Abstract
Small or shrivelled wheat kernels (screenings) that reduce crop value are commonly produced in terminal drought environments. The aim of this study was to establish whether the incorporation of the tiller inhibition (tin) gene would contribute to maintenance of kernel weight and reductions in screenings under terminal water deficit. Five Silverstar near-isogenic lines contrasting in high and low tiller potential and their recurrent Silverstar parent were established at two plant densities under managed terminal water deficit (mild and severe) and irrigated conditions. With irrigation (grain yield of 5.6 t ha–1), kernels of all lines weighed ~31mg, with restricted-tillering (R-tin) lines producing an average 15% lower grain yield. Under both mild and severe terminal water deficit (4.1 t ha–1 and 2.8 t ha–1), free-tillering lines had relatively high screenings ranging from 11.9% to 16.2%. Compared with free-tillering lines, R-tin lines maintained large kernel weight (~29mg kernel–1) and had 29% and 51% fewer screenings under the two stresses, and a significantly greater (+11%) grain yield under mild stress. Higher kernel weights in tin lines were realized even with the greater kernel number per spike. The higher kernel weight of the R-tin lines under stress conditions was associated with greater anthesis biomass and increased stem water-soluble carbohydrates, ensuring more assimilate for later translocation to filling grain. The incorporation of the tin gene into genetic material adapted to the target environments provides scope for improvement in both grain yield and kernel weight, and a reduction in screenings in terminal water deficit environments.
Key words: Dryland agriculture, grain size, tiller inhibition gene, water-soluble carbohydrate, water stress, yield.
Introduction
Crops grown in terminal water deficit environments become increasingly water stressed as the season progresses. Water deficit, particularly after anthesis, is a major limitation to wheat (Triticum aestivum L.) yield, especially in the northern wheatbelt of Australia where crop water use relies strongly on stored soil moisture (Chenu et al., 2011), and in the dry western and southern wheatbelt in years when spring rains finish early. In the northern region, crops are sown into a full or near-full moisture profile, stored soil moisture declines as the season progresses, and the impact of stress on yield components, and therefore yield, will depend on the timing of the onset of stress in relation to crop development and particularly anthesis (e.g. Gallagher et al., 1975; Woodruff and Tonks, 1983; Mitchell et al., 1996; Chapman, 2008). In the driest years on soils with low water storage, pre-anthesis water deficit limits assimilate supply, and therefore growth and development of competing sinks (e.g. leaf area development and floret fertility), reducing kernel number. While post-anthesis water deficit can result in grain abortion during early grain growth, the main impact is to reduce kernel weight. Combined with rising air temperatures during grain filling, water deficit can reduce assimilate supply, resulting in production of small or shrivelled kernels (screenings), unless assimilate supply can be offset by the availability of stored water-soluble carbohydrates (WSCs) (Dreccer et al., 2009). While drought-affected yield directly limits farm profit, the value of the crop is further reduced when small grain screenings are high.
Typically, wheat seedlings tiller profusely in developing a large leaf area early in the season, prior to initiation of the reproductive structures (Duggan et al., 2005). While this may be an advantage to control weeds (by covering the soil quickly), there is a cost if demand for water is so high that crops exhaust soil water before the completion of grain filling. Reducing demand for water early in the season should increase availability of water for post-anthesis crop growth (Richards and Townleysmith, 1987). Restricted tillering has been proposed as a method for reducing leaf area development, thereby slowing canopy growth early in the season (Islam and Sedgley, 1981; Yunusa and Sedgley, 1992). Islam and Sedgley (1981) demonstrated that manually de-tillered wheat utilized less water pre-anthesis to leave more water for post-anthesis growth. However, Duggan et al. (2005) and Yunusa and Sedgley (1992) compared different reduced- and free-tillering near-isogenic lines, and observed either no difference in leaf area index (LAI; southern NSW), or an even greater LAI (Western Australia) in reduced-tillering lines. Both these researchers and others (Marshall and Boyd, 1985; Richards, 1988) have reported that the large individual leaf size of reduced-tillering lines compensated for fewer tillers and therefore water use may not be conserved for use during grain filling (Yunusa and Sedgley, 1992).
In the northern Australian wheatbelt, it was observed that the tiller inhibition (tin) gene when incorporated in the Silverstar genetic background produced a reduced LAI (Mitchell, 2010), in part because the warmer environment accelerates development and therefore the ‘compensation’ by leaf size for reduced tiller number is reduced. Previous experiments (Mitchell, 2010) conducted under conditions of non-limiting water availability post-anthesis, identified tin lines that achieved relatively high grain yield and maintained a high kernel weight. However, the greatest advantage of reduced-tillering lines is likely to occur under terminal water deficit conditions. The objective of the experiment reported here was to assess Silverstar tin and free-tillering counterparts in their ability to maintain kernel weight when grown under a carefully managed terminal water deficit using a rainout shelter facility. The study aimed to examine: (i) whether stem and spike production (i.e. retention) in tin lines was altered by exposure to terminal water deficit; (ii) whether the kernel weight advantage of tin lines was maintained and screenings reduced under terminal water deficit; (iii) whether the tin lines at high plant density were able to maintain a kernel weight advantage under terminal water deficit; (iv) whether tin lines were able to maintain grain yield comparable with free-tillering lines when exposed to a terminal water deficit; and (v) to explain the morphological and physiological basis for differences in kernel weight and grain yield.
Materials and methods
Environment
The experiment was conducted at the University of Queensland Research Farm at Gatton (27°33ʹS, 152°17ʹE, 98 m) in south-east Queensland in 2007, using automatic rainout shelters to create drought conditions when required. Gatton has a subtropical climate with predominantly summer rainfall (784mm average) and mild, dry winters that are slightly warmer than those of the northern region wheatbelt which passes ~100 km to the west. The experimental site was located on an alluvial Lockyer prairie soil [USDA Soil Taxonomy: Fluventic Haplustoll; Isbell (1996)] and consisted of light, black clay becoming brown at ~60–80cm depth. Soil pH tends to be alkaline (~8.2). Powell (1982) has described the soil physical and chemical characteristics in detail. Daily weather data were obtained from a Bureau of Meteorology weather station (040436; Gatton DPI Research Station) located ~400 m from the trial site.
Experimental design and water supply
Six lines representing three contrasting tillering groups were selected based on previous experimental work, and their selection history was given by Mitchell (2010). These were two lines characterized as ‘restricted tillering’ (R-tin lines SsrT17, SsrT65), one line with ‘semi-restricted tillering’ (SR-tin, SsrT16), and three ‘free-tillering’ lines (non-tin lines SsrW35, SsrW47, and cv. Silverstar). Except for the Silverstar parent line, all other lines were BC2F6:8 breeding lines derived by backcrossing a tin donor to the spring wheat variety Silverstar (G. Rebetzke, personal communication).
Three contrasting environments were established: non-water-limiting, irrigated (I), and both mild (MS) and severe (SS) terminal water deficit conditions, the latter having limited water at depth. The experimental plants were machine sown into dry topsoil on 16 May 2007. Access tubes to monitor soil moisture with a neutron moisture meter were inserted the following day. To ensure good plant establishment, all treatments received 65mm irrigation in the first 3 weeks after sowing using overhead sprinklers. After this time, water (rainfall and irrigation) was excluded from the water deficit treatments through the use of two automatic rainout shelters, each covering an area of 281 m2, of which the treatment area comprised 225 m2.
In the area containing the severe stress treatment, there was a slight trend in initial soil moisture content across the experimental site and therefore the replicates were blocked to take this variation into account. At the time of stem elongation, replicate 1 was severely stressed, and it was anticipated that this replicate would fail to produce grain if water was not supplied. Based on initial soil moisture content, it was calculated that there was a difference of 30mm (available) soil moisture between replicate 1 and replicates 2 and 3. Subsequently, on one occasion (62 days after sowing; DAS), T-tape irrigation was used to carefully supply 30mm of water to replicate 1 only. The irrigated regime (located 20 m west of the shelters) received an additional 30mm irrigation at 74 DAS (irrigation was halted at anthesis to avoid lodging), and continued to grow with access to a substantial store (>160mm) of soil water.
Within each water regime, the six lines were grown at two plant densities in a split-plot design with three replicates per treatment. The target plant density was 100 plants m–2 in the low density (LD) treatment, which was consistent with commercial practice, and ~200 plants m–2 in the high density (HD) treatment.
