growing season under ambient conditions, between October and April, new leaf primordia are produced inside the N. tazetta bulb by the vegetative apical meristem, which are 500 μm (±40) in diameter and 300 μm (±15) in height (Fig. 2B). In May–June, after the transition to reproductive development and inflorescence initiation, the meristem becomes dome-like and its size increases to 800 μm (±124) in diameter and 500 μm (±33) in height (Fig. 2C).
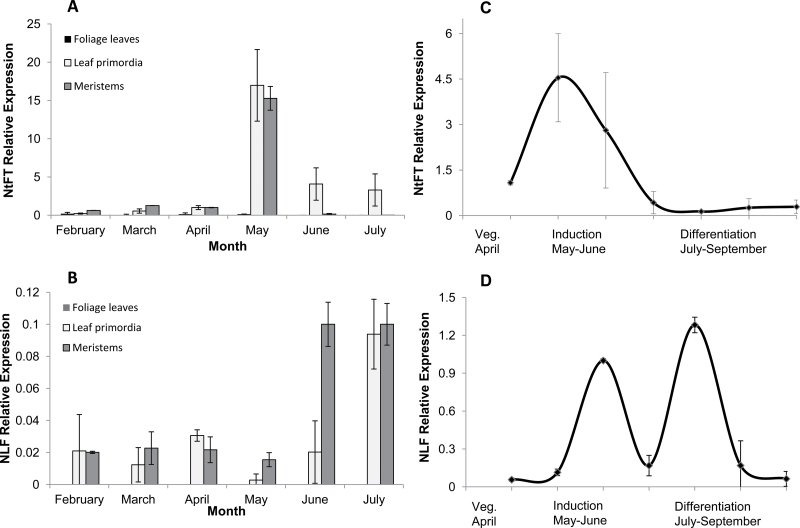
Organ-specific expression patterns of NtFT and NLF during floral transition
To examine the organ-specific expression patterns of NtFT and NLF in N. tazetta, qRT–PCR analysis was performed. During the observation period in February–July 2008, both NtFT and NLF expression was absent in the leaf bases, the mature scales, the basal plate, and the roots.
From February to May, NtFT was expressed at a constant basal level in the mature foliage leaves (Fig. 3A). By mid-May, the foliage leaves had dried up and therefore were not examined any further.
Fig. 3.
Differential experession of NtFT and NLF during florogenesis of Narcissus tazetta cv. Ziva. Samples were normalized against β-actin. (A) Relative expression of NtFT in apical meristems, morphologically defined as vegetative, leaf primordia, and foliage leaves under ambient growth conditions between February and July 2008. In mid-May, the foliage leaves dried up and were not examined any further. (B) Relative expression of NLF in apical meristems, morphologically defined as vegetative, leaf primordia, and foliage leaves under ambient growth conditions between February and July 2008. In mid-May, the foliage leaves dried up and were not examined any further. (C) Relative expression of NtFT at the various stages of florogenesis. Samples of morphologically vegetative meristems, reproductive meristems, and differentiated inflorescences were analysed under ambient conditions in April–September 2005. (D) Relative expression of NLF at the various stages of florogenesis. Samples of morphologically vegetative meristems, reproductive meristems, and differentiated inflorescences were analysed under ambient conditions in April–September 2005.
In the leaf primordia surrounding the apical meristem, NtFT showed a basal expression level from February to April similar to that observed in mature foliage leaves. A sharp increase (~17-fold) in NtFT expression in the leaf primordia was detected in May, followed by a decrease in June–July (Fig. 3A). A similar pattern was detected in the apical meristem: from February to April, only basal expression of NtFT was registered, with a sharp increase in expression (~15-fold) occurring in May, simultaneously with the increase in the leaf primordia (Fig. 3A). NtFT also showed very low basal expression in young scales, which was significantly lower than in foliage leaves and was stable throughout the observation period (data not shown).
NLF was not detected in mature green leaves or any other vegetative tissue at any time during the observation period. However, this gene was expressed at a relatively low level from February to May in vegetative non-differentiated meristems, with a significant increase (~6.5-fold) in June (Fig. 3B). In leaf primordia, NLF expression increased only in July, ~4.5-fold, a month later than in the apical meristems.
Further analysis at the various stages of inflorescence development confirmed a transient increase in NtFT expression with the meristem shift from vegetative to reproductive stage (Fig. 3C). Following inflorescence induction, during its differentiation, NtFT expression in the meristem decreased to basal levels (Fig. 3C). On the other hand, NLF expression increased during inflorescence initiation and again during differentiation. The second significant increase in NLF expression was observed during differentiation of the flower primordia (Fig. 3D).
