Abstract
Bioactive gibberellins (GAs) are involved in many developmental aspects of the life cycle of plants, acting either directly or through interaction with other hormones. Accumulating evidence suggests that GAs have an important effect on root growth; however, there is currently little information on the specific regulatory mechanism of GAs during adventitious root development. A study was conducted on tobacco (Nicotiana tabacum) plants for altered rates of biosynthesis, catabolism, and GA signalling constitutively or in specific tissues using a transgenic approach. In the present study, PtGA20ox, PtGA2ox1, and PtGAI were overexpressed under the control of the 35S promoter, vascular cambium-specific promoter (LMX5), or root meristem-specific promoter (TobRB7), respectively. Evidence is provided that the precise localization of bioactive GA in the stem but not in the roots is required to regulate adventitious root development in tobacco. High levels of GA negatively regulate the early initiation step of root formation through interactions with auxin, while a proper and mobile GA signal is required for the emergence and subsequent long-term elongation of established primordia. The results demonstrated that GAs have an inhibitory effect on adventitious root formation but a stimulatory effect on root elongation.
Key words: Adventitious root formation, auxin–gibberellin interaction, gibberellin, Nicotiana tabacum, root elongation, vascular tissue.
Introduction
Gibberellins (GAs) are tetracyclic diterpenoid phytohormones that control a wide range of growth and developmental processes throughout the plant life cycle, acting either directly or through interactions with other hormones. Recently, impressive advances through molecular studies have elucidated the vital role of GAs in coordinating seed development and germination, seedling growth, stem elongation, leaf development and bud outgrowth, flower induction, and xylem expansion (Stavang et al., 2005; Gabriele et al., 2010; Ikezaki et al., 2010; Mauriat et al., 2011; Nadeau et al., 2011; Zhao et al., 2011). However, understanding the role of GAs in root formation and growth (Osmont et al., 2007) has progressed at a slower rate. This is probably due to the fact that GAs, compared with auxin, often exhibit an indeterminate or have an indistinguishable effect on root development. The effects of exogenous GA and inhibitors related to this hormone on root development vary depending on the plant species and the experiment. However, recent advances in molecular biology and plant physiology open up new horizons for regulation of root growth by GAs in plants. Several lines of evidence support the hypothesis that active GAs are critical for root development.
GAs have been demonstrated to have a positive effect on primary root growth. A variety of GA-deficient mutant plants have been crucial for establishing the role of GAs in primary root development. Study of GA-deficient mutations in Arabidopsis shows that mutants have reduced levels of active GAs in roots compared with wild-type (WT) plants and this is associated with impaired root elongation (Fu and Harberd, 2003; Griffiths et al., 2006; Ueguchi-Tanaka et al., 2007; Willige et al., 2007). The flux of bioactive GAs is regulated by three dioxygenase enzymes, namely GA20-, GA3-, and GA2-oxidases. GA20-oxidases and GA3-oxidases catalyse the final steps in the synthesis of bioactive GAs, whereas GA2-oxidase is the major GA deactivation enzyme (Yamaguchi, 2008). Ectopic expression of the SoGA2ox3, OsGA2ox5, and OsGA2ox6 genes markedly inhibits root elongation in transgenic tobacco. Differences in root growth between WT and transgenic seedlings were most pronounced under continuous light (Lee and Zeevaart, 2005; Lo et al., 2008). Similarly, root morphology of transgenic Arabidopsis seedlings overexpressing CmGA7ox and CmGA3ox1 changed dramatically. CmGA7ox overexpressors develop much longer primary roots compared with WT plants. However, CmGA3ox1 transgenic lines develop more lateral roots that are thicker compared with WT plants (Radi et al., 2006). Recently, molecular studies have advanced our understanding of the regulatory mechanism and clearly suggest that GAs are involved in the control of root cell elongation (Ubeda-Tomas et al., 2008) and cell proliferation (Achard et al., 2009; Ubeda-Tomas et al., 2009). GA biosynthetic mutants are unable to increase their cell production rate and meristem size after germination, indicating that GA signalling controls cell division activity in the root meristem, thereby contributing to the regulation of root meristem growth (Ubeda-Tomas et al., 2009).
Nevertheless, reports from various plant species suggest that in comparison with the primary root, GAs have an inhibitory effect on the regulation of lateral roots and adventitious root development. Several studies have examined GA responses in root formation using transgenic and physiological experiments, and provide insight into its regulatory mechanisms. It was shown that accelerated lateral root or adventitious root formation are common behaviours in GA-deficient mutants (Eriksson et al., 2000; Gou et al., 2010, 2011; El-Sharkawy et al., 2012). Overexpression of GA2ox led to increased lateral root proliferation and transcriptome changes consistent with increased growth and lateral root initiation in the transgenic roots, and RNA interference (RNAi) suppression of these genes led to a decrease in root biomass (Gou et al., 2010, 2011). Correspondingly, a deficiency of GAs in rice increased adventitious root formation, while exogenous application of GA3 suppressed it (Lo et al., 2008). In contrast, overexpression of AtGA20ox1 and exogenous GA applications in aspen led to an increased concentration of bioactive GAs and suppression of lateral and adventitious root formation (Eriksson et al., 2000). A similar effect was also described in citrus plants overexpressing CcGA20ox1 (Fagoaga et al., 2007). Similarly, several studies suggest that inhibitors of GA biosynthesis can stimulate lateral roots (Berova and Zlatev, 2000; Chaney, 2003; Gilman, 2004).
Considerable progress was made recently in elucidation of the molecular basis of GA action. DELLA proteins (DELLAs), major negative regulators of GA signalling, play a central role in GA response and appear to be a point of cross-talk with other signals (Achard et al., 2006; Nemhauser et al., 2006; Harberd et al., 2009). GA response in the endodermis and the effects of GA-regulated DELLAs on root growth have been well characterized recently (Ubeda-Tomas et al., 2012). Blockage of GA signalling via heterologous expression of DELLA-less versions of GAI and RGL1 in Populus elicited an increase in root biomass (Busov et al., 2006; Han et al., 2011). Previously, it was shown that GAs appear to affect cell expansion in the root elongation zone via destabilizing the DELLA-like repressor of GA1. DELLAs restrain growth of organs, such as leaves and primary roots, by first decreasing the rate of division of proliferating cells, then by altering the rate of elongation of differentiated cells (Achard et al., 2009). Targeting the expression of gai (a non-GA-degradable mutant form of GAI) in the root meristem disrupts cell proliferation. Moreover, expressing gai in dividing endodermal cells was sufficient to block root meristem enlargement (Ubeda-Tomas et al., 2009). Subsequent studies revealed that the GA-mediated removal of DELLA repressor proteins only in the endodermis promotes anisotropic growth in the surrounding root tissues (Ubeda-Tomas et al., 2008).