The plot size was 1.80×4 m in the irrigated treatment and 1.32×3 m in the water deficit treatments. Each plot consisted of eight (irrigated) or six (stress treatments) rows spaced at 0.22 m. Plants in the rainout shelters were thinned 3 weeks after sowing to achieve 100 and 200 plants m–2. In the irrigated treatment, this was achieved via different seeding rates adjusted for seed source kernel weight. Plant establishment counts were 114 and 195 plants m–2 for the 100 and 200 plants m–2 treatments, respectively.
Crop management
Prior to sowing, the experimental site had a well-managed, sorghum cover crop to reduce the total soil water store. The sorghum cover crop was slashed and rotary hoed to incorporate the sorghum before deep-ripping to 0.3 m depth. Immediately prior to sowing, the areas were twice cultivated with a rotary hoe. Crop King 88 fertilizer (15.1% N: 4.4 P: 11.5 K: 13.6 S) was applied before sowing at a rate of 300kg ha–1 to minimize nutrient limitation. Aphids were controlled by one application of Rogor® (dimethoate 100g l–1 at a rate of 750ml ha–1) on 19 July.
Measurements
Soil water content
A neutron moisture meter (NMM, model CPN 503 DR Campbell Pacific Nuclear International Inc., USA) was used to monitor the volumetric soil moisture content for four lines (SsrT65, SsrT16, SsrW35, and Silverstar) grown at both LD and HD in the mild stress treatment, and for three lines (SsrT65, SsrW35, and Silverstar) grown at HD in the severe stress treatment. A 2 m long aluminium access tube (0.05 m diameter) was inserted between two adjacent rows in the centre of each plot. NMM readings (at 16 s intervals) were obtained from a depth of 0.3 m to 1.7 m at 0.2 m depth intervals, with initial readings taken at 31 DAS and then three times corresponding closely to biomass harvest times; at 53 (~stem elongation), 88 (~anthesis), and 158 (~after maturity) DAS. Soil cores (0.05 m diameter×2 m length) were taken at the beginning and end of the experimental period for NMM calibration. This calibration was used to convert the NMM count readings to volumetric moisture content measurements. Plant available water (PAW) calculations were based on measurements of drained upper limit of Lockyer Levee Prairie soil sampled nearby (Dalgliesh and Foale, 2005) and the lower limit which was assumed to be the soil moisture remaining in the profile at maturity of the wheat crop.
Leaf water potential, relative water content, and canopy-to-air temperature depression
Mid-day leaf water potential (LWP) measurements were taken on clear sunny days at 47 (irrigated and severe stress treatments only) and 60 DAS (all treatments) for two (SsrT65 and Silverstar) lines at both plant densities. Leaf relative water content was measured on flag leaves from all lines at 84 DAS, where this measurement coincided with anthesis in the mild and severe stress treatments.
Canopy-to-air temperature depression (CTD) was measured on all plots with a hand-held infrared thermometer (Mikron® Infrared Inc., USA, model MI-N14/N15). Measurements were taken between 11:00h and 12:00h at 55 and 84 DAS, and between 09:00h and 10:00h and again at between 15:00h and 16:00h at 60 DAS. Two (4 s average) readings were taken from opposite northern corners of each plot (i.e. with the winter sun behind the observer). In order to avoid detecting soil temperature, the infrared thermometer was held so that the sensor viewed only the canopy at an angle of ~25 ° below the horizontal.
Light interception
Light interception (photosynthetically active radiation, PAR) was measured on three (45, 60, and 82 DAS) occasions using a 0.9 m linear ceptometer (AccuPAR, Decagon Devices Inc., WA, USA). The sensor was inserted below the canopy close to ground level at an ~45 ° angle to the rows. Two measurements were taken below and another above the canopy for each plot. Percentage light interception was expressed as the difference between above and mean below canopy readings.
Plant sampling
Phenology was recorded using the Zadoks decimal code (Zadoks et al., 1974). Anthesis date (DC65) was estimated as when ~50% of heads in a plot contained dehisced anthers. At mid-grain fill, plant height (cm) was determined (mean distance from the base of the plant to the tip of the ear, excluding awns). Loss of green colour from glumes from 50% of plants was scored to determine physiological maturity.
Total biomass and stem number were sampled by quadrats taken at three stages during the growing season. All lines were sampled at the same time for the first harvest at stem elongation (DC31). Anthesis and maturity harvests were taken when each plot had reached anthesis and physiological maturity. Quadrats were sampled to leave at least two guard rows either side and six plants in a row between sampling areas. An area of 0.11 m2 was sampled for the initial harvest which coincided with stem elongation (47 DAS). The area at the anthesis harvest (86–94 DAS) was 0.22 m2, while the area at the maturity harvest (136–152 DAS) was 0.44 m2. At each harvest, stems were cut just below the soil surface so that stem number per plant could be recorded, and above-ground biomass partitioned into stems plus leaf sheath, leaf blade, and spike (when present) before oven-drying at 70 °C for a minimum of 48h. The ratio of maturity spike number to maximum stem number (SPSR) was used to estimate stem loss over the season.
At the anthesis harvest, a subsample of leaf blade was used to estimate leaf area using a laser area meter (CI-203, CID Bio-Science, USA). The leaf material of known area (at least 600cm2) was oven-dried at 70 ° C and specific leaf area estimated for the calculation of LAI. WSC measurements were obtained from grab samples (four grabs per plot, ~10–15 stems per grab) taken from each plot before 11:00h and immediately placed in a 70 °C oven and dried for a minimum of 48h. After drying, leaf blades were removed, and stem and leaf sheath material was ground in a Wiley mill to pass a 1mm screen. These samples were then scanned at 2nm intervals in the 1100–2500nm range by near-infrared reflectance spectroscopy. A subset of lines was selected upon which an anthrone extraction was performed to extract WSCs (van Herwaarden et al., 1998). Total tissue N (0.2g) was also determined on the same samples by combustion analysis (CNS-2000 Combustion Analyser, LECO, USA). Calibration equations were developed for WSC and N on this subset, and the WSC and N concentrations were subsequently predicted (R 2=0.92–0.96) for all samples. Concentrations of stem and leaf sheath combined (for simplicity, referred to as ‘stem’) WSC and N at anthesis and maturity were estimated. The WSC and N were converted to an amount based on total anthesis biomass and then expressed on a per spike (WSCS, NS) and per kernel (WSCK, NK) basis by dividing by maturity spike and kernel number, respectively. Thus, respectively, the WSCS and NS, and WSCK and NK were considered the anthesis WSC and N that were potentially available for translocation to the spike or kernel during the grain-filling period.
At maturity, a small mechanical thresher was used to separate kernels from chaff. Kernels were oven-dried at 40 °C for 4 d and grain yield was expressed per unit ground area. Harvest index (HI) was calculated as the ratio of kernel weight to total above-ground biomass. In the irrigated treatment, plots were machine harvested at maturity after plot end-trimming. Average kernel weight was determined by weighing 300 kernels per plot from a quadrat sample. Kernel number per unit area was determined by dividing grain yield by kernel weight. Percentage grain screenings was determined on all plots according to industry standards [i.e. 40 shakes on Agtator (http://www.graintec.com.au/) with a 2mm slotted sieve].
Statistical analysis
Statistical analysis was conducted using Genstat® software. Significant differences among treatment means were considered at an α of 0.05 (i.e. P < 0.05) unless stated otherwise. Analyses were applied within and across treatments.
An analysis of variance (ANOVA) was conducted on n l lines at n d plant density with n b randomized blocks for each individual treatment. Traits, for example grain yield, were partitioned using the linear statistical model where the observed yield of the ijkth plot was: Y ijk=m+l i+d j+(l d)ij+b k+ε ijk, where Y ijk=yield of the ith line at jth plant density in the kth block; m=the overall mean; l i=effect of the ith line, i=1,...,n l; d j=effect of the jth plant density, j=1,...,n d; b k=effect of the kth block, k=1,...,n b; ε ijk=random error associated with the ijkth plot.
For each trait and sample time, a pooled ANOVA over the irrigated, mild, and severe stress treatments (environments) was also conducted. ANOVA was conducted on n l lines at n d plant density with n b randomized blocks over n e environments (treatments).
The observed yield of the ijklth plot, Yijkl, was represented by the model: Y ijkl=m+e i+(b/e)il=l j+(le)ij+d k+(ld)jk+(led)ijk+ε ijkl, where m=the overall mean; e i=effect of the ith environment, i=1,.....,n e; l j =effect of the jth line, j=1,.....,n l; d k=effect of the kth plant density, j=1,...,n k; (b/e) il=ε1=effect of the lth block within the ith environment, l=1,.....,n b; ε ijkl=ε 2=random error associated with the ijklth plot; and (le)ij, (ld)jk, and (led) ijk are the line by environment, line by density, and line by environment by density interaction and effect for the ith line at the kth density in the jth environment.