Spatial expression of NtFT and NLF in the apical meristem and leaf primordia
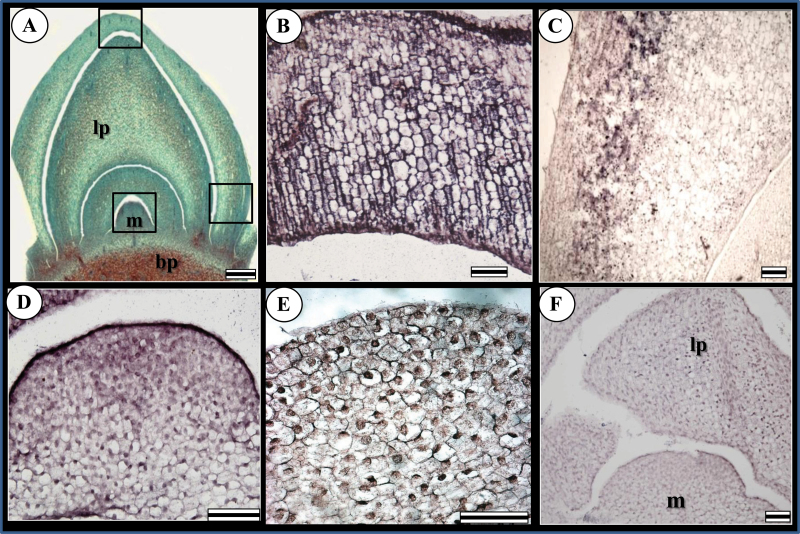
Since the results from qRT–PCR indicated an increase in NtFT and NLF expression in both meristem and leaf primordia in May–June, spatial expression of the two genes in these organs was examined by in situ hybridization (Fig. 4). NtFT expression was observed in both apical meristem and leaf primordia collected in May (Fig. 4B–D). In the leaf primordia, NtFT expression was observed at the tip and in the spongy mesophyll (Fig. 4B, C). In the meristem, NtFT expression was weaker, and appeared mostly in the central zone and upper cell layers (Fig. 4D).
Fig. 4.
In situ hybridization of NtFT and NLF in leaf primordia and apical meristems during transition from vegetative to reproductive development in Narcissus tazetta cv. Ziva; m, meristem; lp, leaf primordia; bp, basal plate. (A) Developing bud inside the bulb consisting of an apical meristem surrounded by three leaf primordia. The tip and side of the outer leaf are marked and shown in B and C, respectively. The meristem is marked and shown in D and E. Bar=500 μm. (B, C) Leaf primordia collected in May. Strong expression of NtFT is observed in all tissues, including epidermis and palisade tissue, and the expression is not restricted to the vascular tissue. Bar=100 μm (D) Meristem collected in May and stained for NtFT. Expression is visible in the central zone of the meristem and in the upper cell layers. Bar=100 μm. (E) Meristem collected in June and stained for NLF. Expression is weakly observed in cells throughout the meristem. Bar = 100 μm. (F) Sense control of meristem and leaf primordia. Bar=100 μm
NLF showed weak expression in cells throughout the meristem (Fig. 4E). Similar expression was observed in the leaf primordia (data not shown).
Expression of NtFT and NLF under storage at different temperatures in the dark
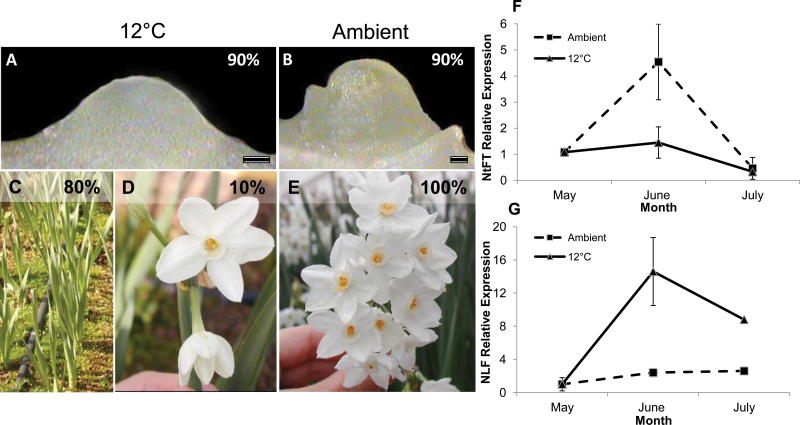
To investigate the effect of light and temperature on NtFT and NLF expression in N. tazetta, the genes’ expression patterns in the meristems with a vegetative morphological appearance were investigated during bulb storage in May–July 2005. NtFT and NLF expression in the meristem was monitored under two temperature regimes: high ambient (25–30 ºC day and 16–22 ºC night) and constant (12 °C). In the bulbs stored at 12 °C, only 10% of the meristems progressed to reproductive development (Fig. 5A), but, after planting, only 80% of the plants showed leaf emergence and all inflorescences in these plants had a significantly lower number of flowers, which were malformed: flowers differentiated five tepals instead of six, and degenerated anthers (Fig. 5C, D). qRT–PCR analysis showed no NtFT expression in the meristems stored at 12 °C during the examination period (Fig. 5F), whereas NLF expression was high (Fig. 5G). In bulbs stored under ambient conditions, >90% of the meristems became reproductive (Fig. 5B), and all of them produced normal flowers after planting in the autumn (Fig. 5E). In these plants, both NtFT and NLF expression increased significantly in June (Fig. 5F, G).
Fig. 5.