A number of physiological observations suggest cross-talk between GA signalling and auxin transport. It has long been known that indole acetic acid (IAA) can stimulate the expression of GA biosynthetic genes in various plants (Wolbang et al., 2001, 2004; Frigerio et al., 2006) and GAs also apparently mediate increased rates of polar IAA transport (Bjorklund et al., 2007). Auxin signalling was shown to induce the degradation of the negative GA signalling element RGA, thereby promoting GA signalling and root growth (Fu and Harberd, 2003). In GA-deficient and GA-insensitive transgenic Populus, modification of a differentially expressed gene encoding two efflux and one influx carrier involved in auxin transport suggests that modulation of lateral root development by GAs is at least partly imparted by polar auxin transport modification (Gou et al., 2010). Recent work implicated GAs and DELLAs in the control of auxin transport and the coordination between cell division and cell differentiation during this stage of root growth and differentiation (Moubayidin et al., 2010).
To improve understanding of the role of GAs in root development, pharmacological and genetic approaches were used to investigate the effects of modification of the levels of bioactive GAs in specific tissues during root development in model tobacco (Nicotiana tabacum) plants. In this study, PtGA20ox and PtGA2ox1 were expressed under the control of two well-studied promoters, namely LMX5 and TobRB7, respectively, leading to expression on the xylem side of the cambial zone in the stem (Bjorklund et al., 2007) and the meristem and central cylinder regions in the root (Yamamoto et al., 1991). The results show that the precise tissue localization of GAs regulates adventitious root initiation and elongation in tobacco. These findings provide a more comprehensive insight into understanding the role of GAs in root growth and development.
Materials and methods
Plant material and chemical treatments
The previously characterized PtGA2ox1, PtGA20ox, and PtGAI genes (Busov et al., 2003; Israelsson et al., 2005; Han et al., 2011) were isolated from 1316 clones of Chinese white poplar (Populus tomentosa Carr.). Genetic studies were carried out using clone W38 from tobacco (N. tabacum cultivar Wisconsin 38).
All plants were grown in hormone-free half-strength MS medium (Murashige and Skoog, 1962) containing 0.7% carrageenan and 2% sucrose, and maintained at 25 °C under a 16:8h photoperiod. For GA, paclobutrazol (PAC), and IAA treatments, preliminary experiments for the WT were carried out to determine suitable concentrations. The WT and transgenic tobacco plants were transferred to media containing 10 μM PAC, 10 μM GA3, and 1 μM IAA, respectively, for 2–3 weeks.
RNA extraction and cDNA synthesis
Plant material was ground to a fine powder with a mortar and pestle in liquid N2. Approximately 100mg of ground tissue were used for RNA extraction, using an SV Total RNA Isolation Kit (Promega, USA). A 2 μg aliquot of total RNA was used to synthesize single-stranded cDNA using the M-MLV reverse transcriptase kit (Promega). cDNA samples were diluted to a total volume of 100 μl.
Plasmid constructs
The open reading frames of PtGA2ox1, PtGA20ox, and PtGAI were amplified from the cDNA of P. tomentosa vegetative tissue using Pyrobest DNA polymerase and the primers described in Supplementary Table S1 available at JXB online. The products were cloned into pYL322-d1-TobRB7, pYL322-d1-CaMV35S, or pYL322-d1-LMX5 and then recombined into pYLTAC380H, which contained the HPTII gene as a selectable marker with the Cre/loxP system.
The constructs were transformed into Agrobacterium strain LBA4404 via the freeze/thaw method (Holsters et al., 1978).
Plant transformation
Nicotiana tabacum plants were transformed with the seven constructs following a previously described protocol (Dayan et al., 2010). The leaves were transferred to fresh solidified MS medium containing 2mg l–1 6-benzyladenine (6-BA), 0.1mg l–1 α-naphthyl acetic acid (NAA), 300mg l–1 cefotaxime, and 25mg l–1 hygromycin B every 2 weeks until shoot regeneration. Regenerated shoots that rooted in the presence of 20mg l–1 hygromycin B were potted. The presence and expression of the inserted genes were confirmed by PCR and reverse transcription–PCR (RT–PCR), respectively. At least three lines with the highest expression of the respective transgene were selected by qPCR with the primers described in Supplementary Table S1 at JXB online. Once again, shoots were regenerated from leaves of these screened plants to produce homozygous and aseptic transgenic plants. Regenerated plants were then transferred to antibiotic-free half-strength MS medium. Three independent lines and at least 10 ramets per line were used for the analysis in all experiments.
Biometric measurements
To measure root development, 4cm cuttings were grown in vitro. Roots from 2- or 3-week-old treated plants were harvested. The number of adventitious roots was counted and the primary root length was measured. Each experiment was repeated three times and performed with at least three events and 10 ramets per event. Two-tailed t-tests were performed with Microsoft Excel software.
Culture of petiole sections in vitro
Petioles of mature leaves were harvested and incubated on plates of MS medium containing 1mg l–1 IAA at 25 °C under a 16:8h photoperiod. For application of GA, leaf sections were incubated on MS medium with 1mg l–1 IAA and 1mg l–1 GA3.
Phytohormone analysis
A 3g aliquot of roots and expanding leaves was harvested from 1-month-old transgenic plants, each line represented by three independent plants. The samples were immediately weighed (fresh), frozen, powdered in liquid nitrogen, and later analysed with a liquid chromatography–mass spectrometry (LC-MS) system in the biotechnology laboratory of the forestry administration of China. The same approach had been previously described (Gou et al., 2010, 2011).
Histology and microscopy
Rooting stem cuttings from WT and transgenic plants were sampled and immediately fixed in FAA solution [containing 5% (v/v) formalin, 5% (v/v) acetic acid, and 50% (v/v) ethanol]. Samples were embedded in paraffin using a method described previously (Nakayama et al., 2002). The sections were stained with safranin fast-green for general tissue structure observation. Sections were analysed using a light microscope (modelBA210; Motic, China).
GenBank accession numbers
Sequence data from this article can be found in the GenBank data libraries under accession numbers: AJ001326 (PtGA20ox), JX102472 (PtGA2ox1), JX102471 (PtGAI), AB125232.1 (NtGA2ox1), and AB016083.1 (NtGA20ox1).
