Abstract
2-Cysteine peroxiredoxins (2-CysPrxs) are ubiquitous and highly abundant proteins that serve multiple functions as peroxidases, chaperones, and thiol oxidases and in redox-dependent cell signalling. The chloroplast protein plays a role in seedling development and protection of the photosynthetic apparatus. This study aimed to unequivocally link conformation and function. To this end, a set of non-tagged site-directed mutagenized At2-CysPrx variants was engineered, which mimicked the conformational states and their specific functions: hyperoxidized form (C54D), reduced form (C54S, C176S), oxidized form (C54DC176K), phosphorylated form (T92D), reduced ability for oligomerization by interfering with the dimer–dimer interface (F84R) and a C-terminally truncated form [ΔC (–20 aa)]. These variants were fully or partly fixed in their quaternary structure and function, respectively, and were analysed for their conformational state and peroxidase and chaperone activity, as well as for their sensitivity to hyperoxidation. The presence of a His6-tag strongly influenced the properties of the protein. The ΔC variant became insensitive to hyperoxidation, while T92D and F84R became more sensitive. The C54D variant revealed the highest chaperone activity. The highest peroxidase activity was observed for the F84R and ΔC variants. Efficient interaction with NADP-dependent thioredoxin reductase C depended on the presence of Cys residues and the C-terminal tail. The results suggest that the structural flexibility is important for the switch between peroxidase and chaperone function and that evolution has conserved the functional switch instead of maximizing a single function. These variants are ideal tools for future conformation-specific studies in vivo and in vitro.
Key words: Chaperone, chloroplast, conformation, peroxidase, peroxiredoxin, thiol switch.
Introduction
2-Cysteine peroxiredoxins (2-CysPrxs) are ubiquitous proteins with conserved functions in all kingdoms of life (Dietz, 2011; Edgar et al., 2012). Additionally, in most organisms the 2-CysPrx homologues are highly abundant proteins. In the erythrocyte, Prdx2 is the third most abundant protein (Wood et al., 2003). In the chloroplast stroma, the two 2-CysPrxs A and B are placed at the 14th and 16th positions in the list of most abundant proteins, respectively (Peltier et al., 2006). The accumulation of hyperoxidized Prxs is a conserved marker of circadian rhythmicity (Edgar et al., 2012). Prx proteins are linked to human diseases; for example, they are key initiators of post-ischaemic inflammation in the brain (Shichita et al., 2012) and play a role in cancer (Neumann et al., 2003; Kim et al., 2008; Chen et al., 2010) and Alzheimer’s disease (Yoshida et al., 2009).
2-CysPrxs display at least four functions, namely as a peroxidase, chaperone, thiol oxidase, and modulator of cell signalling. Essential for the peroxidase function is the highly reactive cysteinyl residue in the active site (peroxidatic Cys, CysP), which reacts with a broad range of hydroperoxides and peroxynitrite (Dietz, 2011). The intermediately formed sulfenic acid of CysP reacts with a second cysteine thiol (resolving Cys, CysR) from the other subunit of the homodimer to build a disulfide bridge. This oxidized disulfide form is regenerated by several chloroplastic thioredoxins (Trxs) and proteins with Trx domains, in particularly NADPH-dependent Trx reductase (TR) with a built-in Trx domain (NTRC), in vitro and in vivo (Pulido et al., 2010). However, the sulfenic acid intermediate is susceptible to further oxidation to sulfinic acid. CysP hyperoxidation switches the function from peroxidase to that of a chaperone. Furthermore, accumulation of the hyperoxidized form shows rhythmicity along with the circadian clock in algae, humans, and plants (O’Neill et al., 2011; Edgar et al., 2012) and coincides with membrane association (König et al., 2003). A high peroxide concentration and lipid hydroperoxides are contributing factors. Additionally, the decameric form of 2-CysPrx is linked to the chaperone function (Jang et al., 2004; Kim et al., 2009). The molecular basis for the functional switch from peroxidase to chaperone is still not completely understood. Sulfiredoxins (Srxs) reduce the hyperoxidized protein in a Mg2+/ATP/thiol reductant-dependent reaction. Due to the limited Srx concentration and slow reaction rate, the regeneration of hyperoxidized 2-CysPrx in vivo is a slow process (Iglesias-Baena et al., 2010).
2-CysPrxs have been suggested to function as a redox-dependent molecular hub within the redox regulatory network. Besides Trx and Trx-like proteins, cyclophilin (Cyp20-3), Srx, fructose-1,6-bisphosphatase, and photosystem II were reported as interacting partners of 2-CysPrx in plants (Caporaletti et al., 2007; Laxa et al., 2007; Muthuramalingam et al., 2009). In addition to the peroxidase and chaperone functions, a role as proximity-based thiol oxidase has been proposed (Gutscher et al., 2009). A precise understanding of the relative significance of these different roles of 2-CysPrx in vivo is still missing, but they are all in line with the suggested role of Prxs as redox sensors within the thiol-disulfide redox regulatory network of the cell (Dietz, 2008).
This work aimed to dissect the functional diversity and link the quaternary structure 2-CysPrx to its specific functions. To this end, 2-CysPrx variants were designed that were sought to mimic distinct quaternary structures and redox states. Fixing the conformation by introducing defined amino acid exchanges should then allow using the variants as gain-of-function tools to investigate their activities and properties undisturbed from redox changes during the catalytic cycle. To this end, the quaternary structure and peroxidase and chaperone activities, as well as the hyperoxidation sensitivity, were analysed for a set of site-directed mutagenized variants. It was shown that, for such an investigation of fine-tuned functional differentiation, non-tagged variants need to be used, as His6-tagged 2-CysPrx displayed behaviour in some assays different from the non-tagged protein. Analyses of the variants suggested that the flexibility of the protein structure is essential for its sensitivity to hyperoxidation and for the switch from the peroxidase to chaperone function.
Materials and methods
Cloning, expression, and purification of proteins
The gene sequence of 2-CysPrxA (At3g11630) was amplified by PCR from cDNA of Arabidopsis thaliana (Columbia) without the signal peptide (N-terminal MAQA) using a forward primer containing an NdeI site and a reverse primer containing an EcoRI site. The PCR product was cloned into the NdeI/EcoRI site of pCRT7/NT-TOPO (Invitrogen) and pET-28a (Merck Millipore). In a similar manner, NTRC was cloned into pET-28a. Site-directed mutagenesis was accomplished by PCR using primers as listed in Supplementary Table S1 (at JXB online) and verified by sequencing (Eurofins MWG Operon, Eberswalde, Germany).
The plasmids were transformed into Escherichia coli BL21(DE3)pLysS, and protein expression was induced with 0.4mM IPTG at an optical density of 0.45–0.6. After incubation overnight at room temperature, the culture was centrifuged at 5000g for 20min. The pellet was stored at –80 °C.
For purification of non-tagged 2-CysPrx wild type (WT) and variants, the pellets from 1 litre cultures were resuspended in 50ml of lysis buffer (40mM Bis-Tris/HCl, pH 6.5). After 30min incubation on ice, the bacteria were lysed by sonication (4×2min, 70% power) and centrifuged at 50 000g for 45min. The supernatant was filtered and loaded on a 5ml HiTrap Q-Sepharose Fast Flow column (GE Healthcare, München, Germany) equilibrated with 40mM Bis-Tris/HCl (pH 5.5). Proteins were eluted in a 0–1M sodium acetate gradient with a flat gradient between 30 and 70%. Samples from the protein-containing fractions were analysed by 15% SDS-PAGE. 2-CysPrx-containing fractions were pooled. The 35–80% (NH4)2SO4 precipitate was resuspended in 25mM HEPES/KOH (pH 8.0) and dialysed against the same buffer overnight. Finally, the protein was purified by gel filtration using a S200 16/60 column (GE Healthcare), equilibrated in 40mM potassium phosphate buffer (pH 7.2). Pooled 2-CysPrx fractions were concentrated using a Vivaspin 20 5000 MWCO centrifugal concentrator (Sartorius, Göttingen, Germany). The protein was frozen in liquid N2 and stored at –80°C. The protein concentration was determined using the extinction coefficient: ε=19940M–1 cm–1 and a theoretical M w of 22 170Da (http://expasy.org/tools/protparam.html).
All His-tagged proteins (His6–2-CysPrx, His6–NTRC, His10–EcTrxA, and His10–EcTR; König et al., 2002) were purified by Ni-NTA affinity chromatography as described by the manufacturer (Qiagen). The calculated parameters of His6–NTRC were: MW=52 282Da and ε=40715M–1 cm–1.
Quaternary structure analysis
2-CysPrx protein (2ml at 5mg ml–1) was loaded on a SEC S200 16/60 (GE Healthcare), or 0.5ml was loaded on a SEC S200 10/300 GL (GE Healthcare). The column was equilibrated with 40mM potassium phosphate buffer (pH 7.2). The elution profile was monitored at an optical density of 280nm.