The effect of high ambient versus low storage temperatures on floral transition and flowering in Narcissus tazetta cv. Ziva. Bulbs were stored in the dark at high ambient temperatures (25–30 ºC day, 16–22 ºC night) or a constant 12 °C between May and September 2005 and then transferred to ambient growth conditions in October. (A) Vegetative meristem. Under storage at 12 °C, 90% of the meristems were not induced to flower and remained vegetative. (B) Reproductive meristem. Under storage at ambient temperatures, 90% of the meristems became reproductive and developed inflorescences. (C) Foliage leaf development after storage at 12 °C and planting in the autumn. Only 80% of the bulbs showed leaf emergence, and this was extremely delayed compared with bulbs stored under ambient conditions. (D) Inflorescence development in bulbs stored at 12 °C. After planting in the autumn, only 10% of the plants developed inflorescences, with a significantly lower number of flowers per inflorescence, as compared with ambient temperatures, and flower abnormalities. (E) Inflorescence development after storage under ambient conditions. All bulbs developed leaves and 100% of the inflorescences were normal. (F) Relative expression of NtFT in meristems of bulbs stored at high ambient temperatures or constant 12 °C between May and July 2005. Samples were normalized against β-actin. (G) Relative expression of NLF in meristems of bulbs stored at high ambient temperatures or constant 12 °C between May and July 2005. Samples were normalized against β-actin.
Effect of seasonal ambient temperature regime on floral transition
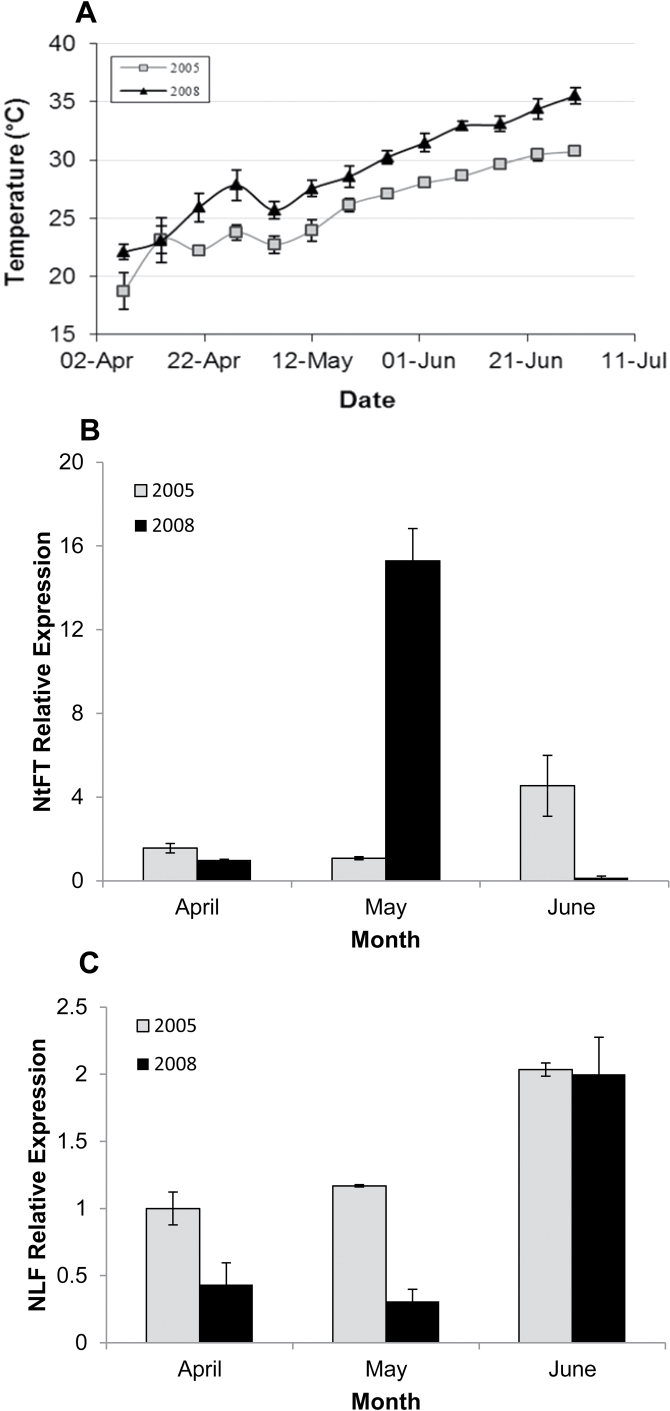
To investigate the effect of seasonal temperature on NtFT and NLF expression, the expression patterns of these genes during two different growing seasons were compared. It should be noted that in 2005, soil temperatures in April–June were close to the seasonal average for this location. In 2008, temperatures in March–May were well above average and, in fact, April 2008 was the hottest April since 1994 (Fig. 6A). Temperatures >25 °C in 2008 were therefore observed on 21 April, in comparison with 2005, when temperatures increased to this level on 19 May. Consequently, the morphological appearance of the reproductive meristem was registered in mid-June in 2008, at least 4 weeks earlier than in 2005.