Results
Effects of GA and auxin and their interaction in adventitious root development
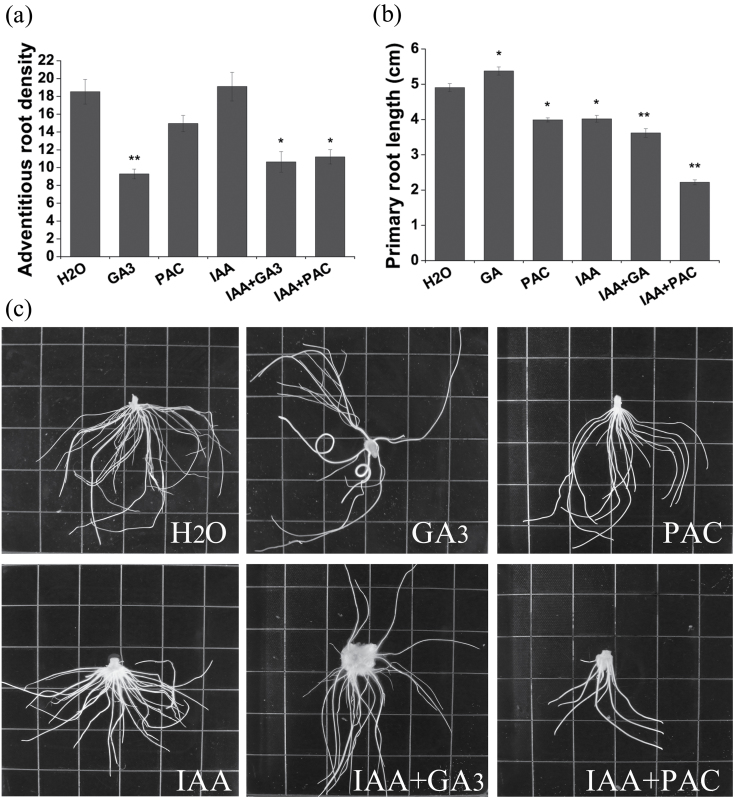
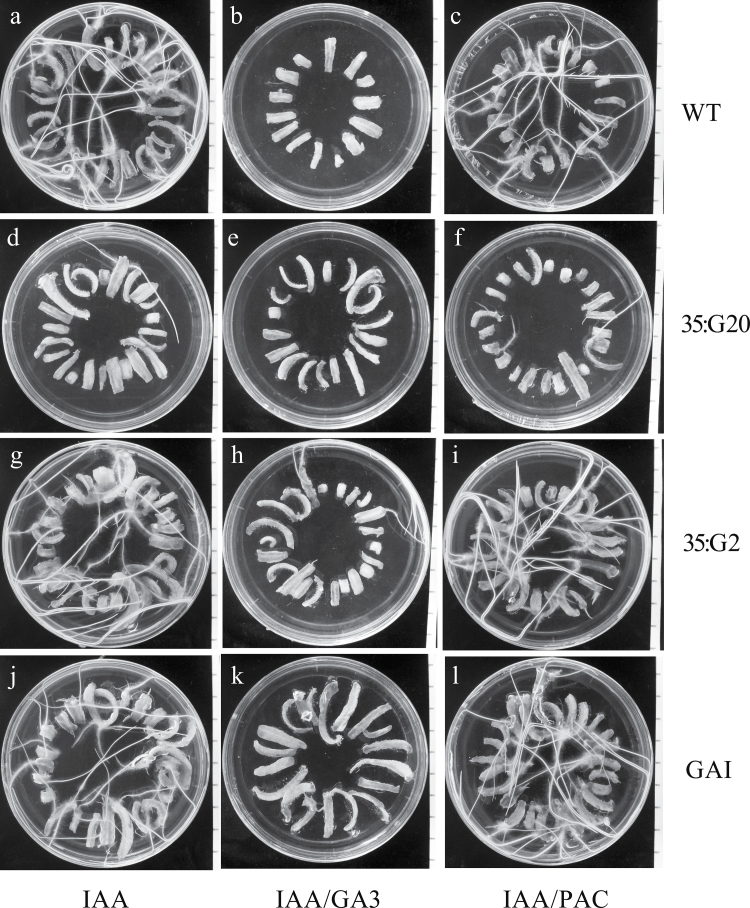
The effects of GA and auxin in regulation of adventitious root formation in WT tobacco stem cuttings were examined. Exogenous application of bioactive GA (GA3) in the medium significantly reduced the total number of adventitious roots in stem cuttings under the control conditions over 3 weeks (Fig. 1a, c). However, application of the GA biosynthesis inhibitor PAC (Rademacher, 2000) also reduced the number of adventitious roots, but to a lesser degree than GA3 (Fig. 1a, c). Interestingly, exogenous application of IAA did not reverse the effects of GA3 and PAC; simultaneous treatment with IAA and PAC has a marked effect on adventitious root formation similar to the individual and combined effects of GA3 and IAA. In fact, WT tobacco stem cuttings with an apical bud can be easily rooted on hormone-free medium (100%). Application of exogenous auxin retarded root development in a dose-dependent manner; root elongation is more sensitive to auxin than root density (Fig. 1). GA3 and PAC have opposite effects on root elongation (Fig. 1b). Treatment with IAA and PAC and with IAA and GA resulted in comparable phenotypes, but through different mechanisms.
Fig. 1.
Effects of GA and auxin on adventitious rooting of tobacco stem cuttings. (a) Average adventitious root number of WT plants grown in vitro on media with different treatments for 3 weeks. (b) Average length of the three longest primary roots of WT plants grown in vitro on media with different treatments for 3 weeks. Bars show the means and standard error over three events (at least 10 ramets per event). * and ** indicate significance as determined by Student’s t-test at P < 0.05 and P < 0.01, respectively. (c) Roots of differently treated plants. The length of the sides of the square grids is 0.5 inches.
For root elongation, exogenous application of GA3 significantly stimulated primary root elongation while PAC had the opposite effect (Fig. 1b). Application of IAA+GA3 significantly reduced primary root length, indicating that an appropriate amount of bioactive GAs and auxin is essential for root elongation (Fig. 1b). Taken together, these observations demonstrate that GAs have a significant effect on adventitious root development in tobacco.
Alteration of GA synthesis and signalling in different tissues using the transgenic approach
To establish a causative relationship between GAs and the observed root phenotypes caused by exogenous application of GA3 and PAC and to gain insight into the specific regulatory mechanism of GAs during adventitious root development, the levels of bioactive GAs were modified in specific tissues of tobacco with PtGA20ox, PtGA2ox1, and PtGAI under the control of the promoters Cauliflower mosaic virus 35S, LMX5, and TobRB7, respectively. Transgenic tobacco plants were generated, namely 35S::PtGA20ox, 35S::PtGA2ox1, 35S::PtGAI, LMX5::PtGA20ox, LMX5::PtGA2ox1, TobRB7::PtGA20ox, and TobRB7::PtGA2ox1 (hereafter referred to as 35:G20, 35:G2, GAI, L:G20, L:G2, T:G20, and T:G2, respectively). These plants exhibited phenotypes similar to those found in earlier studies that used the same technique (Eriksson and Moritz, 2002; Israelsson et al., 2005; Mauriat and Moritz, 2009). 35:G20 plants exhibited the greatest increase in internode length and corresponding plant stature, typical of GA overproduction syndrome, while 35:G2 and GAI plants exhibited dwarfism and had small, dark green leaves, phenotypic hallmarks of GA-deficient mutants. The phenotypes of L:G20 and T:G20 plants resembled that of 35:G20, but to a lesser extent; however, these responses were much lower in the L:G2 and T:G2 plants, which exhibited only a marginal decrease in height relative to WT plants (Fig. 2; Supplementary Fig. S1 at JXB online).