Peroxidase assay
The standard assay employed E. coli Trx as regenerant. 2-CysPrx was added at 2 μM concentration in quartz cuvettes, and the assay was performed at 25°C in 40mM potassium phosphate buffer (pH 7.2), 1mM EDTA, 250 μM NADPH, 6–12 μM E. coli Trx and 10 μM E. coli TR. The reaction was started by the addition of 75 μM H2O2, and the decrease in absorbance due to the oxidation of NADPH was measured at 340nm with a Cary 300 Bio UV/Vis spectrometer (Varian, Middelburg, The Netherlands) for 10min. Rate constants were determined using the Dalziel equation (Nogoceke et al., 1997; König and Fairlamb, 2007).
Peroxidase assay with dithiothreitol (DTT)
Reduction of H2O2 by 2-CysPrx was measured using a ferrous ammonium sulphate/xylenol orange (FOX) assay for H2O2 quantification. The assay was performed at 25 °C and contained 40mM potassium phosphate buffer (pH 7.2), 1mM DTT, and 5 μM protein (2-CysPrx, His6-tagged 2-CysPrx, C176S and F84R). After 10min, the reaction was started by addition of 100 μM H2O2. A volume of 10 μl of the assay was added to 200 μl xylenol orange solution (250mM sulfuric acid, 1M sorbitol, 2.5mM ferrous ammonium sulfate, and 1.25mM xylenol orange in H2O) after different time points (0.5, 1, 2, 3, 4, and 5min). The decrease in H2O2 levels was determined spectrophotometrically by measuring absorbance at 560nm.
Inactivation assay
The reaction conditions were identical to the Trx-dependent peroxidase assay described above with 15 μM E. coli Trx. NADPH oxidation was started after addition of 0.25, 1, 2, 5, and 10mM cumene hydroperoxide, and the decrease in absorbance due to NADPH oxidation was determined by measuring absorbance at 340nm for 5min. Background H2O2 reduction without 2-CysPrx was subtracted. The NADPH consumption and consequently the catalytic turnover per 2-CysPrx molecule at each time point were calculated. The turnover numbers were plotted against the time and the resulting curve fitted to a first-order equation (1) with simple weighting:
| y(t)=A∞ (1 – e –kt) |
The calculated limits were plotted against the cumene hydroperoxide concentration and fitted to an equation describing a single exponential decay curve with offset and statistical weighting:
| y (t)=A0 (overall limit of turnover)×e(–k[COOH]٠) + offset |
Chaperone assay
The chaperone activity of 2-CysPrx WT and variants was measured using citrate synthase as substrate. 2-CysPrx protein (10 μM) was incubated at 45 °C in 40mM potassium phosphate buffer (pH 7.2). After temperature adjustment for 15min, citrate synthase (1 μM) was added and the increase in absorption at 360nm was monitored with a spectrophotometer.
Circular dichroism (CD) spectra
CD spectra of 10 μM 2-CysPrx protein solutions in 100mM potassium phosphate buffer (pH 8) and 5mM NaCl were measured at 20 °C with a quartz microcuvette (light path 0.2×1cm) placed in a J-810 spectropolarimeter (Jasco, Easton, MD, USA). Spectra were measured at a 1nm data pitch and a scanning speed of 50nm min–1 and averaged over four scans. Four spectra for each condition were collected and averaged after subtracting the base line with buffer only. For quantification of secondary structures, the ellipticity of CD spectra was analysed with the software Spectra Manager (Jasco). CD spectra were compared between oxidized and reduced proteins. 2-CysPrx WT and variants were reduced in 100mM potassium phosphate buffer (pH 8) and 5mM NaCl with 10mM DTT at 37 °C for 2h. The proteins were then dialysed overnight in 100mM potassium phosphate buffer (pH 8) and 5mM NaCl supplemented with 1mM DTT.
Isothermal titration microcalorimetry
2-CysPrx variants were reduced in 40mM potassium phosphate buffer (pH 8) with 10mM DTT at 37 °C for 2h and dialysed overnight in 40mM potassium phosphate buffer (pH 8), supplemented with 1mM DTT. All samples were filter sterilized. A VP-ITC (isothermal titration calorimetry) microcalorimeter was adjusted to the following parameters: 25 °C, 10 μcal s–1 reference power, initial offset 60 s, stirring speed 502, fast feedback mode, and ITC equilibration option. Protein was loaded in the syringe, the ITC cuvette was filled with dialysis buffer and 120 nmol of protein was injected in an interval of 3min (Barranco-Medina et al., 2008).
Far-Western dot blot
2-CysPrx protein (5 μl of 0.63, 1.25, 2.5, 5, 10, and 20 μM, WT and variants) was spotted in arrays on nitrocellulose membrane. Following washing in TBS/Tween 20 (TBST) and 1h of blocking (1% skimmed milk in TBST), the membrane was equilibrated in binding buffer [50mM MOPS (pH 6.5), 20% glycerol, 2mM DTT, 10mM MgCl2] containing 20 μg ml–1 of NTRC. Following repeated washing in TBST, the primary antibody (mouse anti-His6, diluted 1:6000 in blocking solution) was incubated for 3h. After washing, alkaline phosphatase-conjugated secondary antibody was added for 2h. Alkaline phosphatase activity was quantified on the membrane in 30ml carbonate buffer (pH 9) containing BCIP/NBT following digitization of the images and using image analysis software. The background signal of neighbouring membrane sections without protein was routinely subtracted. Staining intensities were linear in the concentration range from 1.25 to 10 μM for each variant. NTRC binding of the variants at each concentration was calculated in relation to WT (100%). Finally, NTRC binding was presented as mean±standard deviation (SD) at protein concentrations ranging from 1.25 to 10 μM.
Results
Engineering of 2-CysPrx variants
The aim of the work was to engineer and characterize 2-CysPrx variants mimicking naturally occurring 2-CysPrx conformations. These variants should have fixed quaternary structures or restricted dynamics, which would allow the establishment of firm redox-dependent structure/function relationships. Table 1 shows a compilation of all the variants used in this study, outlining the intended functional or structural characteristics. The decameric quaternary structure of reduced 2-CysPrx was fixed by substituting serine for either CysP or CysR as shown previously for barley 2-CysPrx (König et al., 2003) (Supplementary Fig. S1 at JXB online). The variant lacking CysR (C176S) should have enhanced proximity-based thiol oxidase capacity as a consequence of the expanded life time of the sulfenic acid intermediate (Gutscher et al. 2009). Substitution of CysR (C176S) should affect the interaction with Trx-like proteins (König et al., 2003). The negative charge introduced during sulfinic acid formation in the hyperoxidized 2-CysPrx might be simulated by the negatively charged aspartic acid (C54D). This type of site-directed mutagenesis is often used to functionally mimic thiol hyperoxidation (Wang et al. 2012). The oxidized 2-CysPrx forms a disulfide bridge between CysP and CysR. Here, a salt bridge between aspartic acid and the positively charged lysine was hypothesized to imitate the oxidized disulfide form (C54DC176K). Introducing the Lys–Asp salt bridge instead of Cys208 and Cys272 successfully restored the disulfide-dependent state of CadC, a regulator of E. coli gene expression (Tetsch et al. 2011). The C-terminal tail of one homodimer subunit embraces the other subunit and the redox cycle of 2-CysPrx involves extensive changes in quaternary structure. A shortened C-terminal tail should increase the flexibility and the speed of the change in quaternary structure during the catalytic cycle and affect the sensitivity to hyperoxidation. The homologue of tryptophane residue W180 was shown to be essential for the quaternary structure in barley 2-CysPrx (König et al., 2003). The neighbouring lysine residue (K181) potentially forms a salt bridge with a negatively charged amino acid residue near the catalytic centre, thereby stabilizing the loop that contains CysR (Supplementary Fig. S1). The remaining residues of the C terminus should be less important for the peroxidase activity and therefore a variant lacking the last 20 aa was designed (ΔC). The stability of the dimer–dimer interface in 2-CysPrxs is mainly based on hydrophobic interactions (Matsumura et al., 2008). Replacing the hydrophobic Phe by a charged residue in this interface should impede the interaction in the dimer–dimer interface and stabilize the dimeric form (F84R). Recently, it was found that Oryza sativa 2-CysPrx is phosphorylated (Chen et al., 2011). Plant 2-CysPrxs have the conserved threonine residue T92. This residue is also found in other organisms (Supplementary Fig. S1). In a human cell line, the threonine residue was found to be phosphorylated as well. Human Prdx1 variant T90D eluted only in the fraction containing high-molecular-weight protein (Jang et al., 2006). Prdx1 T90D displayed very low peroxidase activity and showed markedly increased chaperone activity. The variant T92D where aspartic acid replaces threonine should simulate the negative charge introduced by threonine phosphorylation. N-terminally His6-tagged 2-CysPrx has been used in nearly all studies without thorough testing of whether the charged tag affects the conformational dynamics, catalytic activities or redox-linked properties. As we were interested in major but also subtle modifications, the influence of the His6 tag was investigated in parallel.
Table 1.