Fig. 6.
Relative expression of NtFT and NLF under different temperature regimes recorded in April–July 2005 and 2008. Samples were normalized against β-actin. (A) Soil temperature recorded at 10cm depth in the open field in April–July 2005 and 2008. (B) Relative expression of NtFT in apical meristems, morphologically defined as vegetative. (C) Relative expression of NLF in apical meristems, morphologically defined as vegetative.
In agreement with morphological observations, the increase in NtFT expression in 2008 occurred at the beginning of May (Fig. 6B), whereas no difference was found in the temporal expression of NLF. In both bulb populations, sampled in 2005 and 2008, a marked increase in NLF was observed in meristems in June (Fig. 6C).
Discussion
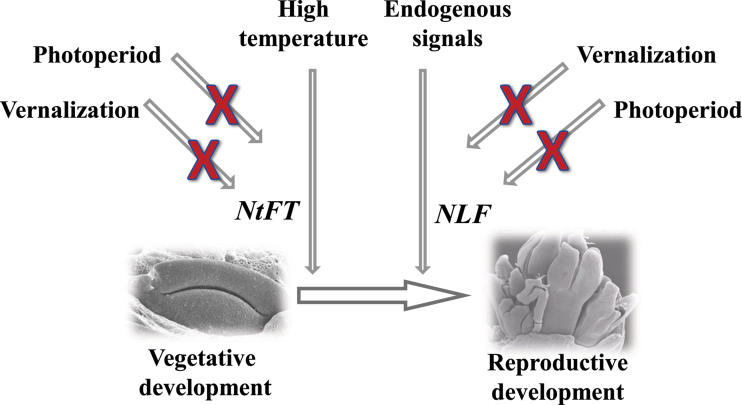
Extensive development of geophytes as ornamental crops has led to the generation of a considerable amount of research data on their flowering physiology. However, only limited information on the genetic control of floral transition is available in herbaceous perennial plants in general, and geophytes in particular (Townsend et al., 2006; Albani and Coupland, 2010; Kamenetsky et al., 2012). Following major breakthroughs in understanding flowering biology in model species, the homologues of several key flowering genes have also been found in geophyte and herbaceous species. For example, LFY homologues have been isolated from Allium sativum (gaLFY; Rotem et al., 2007, 2011), N. tazetta (NLF; Noy-Porat et al., 2010), and Aquilegia formosa (AqLFY; Ballerini and Kramer, 2011). FT-like AcFTL has been found in onion (Allium cepa) (Taylor, 2009; Taylor et al., 2010). In this report, first evidence is provided of the expression of two key flowering genes, NtFT and NLF, in different plant organs of N. tazetta under various environmental conditions. A major distinction in flowering control in this geophyte from the known paradigms for model plants is presented in Fig. 7.
Fig. 7.
Proposed scheme for environmental and molecular control of floral transition in Narcissus tazetta. Floral initiation is probably not stimulated by photoperiodic signal or low temperatures (vernalization). High temperatures at the end of the growth period induce expression of the FT homologue NtFT in leaf primordia and the apical meristem inside the bulb, followed by floral initiation and meristem transition to the reproductive stage. The LFY homologue NLF might be regulated differently from NtFT, and does not act in the same signalling cascade. NLF expression is not induced directly by ambient temperature, and under this pathway might not regulate floral transition but acts in later stages. However, it might regulate floral transition under an endogenous signalling cascade.
High temperature provides a flowering signal in N. tazetta
The common paradigm of ‘florigen’ movement from foliage leaves to the apical meristem, developed for model plants in the context of the photoperiodic pathway (Kobayashi and Weigel, 2007; Turck et al., 2008; Xu et al., 2012), is not always confirmed in other species. FT expression following temperature signals may be different from that observed following photoperiodic signals in model plants. For example, Citrus flowering is induced by low ambient temperature and, during floral induction, mRNA levels of the FT homologue CiFT3 increase in stems, paralleling the decrease in temperature (Nishikawa et al., 2007). In addition, in adult citrus under inductive temperatures, leaves are not necessary for floral initiation (Wilkie et al., 2008). In the perennial herbaceous A. formosa, the FT homologue AqFT is expressed before the transition to flowering under both long-day and short-day conditions. Although vernalization is critical to flowering in Aquilegia, low temperature is not strictly required for the transcriptional activation of AqFT (Ballerini and Kramer, 2011). In the present experiments, floral induction in N. tazetta occurred either when bulbs remained underground with no foliage leaves or active roots, or during bulb storage at high temperatures in complete darkness. Therefore, the light signal was not perceived by the foliage leaves or other plant organs prior to meristem transition. Peak NtFT expression was recorded in the apical meristem in May, prior to visible morphological changes (Fig. 3A). The temporal analysis of gene expression implied that NtFT is regulated by temperature and its expression correlates with timing of floral induction. A comparison of flower initiation under different temperature regimes during two growing seasons (Fig. 6) showed that an earlier rise in temperature causes earlier NtFT up-regulation and floral transition. Therefore, it is argued that high soil temperatures at the end of the vegetative period (April–May) affect the expression of NtFT in the quiescent renewal bud of N. tazetta and that NtFT up-regulation marks the time point of floral induction within the bulb (Fig. 7).