Fig. 2.
Phenotypic characterizations of WT and transgenic plants. One-month-old WT and transgenic plants grown in vitro are pictured. The top and bottom panels show the same plants with leaves and defoliated, respectively. The transgenic plants were significantly affected in terms of internode elongation. Bar=10cm in the top panel and 5cm in the bottom panel. WT, wild-type; 35:G20, 35S::PtGA20ox transgenic plants; 35:G2, 35S::PtGA2ox1 transgenic plants; T:G20, TobRB7::PtGA20ox transgenic plants; T:G2, TobRB7::PtGA2ox1 transgenic plants; L:G20, LMX5::PtGA20ox transgenic plants; L:G2, LMX5::PtGA2ox1 transgenic plants; GAI, 35S::PtGAI transgenic plants. (This figure is available in colour at JXB online.)
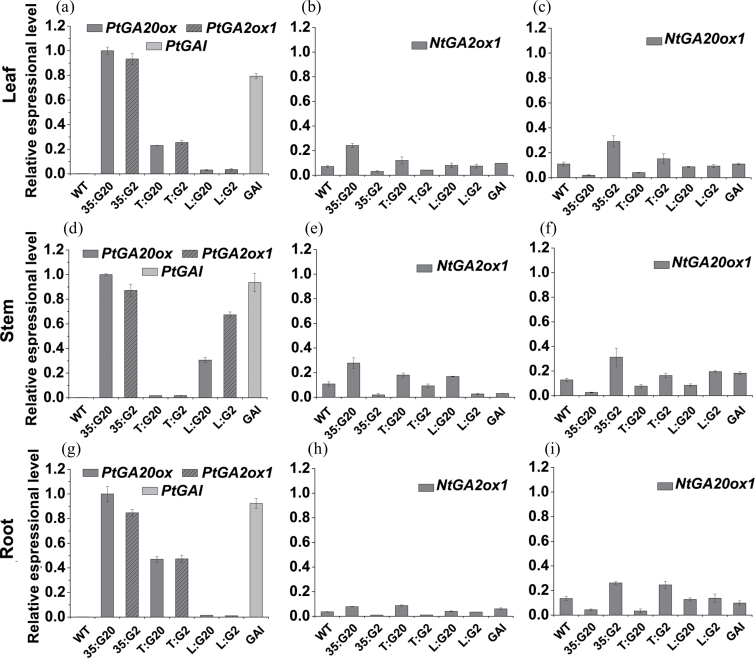
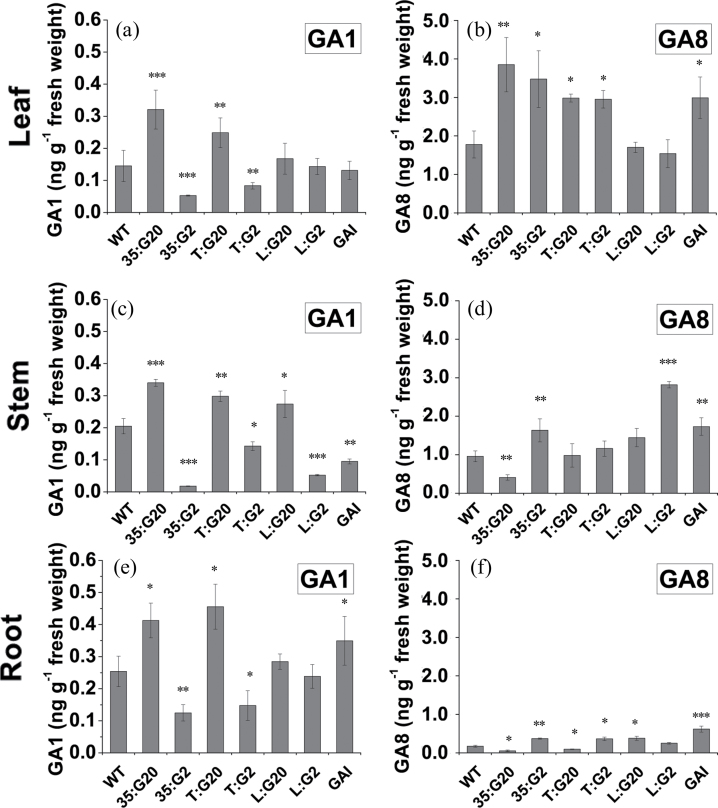
To determine if the phenotype observed in transgenic plants corresponds to expression changes in exogenous genes and GA concentrations, the expression patterns of exogenous genes and their orthologues in the leaves, stems, and roots of WT and transgenic plants were assessed by qPCR (Fig. 3). A feedback and feed-forward regulatory mechanisms of the tobacco GA genes were identified as expected. Because all these genes are responsive to active GAs, the expression changes are the result of active GA content alteration. The GA concentrations were assessed using an LC-MS system (Fig. 4; Supplementary Fig. S2 at JXB online). The changes in concentration of bioactive GA1 closely mirrored the observed phenotypic changes in the respective organs of predominant expression (Fig. 4). Interestingly, concentrations of GA1 also increased and decreased in stems of T:G20 and T:G2 plants, respectively (Fig. 4c), and feedback regulation of NtGA20ox1 (Fig. 3f) and NtGA2ox1 (Fig. 3e) was observed; however, PtGA20ox and PtGA2ox1 expression was not detected in the stems (Fig. 3c). It is thought that this gene affects GA biosynthesis in other tissues, and the high levels of GA1 in the other tissues move in a non-polar fashion to the stem. Analysis of the effect of excision of developing and mature leaves from cuttings on stem elongation indicated that these signals were not derived from the developing and mature leaves (Supplementary Fig. S3). Bioactive GA4 exhibited more complex changes (Supplementary Fig. S2); however, it is generally a minor active GA in tobacco. Therefore, the phenotype of the transgenic plants is related more to changes in GA1 concentration. The inactive GA8, converted from GA1, is the most abundant gibberellin in both wild-type and transgenic plants. It is noteworthy that the GA1 content is relatively stable in roots, stems, and leaves; however, very substantial increases in GA8 content from roots to leaves were identified in both WT and transgenic plants (Fig. 4b, d, f).
Fig. 3.