2-CysPrx variants generated and characterized in this study
| 2-CysPrx variant | Mimicked 2-CysPrx conformation |
|---|---|
| Wild type (WT) | Redox-variable conformation |
| C54D | Hyperoxidized form |
| T92D | Phosphorylated form |
| C54S | Reduced form, interaction with Trx-like proteins |
| C176S | Reduced form with putative thiol oxidase function |
| ΔC (–15 aa) | Peroxidase with a loss of sensitivity to hyperoxidation |
| C54DC176K | Oxidized form |
| F84R | Reduced aggregation capacity, increased sensitivity to hyperoxidation |
| WT, N-6×His | Enhanced flexibility by limited steric constraints |
Cloning, expression, and purification
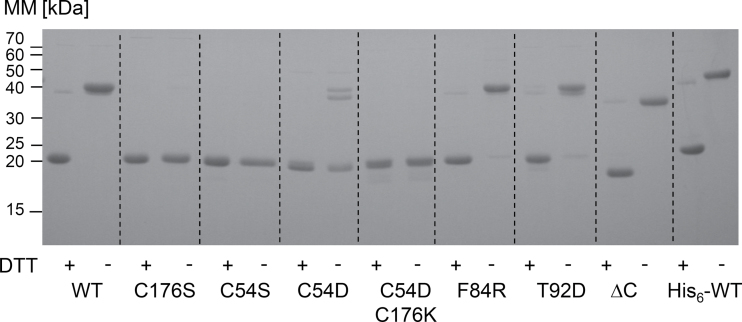
The bioinformatics-predicted plastid transit peptide consists of 82 aa (TargetP) starting with the amino acids MAS/C (Supplementary Fig. S1). Mass spectrometric data of At-2-CysPrx available in the Plant Proteome Database (PPDB, http://ppdb.tc.cornell.edu/) as well as N-terminal sequencing of the pea 2-CysPrx suggest the N-terminal cleavage of the 2-CysPrx signal peptide before Ala67 (AQA…) (Caporaletti et al., 2007). Therefore, tag-free 2-CysPrx was expressed starting with the N-terminal amino acids MAQA. WT and variants were expressed heterologously in E. coli with high yield. WT 2-CysPrx was also expressed with an N-terminal His6-tag. The calculated low isoelectric point of pI=4.91 of 2-CysPrx A was exploited for a first efficient purification step by anion exchange chromatography. All variants were isolated with purities >90% after this step. Following ammonium sulfate precipitation, a final purification was achieved by size exclusion chromatography. Analysis by SDS-PAGE demonstrated the successful purification of all 2-CysPrx A variants (Fig. 1). In solution, 2-CysPrx forms stable dimers as the smallest unit that can aggregate in solution to form doughnut-shaped decamers. Under reducing and denaturing conditions of SDS-PAGE analysis, the reduced dimer decayed to monomers (22.4kDa). However, a small fraction of reduced dimers was still seen as dimers (44.8kDa) (Fig. 1). The oxidized dimers were covalently linked and could be clearly distinguished from the reduced monomer. The variants C54S, C176S, and C54DC176K did not form dimers under reducing and non-reducing conditions. Interestingly, a significant double band of C54D at the size of the dimer was seen in the absence of DTT. It can be speculated that a negative charge in the active site, either due to hyperoxidation to sulfinic acid or here due to the aspartic acid, activated the second cysteine C176 from the same subunit to form a disulfide bridge with other proteins or with another 2-CysPrx C54D dimer. Alternatively, the pseudo-hyperoxidized state might allow for very persistent hydrophobic interactions, which in part are resistant to SDS during SDS-PAGE separation. N-terminal sequencing could provide definite answers as to a possible truncation of the C54D variant.
Fig. 1.
SDS-PAGE analysis of purified recombinant proteins. All proteins were reduced with 20mM DTT (+) or left untreated (–) and denatured in loading buffer at 95 °C for 10min. The redox state was fixed by incubating the proteins with 100mM N-ethylmaleimide. For each lane, 2 μg of each protein was loaded. MM, molecular mass markers (kDa).
Quaternary structure
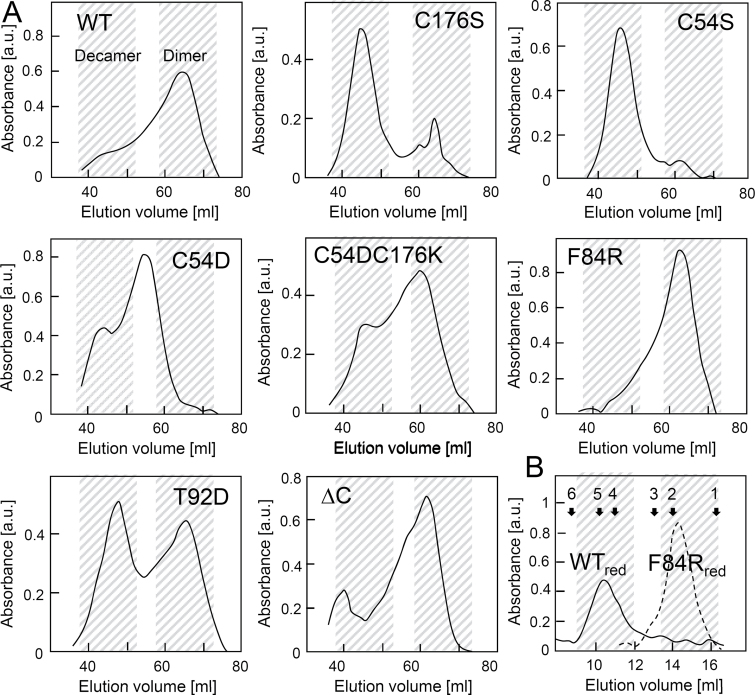
2-CysPrx undergoes major conformational changes during the catalytic thiol-disulfide and non-catalytic hyperoxidation cycles, respectively (König et al., 2003). Oxidation destabilizes the dimer–dimer interface and causes preferential formation of dimers. Reduction of 2-CysPrx triggers the oligomerization to a doughnut-shaped form, which most often assembles as decamer consisting of five dimers. The elution profile after size fractionation under non-reducing conditions tentatively allowed us to distinguish the dimer eluting at its maximum at 64ml, the decamer at 46ml and intermediate or abnormal forms (Fig. 2A). The results could be assigned to three distinct patterns: first, WT, F84R, and the ΔC variants eluted almost exclusively as dimers; secondly, the CysP and CysR variants C54S and C176S predominantly formed decamers like the reduced protein (Fig. 2A, B) (König et al., 2002). In contrast, the reduced F84R variant eluted as a dimer (Fig. 2B), suggesting that formation of the reduced decamer was impeded in this variant. C54D eluted as an oligomer at 55ml that was larger than the dimers but smaller than the decamers. The third group contained the salt-bridged C54DC176K and the pseudo-phosphorylated T92D variant, and eluted in a mixed manner as a dimer and decamer.
Fig. 2.
Size-exclusion chromatography elution profiles of 2-CysPrx WT and variants. (A) Two ml of 5mg protein ml–1 was separated on an S200 16/60 size-exclusion chromatography column using 40mM potassium phosphate (pH 7.2) as running buffer. (B) 2-Cys Prx WT and F84R were reduced for 2h with 10mM DTT and analysed using an S200 10/300 gel filtration column using 40mM potassium phosphate (pH 7.2) as running buffer. Standard proteins were used for calibration: 1, chymotrypsin (25kDa); 2, 2-Cys Prx WTox; 3, ovalbumin (44kDa); 4, aldolase (158kDa); 5, 2-Cys Prx C54S; 6, catalase (232kDa).
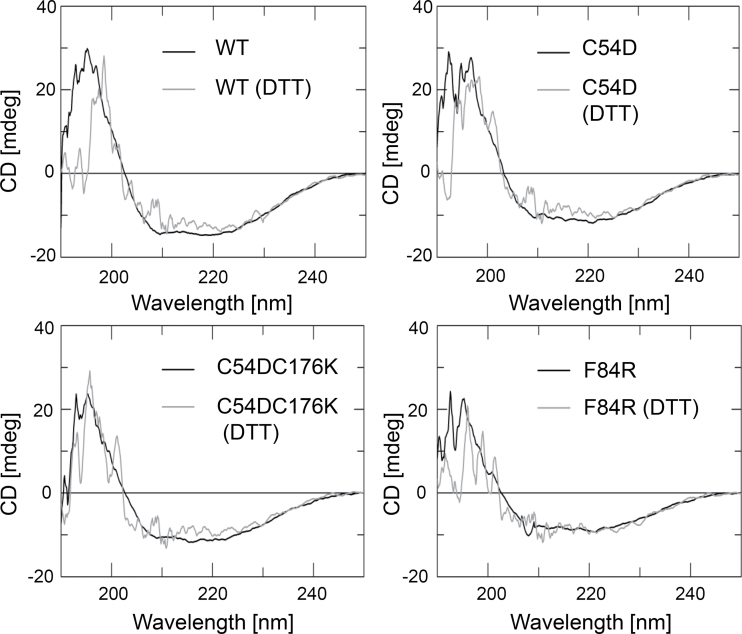
CD spectra depend on secondary and tertiary structures (Fig. 3, Table 2, and Supplementary Fig. S2 at JXB online). The eight variants were analysed for altered CD in the reduced and oxidized state. In all proteins except C54DC176K and C176S, oxidation increased the α-helical proportion at the expense of β-turns and other more random structures. Thus, C54DC176K in the reduced state was already close to the α-helical arrangement realized in the oxidized WT, suggesting at least partial establishment of the salt bridge and an arrangement mimicking the oxidized form. C54D revealed the highest percentage of helical arrangement among all variants both under reducing and non-reducing conditions, confirming the expectation that introducing the negative charge fosters the compact multimeric state. C176S lacked any redox-dependent change in α-helical assembly, but random and β-turns decreased upon oxidation. This is interesting as it indicates that oxidation of Cys54 triggers a rearrangement of the protein that probably eases disulfide bridge formation. The ΔC variant behaved in a peculiar way as the structure was rearranged upon oxidation in a manner that was much more profound than in any other variant, only approximated by T92D. Apparently a negative charge at aa 92, as possibly introduced by phosphorylation, strongly affects thiol redox-dependent conformational rearrangements. The conformation of the last variant with a charge at the dimer–dimer interface, F84R, was WT-like in the oxidized state but differed from WT as expected in the reduced state where WT 2-CysPrx assembles to oligomers, which was impeded in the F84R variant.