Additional analyses were conducted in which lines were partitioned into allelic (tin versus free-tillering) contrasts, and orthogonal contrasts were used to test for statistical significance between tillering groups [see Mitchell (2010) for an explanation of tillering group contrasts] of lines (R-tin versus SR-tin vs. free-tillering). Results throughout this paper are reported on a tillering group basis contrasting the R-tin, SR-tin, and free-tillering (W) lines, and also reported on a line basis. Associations between traits, such as the components of grain yield, were determined using phenotypic correlations (on a line-mean basis).
Results
Weather and soil water status
The irrigated treatment received 171mm of rainfall during the season. Around 70mm of this rain occurred between 90 and 100 DAS which coincided with anthesis for all genotypes in the irrigated treatment. Daily maximum and minimum air temperature ranged between 14 and 36 °C and 1 and 18 °C, respectively. Temperatures tended to decline until 50 DAS (early July) and thereafter increased up to maturity, with maximum daily temperature frequently exceeding 30 °C in the latter part of grain filling. No frosts occurred.
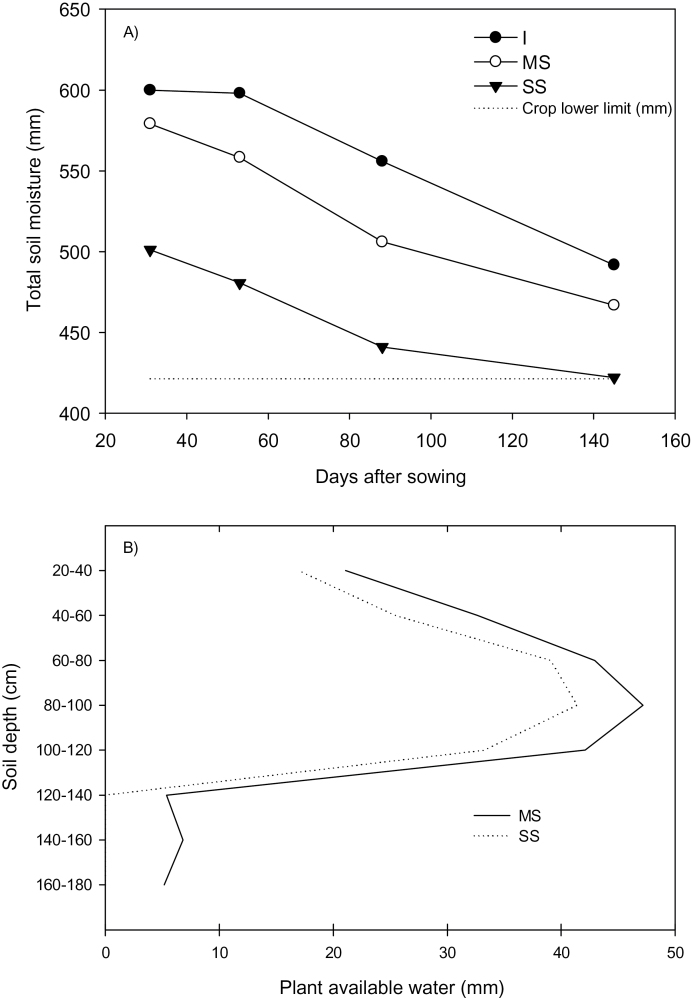
As the season progressed, the total soil moisture in the profile declined (Fig. 1A) even in the irrigated treatment which had received an additional 200mm water (rainfall and irrigation) during the season. As planned, the two rainout shelter treatments differed in initial PAW, with the mild and severe stress treatments beginning with 203mm and 125mm, respectively. The 78mm difference in moisture content was due to the presence of 5–9mm more PAW in each layer of the profile of the mild stress compared with severe stress treatments, and the severe stress had almost no available water below 120cm (Fig. 1B).
Fig. 1.
(A) Total soil moisture (mm) in the soil profile (20–180cm) on four occasions in the 2007 Gatton irrigated (C), mild (MS) and severe (SS) stress treatments; and (B) plant available water (mm) in the soil profile (20–180cm) 53 DAS in the 2007 Gatton mild (MS) and severe (SS) stress treatments.
By 88 DAS (~anthesis), 65% and 76% of total water extraction for the season had occurred in the mild and severe stress treatment, respectively. Lines grew on a progressively depleting soil moisture profile, a typical terminal drought pattern, with lines in the mild stress treatment able to access more water post-anthesis than those in the severe stress treatment. Water use at individual depths for the period between 31 and 144 DAS in the mild stress treatment identified significant tillering group contrasts at 150cm (P=0.06) and 170cm (P=0.07) depths, in which the SR-tin (SsrT16) line extracted 4mm more water than free-tillering lines at 150cm, and 2mm more than R-tin (SsrT65) at 170cm. No among-line water use differences were identified between initial sampling and stem elongation (31–53 DAS) for individual depths, but between stem elongation and anthesis (53–88 DAS) line differences existed at 30, 130, and 150cm, and tillering group contrasts were also significant at 50cm and 110cm. At all depths except 50cm, SsrT16 extracted a greater amount (ranging from 1.8mm to 5.9mm) of water than other lines. At 50cm, SsrT65 extracted 3mm more water than SsrT16 (P < 0.05).
In the severe stress treatment, analysis at individual depths indicated slight differences (P=0.08) at 130cm depth between stem elongation and anthesis; SsrT65 extracted 8.4mm, Silverstar 2.5mm, while SsrW35 extracted 6.5mm water. However, due to large errors associated with calculation of overall water use (coefficients of variation ranging from 8% to 39%), there were no significant density, line, or tin allele differences in total water use between any measurement time in the mild or severe stress treatment. Despite statistical non-significance in total water use, it is worthwhile examining the water extraction trends as small differences in extraction at different depths can contribute to significant differences in biomass and yield (Manschadi et al., 2006). Overall, in the mild stress treatment, the tin lines extracted 8mm more water than free-tillering lines between stem elongation and maturity (53–144 DAS) (Table 1A). In the severe stress treatment, SsrW35 extracted 126mm, which was 6mm more than SsrT65; this was due to an 11mm difference in soil water extraction between stem elongation and anthesis (53–88 DAS; Table 1B). However, between anthesis and maturity (88–144 DAS), SsrT65 extracted 5mm more than SsrW35.
Table 1.
Total soil moisture extracted (WU, mm) between sampling times (DAS) of (A) four lines (averaged across density) grown in the mild stress treatment; and (B) three lines grown in the severe stress treatment (HD and replicates 2 and 3 only)
| Line | Tiller group | WU (mm) | ||||
|---|---|---|---|---|---|---|
| FI–A | A–M | FI–M | ||||
| 31–53 DAS | 53–88 DAS | 88–144 DAS | 53–144 DAS | 31–144 DAS | ||
| (A) Mild stress | ||||||
| SsrT65 | R | 23 | 52 | 40 | 92 | 115 |
| SsrT16 | SR | 26 | 58 | 41 | 99 | 125 |
| Mean | tin | 25 | 55 | 41 | 96 | 120 |
| SsrW35 | W | 23 | 47 | 37 | 84 | 107 |
| Silverstar | W | 25 | 53 | 38 | 90 | 115 |
| Mean | W | 24 | 50 | 38 | 87 | 111 |
| Overall mean | 24 | 52 | 39 | 91 | 115 | |
| CV (%) | 22.9 | 22.5 | 25.3 | 20.1 | 17.9 | |
| (B) Severe stress | ||||||
| SsrT65 | R | 33 | 57 | 30 | 87 | 120 |
| SsrW35 | W | 33 | 68 | 25 | 93 | 126 |
| Silverstar | W | 26 | 54 | 25 | 78 | 106 |
| Mean | 30 | 60 | 27 | 86 | 117 | |
| CV (%) | 7.7 | 21.3 | 37.5 | 18.0 | 22.9 | |
CV, coefficient of variation; FI, floral initiation; A, anthesis; M, maturity.