Previous reports have suggested a possible role for ambient temperatures in flower transition. In Arabidopsis, elevated temperatures (>23 °C) were shown to induce flowering as efficiently as long days. The process can be regulated by genes belonging to the autonomous pathway, and perhaps by histone modification and microRNA abundance as well (Kumar and Wigge, 2010; Lee et al., 2010; McClung and Davis, 2010). Consistent with the present findings, most studies on ambient temperature signalling suggest that FT is this pathway’s target gene (Blazquez et al., 2003; Halliday et al., 2003; Samach and Wigge, 2005; Balasubramanian et al., 2006; Kumar and Wigge, 2010).
On the other hand, the second key gene, NLF, might not be regulated directly by temperature, since its expression was registered in the renewal bud independent of the temperature regime (Fig. 6), and was not down-regulated during bulb storage at 12 °C (Fig. 5). It is proposed that NLF expression is not regulated by photoperiod or temperature, but might be affected by an endogenous signal (Fig. 7). For comparison, in Arabidopsis, LFY is known to be the target of several endogenous signals, such as age (Wang et al., 2009) and gibberellin (Blazquez and Weigel, 2000; Mutasa-Gottgens and Hedden, 2009). In agreement with the known functions of FT and LFY in Arabidopsis (Kobayashi and Weigel, 2007), it is argued that in Narcissus, NtFT and NLF might act in parallel signalling flows, rather than in a downstream cascade. However, the present results suggest that under ambient temperature NLF does not take part in the floral transition, but is up-regulated slightly later, at the initiation stage (see also Noy-Porat et al. 2010).
Organography and spatial patterns of NtFT and NLF expression
Numerous studies (Carmona et al., 2002; Wada et al., 2002; Hsu et al., 2006; Hattasch et al., 2008; Igasaki et al., 2008) have shown that transcription of FT and LFY homologues in perennial plants coincides with flower induction, and that these genes are involved in floral meristem formation. The ‘florigen’ theory states that the light signal is perceived in the leaves, leading to the formation of FT mRNA (Corbesier et al., 2007; Turck et al., 2008). Surprisingly, however, in Narcissus, both NtFT and NLF were expressed in meristems and leaf primordia within the bulb, but not in foliage leaves or other mature vegetative organs (Fig. 3; Noy-Porat et al., 2010). NtFT expression was found mainly in the central zone of the meristem, prior to its shift to reproductive development. At this stage, the vascular system of the reproductive organs is not differentiated. Therefore, NtFT is assumed to play a key role in the meristem transition to reproductive development, but NtFT mRNA is transcribed in the renewal bud inside the bulb and is not translocated from other organs. On the other hand, NLF is up-regulated in the apical meristem later than NtFT and might be involved in several stages of florogenesis, from the meristem transition to flower differentiation and gametogenesis (Noy-Porat et al., 2010; Fig. 3D). Similar activity has been demonstrated for LFY homologues in garlic (Rotem et al., 2011). In Arabidopsis, LFY is expressed throughout the development of floral meristems and also activates different floral organ identity genes in distinct patterns within the flower. This seems to result from interactions between the globally expressed LFY and cofactors expressed in more spatially restricted domains (Krizek and Fletcher, 2005; Moyroud et al., 2010).
In addition to florogenesis, FT and LFY homologues might be involved in a range of plant growth processes. Shalit et al. (2009) showed that in the perennial tomato, SFT, the respective orthologue of FT, regulates diverse growth processes, such as flowering, growth and termination of typical perennial plant cycles, leaf maturation, growth of stems, and the formation of abscission zones. The FT homologues have been suggested to control seasonal growth cessation as well as flowering in Populus and Norway spruce (Picea abies) (Bohlenius et al., 2006; Gyllenstrand et al., 2007; Olsen, 2010). FT-like proteins have also been suggested to regulate potato tuberization (Abelenda et al., 2011). In Narcissus, the elevated expression of NtFT in leaf primordia suggests a role in leaf development (Figs 3, 4). Storage at 12 °C prevented NtFT expression and also negatively affected leaf elongation after planting (Fig. 5). It is therefore possible that NtFT down-regulation at 12 °C inhibits both flower induction and leaf development. Further studies might also reveal a possible role for FT homologues in dormancy induction and the bulbing process (Okubo, 2012).
LFY homologues have been shown to play a significant role in compound leaf development of Medicago truncatula (Wang et al., 2008), and have also been detected in leaf primordia in Vitis (Carmona et al., 2002), tomato (Molinero-Rosales et al., 1999), radish (Oshima and Nomura, 2008), Populus (Rottmann et al., 2000), and Eucalyptus (Southerton et al., 1998). Similarly, the present findings show that NLF might be involved in leaf or scale development within the bulb.