Fig. 3.
Secondary structure analysis of 2-CysPrx WT and variants using CD spectroscopy. A concentration of 10 μM non-treated and oxidized and reduced (10mM DTT) 2-CysPrx WT and variants was analysed. The spectra of the other variants are presented in Supplementary Fig. S2.
Table 2.
Analysis of CD spectra recorded from reduced and oxidized 2-CysPrx
Data are means±SD of four independent experiments. 2-Cys Prx variants were analysed under non-reducing and reducing conditions. Numbers show the percentage of the respective structures.
| WT | C54D | C54S | C176S | T92D | F84R | C54D/ C176K | ΔC | |
|---|---|---|---|---|---|---|---|---|
| Non-reducing | ||||||||
| Helix | 38±10 | 57±5 | 51±4 | 38±5 | 38±4 | 41±3 | 34±7 | 80±6 |
| β-sheet | 11±3 | 0±0 | 0±0 | 14±8 | 21±5 | 19±2 | 21±7 | 0±0 |
| Turn | 27±3 | 29±3 | 39±2 | 30±3 | 25±3 | 22±4 | 25±1 | 2±2 |
| Random | 24±4 | 14±3 | 10±2 | 18±1 | 16±2 | 17±1 | 19±2 | 18±4 |
| Reducing | ||||||||
| Helix | 28±4 | 42±0 | 29±2 | 33±1 | 33±9 | 32±2 | 31±8 | 18±3 |
| β-sheet | 0±0 | 2±2 | 0±0 | 0±0 | 6±6 | 0±0 | 2±3 | 61±9 |
| Turn | 36±3 | 40±6 | 39±7 | 41±4 | 37±6 | 37±4 | 37±4 | 6±6 |
| Random | 36±6 | 16±4 | 32±9 | 27±5 | 24±6 | 31±7 | 30±10 | 15±6 |
Oligomer–dimer transition by ITC
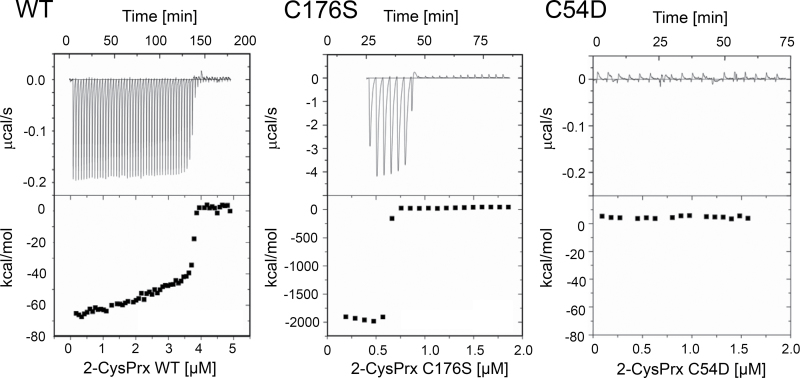
ITC is a powerful technique to analyse the oligomer–dimer transition of reduced 2-CysPrxs by quantifying the thermodynamics of its dissociation (Barranco-Medina et al., 2008). Only the reduced form displayed this fast dissociation behaviour. The oxidized and hyperoxidized protein showed only background heat dilution. Injected oligomers disintegrate into dimers below a critical transition concentration (CTC) accompanied by a release of heat; thus, oligomerization is an endothermic reaction. Above the CTC, no dissociation is recorded. Here, the untagged At2-CysPrx displayed a CTC of 3.7 μM, which was about 2.5-fold higher than previously reported for His-tagged At2-CysPrx (Barranco-Medina et al. 2008) (Fig. 4, Table 3). The C176S variant showed a lower CTC of 0.63 μM, suggesting that the oligomerized state is stabilized in this variant. The released heat (ΔH) was 22-fold increased in comparison with WT. The C54D variant showed only background heat dilution and no dissociation, and thus a CTC could not be determined.
Fig. 4.
Quantification of the oligomer–dimer transition by isothermal titration microcalorimetry. Reduced His6-At2-CysPrx WT forms oligomers which have been shown to disintegrate into dimers below a critical dissociation concentration (CTC) (Barranco-Medina et al., 2008). Here, the thermodynamics of 2-CysPrx dissociation were quantified by multiple injections of highly concentrated reduced 2-CysPrxs (1mM DTT, 40mM potassium phosphate) for WT (left panel), C176S (middle panel), and C54D (right panel). The data are summarized in Table 3.
Table 3.
Analysis of 2-CysPrx WT, C54D and C176S dissociation by ITC
Proteins were titrated under reducing conditions (40mM potassium phosphate buffer, 1mM DTT); thus, the protein solubilization buffer and vessel buffer were identical after extensive dialysis. Critical transition concentrations (CTC) and dissociation enthalpy (ΔH m, kcal/mol) were calculated. Data are means±SD for WT (n=4), C54D (n=6), and C176S (n=5).
| 2-CysPrx | CTC (μM) | ΔHm (kcal/mol) |
|---|---|---|
| WT | 3.73±0.73 | 72±17 |
| C54D | No dissociation | – |
| C176S | 0.63±0.03 | 1614±176 |
Peroxidase activity
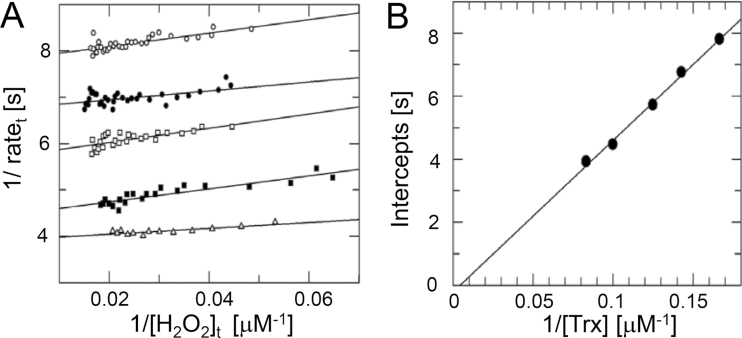
2-CysPrx reacts with two redox substrates in each reaction cycle, reacting first with the peroxide substrate. Upon CysP oxidation, an intermediate sulfenic acid is formed, which then converts to the disulfide bridge with CysR. Secondly, the disulfide bridge is reduced by a redox transmitter such as NTRC, Trx-x or CDSP32, to complete the peroxidatic redox cycle. Participation of two substrates implies that the kinetics of 2-CysPrx is well described by a ‘ping-pong’ mechanism (Nogoceke et al., 1997). Using the integrated Dalziel equation, it is possible to independently determine the rate constants of the hydroperoxide reduction (Fig. 5A) and regeneration by Trx, respectively (Fig. 5B). The best regeneration system of plant 2-CysPrx in vitro is the coupled E. coli Trx/thioredoxin reductase system (Muthuramalingam et al., 2009) with NADPH as reductant. The regeneration by Trx remained the limiting step during the reaction cycle in all variants irrespective of the introduced modification (Table 4). Both variants with a variation at the N (His6) or C (ΔC) terminus failed to saturate, and accordingly a V max value could not be determined. The His6-tagged protein revealed nearly twice the rate constant of hydroperoxide reduction compared with the WT. The reaction rate constant of T92D was indistinguishable from that of WT. The K M value towards H2O2 as well as towards Trx was strongly increased. The hydroperoxide-dependent rate constant of F84R was greatly increased, indicating that the rearrangement of the quaternary structure during transition from dimer to decamer decelerates the reaction in the WT. In a converse manner, the Trx-dependent rate constant was reduced, as was the K M value towards Trx.
Fig. 5.
Kinetic analysis of 2-CysPrx WT and variants. Representative data are shown for WT. Linear plot of the integrated Dalziel rate equation for a two-substrate reaction. Activity of 2-CysPrx was determined with 75 μM hydrogen peroxide and varying concentrations of E. coli Trx (6 μM, open circles; 8 μM, filled circles; 10 μM, filled squares; 12 μM, open squares) as described in Materials and methods. (B) Secondary Dalziel plot. The data are summarized in Table 4.
Table 4.