Leaf water potential, relative water content, and canopy-to-air-temperature depression
As early as 47 DAS, the mean LWP at noon was higher in the irrigated (–1.1MPa) than in the severe stress (–1.7MPa) treatment (P < 0.05). At 60 DAS, the severity of stress as measured by LWP had increased in all treatments, with lines in the irrigated treatment maintaining –1.6MPa compared with the mild stress (–2.2MPa), and those in severe stress were at –3.1MPa. Lines at LD maintained a 0.60MPa higher LWP than those at HD in both mild and severe stress treatments. However, within water treatments at 60 DAS, lines had similar LWPs (P > 0.05). Similarly, only water treatment differences were significant for leaf relative water content at 84 DAS (~anthesis), in which the irrigated lines maintained an average of 89% leaf relative water content, while mild and severe stress had 79% and 77%, respectively. No tillering group/line, density, or interaction effects were observed for relative water content.
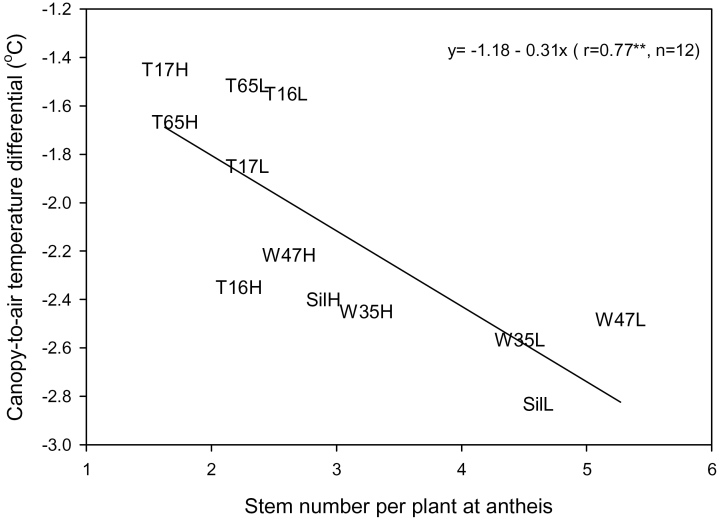
The mean CTD of lines in the severe stress (–2.5 °C) treatment was markedly less (more stressed) than that of lines grown in the mild stress (–4.4 °C) and irrigated (–5.5 °C) treatments. There was no change in average CTD prior to anthesis (in the period 55–84 DAS) for the treatments. Line and tin allele differences for CTD were not significant (P > 0.05) in the stress treatments but were significant for the irrigated (P < 0.05) treatment at 09:00h (when relative humidity was 48.9% and air temperature 10.9 °C) on day 60 (Fig. 2). At this measurement, the free-tillering lines, averaged across densities, were able to maintain larger (–2.5 °C) depressions than the tin lines (–1.7 °C). In particular, Silverstar had significantly greater depressions than the R-tin lines (SsrT65 and SsrT17), implying that when water was available, the Silverstar canopy was transpiring to a greater extent. In the irrigated treatment, there was a significant linear relationship between the 09:00h CTD at 60 DAS and stem number per plant at both 47 DAS (r 2=0.72) and anthesis (r 2=0.59; Fig. 2). The free-tillering (W) lines producing higher stem numbers per plant maintained higher CTD and therefore higher transpiration, particularly at LD.
Fig. 2.
Relationship between canopy-to-air temperature depression (°C) at 09:00h 60 DAS and anthesis stem number per plant of six Silverstar lines [R-tin (T17, T65), SR-tin (T16), and free tillering (W35, W47, and Silverstar)] at low (L) and high (H) plant density in the 2007 Gatton irrigated treatment.
Stem and spike number
Combined analysis across treatments for stem number per plant at stem elongation, maximum stem number per m2, and maturity spike number per m2 indicated highly significant (P < 0.01) differences within the environment, line, tillering group, and density treatments, with no significant interaction effect (Table 2A).
Table 2.
(A) Stem number per plant at stem elongation (SNPL); (B) maximum stem number per m2 (SN); and (C) maturity spike number per m2 (MSPN) of six Silverstar lines at low (LD) and high (HD) plant density, and across densities for each tillering group in the irrigated, mild, and severe stress treatments
| Line | Group | Irrigated | Mild Stress | Severe stress | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LD | HD | Group mean | LD | HD | Group mean | LD | HD | Group mean | ||
| (A) SNPL | ||||||||||
| SsrT17 | R | 3 | 2.3 | 2.4 | 2.1 | 1.7 | 2.2 | |||
| SsrT65 | R | 3 | 2.5 | 2.7 b | 2.5 | 1.7 | 2.1 c | 1.8 | 1.9 | 1.9 c |
| SsrT16 | SR | 3.4 | 2.8 | 3.1 b | 3.6 | 2.5 | 3.0 b | 3.7 | 2.3 | 3.0 b |
| SsrW35 | W | 4.7 | 3.4 | 4.4 | 3.9 | 4.7 | 3.4 | |||
| SsrW47 | W | 5.1 | 3.1 | 5.2 | 3.4 | 3.4 | 3.1 | |||
| Silverstar | W | 4.4 | 3.6 | 4.1 a | 4.3 | 3.3 | 4.1 a | 4.4 | 3.1 | 3.7 a |
| Mean | 4.0 | 2.9 | 3.4 | 3.7 | 2.8 | 3.3 | 3.3 | 2.7 | 3.0 | |
| (B) SN | R | 302 | 467 | 384 c | 303 | 395 | 349 c | 191 | 411 | 300 c |
| SR | 443 | 635 | 538 b | 380 | 548 | 463 b | 396 | 563 | 485 b | |
| W | 603 | 659 | 631 a | 598 | 734 | 666 a | 532 | 686 | 608 a | |
| (C) MSPN | R | 231 | 422 | 327 b | 261 | 308 | 285 c | 171 | 306 | 238 b |
| SR | 414 | 586 | 500 a | 307 | 443 | 375 b | 309 | 445 | 377 a | |
| W | 468 | 616 | 542 a | 419 | 552 | 485 a | 346 | 421 | 384 a | |
Means within irrigation treatments followed by different letter are statistically different at P < 0.05.
Under favourable (irrigated) conditions, the free-tillering lines produced an average 4.7 stems per plant at maturity (Table 2A). The R-tin (SsrT17 and SsrT65) and SR-tin (SsrT16) lines produced 3.0 and 3.4 stems, respectively, per plant. At HD, the R-tin lines produced 2.4 stems per plant, which was only 51% of that produced by free-tillering lines at LD. Under mild and severe stress, the R-tin lines had 0.6 and 0.8 fewer stems per plant than in the irrigated treatment (22% and 35% reduction), and free-tillering lines were reduced 10% in stem number in the severe stress treatment (compared with irrigated). There was no effect of water deficit on stem number per plant at stem elongation for SR-tin lines in either mild or severe stress, or for free-tillering lines in the mild stress treatment.
On an area basis in the irrigated treatment, LD produced 23% fewer stems than HD (587 stems m–2; Table 2B). On average, the irrigated treatment produced 463 spikes m–2 while mild and severe stress produced 400 and 334 spikes m–2, respectively (Table 2C). While the spike number of R-tin lines grown at HD ranged from 10% to 26% fewer than produced by free-tillering lines grown at LD, the SR-tin lines at HD produced between 6% and 29% more spikes per m2 compared with the free-tillering lines at LD
The ratio of maturity spike to maximum stem number (SPSR) was used as an indication of stem loss over the season. Combined analysis for SPSR indicated a significant effect of treatment, tillering group, and the interaction between tillering group and treatments. The SPSR was 0.88 in the irrigated, 0.79 in the mild stress, and 0.75 in severe stress, indicating fewer maturity spikes as water stress increased. In the irrigated treatment, free-tillering and R-tin lines maintained similar SPSR (0.87 versus 0.85), with SR-tin lines maintaining a high (0.93) ratio. However, SPSR of free-tillering lines reduced to 0.73 and 0.64 under mild and severe stress, respectively, while R-tin lines were able to maintain high (0.83) SPSR.
Plant height and phenology
At maturity, lines in the irrigated treatment were taller (90cm) than those in the mild (74cm) and severe (62cm) stress treatments. Line differences were significant only because SsrT17 (71cm) was on average 4cm shorter than all other lines (75cm). Tillering group differences were not significant (P > 0.05), nor was the effect of plant density. In the irrigated treatment, the R-tin lines reached anthesis 3 d earlier (91 versus 94 DAS) than did SR-tin and free-tillering lines. Water deficit hastened development, and anthesis occurred 4 d and 6 d earlier in the mild (87 DAS) and severe stress (85 DAS) treatments, respectively, with the relative performance of lines being consistent across treatments (data not shown). In the stress treatments, anthesis was 1–2 d earlier at HD than for LD. The density effect was greatest in the severe stress treatment, and it was only in the severe stress treatment that significant (P < 0.01) line by density interaction effects were observed, due largely to the delay in days to flowering in LD for SsrT65 (8 d delay) and Silverstar (4 d delay).