Expression levels of PtGA20ox, PtGA2ox1, PtGAI, and their orthologous genes in leaves, stems, and roots of wild-type and transgenic tobacco. (a–i) qRT–PCR analysis of PtGA20ox, PtGA2ox1, PtGAI (a, d, i), NtGA20ox1 (b, e, h), and NtGA2ox1 (c, f, i) in leaves (a, b, c), stems (e, d, f), and roots (g, h, i), respectively. The expression levels are relative to the Ntactin, and to the PtGA20ox of 35:G20 value (set at 1). Values are means ±SE of three biological replicates.
Fig. 4.
Gibberellin concentration in leaves, stems, and roots of wild-type and transgenic tobacco. Wild-type and transgenic plants were grown in vitro on hormone-free medium for 4 weeks. GA1 (a, c, e) and GA8 (b, d, f) concentrations in stems (c, d), roots (e, f), and leaves (a, b) were assessed using an LC-MS system. Values are means ±SE of three biological replicates.
These results indicate that different tissues may play disparate roles in GA homeostasis in the whole plant, and that exogenous gene expression induces homeostasis responses.
Down-regulation of GA level in the vasculature but not in roots altered adventitious rooting
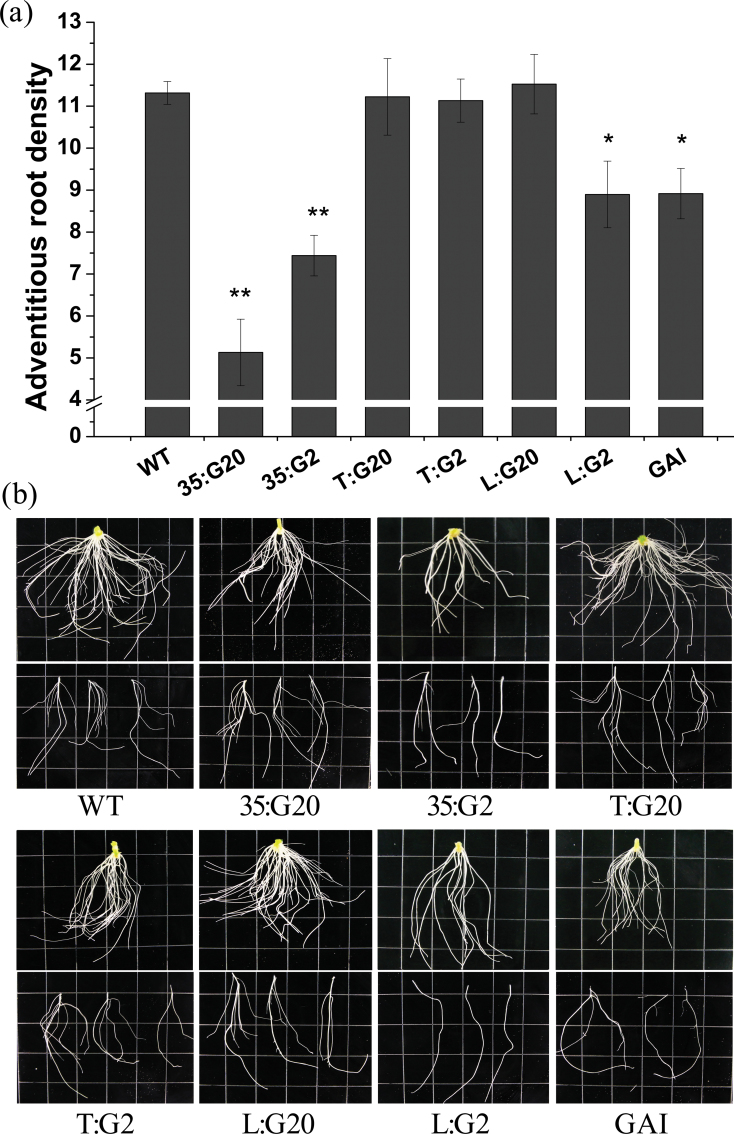
Stem cuttings of transformed lines and WT controls were cultured in the same medium for 2 weeks to examine rooting ability. Similar to the effect produced by exogenous application of GA3 and PAC, constitutive overexpression of PtGA20ox and PtGA2ox1 significantly reduced root number to varying degrees (Fig. 5).
Fig. 5.
The primary and lateral roots of wild-type and transgenic plants. (a) Average primary root density of wild-type and transgenic plants. Wild-type and transgenic plants were grown in vitro on hormone-free medium for 2 weeks. Adventitious roots were harvested and quantitatively analysed. Bars show the means and standard errors over three events (at least 10 ramets per event). (b) The phenotype of primary and lateral roots of wild-type and transgenic plants. The length of the sides of the square grids is 0.5 inches.
Interestingly, not all of the combinations of the PtGA20ox and PtGA2ox1 genes with the two tissue-specific promoters resulted in similar changes in rooting ability in overexpressing plants. Neither T:G20 nor T:G2 plants showed any obvious phenotypic changes in root number compared with the WT (Fig. 5). However, L:G2 and GAI transformants had similar rooting traits to 35:G2 (Fig. 5). This indicates that the presence of GAs in the vasculature is necessary for adventitious root formation.
Additionally, the results indicate that GA plays a role in lateral root development (Fig. 5b) and root tip diameter regulation. Lateral root development was delayed for more than a week in L:G2 plants. Root tips of transgenic plants were observed under a microscope (n >150). The results indicated that GA negatively regulates the root tip diameter (data not shown). The root tip diameters of 35:G20 and 35:G2 were 314±44nm and 367±49nm, respectively, compared with 338±34nm in the WT. This difference can be reversed by exogenous application of GA3 or PAC.
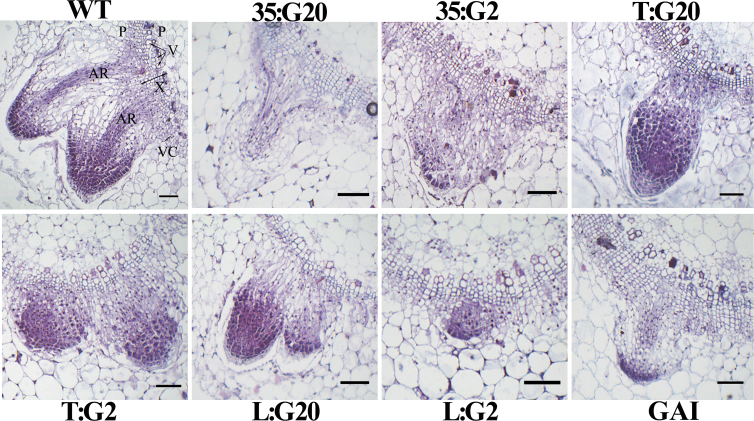
To improve understanding of the role of GAs in the vasculature during root formation, the initiation and development of adventitious roots in tobacco were examined histologically. The results showed that in both WT and transgenic plants, all adventitious roots originated from the vascular cambium (Fig. 6). These findings reveal that a threshold level of GAs is required for adventitious root development.