Kinetic properties of 2-CysPrx WT and variants
Peroxidase activity of the variants that possessed CysP was analysed with five different concentrations of EcTrx and 75 μM hydrogen peroxide. The data were analysed using the Dalziel equation. Additionally, the apparent peroxidase activity of 5 μM 2-CysPrx and variants was analysed using 1mM DTT as reductant and 100 μM hydrogen peroxide. All data are means±SD (n=3). ND, not determined.
| 2-CysPrx | k 1 (H2O2) (104 M-1 s–1) | k 2 (Trx) (104 M-1 s–1) | V max (s–1) | K M (H2O2), µM-1 | K M (Trx) (µM) | DTT (V max app, min–1) |
|---|---|---|---|---|---|---|
| WT | 3.23±0.02 | 2.24±0.63 | 0.38±0.13 | 12±4 | 17±1 | 0.31±0.10 |
| His6 variant | 6.35±1,66 | 2.26±0.29 | > | – | – | 1.20±0.07 |
| ΔC | 2.64±0,17 | 2.16±1.15 | > | – | – | ND |
| T92D | 2.7±0.6 | 2.0±0.7 | 0.52±0.25 | 83±26 | 105±24 | ND |
| F84R | 8.3±1.0 | 1.14±0.21 | 0.70±0.42 | 8.8±6.1 | 59±26 | 0.36±0.04 |
| C176S | ND | ND | ND | ND | ND | 0.24±0.10 |
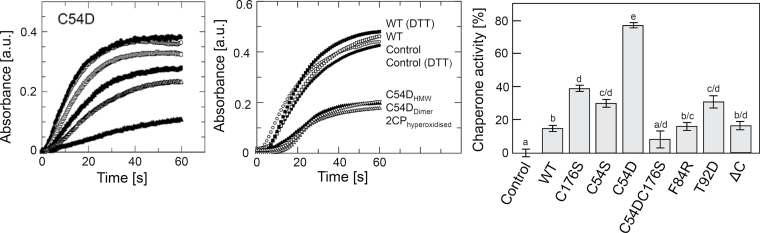
Chaperone function
Previous studies have shown that hyperoxidized 2-CysPrx functions as general chaperone. Citrate synthase (CS) aggregates at high temperature, as observable by light scattering or as an absorbance change at 360nm. Hyperoxidized At2-CysPrx protects CS from heat denaturation (Muthuramalingam et al., 2009). Here, in a similar assay, the 2-CysPrx variants were analysed for chaperone activity (Fig. 6, left panel). Only hyperoxidized WT displayed chaperone activity but not the reduced or oxidized protein (Fig. 6, middle panel). The C54D variant showed similar concentration-dependent chaperone activity as the hyperoxidized WT protein, confirming that this variant is mimicking the hyperoxidized protein (Fig. 6, middle panel). Chaperone activity is not just linked to hyperoxidation but was brought into the context of concomitant hyperaggregate formation. Oxidized plant 2-CysPrx exists mainly as a dimer, unlike homologous proteins from other organisms (Cao et al., 2011). The C54D variant predominantly eluted in chromatographic fractions that corresponded to multimers. However, C54D protein from fractions corresponding to low molecular mass as well as from fractions corresponding to the size of an oligomer revealed similar chaperone activity. The other 2-CysPrx variants displayed chaperone activity in a decreasing order of C54D > C176S > C54S > T92D. The C54DC176K and F84R variants lacked any appreciable chaperone activity. Interestingly, 2-CysPrx showed higher chaperone activity using HEPES buffer instead of potassium phosphate buffer (Fig. 6, middle and right panels).
Fig. 6.
Chaperone activity. The light scattering due to thermal aggregation at 45 °C of citrate synthase (1 μM) was visualized by using a spectrophotometer at 360nm. Left panel: chaperone activity of 2-CysPrx C54D in 40mM potassium phosphate buffer (pH 7.2) at the concentrations of 0 (●), 0.5 (◯), 1 (□), 2 (■), 4 (△) and 10 (▲) μM. Middle panel: chaperone activity of oxidized WT (□), reduced WT (■), hyperoxidized WT (△) and non-treated C54D variant at concentrations of 2 μM in 50mM HEPES buffer (pH 7.0). C54D from fractions eluted by size-exclusion chromatography corresponded to a decamer (▽) or lower molecular mass (▲). The controls contained no 2-CysPrx (◯) or 10mM DTT (●). Right panel: chaperone activities of variants at 10 μM in 40mM potassium phosphate buffer (pH 7.2). Data are means ±standard error (n=10). Different letters indicate the significance of groups at P ≤0.05 determined using Student’s t-test.
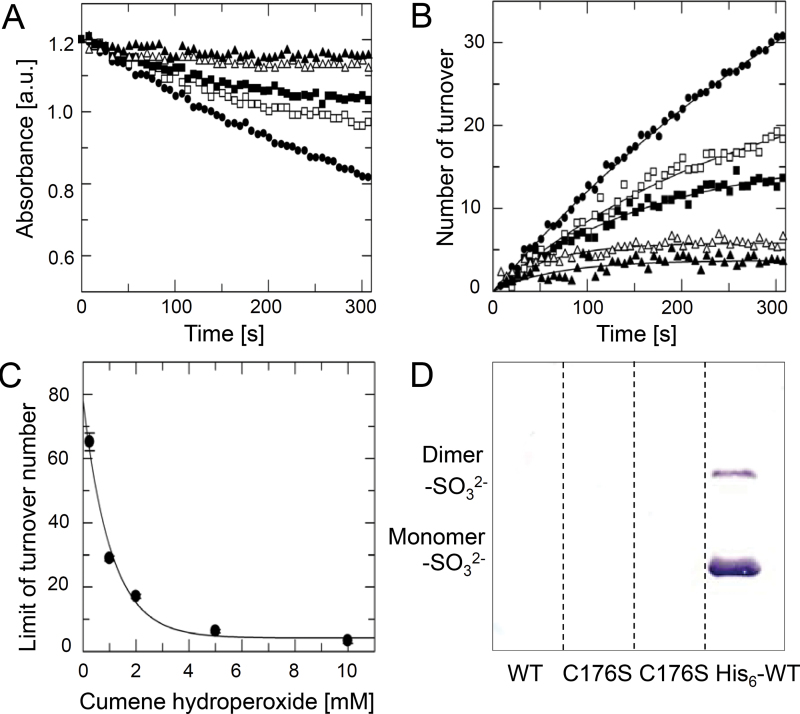
Sensitivity to hyperoxidation
Hyperoxidized 2-CysPrx accumulates in vivo as a consequence of severe environmental stress conditions (König et al., 2003). Recently, it was reported that the appearance of hyperoxidized 2-CysPrx may serve as a transcription-independent biomarker for the functional circadian clock (Edgar et al., 2012). Here, an assay was established to quantify the hyperoxidation sensitivity of the variants to get a better understanding of the switch between peroxidase and chaperone activity. Complex hydroperoxides such as cumene hydroperoxide or even more lipid hydroperoxides are known to trigger hyperoxidation (König et al., 2003). 2-CysPrx variants were exposed to various concentrations of cumene hydroperoxide in the presence of the E. coli Trx/TR regeneration system. The consumption of NADPH was monitored (Fig. 7A), and the number of cumulated turnovers per 2-CysPrx subunit was calculated (Fig. 7B). The data could be fitted to a saturation curve. The calculated cumulated turnover numbers were plotted against the corresponding cumene hydroperoxide concentrations and the data fitted to an exponential decay curve with offset (Fig. 7C). The ΔC variant maintained very high peroxidase activities at high cumene hydroperoxide concentrations, consistent with the lack of saturation (Table 5) already observed in the peroxidase assay. Apparently, the ΔC variant was insensitive to hyperoxidation. The hyperoxidation sensitivity of WT and His6-tagged protein at very low hydroperoxide concentration was similar, each with a cumulated turnover of about 200 peroxidative cycles per protein subunit. However the rate constant of hyperoxidation was higher than for WT. The offset of over 100 turnovers suggested unspecific hydroperoxide binding, which was seen in the peroxidase assay. The variants T92D and F84R showed considerably decreased cumulated turnover numbers in comparison with WT, and thus high rate constants of hyperoxidation and low offsets. In comparison with F84R, T92D had a higher rate constant and a higher offset, as well as higher K M values towards cumene hydroperoxide and Trx, respectively.
Fig. 7.
Hyperoxidation sensitivity of 2-CysPrx WT and variants. Representive data are shown for 2-CysPrx F84R. Similar assay conditions were applied as used for the peroxidase activity. A fixed concentration of 15 μM Ec-Trx was used, and the reaction was started with various concentrations (0.5, 1, 2, 5, and 10mM) of cumene hydroperoxide. (A) The NADPH consumption was monitored over 5min. (B) The number of turnovers per 2-CysPrx molecule was calculated and plotted against time. The data were fitted to an equation describing a saturation curve. (C) The calculated limits of turnover were plotted against cumene hydroperoxide concentrations. Data were fitted to an equation describing an exponential curve with offset. The calculated parameters are displayed in Table 5. (D) Western blot analysis of hyperoxidized 2-CysPrx using antiserum against sulfinic acid 2-CysPrx (2-CysPrx-SO3 2–). WT, C176S and His6-WT were reduced with 10mM DTT and treated three times with 2mM H2O2 for 15min at 37 °C. Untreated C176S was loaded in lane 2. One microgram of each 2-CysPrx variant was loaded per lane.
Table 5.