In conclusion, high temperature is required for floral induction inside the bulb of N. tazetta during the summer quiescent period, while the photoperiodic signal is probably not essential for flower transition. These findings expand our understanding of the flowering process in various life forms, and open up the use of Narcissus as an alternative perennial plant model for studies of flowering control.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056 [DOI] [PubMed] [Google Scholar]
- Abelenda JA, Navarro C, Prat S. 2011. From the model to the crop: genes controlling tuber formation in potato. Current Opinion in Biotechnology 22, 287–292 [DOI] [PubMed] [Google Scholar]
- Albani MC, Coupland G. 2010. Comparative analysis of flowering in annual and perennial plants. Current Topics in Developmental Biology 91, 323–348 [DOI] [PubMed] [Google Scholar]
- An HL, Roussot C, Suarez-Lopez P, et al. 2004. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131, 3615–3626 [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. 2006. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genetics 2, 980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini ES, Kramer EM. 2011. Environmental and molecular analysis of the floral transition in the lower eudicot . Aquilegia formosa. EvoDevo 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlloch R, Berbel A, Serrano-Mislata A, Madueño F. 2007. Floral initiation and inflorescence architecture: a comparative view. Annals of Botany 100, 659–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez MA, Ahn JH, Weigel D. 2003. A thermosensory pathway controlling flowering time in Arabidopsis thaliana . Nature Genetics 33, 168–171 [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Weigel D. 2000. Integration of floral inductive signals in Arabidopsis. Nature 404, 889–892 [DOI] [PubMed] [Google Scholar]
- Bohlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O. 2006. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312, 1040–1043 [DOI] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C. 2004. Multiple pathways in the decision to flower: enabling, promoting, and resetting. The Plant Cell 16, S18–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona MJ, Cubas P, Martinez-Zapater JM. 2002. VFL, the grapevine FLORICAULA/LEAFY ortholog, is expressed in meristematic regions independently of their fate. Plant Physiology 130, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Coupland G. 2006. The quest for florigen: a review of recent progress. Journal of Experimental Botany 57, 3395–3403 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang SH, et al. 2007. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033 [DOI] [PubMed] [Google Scholar]
- Dulberger R. 1967. Pollination systems in plants of Israel: heterostyly. Dissertation, The Hebrew University of Jerusalem.
- Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M. 2005. Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Research 14, 703–712 [DOI] [PubMed] [Google Scholar]
- Gyllenstrand N, Clapham D, Kallman T, Lagercrantz U. 2007. A Norway spruce FLOWERING LOCUS T homolog is implicated in control of growth rhythm in conifers. Plant Physiology 144, 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. 2003. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. The Plant Journal 33, 875–885 [DOI] [PubMed] [Google Scholar]
- Hanks GR. 2002. Narcissus and daffodil: the genus Narcissus. London: Taylor & Francis [Google Scholar]
- Hattasch C, Flachowsky H, Kapturska D, Hanke MV. 2008. Isolation of flowering genes and seasonal changes in their transcript levels related to flower induction and initiation in apple (Malus domestica). Tree Physiology 28, 1459–1466 [DOI] [PubMed] [Google Scholar]
- Hayama R, Agashe B, Luley E, King R, Coupland G. 2007. A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in Pharbitis . The Plant Cell 19, 2988–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Dean C. 2004. Control of Arabidopsis flowering: the chill before the bloom. Development 131, 3829–3838 [DOI] [PubMed] [Google Scholar]
- Hofer J, Turner L, Hellens R, Ambrose M, Matthews P, Michael A, Ellis N. 1997. UNIFOLIATA regulates leaf and flower morphogenesis in pea. Current Biology 7, 581–587 [DOI] [PubMed] [Google Scholar]
- Hou CJ, Yang CH. 2009. Functional analysis of FT and TFL1 orthologs from orchid (Oncidium Gower Ramsey) that regulate the vegetative to reproductive transition. Plant and Cell Physiology 50, 1544–1557 [DOI] [PubMed] [Google Scholar]
- Hsu CY, Liu YX, Luthe DS, Yuceer C. 2006. Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. The Plant Cell 18, 1846–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igasaki T, Watanabe Y, Nishiguchi M, Kotoda N. 2008. The FLOWERING LOCUS T/TERMINAL FLOWER 1 family in Lombardy poplar. Plant and Cell Physiology 49, 291–300 [DOI] [PubMed] [Google Scholar]
- Jaakola L, Pirttila AM, Halonen M, Hohtola A. 2001. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Molecular Biotechnology 19, 201–203 [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. 2007. FT protein acts as a long-range signal in Arabidopsis. Current Biology 17, 1050–1054 [DOI] [PubMed] [Google Scholar]