Fig. 6.
The anatomy of root initiation in stem cuttings of wild-type control and transgenic tobacco plants. Wild-type and transgenic plants were grown in vitro on hormone-free medium for 5 d and then harvested for analysis. It is evident that all adventitious roots originated from the vascular cambium in both wild-type and transgenic plants, and proper GA localization in this zone is required for development of adventitious roots. X, xylem; P, phloem; VC, vascular cambium; V, vessels; AR, adventitious root. Scale bars represent 100 μm. (This figure is available in colour at JXB online.)
Up-regulation of GA level in the vasculature arrests adventitious root formation in conjunction with high levels of auxin
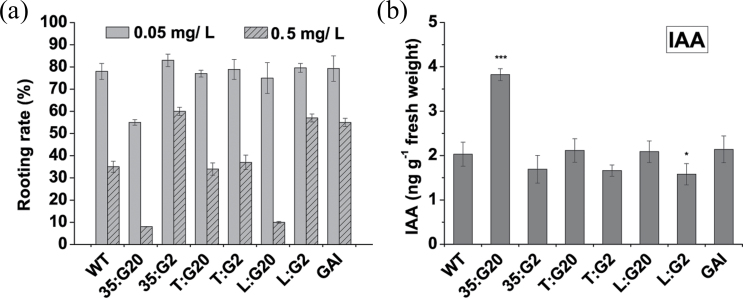
A remaining open question was why both L:G20 and T:G20 did not display similar effects to 35:G20 and whether GAs have an inhibitory effect on adventitious root formation in conjunction with auxin or polar auxin transport. To answer this, the rooting ability of these transgenic plants grown in media supplemented with exogenous auxin was examined. The expected results were obtained using high levels of NAA-treated L:G20 plants, in terms of the substantial decreases in the adventitious rooting rate (Fig. 7a). Intriguingly, application of high levels of IAA (1mg l–1) did not produce similar results (data not shown). However, a very substantial increase in endogenous IAA levels occurred in the lowest stem section in 35:G20 plants (Fig. 7b). High levels of IAA or NAA and GA result in arrest of adventitious root formation with greater callus formation at the base of the stem cutting (Supplementary Fig. S4 at JXB online). Considering the promoter used, it was no surprise that the phenotype of the L:G20 plants did not show any major modifications under normal conditions. Auxin is synthesized in the apical meristems of shoots and in young leaves, and moves in a polar fashion, preferentially downward, through phloem parenchyma cells of the stem to the base of the plant (Jacobs and Gilbert, 1983), and the effect of GAs on polar auxin transport is not found on the xylem side of the cambial zone.
Fig. 7.
Effect of exogenous NAA application on adventitious root development and endogenous IAA concentration in the lowest stem section of transgenic and wild-type plants. (a) The rooting rate of stem cuttings of wild-type control and transgenic tobacco plants after 3 weeks of NAA treatment. Bars represent the means and standard errors of three independent experiments where each experiment included at least 10 ramets per event. (b) IAA concentrations of the three lowest internodes were assessed using an LC-MS system. Values are means ±SE of three biological replicates.
GA inhibited adventitious root formation by repressing primordium initiation
When petiole sections from wild-type plants were incubated on MS medium containing 1mg l–1 IAA, adventitious roots were often regenerated from the base of the sections. The formation of adventitious roots from petioles and midvein sections was inhibited by GA3 and overexpression of PtGA20ox (Fig.8; Supplementary Fig. S5 at JXB online). When petiole sections of WT, 35:G2, and GAI plants were incubated under the above-described conditions with the addition of GA3, root regeneration was significantly inhibited. Moreover, WT and 35:G2 petiole sections from plants pre-cultured in GA-containing medium were similarly inhibited in terms of root regeneration (Supplementary Fig. S5); incubation of 35:G20 petiole sections on PAC-containing medium did not relieve this inhibition (Fig. 8).
Fig. 8.
GA inhibited adventitious root formation from petioles and midvein sections of transgenic and wild-type plants. The petioles and midvein sections from the transgenic and wild-type plants were cut into 10mm long segments and cultured for 3 weeks on MS medium. The concentrations of IAA, GA3, and PAC were each 1mg l–1. The explants did not generate roots on hormone-free medium. Bars=10mm.
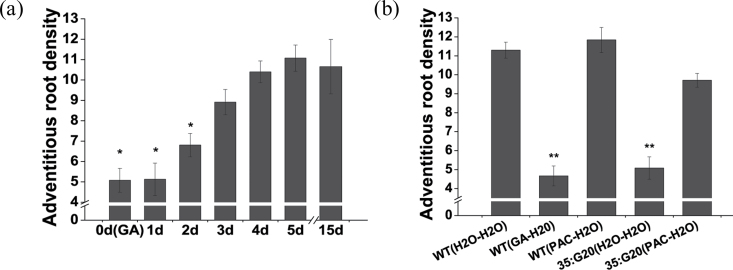
To investigate whether the effect of GAs on adventitious root formation was a result of the suppressed initiation of root primordia or the arrest of already initiated root primordia, WT plant cuttings were transferred from hormone-free medium to a GA-containing medium in the first 0–5 d after pre-culture. Quantitative analysis of primary root numbers showed that the inhibitory effect of GAs dramatically attenuated to normal levels when the pre-culture time was >3 d (Fig. 9a). In addition, WT plant cuttings in hormone-free medium transferred from GA-containing medium still showed a significant GA-mediated inhibitory effect. Pre-culture in PAC-containing medium rescued root formation in 35:G20 plants (Fig. 9b).
Fig. 9.
The inhibitory effect of GA is manifested in the early stages of adventitious root formation. (a) Quantitative analysis of adventitious root numbers of wild-type plant cuttings transferred from hormone-free medium to a GA-containing medium in the first 0–5 d of a 14 d period. (b) Quantitative analysis of adventitious root numbers of wild-type and 35:G20 plant cuttings grown on hormone-free medium transferred to GA-containing medium or PAC-containing medium.
Taken together, these data suggest that the deleterious action of GAs is manifested at a very early stage of adventitious root formation and established primordia were least affected by GAs.