Light interception, WSC, and grain yield
At 60 DAS, ~3 weeks prior to anthesis, the R-tin lines were intercepting ~10% less light than SR-tin and free-tillering lines in all treatments (Table 3). In the irrigated treatment, SR-tin and free-tillering lines intercepted up to 97% light, while under mild and severe stress light interception was reduced to 92% and 82%, respectively. With increasing severity of stress, average LAI at anthesis reduced from a high of 5.6 in the irrigated, to 4.0 and 2.9 in the mild and severe stress, respectively (Table 3). In the irrigated treatment, an LAI of 7.2 was reached by free-tillering lines at anthesis, which was significantly greater than that produced by both the SR-tin (5.7) and R-tin (3.9) lines. In the mild and severe stress treatments, the SR-tin lines produced a similar LAI to free-tillering lines of 4.8 and 3.5, respectively. However, this was not the case for the LAI of R-tin lines which produced 39% and 40% less LAI than free-tillering lines.
Table 3.
Summary of traits related to biomass accumulation of R-tin, SR-tin, and free-tillering (W) Silverstar lines averaged across density in the irrigated, mild, and severe stress treatments
| Trait | Irrigated | Mild stress | Severe stress | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R-tin | SR-tin | W | R-tin | SR-tin | W | R-tin | SR-tin | W | |
| LI (%) 60 DAS | 88 b | 96 a | 97 a | 82 b | 92 a | 91 | 69 b | 81 a | 82 a |
| LAI (m2 m–2) | 3.88c | 5.74 b | 7.15 a | 2.90 b | 4.42a | 4.76a | 2.12b | 3.01 a,b | 3.53 a |
| CGR (g m–2 d–1) | 12.6 a | 12.9 a | 14.5 a | 13.0 a | 15.1 a | 14.0 a | 9.7 a | 12.0 a | 11.0 a |
| WSC (mg g–1) | 113 a | 74 b | 44 c | 236 a | 207 a | 206 a | 276 a | 256 b | 242 b |
| TDMf (g m–2) | 167 a | 178 a | 176 a | 124 a | 138 a | 135 a | 86 b | 105 a | 100 a |
| TDMa (g m–2) | 721 b | 780 a,b | 852 a | 598 b | 754 a | 711 a | 446 a | 570 a | 536 a |
| TDMm (g m–2) | 1321 b | 1401 b | 1632 a | 1114 a | 958 b | 1128 a | 635 a | 803 a | 739 a |
LI, light interception 60 d after sowing; LAI, leaf area index; CGR, crop growth rate between floral initiation and anthesis; WSC, stem water soluble carbohydrates at anthesis; TDM, total dry matter at floral initiation (f), anthesis (a), and maturity (m).
Means within irrigation treatments followed by the same letter are not statistically different at P=0.05.
There was no significant variation among lines or tillering groups in crop growth rate up to anthesis in any of the treatments, with the average crop growth rate being similar in the irrigated and mild stress treatments (13.6 and 13.8g m–2 d–1), but reduced to 10.8g m–2 d–1 under severe stress conditions. Stem WSC was greatest in the stress treatments, with an average of 256mg g–1 produced in the severe and 216mg g–1 in the mild stress (Table 3). Lines in the irrigated treatment produced stem WSC of 72mg g–1. Under mild and severe stress, R-tin lines produced, respectively, 15% and 14% greater WSC than free-tillering lines. By anthesis, biomass production in the irrigated treatment was greatest in free-tillering lines, and this difference was maintained through to maturity (Table 3). However, by maturity in the mild and severe stress treatments, there was no significant difference in total biomass between R-tin and free-tillering lines.
Within the mild and severe stress treatments, there was a significant (P < 0.05 for mild stress and P=0.06 for severe stress) effect of plant density on grain yield, in which HD achieved 12% and 29% greater grain yield than at LD (Table 4A). Line and tillering group differences were significant (P < 0.05) in the irrigated and mild stress treatments but not in the severe stress treatment. Within the irrigated treatment, averaged across densities, R-tin and SR-tin lines produced 15% and 24% less grain yield than free-tillering (610g m–2) lines. However, SsrT17 at HD (571g m–2) achieved relatively high grain yield, and this was not significantly different from free-tillering lines at LD (596–643g m–2). The free-tillering line SsrW35 at HD produced the highest grain yield of 670g m–2.
Table 4.
(A) Grain yield (GY; g m–2) of six Silverstar R-tin (SsrT17 and SsrT65), SR-tin (SsrT16), and free-tillering (SsrW35, SsrW47, and Silverstar) lines grown at low (LD) and high (HD) plant density, and across tillering groups for (B) harvest index (HI) in the irrigated, mild and severe treatments
| Line | Group | Irrigated | Mild stress | Severe stress | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LD | HD | Group mean | LD | HD | Group mean | LD | HD | Group mean | ||
| (A) GY | ||||||||||
| SsrT17 | R | 501 | 571 | 479 | 398 | 263 | 192 | |||
| SsrT65 | R | 518 | 478 | 517 b | 412 | 485 | 444 a | 153 | 412 | 255 a |
| SsrT16 | SR | 400 | 527 | 463 b | 358 | 335 | 347 c | 283 | 330 | 306 a |
| SsrW35 | W | 619 | 670 | 388 | 436 | 255 | 438 | |||
| SsrW47 | W | 596 | 577 | 307 | 419 | 253 | 272 | |||
| Silverstar | W | 643 | 558 | 610 a | 385 | 499 | 406 b | 266 | 247 | 288 a |
| Mean | 546 | 563 | 555 | 388 | 429 | 409 | 245 | 315 | 280 | |
| (B) HI | R | 0.45 a | 0.41 a | 0.39 a | ||||||
| SR | 0.38 b | 0.36 b | 0.38 a | |||||||
| W | 0.42 a | 0.36 b | 0.39 a | |||||||
| Mean | 0.42 | 0.38 | 0.39 | |||||||
Means within irrigation treatments followed by the same letter are not statistically different at P=0.05.
Relative to the irrigated treatment, grain yield in the mild stress treatment was reduced by 26% overall, with grain yield ranging from 307 to 499g m–2. However, the greatest yield reduction of 33% was observed for the free-tillering lines, while only 14% and 25% yield reduction was noted for R-tin and SR-tin, respectively. In the mild stress treatment, the R-tin lines (SsrT17 and SsrT65) outyielded free-tillering lines by 11% and produced relatively high grain yield at both LD and HD. In contrast, averaged across densities, SR-tin produced the lowest yield (347g m–2). Mean grain yield in the severe stress (280g m–2) treatment was reduced to 50% of that of the irrigated treatment with no significant (P > 0.05) line, tillering group, density, or interaction effects.
Line differences in HI were significant in the irrigated and mild stress treatment (Table 4B). In the irrigated treatment, R-tin lines produced the highest HI (0.45), followed by free-tillering (0.42) and SR-tin (0.38) lines. The HI decreased in the mild stress treatment; however, the R-tin lines maintained 17% higher HI than the SR-tin (0.36) and free-tillering lines. There was no significant line or tillering group difference in the severe stress treatment, which produced an HI of 0.38–0.39.
Kernel weight and screenings
In the irrigated treatment, average kernel weight was 31mg, with no evidence of significant (P > 0.05) line, density, or line by density interaction effects (Table 5). However, in the stress treatments, highly significant (P < 0.01) line, tillering group, and density differences were observed for kernel weight. Density by tillering group interactions were significant (P=0.06) between R-tin and free-tillering lines in both mild and severe stress treatments; that is, the kernel weight of R-tin lines decreased with increased plant density to a greater extent than in free-tillering lines; however, R-tin lines were still able to maintain higher kernel weights (Table 5). Relative to the irrigated treatment, kernel weight was reduced by 21% and 25% in the mild (25mg) and severe (23mg) stress treatments. However, in both stress treatments, R-tin lines produced kernel weights 18% and 20% greater than free-tillering lines. The R-tin lines consistently maintained higher kernel weights than the SR-tin line which tended to perform more like the free-tillering lines. On average, as density increased from 100 to 200 plants m–2, kernel weight decreased from 26 to 24mg and from 26 to 22mg in the mild and severe stress treatments, respectively. This strong effect of density was largely due to the high kernel weight of the R-tin lines at LD, whose relative performance was maintained at HD.
Table 5.