- Jang S, Torti S, Coupland G. 2009. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. The Plant Journal 60, 614–625 [DOI] [PubMed] [Google Scholar]
- Jung C, Muller AE. 2009. Flowering time control and applications in plant breeding. Trends in Plant Science 14, 563–573 [DOI] [PubMed] [Google Scholar]
- Kamenetsky R, Zaccai M, Flaishman MA. 2012. Florogenesis. In: Kamenetsky R, Okubo H, eds. Ornamental geophytes: from basic science to sustainable production. Boca Raton, FL: CRC, Taylor and Francis Group, 197–232 [Google Scholar]
- Kanno A, Nakada M, Akita Y, Hirai M. 2007. Class B gene expression and the modified ABC model in nongrass monocots. Scientific World Journal 7, 268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Pajoro A, Angenent GC. 2010. Regulation of transcription in plants: mechanisms controlling developmental switches. Nature Reviews Genetics 11, 830–842 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Ono N, Hayashi Y, et al. 2011. FLOWERING LOCUS T regulates stomatal opening. Current Biology 21, 1232–1238 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Weigel D. 2007. Move on up, it’s time for change—mobile signals controlling photoperiod-dependent flowering. Genes and Development 21, 2371–2384 [DOI] [PubMed] [Google Scholar]
- Koike Y, Ohbiki A, Mori G, Imanishi H. 1994. Effects of bulb storage temperatures and duration on the flowering of Narcissus tazetta var. Chinensis. Journal of the Japanese Society for Horticultural Science 63, 639–644 [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M. 2002. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant and Cell Physiology 43, 1096–1105 [DOI] [PubMed] [Google Scholar]
- Komiya R, Yokoi S, Shimamoto K. 2009. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136, 3443–3450 [DOI] [PubMed] [Google Scholar]
- Krizek BA, Fletcher JC. 2005. Molecular mechanisms of flower development: an armchair guide. Nature Reviews Genetics 6, 688–698 [DOI] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA. 2010. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140, 136–147 [DOI] [PubMed] [Google Scholar]
- Lee H, Yoo SJ, Lee JH, Kim W, Yoo SK, Fitzgerald H, Carrington JC, Ahn JH. 2010. Genetic framework for flowering-time regulation by ambient temperature-responsive miRNAs in Arabidopsis. Nucleic Acids Research 38, 3081–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hong SM, Yoo SJ, Park OK, Lee JS, Ahn JH. 2006. Integration of floral inductive signals by flowering locus T and suppressor of overexpression of Constans 1. Physiologia Plantarum 126, 475–483 [Google Scholar]
- Li CY, Zhang K, Zeng XW, Jackson S, Zhou Y, Hong YG. 2009. A cis element within Flowering Locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. Journal of Virology 83, 3540–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz E, Eshed Y. 2006. Universal florigenic signals triggered by FT homologues regulate growth and flowering cycles in perennial day-neutral tomato. Journal of Experimental Botany 57, 3405–3414 [DOI] [PubMed] [Google Scholar]
- Lin MK, Belanger H, Lee YJ, et al. 2007. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. The Plant Cell 19, 1488–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR, Davis SJ. 2010. Ambient thermometers in plants: from physiological outputs towards mechanisms of thermal sensing. Current Biology 20, R1086–R1092 [DOI] [PubMed] [Google Scholar]
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T. 2008. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana . Nature Genetics 40, 1489–1492 [DOI] [PubMed] [Google Scholar]
- Molinero-Rosales N, Jamilena M, Zurita S, Gomez P, Capel J, Lozano R. 1999. FALSIFLORA, the tomato orthologue of FLORICAULA and LEAFY, controls flowering time and floral meristem identity. The Plant Journal 20, 685–693 [DOI] [PubMed] [Google Scholar]
- Moyroud E, Kusters E, Monniaux M, et al. 2010. LEAFY blossoms. Trends in Plant Science 15, 346–352 [DOI] [PubMed] [Google Scholar]
- Mutasa-Gottgens E, Hedden P. 2009. Gibberellin as a factor in floral regulatory networks. Journal of Experimental Botany 60, 1979–1989 [DOI] [PubMed] [Google Scholar]
- Nishikawa F, Endo T, Shimada T, Fujii H, Shimizu T, Kobayashi Y, Araki T, Omura M. 2010. Transcriptional changes in CiFT-introduced transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Tree Physiology 30, 431–439 [DOI] [PubMed] [Google Scholar]
- Nishikawa F, Endo T, Shimada T, Fujii H, Shimizu T, Omura M, Ikoma Y. 2007. Increased CiFT abundance in the stem correlates with floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.). Journal of Experimental Botany 58, 3915–3927 [DOI] [PubMed] [Google Scholar]
- Noy-Porat T, Flaishman MA, Eshel A, Sandler-Ziv D, Kamenetsky R. 2009. Florogenesis of the Mediterranean geophyte Narcissus tazetta and temperature requirements for flower initiation and differentiation. Scientia Horticulturae 120, 138–142 [Google Scholar]
- Noy-Porat T, Kamenetsky R, Eshel A, Flaishman MA. 2010. Temporal and spatial expression patterns of the LEAFY homologue NLF during florogenesis in Narcissus tazetta . Plant Science 178, 105–113 [Google Scholar]