A mobile GA signal is required for the emergence and subsequent long-term elongation of adventitious roots
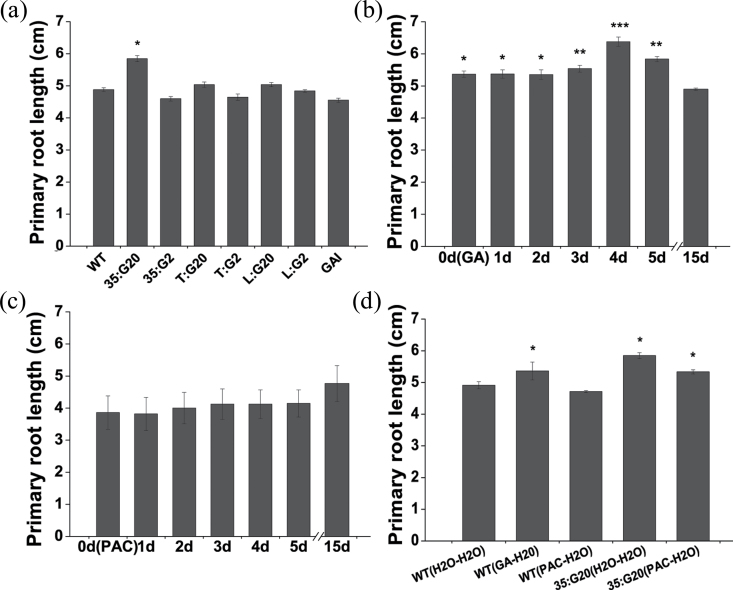
When each of the above-mentioned GA mutants was examined with regard to root elongation, it was noted that mutants containing PtGA2ox1 or PtGAI exhibited compromised primary root elongation compared with the WT control. In contrast, all transgenic types of PtGA20ox expressers displayed an increase in primary root length relative to the WT control (Fig. 10a, d). Although these results are not significant except for 35:G20 plants, they were reproducible; furthermore, exogenous application of GA3 and PAC had similar effects (Fig. 1b). The distinct effects of GAs on tissue-specific expressers in terms of adventitious root formation and elongation suggest that root elongation relative to root formation is a long-term process and GAs are mobile elongation-promoting signals.
Fig. 10.
GAs are required for the emergence and subsequent long-term elongation of adventitious roots. (a) Average length of the three longest primary roots of wild-type and transgenic plants. Transgenic plants were grown in vitro on hormone-free medium for 2 weeks. Adventitious roots were harvested and quantitatively analysed. (b) Quantitative analysis of the three longest primary roots of wild-type plant cuttings transferred from hormone-free medium to a GA-containing medium in the first 0–5 d of a 14 d period. (c) Quantitative analysis of the three longest primary roots of wild-type plant cuttings transferred from hormone-free medium to a PAC-containing medium in the first 0–5 d of a 14 d period. (d) Quantitative analysis of adventitious root length of wild-type and 35:G20 plant cuttings grown on hormone-free medium transferred to GA-containing medium or PAC-containing medium.
Further evidence confirmed this view; WT plant cuttings transferred from hormone-free medium to a GA-containing medium in the first 0–5 d after pre-culture did not show a decrease in root length (Fig. 10b). Upon application of PAC rather than GAs, cuttings of treated plants exhibited gradual and slight PAC-mediated inhibition of root elongation (Fig. 10c, d).
Discussion
Recent advances in molecular biology and plant physiology show that active GAs are important regulatory phytohormones in root development. Accumulating evidence suggests that GAs have an opposite effect on primary root growth (Fu and Harberd, 2003; Griffiths et al., 2006; Ueguchi-Tanaka et al., 2007; Willige et al., 2007) relative to lateral root and adventitious root development (Eriksson et al., 2000; Gou et al., 2010, 2011; El-Sharkawy et al., 2012). To gain insight into the specific regulatory mechanism of GAs during root development, tobacco plants were examined for altered GA synthesis, catabolism, and signalling in different tissues using a transgenic approach. It was demonstrated that GAs have an inhibitory effect on adventitious root formation but a stimulatory effect on root elongation.
In this study, striking differences were found between transgenic tobacco and WT plants regarding adventitious root development. On the one hand, GA inhibits adventitious root formation. GA20ox overexpressers had poorer rooting capacity than their control counterparts; this was reported previously in trees (Eriksson et al., 2000; Gou et al., 2011). However, the negative effect on rooting efficiency during initial establishment of young plantlets in the growth chamber did not significantly affect root growth at later stages (Eriksson et al., 2000). Moreover, consistent with previous observations (Smith and Fedoroff, 1995; Busov et al., 2006) of the response to GA3 treatment, the formation of adventitious roots in the WT, GA2ox-, and GAI-overexpressing plants was reduced dramatically. Therefore, a plausible explanation for the phenotype is that GAs negatively regulate the early initiation step of root formation, while PAC inhibits the emergence (and hence scoring) of roots, rather than their initiation. Consistent with this hypothesis, the distinct effects of GAs depend on the time of application. While it is an inhibitor during the pre-initiation phase, it strongly enhances rooting if applied at a time coincident with the first observable stage of root initiation (Smith and Thorpe, 1975). GA treatment seemed mainly to reduce intraprimordial cell division, the continued development of which the established primordia mostly depend on (Haissig, 1972). A previous study demonstrated that GAs primarily suppress lateral root formation at the early stages of lateral root primordium initiation (Gou et al., 2010). It is believed that reduced GA levels are necessary for maintenance of undetermined meristem cell fate (Sakamoto et al., 2001), whereas high GA concentrations promote cell differentiation and expansion (Jasinski et al., 2005; Shani et al., 2006). In contrast, GAs have a stimulatory effect on root elongation. Increases in endogenous GA content can induce significant increases in root elongation. Conversely, reduction of GA levels results in a decreased root growth rate (Lee and Zeevaart, 2005; Lo et al., 2008; Rieu et al., 2008). GA biosynthesis inhibitors are also known to inhibit root elongation, which is overcome by application of GA, providing further evidence that GAs are important for root growth (Yaxley et al., 2001). Subsequent studies revealed that regulation of GA-dependent elongation is restricted to cells of the root meristem and is not observed in the elongation or differentiation zone of the root (Ubeda-Tomas et al., 2008, 2009; Achard et al., 2009; Willige et al., 2011).