Kernel weight (mg) of six Silverstar R-tin (SsrT17 and SsrT65), SR-tin (SsrT16), and free-tillering (W; SsrW35, SsrW47 and Silverstar) lines grown at low (LD) and high (HD) plant density, and across density for each tillering group in the irrigated, mild stress, and severe stress treatments
| Line | Group | Irrigated | Mild stress | Severe stress | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LD | HD | Group mean | LD | HD | Group mean | LD | HD | Group mean | ||
| SsrT17 | R | 32.1 | 29.3 | 29.3 | 24.5 | 27.8 | 24.2 | |||
| SsrT65 | R | 33.7 | 33.7 | 32.2 a | 30.2 | 26.8 | 27.7 a | 29.3 | 23.6 | 26.2 a |
| SsrT16 | SR | 31.6 | 29.2 | 30.4 a | 24.8 | 22.7 | 23.7 b | 22.9 | 21.7 | 22.3 b |
| SsrW35 | W | 30.4 | 32.2 | 25.1 | 22.3 | 21.8 | 22.5 | |||
| SsrW47 | W | 30.8 | 31.5 | 23.2 | 23.2 | 22.6 | 21.5 | |||
| Silverstar | W | 32.1 | 30.6 | 31.3 a | 23.9 | 23.1 | 23.5 b | 22.2 | 20.1 | 21.8 b |
| Mean | 31.8 | 31.1 | 31.4 | 26.1 | 23.8 | 24.9 | 24.4 | 22.3 | 23.4 | |
Means within irrigation treatments followed by the same letter are not statistically different at P=0.05.
Relative to the other traits, there was large variation for kernel screenings (coefficient of variation of 46–70%). The irrigated treatment produced significantly fewer screenings (4.7%) than the mild (11%) and severe (13%) stress treatments (Table 6). In the irrigated treatment, R-tin lines produced slightly more (P > 0.05) screenings than the free-tillering lines, probably due to the larger numbers of higher order kernels per spike (Mitchell, 2010). In contrast, in the mild stress treatment, R-tin (8.3%) lines produced significantly (P < 0.05) fewer screenings than SR-tin (10%) and free-tillering (13%) lines. The R-tin lines in the severe stress treatment produced the fewest screenings at LD and HD (6.2% and 8.9%, respectively), while the free-tillering lines produced between 11.9% and 16.2% with an average of 14.5% screenings, which was not significantly different from the SR-tin line (16.8%).
Table 6.
Summary of traits (LA, leaf area; TDMa, anthesis total dry matter; WSC, water soluble carbohydrate; N, nitrogen; KNS, kernel number per spike; and SCR, screenings) related to kernel weight of R-tin, SR-tin, and free-tillering (W) Silverstar lines averaged across density in the irrigated, mild, and severe stress treatments
| Trait | Irrigated | Mild stress | Severe stress | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R-tin | SR-tin | W | R-tin | SR-tin | W | R-tin | SR-tin | W | |
| LA (cm2 stem–1) | 144 a | 135 a | 111 b | 89 a | 94 a | 71 b | 86 a | 72 b | 60 c |
| TDMa (g spike–1) | 2.59 a | 1.66 b | 1.69 b | 2.16 a | 2.1 a | 1.52 b | 2.0 a | 1.53 b | 1.41 b |
| WSC (mg g–1) | 113 a | 74 b | 44 c | 236 a | 207 a | 206 a | 276 a | 256 b | 242 b |
| WSC (g spike–1) | 0.16 a | 0.07 b | 0.04 b | 0.25 a | 0.23 a | 0.15 b | 0.27 a | 0.20 b | 0.17 b |
| N (%) | 1.24 b | 1.45 a | 1.55 a | 1.15 a | 1.23 a | 1.24 a | 1.24 b | 1.23 a,b | 1.40 a |
| N (mg spike–1) | 16.7 a | 12.5 b | 13.7 b | 12.5 a | 13.3 a | 9.5 b | 12.3 a | 9.9 b | 9.9 b |
| KNS | 58.0 a | 32.0 b | 38.8 b | 59.0 a | 40.0 b | 36.6 b | 41.8 a | 36.5 a,b | 33.4 b |
| SCR (%) | 6.5 a | 4.0 b | 3.6 b | 8.3 b | 10.1 a | 13.0 a | 7.6 b | 16.8 a | 14.5 a |
Means within irrigation treatments followed by the same letter are not statistically different at P=0.05.
Combined across treatments, a highly significant negative relationship was observed between screenings and kernel weight, in which 81% of the variation in screenings was accounted for by differences in kernel weight (data not shown).
Discussion
tin-containing lines produced fewer spikes but maintained spike number under stress
Tiller production is dependent on genetics and environment, including management, and consequently variation for tillering is high (Innes et al., 1981). The presence of the tin gene provides a genetic control on the maximum stem number produced by a plant, but this varied from line to line depending on unknown ‘modifier genes’ controlling the level of tin expression to affect the numbers of stems produced per plant. In the current treatments, lines were classified as restricted (R-) or semi-restricted (SR-) tin, or free-tillering (W) lines, based on the maximum stem number attained per plant (Mitchell, 2010). As expected, the maximum stem number per plant was obtained at LD, and declined at HD particularly in the free-tillering lines (Table 2). The relative ranking of lines for stem number per plant was consistent across water treatments and densities. With increasing water deficit, there was no reduction in stem number per plant at stem elongation for the SR-tin and free-tillering lines, but a small reduction for R-tin lines. However, the overall numbers of stems produced by the free-tillering and SR-tin lines in the irrigated treatment in the current experiment were relatively low (4.1 and 3.1, respectively). At Gatton in 2006, the same free-tillering and SR-tin lines produced an average of 5.8 and 4.0 stems per plant, and only 46% of stems produced by free-tillering lines at stem elongation remained to produce a spike at maturity (Mitchell, 2010). In contrast, the stem production per plant in R-tin was consistent across both years. The production of fewer stems in 2007 was probably due to warmer night temperatures between sowing and stem elongation (9.3 versus 6.6 °C for 2007 and 2006, respectively), which hastened plant development and tended to reduce stem production per plant (e.g. Kirby and Ellis, 1980). As a consequence of the relatively low stem numbers generated per plant in free-tillering lines in 2007, the difference between R-tin and free-tillering was not as great as in 2006 (Mitchell, 2010) The loss of stems over the growing season was not as high in 2007, and consequently the ratio of maturity spike number to maximum stem number produced (SPSR) was higher in 2007 than in 2006. It is anticipated that the moderate decline in SPSR that did occur for the free-tillering lines as severity of stress increased would be even greater if stem production in free-tillering lines had been greater. In contrast, the R-tin and SR-tin lines maintained a consistently high SPSR in the mild and severe stress treatments. Thus, further experimentation in environments and years with relatively lower temperatures during the vegetative phase is required to test the maximum advantage that tin lines may have in terms of maintenance of high SPSRs.
tin lines maintained kernel weight and have reduced screenings
There was strong evidence from response to irrigated and stress conditions herein that kernel weight was largely decreased by high spike number per m2, as found by others (Sharma and Anderson, 2004) and in regional trials of these lines (Mitchell et al., 2012). However, R-tin lines always maintained higher kernel weight than free-tillering lines. At high spike density, under terminal water deficit, the kernel weight advantage of R-tin lines reduced to 11.7% while at low density this advantage was as large as 28.6% (Table 5). At a moderate spike density which did not compromise the ability of R-tin to produce high kernel number per spike and therefore maintain grain yield, kernel weight was still 18% greater than for free-tillering lines. Thus, under mild stress conditions, R-tin lines have the opportunity to exploit greater assimilate and resource availability per spike, with kernel number per spike and maintenance of kernel weight potentially offsetting reductions in spike number (Tables 6, 7).
Table 7.