Sensitivity of 2-CysPrx WT and variants to inactivation by hyperoxidation
The initial rate in the TR/Trx system was determined with 50 μM cumene hydroperoxide (COOH). Data are means ±SD (n=3).
| 2-CysPrx | Initial rate (s–1 H2O2)b | Initial rate (s–1 COOH) | Overall limit of turnover | COOH concentration reducing turnover to half (mM) | Offset |
|---|---|---|---|---|---|
| WT | 0.23±0.01 | 0.44±0.01 | 193±35 | 0.17±0.11 | -5.5±51 |
| His6- | 0.26±0.00 | 0.34±0.01 | 230±19 | 0.27±0.07 | 105±20 |
| ΔC | 0.31±0.03 | 0.38±0.03 | >a | >a | >a |
| T92D | 0.14±0.01 | 0.18±0.01 | 73±12 | 1.47±0.37 | 20.0±2.3 |
| F84R | 0.14±0.01 | 0.19±0.01 | 74±10 | 0.95±0.17 | 4.1±1.3 |
a No inactivation but enhanced peroxidase activity of the ΔC-variant was observed.
b The initial rate was determined with 75 μM hydrogen peroxide.
C176S lacks the CysR that is essential for disulfide bridge generation between CysP and CysR and subsequent regeneration by Trx. The remaining CysP in the active site enables hydroperoxide reduction. C176S can be reduced in vitro by DTT. With DTT as the reducing agent, the non-tagged C176S displayed a similar peroxidase activity as WT (Table 4). Hyperoxidation of C176S and WT was not detected in this assay. Hyperoxidized 2-CysPrx could be detected by Western blotting using antiserum against sulfinic acid CysP. Only His6-tagged 2-CysPrx was hyperoxidized in the presence of 2mM H2O2 with DTT as reducing reagent (Fig. 7D). The untagged variants were insensitive to hyperoxidation under these conditions.
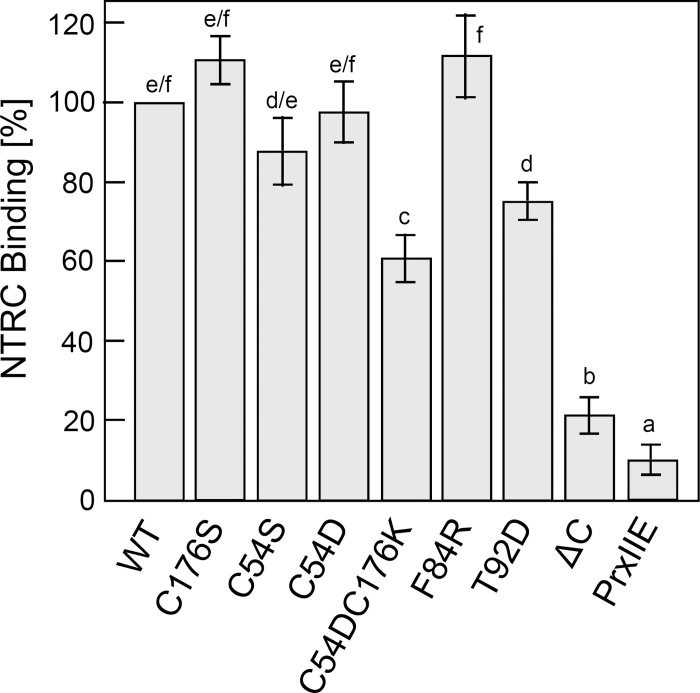
2-CysPrx variants as tool for analysing the interacting partner
The 2-CysPrx variants are proposed to be instrumental for finding and studying the redox conformation-specific interaction with other proteins. NTRC transfers reducing equivalents to 2-CysPrx (Kirchsteiger et al., 2009; Muthuramalingam et al., 2009; Pulido et al., 2010). In vivo studies have shown that NTRC is important for the reduction of 2-CysPrx, especially during the night (Pulido et al., 2010). NTRC is an established interacting partner of 2-CysPrx. Here, the 2-CysPrx variants were used to study the interaction with NTRC by far-Western blot analysis, which is a simple and widely applicable in vitro method to assess stable protein–protein interactions (Fig. 8). The 2-CysPrx variants in various concentrations were directly applied on a membrane and incubated with His6-tagged NTRC. After washing, bound NTRC was detected by immunostaining with anti-oligo-His antibody. The thiol-containing PrxIIE was used as a control for non-specific binding and showed only slight background interaction. WT and the C176S, C54S, C54D and F84R variants showed similar binding of NTRC. All these variants contained at least one cysteine residue, whereas binding of the cysteine-free variant C54DC176K was considerably reduced. Interestingly, binding of the T92D variant was also reduced. The variant with a truncated C-terminal tail showed the lowest binding affinity. The results suggest that both cysteine residues enhance the interaction but that the C-terminal tail is especially important for the NTRC–2-CysPrx interaction.
Fig. 8.
Interaction of At-NTRC with 2-Cys Prx WT and variants as determined by far-Western blotting. Various 2-Cys Prx variants in six different concentrations were spotted on a nylon membrane. The membrane was overlayed with reduced His6-NTRC and after several washing steps, the interacting His6-NTRC was visualized by immunodetection using anti-His6 antiserum. The experiment was repeated four times. Staining intensity was quantified in digitized images using gel scanning software. Background staining was subtracted. Staining intensities in the linear data range were related to WT (100%). Data represent means±SD (n=4). Different letters indicate the significance of groups at P ≤0.05 determined using Student’s t-test.
Discussion
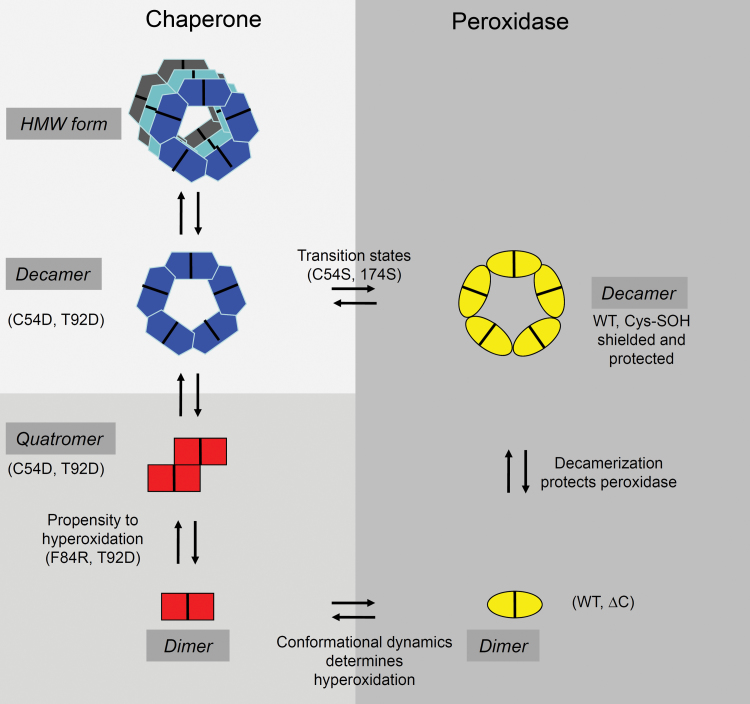
2-CysPrx serves two conserved and thus principle functions as an alkyl hydroperoxide-detoxifying peroxidase and chaperone (Muthuramalingam et al., 2009; König et al., 2012). This work aimed to separate these functions by forcing the protein into specific states. In addition to previously described mutants lacking CysP or CysR, five novel variants were designed that adopted specific conformational states and thus favoured the peroxidase function (ΔC), or mimicked the hyperoxidized chaperone (C54D), the oxidized inactive protein (C54DC176K), the reduced dimeric protein, which usually would accumulate only to a concentration of about 1–2 μM (F84R), and the phosphorylated protein (T92D). Fig. 9 provides a summary of the results observed in this study and discussed in the following sections.
Fig. 9.
Summarizing scheme of the results and conclusions. 2-CysPrx adopts two principle different conformations, one with a peroxidase function and one with a chaperone function. The variants introduced in this study arrested certain conformations (C54S, C176S, C54D, C54DC176K, and F84R) and/or modified the flexibility of the protein (ΔC, C176S, and T92D). Hyperoxidation of C54 switches off the peroxidase and switched on the chaperone activity. However, hyperoxidation is not essential for chaperone activity (T92D, C54S, and C176S). This allowed us to suggest the function–structure relationships as indicated in the scheme. See text for further details. (This figure is available in colour at JXB online.)
Variants as tools for analysing specific conformational states and functions
All catalytically active variants were compared with non-tagged At2-CysPrx WT protein. WT protein showed a peroxidase function with kinetic parameters for H2O2 reduction (k cat/K M: 3.23±0.02×104 M–1 s–1) similar to those described for Pisum sativum 2-CysPrx (k cat/K M: 2.5×104 M–1 s–1) (Bernier-Villamor et al., 2004). Like the reduced WT, both mutants lacking CysP or CysR eluted as decamers after size-exclusion chromatography. This result was in agreement with previous reports for barley 2-CysPrx (König et al., 2002). However, unlike the reduced WT, both variants displayed significant chaperone activity. The C54D variant with its negative charge in the catalytic centre acted as a chaperone, as reported previously for the hyperoxidized human 2-CysPrx (Jang et al., 2004). The C54D oligomer was unable to dissociate like the hyperoxidized His6-tagged 2-Cys Prx (Barranco-Medina et al., 2008). These results confirmed that the C54D variant mimics the hyperoxidized state and can be used to analyse 2-CysPrx chaperone function.