- Okubo H. 2012. Dormancy. In: Kamenetsky R, Okubo H, eds. Ornamental geophytes: from basic science to sustainable production. Boca Raton, FL: CRC, Taylor and Francis Group, 233–260 [Google Scholar]
- Olsen JE. 2010. Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Molecular Biology 73, 37–47 [DOI] [PubMed] [Google Scholar]
- Oshima S, Nomura K. 2008. RsLFY, a LEAFY homologue gene in radish (Raphanus sativus), is continuously expressed in vegetative, reproductive and seed development. Plant Biotechnology 25, 579–582 [Google Scholar]
- Pin PA, Nilsson O. 2012. The multifaceted roles of FLOWERING LOCUS T in plant development. Plant, Cell and Environment 35, 1742–1755 [DOI] [PubMed] [Google Scholar]
- Rotem N, David-Schwartz R, Peretz Y, Sela I, Rabinowitch HD, Flaishman M, Kamenetsky R. 2011. Flower development in garlic: the ups and downs of gaLFY expression. Planta 233, 1063–1072 [DOI] [PubMed] [Google Scholar]
- Rotem N, Shemesh E, Peretz Y, Akad F, Edelbaum O, Rabinowitch HD, Sela I, Kamenetsky R. 2007. Reproductive development and phenotypic differences in garlic are associated with expression and splicing of LEAFY homologue gaLFY. Journal of Experimental Botany 58, 1133–1141 [DOI] [PubMed] [Google Scholar]
- Rottmann WH, Meilan R, Sheppard LA, Brunner AM, Skinner JS, Ma CP, Cheng SP, Jouanin L, Pilate G, Strauss SH. 2000. Diverse effects of overexpression of LEAFY and PTLF, a poplar (Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. The Plant Journal 22, 235–245 [DOI] [PubMed] [Google Scholar]
- Samach A, Wigge PA. 2005. Ambient temperature perception in plants. Current Opinion in Plant Biology 8, 483–486 [DOI] [PubMed] [Google Scholar]
- Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y, Lifschitz E. 2009. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proceedings of the National Academy of Sciences, USA 106, 8392–8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southerton SG, Strauss SH, Olive MR, Harcourt RL, Decroocq V, Zhu XM, Llewellyn DJ, Peacock WJ, Dennis ES. 1998. Eucalyptus has a functional equivalent of the Arabidopsis floral meristem identity gene LEAFY. Plant Molecular Biology 37, 897–910 [DOI] [PubMed] [Google Scholar]
- Tan FC, Swain SM. 2006. Genetics of flower initiation and development in annual and perennial plants. Physiologia Plantarum 128, 8–17 [Google Scholar]
- Taylor K. 2009. Biological Flora of the British Isles: Urtica dioica L. Journal of Ecology 97, 1436–1458 [Google Scholar]
- Taylor A, Massiah AJ, Thomas B. 2010. Conservation of Arabidopsis thaliana photoperiodic flowering time genes in onion (Allium cepa L.). Plant and Cell Physiology 51, 1638–1647 [DOI] [PubMed] [Google Scholar]
- Townsend T, Albani M, Wilkinson M, Coupland G, Battey N. 2006. The diversity and significance of flowering in perennials. In: Ainsworth C, ed. Flowering and its manipulation. Oxford: Blackwell Publishing, 181–201 [Google Scholar]
- Turck F, Fornara F, Coupland G. 2008. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annual Review of Plant Biology 59, 573–594 [DOI] [PubMed] [Google Scholar]
- Wada M, Cao QF, Kotoda N, Soejima J, Masuda T. 2002. Apple has two orthologues of FLORICAULA/LEAFY involved in flowering. Plant Molecular Biology 49, 567–577 [DOI] [PubMed] [Google Scholar]
- Wang HL, Chen JH, Wen JQ, Tadege M, Li GM, Liu Y, Mysore KS, Ratet P, Chen RJ. 2008. Control of compound leaf development by FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago truncatula . Plant Physiology 146, 1759–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Czech B, Weigel D. 2009. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana . Cell 138, 738–749 [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O. 1995. A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495–500 [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. 2005. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309, 1056–1059 [DOI] [PubMed] [Google Scholar]
- Wilkie JD, Sedgley M, Olesen T. 2008. Regulation of floral initiation in horticultural trees. Journal of Experimental Botany 59, 3215–3228 [DOI] [PubMed] [Google Scholar]
- Xu F, Rong X, Huang X, Cheng S. 2012. Recent advences of FLOWERING LOCUS T gene in higher plants. International Journal of Molecular Sciences 13, 3773–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahel H, Sandler D. 1986. Retarding the flowering of Narcissus tazetta cv. ‘Ziva’. Acta Horticulturae 177, 189–195 [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. 2006. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proceedings of the National Academy of Sciences, USA 103, 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD. 2007. FT protein, not mRNA, is the phloem mobile signal for flowering. In: Taiz L, Zeiger E, eds. Plant physiology. Sunderland, MA: Sinauer Associates Inc, 1–8 [Google Scholar]
- Zhang JZ, Li ZM, Yao JL, Hu CG. 2009. Identification of flowering-related genes between early flowering trifoliate orange mutant and wild-type trifoliate orange (Poncirus trifoliata L. Raf.) by suppression subtraction hybridization (SSH) and macroarray. Gene 430, 95–104 [DOI] [PubMed] [Google Scholar]