Comparison of traits contributing to (A) grain yield and (B) kernel weight in R-tin (SsrT17) at low (LD) and high (HD) density and free-tillering SsrW35 at low density in the irrigated, mild, and severe stress treatments
| Irrigated | Mild stress | Severe stress | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SsrT17 | SsrW35 | SsrT17 | SsrW35 | SsrT17 | SsrW35 | ||||
| LD | HD | LD | LD | HD | LD | LD | HD | LD | |
| (A) Yield analysis | |||||||||
| Stem number plant–1 | 3.0 b | 2.3 b | 4.7 a | 2.4 b | 2.1 b | 4.4 a | 1.7 b | 2.2 b | 4.7 a |
| Maximum stem number m–2 | 319 b | 476 a | 588 a | 286 c | 377 b | 528 a | 194 b | 441 a | 513 a |
| Spike number m–2 | 227 b | 489 a | 492 a | 285 b | 225 b | 425 a | 171 b | 301 a | 336 a |
| Kernel number head–1 | 79 a | 45 b | 44 b | 58 a | 69 a | 37 b | 56 a | 26 b | 35 b |
| Kernel number m–2 | 15 670 b | 19 570 a,b | 20 363 a | 16 417 a | 15 630 a | 15 530 a | 9779 a | 8087 a | 11 697 a |
| Kernel weight (mg) | 32.1 a | 29.3 a | 30.4 a | 29.3 a | 25.6 a | 25.1 a | 27.8 a | 24.2 a | 21.8 a |
| Grain yield (g m–2) | 501 b | 571 a,b | 619 a | 479 a | 398 b | 388 b | 263 a | 192 a | 255 a |
| (B) Kernel weight analysis | |||||||||
| Anthesis leaf area (cm2 stem–1) | 125 a | 139 a | 133 a | 86 a | 94 a | 72 a | 106 a | 76 b | 59 b |
| Anthesis total dry matter (g spike–1) | 3.5 a | 1.6 b | 2.0 b | 2.2 a | 2.2 a | 1.7 a | 2.2 a | 1.3 b | 1.4 b |
| Nitrogen (mg spike–1) | 22.2 a | 10.5 a | 15.5 a | 13.0 a | 11.3 a | 10.3 a | 14.7 a | 8.0 a | 10.1 a |
| WSC (mg spike–1) | 260 a | 105 b | 54 c | 298 a | 249 a | 135 b | 319 a | 223 b | 202 b |
| Screenings (%) | 6.9 a | 8.9 a | 4.1 a | 5.8 b | 12.2 a | 12.7a | 7.3 b | 10.5 a,b | 15.5 a |
WSC, water-soluble carbohydrate.
Means within irrigation treatments followed by the same letter are not statistically different at P=0.05.
In the following paragraphs, the phenotypic correlations among traits are reported to help explain the ability of tin lines to maintain high kernel weight. To illustrate this further, the example was used of the highest yielding, free-tillering (SsrW35) line under irrigated conditions at LD and this was compared with R-tin (SsrT17) at LD and HD under irrigated, mild, and severe stress conditions (Table 7). SsrT17 and SsrW35 achieved comparable kernel weight under non-limiting water and mild stress conditions. When subjected to severe water deficit, the kernel weight advantage of SrT17 was 11%, which significantly reduced screenings by 32% compared with SsrW35 (16%) at LD. At LD, SsrT17 maintained comparable kernel weight with that under irrigated conditions in the mild stress treatment, which was 17% greater than SsrW35, and in the severe stress treatment, kernel weight of SsrT17 was 28% greater than that of SsrW35. This maintenance of large kernel weight in SsrT17 led to a reduction in screenings of 54% and 53% in the mild stress and severe stress treatments, respectively (Table 7).
In the mild and severe stress treatments, kernel weight was negatively associated with stem number per m2, and this was maintained through to maturity spike number per m2 (r 2=0.55 and 0.74). This follows from the strong, negative relationships (r 2=0.45 and 0.72) for stem number per m2 at stem elongation and anthesis leaf area per stem in mild and severe stress, respectively. By stem elongation, SsrT17 had only produced, respectively, 54% and 38% of the stems produced by SsrW35 (528 and 513 stems m–2) in the mild and severe stress treatments, and this was reflected in a 19% and 80% greater leaf area per stem. Leaf area per stem was positively associated with anthesis total dry matter per spike (r 2=0.86 in both stress treatments), and SsrT17 produced 29% and 57% greater anthesis total dry matter per spike compared with SsrW35. In the mild and severe stress treatments, anthesis total dry matter per stem accounted for 66% and 92% of variation in kernel weight, respectively. The tin lines always maintained higher kernel weight than free-tillering lines, and under severe stress conditions the association between maximum stem number per plant and kernel weight was significant (P < 0.05), with each stem reducing kernel weight by ~1.7mg, and by 1.2mg per stem in the mild stress treatment (P=0.08). There also existed a strong positive association between anthesis total dry matter per spike and anthesis WSC content available for translocation to the spike (r 2=0.76) across both stress treatments. At LD, SsrT17 had 298mg and 319mg of WSC available per spike in mild and severe stress, respectively, ~121% and 58% more than SsrW35 in the same treatments. The anthesis WSC available for translocation to the spike was positively associated (r 2=0.46 and 0.78) with kernel weight in both the mild and severe stress treatments. None of the above associations were significant in the irrigated treatment.
In the stress treatments, significant associations were identified between kernel weight and post-anthesis water use per stem (r 2=0.69 and 0.92), with R-tin lines having higher kernel weight and higher post-anthesis water use compared with free-tillering lines. Water use per spike was 51% greater for SsrT65 than for SsrW35, and, furthermore, post-anthesis, SsrT65 had a 39% greater water use per spike than SsrW35; that is, the low stem number per plant (at stem elongation) produced by the R-tin lines contributed to high anthesis total dry matter per spike and high WSCs, with more of this available for translocation to spikes.
The R-tin lines had accumulated relatively high levels of WSCs by anthesis and therefore had more WSC reserves per spike and kernel. A similar observation was made by Dreccer et al. (2013) working on free-tillering lines in that the lines producing fewer tiller and spike number tended to have higher WSCs.
In the experiment herein, the lines with the lowest CTDs (transpiring less) also tended to be the tin lines. Under favourable conditions, Miralles et al. (2007) found that in free-tillering lines, the increased kernel number per spike was a result of continued development of distal floret primordia within the spikelet, and suggested that this was probably due to greater carbohydrate (14%) and nitrogen (50%) acquisition by the spikes at anthesis. Reynolds et al. (2005) and Rebetzke et al. (2008) also concluded that high kernel number per spike was associated with greater partitioning of assimilates to the spike. The free-tillering lines developed a smaller leaf area per stem but a larger LAI, used water more quickly early in the season (lower canopy temperatures), and were therefore unable to maintain processes such as the translocation of assimilate to grain, resulting in small kernels and high screenings. Consistent with the findings of others (e.g. Fischer and Kohn, 1966; van Herwaarden et al., 1998), in a terminal water deficit environment, high water use pre-anthesis results in reduced soil water and therefore assimilation potential during grain filling. It appears that physiological and biochemical processes were maintained to a greater degree in the R-tin compared with free-tillering lines and, when combined with a larger assimilate reserve (total dry matter, WSC, and nitrogen) per spike, R-tin lines were able to produce a greater kernel weight and to reduce kernel screenings.
tin lines produced yield comparable with free-tillering lines in terminal water stress environments
Under mild terminal water deficit at low (commercial) density, the R-tin lines produced significantly higher grain yield than other lines. The R-tin lines with their reduced tillering produced relatively low stem number per unit area and LAI, yet crop growth rate up to anthesis was not compromised (Tables 2, 3). Therefore R-tin produced only marginally lower anthesis total dry matter per unit area, but considerably greater anthesis dry matter (and WSC) per spike. In contrast, the free-tillering lines produced more stems per plant at stem elongation, leading to higher stem number per unit area. The resulting high LAI increases demand for pre-anthesis water. The cooler canopies of the free-tillering lines indicate the potential for greater water use early in the season, and the likely reduction in available soil water post-anthesis.
Line differences in water extraction were observed at individual depths, and previous research in wheat has found that each additional millimetre of water extracted post-anthesis in these types of environments can generate an extra 55kg ha–1 of grain yield (Manschadi et al., 2006; Christopher et al., 2008). While there was a tendency for tin to have greater water extraction at depth (Table 1) during grain filling, the differences in water use pattern here were not conclusive. The resource availability per spike was greater for R-tin lines due to low stem number per plant and therefore reduced intraplant competition between stems for available resources. According to Donald (1968), for a cereal to be high yielding the individual plants in a crop should be ‘weak’ competitors—in other words they should produce less vegetative biomass and fewer infertile tillers. It appears that the higher productivity on a stem basis provided R-tin lines with a greater ‘buffering’ capacity and consequently yield stability in stress environments. As noted above, it appears that for R-tin lines to reach their genetic yield potential they need the opportunity to exploit plasticity within the spike, and this cannot be achieved at high spike densities traditionally targeted in high yielding environments. The high yield (5.6 t ha–1) achieved in the irrigated treatment is relatively infrequent in the northern production region, and the mean grain yield (2.1–2.6 t ha–1) of experiments at Kingsthorpe and Emerald presented in Mitchell et al. (2012) are more comparable with that expected by farmers in the northern region of the Australian wheatbelt (Chenu et al., 2011). The evidence from treatments conducted under both favourable and stress conditions is that for R-tin lines to reach yield potential, spike density needs to be optimized for the target environment. Typically for free-tillering lines in the northern area, this has been ~300 spikes m–2, and evidence from the current experiments suggests that, at this spike density, R-tin lines are able to maintain a higher kernel weight, without compromising yield under both favourable and severe stress conditions. However, to achieve this density, the R-tin lines would need to be sown at a greater density than the current practice.