The C54DC176K variant, which was designed to simulate the oxidized dimer by introducing a salt bridge, displayed no significant chaperone function and, due to the loss of cysteines, no peroxidase function. Size-exclusion chromatography indicated that this variant existed as a mixture of dimers and decamers in solution. Apparently, this variant was at least partly mimicking the oxidized protein, which occurs within the peroxidase cycle. The F84R variant in the oxidized as well as the reduced form showed the dimeric conformation. The protein displayed an enhanced reaction rate constant and a lower K M value towards hydrogen peroxide, resulting in a higher sensitivity to hyperoxidation. The disturbance of the dimer–dimer interface in the F84R variant destabilized the reduced decamer conformation and facilitated hyperoxidation. The T92D variant displayed a similar peroxidase function and sensitivity to hyperoxidation as the F84R variant. Size-exclusion chromatography analysis suggested that this variant exists as a dimer and various forms of aggregated dimers up to decamers in solution. The peroxidase activity of the ΔC variant was similar to WT at low, in vivo-relevant hydroperoxide concentrations. Due to the loss of saturation, the variant displayed strongly increased peroxidase activity at higher hydroperoxide concentrations. In addition, this variant became tolerant to hyperoxidation and displayed no significant chaperone function. The dimeric conformation of the oxidized ΔC variant and the results of catalytic and regeneration rates suggested that elimination of the flexible C terminus converts 2-CysPrx into a more efficient peroxidase. Apparently, the C terminus is the major determinant of the slow peroxidase cycle. Thus, the maintenance of the C-terminal extension appears to be a prerequisite to enable the functional switch from peroxidase to chaperone. Analysis of C-terminally truncated Schizosaccharomyces pombe 2-CysPrx has illustrated that the positive charge of the conserved residue Lys191 is a major factor contributing to the hyperoxidation sensitivity (Koo et al., 2002). It was concluded that the optimization of peroxidase function was not a major driving force during evolution.
NTRC–2-CysPrx interaction
NTRC has been shown to efficiently re-reduce oxidized 2-CysPrx in vitro (Perez-Ruiz et al., 2006) and in vivo (Pulido et al., 2010). The interaction is sufficiently strong to be visualized by FRET analysis in transfected protoplasts (Muthuramalingam et al., 2009) and could also be visualized in overlay assays in this work. The interaction was low under reducing conditions for the C54DC176K and ΔC variants. These results concur with the hypothesis that NTRC preferentially interacts with the reduced or hyperoxidized 2-CysPrx and that this interaction is mediated by the C-terminal extension. Association of NTRC with the decameric form and not with the oxidized dimer, as simulated in the C54DC176K variant, could facilitate re-reduction of 2-CysPrx in the regular peroxidase cycle. Under more oxidizing conditions, NTRC might be released from 2-CysPrx in order to preferentially resume other redox regulatory functions (Cejudo et al., 2012). The results exemplarily show that the set of variants constructed in this work should allow straightforward investigation of the molecular basis underlying 2-CysPrx interactions, e.g. with photosystem II, Cyp20-3, or fructose-1,6-bisphosphatase (Laxa et al., 2007; Caporaletti et al., 2007; Muthuramalingam et al., 2009) or other as yet unknown interacting proteins.
Quaternary structure-dependent functions
The reduced WT, C176S and C54D forms all assembled as decamers but showed different CTCs in the ITC dissociation experiment and also different chaperone activities in a reciprocal order. These results support previous suggestions (Barranco-Medina et al., 2008; Muthuramalingam et al., 2009) that there might exist distinct decameric states with different structural and functional properties, namely the reduced decamer favouring peroxidase activity, the decamer with strong chaperone function but in addition intermediate conformational states. The chaperone function of the oxidized T92D variant and non-hyperoxidized C54S and C176S variants implied that the chaperone activity did not necessarily depend on the hyperoxized CysP in the protein. Hyperoxidation only represents one possibility for switching the function from peroxidase to chaperone. Furthermore, chaperone activity was not limited to the decameric or high-molecular-weight forms of 2-CysPrx as seen for the C54D and T92D variant. The presented data suggest that there are two principle conformations, which do not depend exclusively on the dimer or decamer form or on the redox state (Fig. 9). One conformation can be assigned to the chaperone function and the other to the peroxidase function.
CysP and CysR are spaced about 13 Å apart in the reduced decamer (Cao et al., 2011). This distance is closed upon oxidation, which requires structural flexibility. The dimer–dimer interface is modified in F84R, which impeded decamerization in the reduced state. Exclusive elution of F84R as a dimer during size-exclusion chromatography confirmed this hypothesis. It appears that this disturbance of the dimer–dimer interface with the concomitant permanent adoption of the dimeric conformation eased the accessibility of CysP and allowed a faster reaction with hydroperoxides. It may be hypothesized that eased accessibility increases the sensitivity of CysP to hyperoxidation compared with the WT, which adopts the decameric form in the reduced state. These observations are in line with the known dynamics of 2-CysPrx in the peroxidase cycle. Hyperoxidation sensitivity of F84R provides an explanation as to why the extremely high cooperativity in decamer formation of the reduced 2-CysPrx (Barranco-Medina et al., 2008; Barranco-Medina et al., 2009) might be beneficial: the rapid decamer assembly lowers the rate of hyperoxidation in the cell. Furthermore, it is hypothesized that the velocity with which the conformational switch occurs between the oxidized dimer and the reduced decamer is the major determinant of the rate of hyperoxidation.
Apparently, the structure of 2-CysPrx is highly flexible. Changes in the redox state of CysP and CysR strongly affect the equilibrium between the different conformational states. In addition, other post-translational modifications also modulate the functional state of 2-CysPrx and contribute to the switch between peroxidase and chaperone activity. Described mechanisms for human 2-CysPrxs include phosphorylation (Rhee et al., 2012), glutathionylation (Chae et al., 2012), acetylation (Seo et al., 2009) and proteolysis (Koo et al., 2002). It is suggested that post-translational modifications allow the fine-tuning of 2-Cys Prx function in vivo. Furthermore, previous studies have suggested that pH, temperature, and salt concentrations influence the conformation (Bernier-Villamor et al., 2004). In a proteomics approach, rice 2-CysPrx was detected as being reversibly phosphorylated, with heat as the trigger of dephosphorylation (Chen et al., 2011). Introduction of a negative charge at Thr92 mimicking phosphorylation increased the sensitivity of 2-CysPrx to hyperoxidation. It is tempting to speculate that phosphorylation and hyperoxidation might be linked in vivo to control 2-CysPrx structure and function. There are several indications that the mammalian cell cycle and the circadian network are coupled (Gerard and Goldbeter, 2012). The data presented here suggest that future studies should address the question of whether other interacting molecules and proteins influence this delicate structure–function equilibrium of 2-CysPrx.
Outlook
This study introduced a collection of 2-CysPrx variants that reliably separate the peroxidase and chaperone functions by adopting defined conformations (Table 1). These variants will be valuable tools in future studies and will allow us to address and differentiate the role of 2-CysPrx in cell signalling and antioxidant defence. Our data provide evidence that it is feasible to obtain variants with enhanced peroxidase or chaperone function. However, evolution has favoured a multifunctional 2-CysPrx protein that switches between the high-affinity but low-turnover peroxidase function and the low-efficiency chaperone function. There is a need in plants, but also in mammals and other organisms, to explore the reasons for this delicate multifunctionality beyond the reported positive effects of, for example, overexpression of 2-CysPrx on oxidative stress and heat tolerance (An et al., 2011; Kim et al., 2011).
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. 1. Multiple sequence alignment of 2-Cys Prx proteins from various organisms.
Supplementary Fig. 2. Secondary structure analysis of 2-CysPrx variants using circular dichroism spectroscopy.
Supplementary Table S1. Primers used for cloning of 2-CysPrx and variants.
Acknowledgements
The work was supported by the Deutsche Forschungsgemeinschaft (DFG, DI 346/14-1).
Glossary
Abbreviations:
- 2-CysPrx
2-cysteine peroxiredoxin
- CDSP32
chloroplast drought-specific protein of 32 kDa
- CS
citrate synthase
- NTRC
NADPH-dependent thioredoxin reductase with built-in thioredoxin domain
- Prx
peroxiredoxin
- Srx
sulfiredoxin
- TR
thioredoxin reductase
- Trx
thioredoxin.