Conclusions
Genetic control of stem number provides a management tool for use in plant improvement. While the experimental results herein are from one year of field experiments only, the performance of multiple lines in targeted environments evaluated reduces the potential confounding with random year and site factors. It is anticipated that the advantages of tin in maintaining kernel weight and reducing screenings would be further highlighted in environments that encourage tillering of free-tillering lines, and this needs to be confirmed with further experimentation. Reducing the potential ‘wasteful’ stem production and associated resources utilized therein, and yet ensuring no loss in the crop’s ability to capture resources (i.e. controlling and optimizing stem number, and thereby leaf area), improves the efficiency with which resources are used by the crop to maintain grain yield and kernel size. The results expressed on a per spike basis clearly indicate that the R-tin lines have both greater water use during grain filling and greater translocation of WSCs. Together, these appear to contribute to higher kernel number and kernel weight per spike, suggesting that the R-tin lines are more efficient in their use of captured resources over the duration of the season. The genetic restriction of tiller production appears to ensure that more assimilate was available at the time of spike initiation, and spike growth and development. With fewer competing sinks (meristems), more assimilate is available per spike to produce heavier spikes at anthesis and maturity, and maintain large kernel weight. Thus, the incorporation of the tin gene into genetic material adapted to the target environment with optimization of spike density is likely to lead to maintenance of kernel weight and reductions in screenings in terminal water deficit environments.
Acknowledgements
Thanks are due to Tod Eadie, Greg Roberts, Shelley Quinn, Mary Anne Awasi, and Neil Wiltshire for technical support with data collection. The authors gratefully acknowledge discussions and feedback provided by Allan Rattey and Anthony van Herwaarden. The senior author was supported by an Australian Postgraduate Award. This research was jointly funded by Australia’s Commonwealth Scientific and Industrial Research Organisation and the Grains Research and Development Corporation (CSP00053), and is gratefully acknowledged.
References
- Chapman SC. 2008. Use of crop models to understand genotype by environment interactions for drought in real-world and simulated plant breeding trials. Euphytica 161, 195–208 [Google Scholar]
- Chenu K, Cooper M, Hammer GL, Mathews KL, Dreccer MF, Chapman SC. 2011. Environment characterization as an aid to wheat improvement: interpreting genotype–environment interactions by modelling water-deficit patterns in North-Eastern Australia. Journal of Experimental Botany 62, 1743–1755 [DOI] [PubMed] [Google Scholar]
- Christopher JT, Manschadi AM, Hammer GL, Borrell AK. 2008. Developmental and physiological traits associated with high yield and stay-green phenotype in wheat. Australian Journal of Agricultural Research 59, 354–364 [Google Scholar]
- Dalgliesh N, Foale M. 2005. Soil matters: monitoring water and soil nutrient in dryland farming. Collingwood, Australia: CSIRO Publishing; [Google Scholar]
- Donald CM. 1968. Breeding of crop ideotypes. Euphytica 17, 385–403 [Google Scholar]
- Dreccer MF, Chapman SC, Rattey AR, Neal J, Song YH, Christopher JT, Reynolds M. 2013. Developmental and growth controls of tillering and water-soluble carbohydrate accumulation in contrasting wheat (Triticum aestivum L.) genotypes: can we dissect them? Journal of Experimental Botany 64, 143–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreccer MF, van Herwaarden AF, Chapman SC. 2009. Grain number and grain weight in wheat lines contrasting for stem water soluble carbohydrate concentration. Field Crops Research 112, 43–54 [Google Scholar]
- Duggan BL, Richards RA, van Herwaarden AF, Fettell NA. 2005. Agronomic evaluation of a tiller inhibition gene (tin) in wheat. I. Effect on yield, yield components, and grain protein. Australian Journal of Agricultural Research 56, 169–178 [Google Scholar]
- Fischer RA, Kohn GD. 1966. Soil water relations and relative turgidity of leaves in wheat crop. Australian Journal of Agricultural Research 17, 269–280 [Google Scholar]
- Gallagher JN, Biscoe PV, Scott RK. 1975. Barley and its environment. 5. Stability of grain weight. Journal of Applied Ecology 12, 319–336 [Google Scholar]
- Innes P, Blackwell RD, Austin RB, Ford MA. 1981. The effects of selection for number of ears on the yield and water economy of winter-wheat. Journal of Agricultural Science 97, 523–532 [Google Scholar]
- Isbell R. 1996. The Australian soil classification. Collingwood, Australia: CSIRO Publishing; [Google Scholar]
- Islam TMT, Sedgley RH. 1981. Evidence for a uniculm effect in spring wheat (Triticum aestivum L) in a mediterranean environment. Euphytica 30, 277–282 [Google Scholar]
- Kirby EJM, Ellis RP. 1980. A comparison of spring barley grown in England and in Scotland. 1. Shoot apex development. Journal of Agricultural Science 95, 101–110 [Google Scholar]
- Manschadi AM, Christopher J, Devoil P, Hammer GL. 2006. The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823–837 [DOI] [PubMed] [Google Scholar]
- Marshall C, Boyd WJR. 1985. A comparison of the growth and development of biculm wheat lines with freely tillering cultivars. Journal of Agricultural Science 104, 163–171 [Google Scholar]
- Miralles DJ, Slafer GA. 2007. Sink limitations to yield in wheat: how could it be reduced? Journal of Agricultural Science 145, 139–149 [Google Scholar]
- Mitchell JH. 2010. Evaluation of reduced-tillering (tin gene) wheat lines for water limiting environments in Northern Australia. PhD Thesis, The University of Queensland, Australia
- Mitchell JH, Chapman SC, Rebetzke GJ, Bonnett DG, Fukai S. 2012. Evaluation of a reduced-tillering (tin) gene in wheat lines grown across different production environments. Crop and Pasture Science 63, 128–141 [Google Scholar]
- Mitchell JH, Fukai S, Cooper M. 1996. Influence of phenology on grain yield variation among barley cultivars grown under terminal drought. Australian Journal of Agricultural Research 47, 757–774 [Google Scholar]
- Powell B. 1982. Soils of the Gatton Research Station. Queensland Department of Primary Industries Bulletin, QB82005, 21 [Google Scholar]
- Rebetzke GJ, van Herwaarden AF, Jenkins C, Weiss M, Lewis D, Ruuska S, Tabe L, Fettell NA, Richards RA. 2008. Quantitative trait loci for water-soluble carbohydrates and associations with agronomic traits in wheat. Australian Journal of Agricultural Research 59, 891–905 [Google Scholar]
- Reynolds MP, Pellegrineschi A, Skovmand B. 2005. Sink-limitation to yield and biomass: a summary of some investigations in spring wheat. Annals of Applied Biology 146, 39–49 [Google Scholar]
- Richards RA. 1988. A tiller inhibitor gene in wheat and its effect on plant-growth. Australian Journal of Agricultural Research 39, 749–757 [Google Scholar]
- Richards RA, Townleysmith TF. 1987. Variation in leaf-area development and its effect on water-use, yield and harvest index of droughted wheat. Australian Journal of Agricultural Research 38, 983–992 [Google Scholar]
- Sharma DL, Anderson WK. 2004. Small grain screenings in wheat: interactions of cultivars with season, site, and management practices. Australian Journal of Agricultural Research 55, 797–809 [Google Scholar]
- van Herwaarden AF, Farquhar GD, Angus JF, Richards RA, Howe GN. 1998. ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser—I. Biomass, grain yield, and water use. Australian Journal of Agricultural Research 49, 1067–1081 [Google Scholar]
- Woodruff DR, Tonks J. 1983. Relationship between time of anthesis and grain-yield of wheat genotypes with differing developmental patterns. Australian Journal of Agricultural Research 34, 1–11 [Google Scholar]
- Yunusa IAM, Sedgley RH. 1992. Reduced tillering spring wheats for heavy textured soils in a semiarid mediterranean environment. Journal of Agronomy and Crop Science 168, 159–168 [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. Decimal code for growth stages of cereals. Weed Research 14, 415–421 [Google Scholar]