References
- An BC, Lee SS, Lee JT, Hong SH, Wi SG, Chung BY. 2011. Engineering of 2-Cys peroxiredoxin for enhanced stress-tolerance. Molecules and Cells 32, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barranco-Medina S, Kakorin S, Lazaro JJ, Dietz KJ. 2008. Thermodynamics of the dimer–decamer transition of reduced human and plant 2-cys peroxiredoxin. Biochemistry 47, 7196–7204 [DOI] [PubMed] [Google Scholar]
- Barranco-Medina S, Lazaro JJ, Dietz KJ. 2009. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Letters 583, 1809–1816 [DOI] [PubMed] [Google Scholar]
- Bernier-Villamor L, Navarro E, Sevilla F, Lazaro JJ. 2004. Cloning and characterization of a 2-Cys peroxiredoxin from Pisum sativum. Journal of Experimental Botany 55, 2191–2199 [DOI] [PubMed] [Google Scholar]
- Cao ZB, Tavender TJ, Roszak AW, Cogdell RJ, Bulleid NJ. 2011. Crystal structure of reduced and of oxidized peroxiredoxin IV enzyme reveals a stable oxidized decamer and a non-disulfide-bonded intermediate in the catalytic cycle. Journal of Biological Chemistry 286, 42257–42266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaletti D, D’Alessio AC, Rodriguez-Suarez RJ, Senn AM, Duek PD, Wolosiuk RA. 2007. Non-reductive modulation of chloroplast fructose-1,6-bisphosphatase by 2-Cys peroxiredoxin. Biochemical and Biophysical Research Communications 355, 722–727 [DOI] [PubMed] [Google Scholar]
- Cejudo FJ, Ferrandez J, Cano B, Puerto-Galan L, Guinea M. 2012. The function of the NADPH thioredoxin reductase C-2-Cys peroxiredoxin system in plastid redox regulation and signalling. FEBS Letters 586, 2974–2980 [DOI] [PubMed] [Google Scholar]
- Chae HZ, Oubrahim H, Park JW, Rhee SG, Chock PB. 2012. Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators. Antioxidants & Redox Signaling 16, 506–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MF, Lee KD, Yeh CH, Chen WC, Huang WS, Chin CC, Lin PY, Wang JY. 2010. Role of peroxiredoxin I in rectal cancer and related to p53 status. International Journal of Radiation Oncology, Biology, Physics 78, 868–878 [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang W, Zhang B, Zhou J, Wang Y, Yang Q, Ke Y, He H. 2011. Phosphoproteins regulated by heat stress in rice leaves. Proteome Science 9, 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ. 2008. Redox signal integration: from stimulus to networks and genes. Physiologia Plantarum 133, 459–468 [DOI] [PubMed] [Google Scholar]
- Dietz KJ. 2011. Peroxiredoxins in plants and cyanobacteria. Antioxidants & Redox Signaling 15, 1129–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Green EW, Zhao YW, et al. 2012. Peroxiredoxins are conserved markers of circadian rhythms. Nature 485, 459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C, Goldbeter A. 2012. Entrainment of the mammalian cell cycle by the circadian clock: modeling two coupled cellular rhythms. PLoS Computational Biology 8, e1002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y, Dick TP. 2009. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. Journal of Biological Chemistry 284, 31532–31540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Baena I, Barranco-Medina S, Lazaro-Payo A, Lopez-Jaramillo FJ, Sevilla F, Lazaro JJ. 2010. Characterization of plant sulfiredoxin and role of sulphinic form of 2-Cys peroxiredoxin. Journal of Experimental Botany 61, 1509–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HH, Kim SY, Park SK, et al. 2006. Phosphorylation and concomitant structural changes in human 2-Cys peroxiredoxin isotype I differentially regulate its peroxidase and molecular chaperone functions. FEBS Letters 580, 351–355 [DOI] [PubMed] [Google Scholar]
- Jang HH, Lee KO, Chi YH, et al. 2004. Two enzymes in one: two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- Kim JH, Bogner PN, Baek SH, Ramnath N, Liang P, Kim HR, Andrews C, Park YM. 2008. Up-regulation of peroxiredoxin 1 in lung cancer and its implication as a prognostic and therapeutic target. Clinical Cancer Research 14, 2326–2333 [DOI] [PubMed] [Google Scholar]
- Kim MD, Kim YH, Kwon SY, Jang BY, Lee SY, Yun DJ, Cho JH, Kwak SS, Lee HS. 2011. Overexpression of 2-cysteine peroxiredoxin enhances tolerance to methyl viologen-mediated oxidative stress and high temperature in potato plants. Plant Physiology and Biochemistry 49, 891–897 [DOI] [PubMed] [Google Scholar]
- Kim SY, Jang HH, Lee JR, et al. 2009. Oligomerization and chaperone activity of a plant 2-Cys peroxiredoxin in response to oxidative stress. Plant Science 177, 227–232 [Google Scholar]
- Kirchsteiger K, Pulido P, Gonzalez M, Cejudo FJ. 2009. NADPH thioredoxin reductase C controls the redox status of chloroplast 2-Cys peroxiredoxins in Arabidopsis thaliana. Molecular Plant 2, 298–307 [DOI] [PubMed] [Google Scholar]
- König J, Baier M, Horling F, Kahmann U, Harris G, Schurmann P, Dietz KJ. 2002. The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the redox-hierarchy of photosynthetic electron flux. Proceedings of the National Academy of Sciences, U S A 99, 5738–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J, Fairlamb AH. 2007. A comparative study of type I and type II tryparedoxin peroxidases in Leishmania major. FEBS Journal 274, 5643–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J, Lotte K, Plessow R, Brockhinke A, Baier M, Dietz KJ. 2003. Reaction mechanism of plant 2-Cys peroxiredoxin. Role of the C terminus and the quaternary structure. Journal of Biological Chemistry 278, 24409–24420 [DOI] [PubMed] [Google Scholar]
- König J, Muthuramalingam M, Dietz KJ. 2012. Mechanisms and dynamics in the thiol/disulfide redox regulatory network: transmitters, sensors and targets. Current Opinion in Plant Biology 15, 261–268 [DOI] [PubMed] [Google Scholar]
- Koo KH, Lee S, Jeong SY, Kim ET, Kim HJ, Kim K, Song K, Chae HZ. 2002. Regulation of thioredoxin peroxidase activity by C-terminal truncation. Archives of Biochemistry and Biophysics 397, 312–318 [DOI] [PubMed] [Google Scholar]
- Laxa M, König J, Dietz KJ, Kandlbinder A. 2007. Role of the cysteine residues in Arabidopsis thaliana cyclophilin CYP20-3 in peptidyl-prolyl cis-trans isomerase and redox-related functions. Biochemical Journal 401, 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T, Okamoto K, Iwahara S, Hori H, Takahashi Y, Nishino T, Abe Y. 2008. Dimer–oligomer interconversion of wild-type and mutant rat 2-Cys peroxiredoxin: disulfide formation at dimer-dimer interfaces is not essential for decamerization. Journal of Biological Chemistry 283, 284–293 [DOI] [PubMed] [Google Scholar]
- Muthuramalingam M, Seidel T, Laxa M, Nunes de Miranda SM, Gartner F, Stroher E, Kandlbinder A, Dietz KJ. 2009. Multiple redox and non-redox interactions define 2-Cys peroxiredoxin as a regulatory hub in the chloroplast. Molecular Plant 2, 1273–1288 [DOI] [PubMed] [Google Scholar]
- Neumann CA, Krause DS, Carman CV, et al. 2003. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424, 561–565 [DOI] [PubMed] [Google Scholar]
- Nogoceke E, Gommel DU, Kiess M, Kalisz HM, Flohe L. 1997. A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biological Chemistry 378, 827–836 [DOI] [PubMed] [Google Scholar]
- O’Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, Reddy AB, Millar AJ. 2011. Circadian rhythms persist without transcription in a eukaryote. Nature 469, 554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier JB, Cai Y, Sun Q, Zabrouskov V, Giacomelli L, Rudella A, Ytterberg AJ, Rutschow H, van Wijk KJ. 2006. The oligomeric stromal proteome of Arabidopsis thaliana chloroplasts. Molecular & Cellular Proteomics 5, 114–133 [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz JM, Spinola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ. 2006. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 18, 2356–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido P, Spinola MC, Kirchsteiger K, Guinea M, Pascual MB, Sahrawy M, Sandalio LM, Dietz KJ, Gonzalez M, Cejudo FJ. 2010. Functional analysis of the pathways for 2-Cys peroxiredoxin reduction in Arabidopsis thaliana chloroplasts. Journal of Experimental Botany 61, 4043–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Woo HA, Kil IS, Bae SH. 2012. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. Journal of Biological Chemistry 287, 4403–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Lim JC, Lee DY, et al. 2009. Novel protective mechanism against irreversible hyperoxidation of peroxiredoxin: Nα-terminal acetylation of human peroxiredoxin II. Journal of Biological Chemistry 284, 13455–13465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Hasegawa E, Kimura A, et al. 2012. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nature Medicine 18, 911–917 [DOI] [PubMed] [Google Scholar]
- Tetsch L, Koller C, Dönhöfer A, Jung K. (2011). Detection and function of an intramolecular disulfide bond in the pH-responsive CadC of Escherichia coli. BMC Microbiology 11, 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gibney PA, West JD, Morano KA. 2012. The yeast Hsp70 Ssa1 is a sensor for activation of the heat shock response by thiol-reactive compounds. Molecular Biology of the Cell 23, 3290–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ZA, Schroder E, Harris JR, Poole LB. 2003. Structure, mechanism and regulation of peroxiredoxins. Trends in Biochemical Sciences 28, 32–40 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Yoshikawa A, Kinumi T, Ogawa Y, Saito Y, Ohara K, Yamamoto H, Imai Y, Niki E. 2009. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiology of Aging 30, 174–185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.