Little information on the specific regulatory mechanism of GAs during adventitious root development is available. Studies on the effect of GAs on plant growth and development have been hindered by their low abundance and variation in form, time, and localization (El-Sharkawy et al., 2012). Modifying the levels of bioactive GAs in specific tissues by the expression of genes encoding enzymes involved in GA biosynthesis and catabolism provides an alternative approach to such investigations (Mauriat et al., 2011). To gain new insights into the role of GAs, transgenic plants with increased rates of biosynthesis and catabolism of GAs in specific parts of the vascular tissue and the root meristem, respectively, were investigated. The results showed that the presence of GAs in the vasculature is necessary for adventitious root formation. The main reason for this is that in both WT and transgenic plants, all adventitious roots originate from the vascular cambium and this process requires GA biosynthesis and DELLA repressor-mediated GA signalling. Earlier research suggested that the presence of bioactive GAs in the xylem is crucial for adventitious rooting (Mauriat et al., 2011). As mentioned above, GA treatment has an inhibitory effect on adventitious root formation; interestingly, the GA1 levels are increased in all G20 lines but the effect on adventitious root density is just shown for 35:G20. The L:G20 and T:G20 lines have no reduction in adventitious root density. Previous studies had suggested that the GAs cannot transfer freely between different cell layers of the stem (Bjorklund et al., 2007), and their concentration is not distributed evenly between different types of cells in stem (Israelsson et al., 2005). In addition, the GAs have been shown to be a mobile signal (Ragni et al., 2011), and a new homeostasis will soon be achieved with the environmental change (Benschop et al., 2006; Toh et al., 2008). Therefore, as the sources of signal were different, it is speculated that the mechanisms in L:G20 and T:G20 plants are also different. These unexpected results regarding L:G20 may be due to interactions between GAs and auxin because of the well-known cross-talk between auxin and GA signalling at both the molecular and phenotypic levels (Wolbang and Ross, 2001; Bjorklund et al., 2007; Weiss and Ori, 2007). This case, therefore, raised the question as to whether the inhibitory effect of GAs on adventitious root formation is in conjunction with auxin, polar auxin transport, or both. Auxin moves down through phloem parenchyma cells to the base of the plant (Jacobs and Gilbert, 1983); therefore, GAs do not affect polar auxin transport located on the xylem side of the cambial zone. Based on the observation that NAA-treated L:G20 plants showed very substantial decreases in the total number of adventitious roots, the results support the hypothesis that the up-regulation of GAs, and the consequent auxin transport enhancement, and auxin–gibberellin interactions in the vasculature, is causative of the 35:G20 phenotypes reported in the previous study. Therefore, the relationship between GAs and auxin appears to be both complex and context specific. Much progress has recently been made in corroborating auxin’s promotion of root growth by enhancing the GA-induced destabilization of RGA, and perhaps of other DELLA proteins (Fu and Harberd, 2003; Gou et al., 2010; Moubayidin et al., 2010). However, the degree to which the specific effects of GAs are involved in root development remains unclear. Indeed, despite recent progress, there remains much to be learnt about these issues.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Phenotypic characterizationof WT and transgenic plants grown in the greenhouse.
Figure S2. Gibberellin concentrations in leaves, stems, and roots of wild-type and transgenic tobacco.
Figure S3.The effects of excision of developing and mature leaves on stem elongation.
Figure S4. The high levels of IAA or NAA and GA arrest adventitious root formation with more callus formation at the base of the stem cutting.
Figure S5. The formation of adventitious roots from petioles and midvein sections of WT, 35:G20, and 35:G2 plants pre-cultured in GA3 or PAC-containing media.
Table S1. Sequence information of PCR primers used for plasmid construction and qRT–PCR.
Acknowledgements
The authors acknowledge Aimin Wu for comments on the article and for LMX5 promoters. This work was supported by the Fundamental Research Funds for the Central Universities of China (JD2010-4).
Glossary
Abbreviations:
- GA
gibberellic acid, gibberellin
- GAI
35S::PtGAI
- 35:G20
35S::PtGA20ox
- 35:G2
35S::PtGA2ox1
- L:G20
LMX5::PtGA20ox
- L:G2
LMX5::PtGA2ox1
- NAA
α-naphthyl acetic acid
- T:G20
TobRB7::PtGA20ox
- T:G2
TobRB7::PtGA2ox1.
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. 2006. Integration of plant responses to environmentally activated phytohormonal signals. Science 311, 91–94 [DOI] [PubMed] [Google Scholar]
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GT, Genschik P. 2009. Gibberellin signaling controls cell proliferation rate in Arabidopsis . Current Biology 19, 1188–1193 [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Bou J, Peeters AJ, Wagemaker N, Guhl K, Ward D, Hedden P, Moritz T, Voesenek LA. 2006. Long-term submergence-induced elongation in Rumex palustris requires abscisic acid-dependent biosynthesis of gibberellin1. Plant Physiology 141, 1644–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berova M, Zlatev Z. 2000. Physiological response and yield of paclobutrazol treated tomato plants (Lycopersicon esculentum Mill.). Plant Growth Regulation 30, 117 [Google Scholar]
- Bjorklund S, Antti H, Uddestrand I, Moritz T, Sundberg B. 2007. Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. The Plant Journal 52, 499–511 [DOI] [PubMed] [Google Scholar]
- Busov VB, Meilan R, Pearce DW, Ma C, Rood SB, Strauss SH. 2003. Activation tagging of a dominant gibberellin catabolism gene (GA 2-oxidase) from poplar that regulates tree stature. Plant Physiology 132, 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busov V, Meilan R, Pearce DW, Rood SB, Ma C, Tschaplinski TJ, Strauss SH. 2006. Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus. Planta 224, 288–299 [DOI] [PubMed] [Google Scholar]
- Chaney WR. 2003. Tree growth retardants: arborists discovering new uses for an old tool. Tree Care Industry 54, 2–6 [Google Scholar]
- Dayan J, Schwarzkopf M, Avni A, Aloni R. 2010. Enhancing plant growth and fiber production by silencing GA 2-oxidase. Plant Biotechnology Journal 8, 425–435 [DOI] [PubMed] [Google Scholar]
- El-Sharkawy I, El KW, Prasath D, Fernandez H, Bouzayen M, Svircev AM, Jayasankar S. 2012. Identification and genetic characterization of a gibberellin 2-oxidase gene that controls tree stature and reproductive growth in plum. Journal of Experimental Botany 63, 1225–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson ME, Israelsson M, Olsson O, Moritz T. 2000. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nature Biotechnology 18, 784–788 [DOI] [PubMed] [Google Scholar]
- Eriksson ME, Moritz T. 2002. Daylength and spatial expression of a gibberellin 20-oxidase isolated from hybrid aspen (Populus tremula L.×P. tremuloides Michx.). Planta 214, 920–930 [DOI] [PubMed] [Google Scholar]
- Fagoaga C, Tadeo FR, Iglesias DJ, Huerta L, Lliso I, Vidal AM, Talon M, Navarro L, Garcia-Martinez JL, Pena L. 2007. Engineering of gibberellin levels in citrus by sense and antisense overexpression of a GA 20-oxidase gene modifies plant architecture. Journal of Experimental Botany 58, 1407–1420 [DOI] [PubMed] [Google Scholar]
- Frigerio M, Alabadi D, Perez-Gomez J, Garcia-Carcel L, Phillips AL, Hedden P, Blazquez MA. 2006. Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis . Plant Physiology 142, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Harberd NP. 2003. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421, 740–743 [DOI] [PubMed] [Google Scholar]
- Gabriele S, Rizza A, Martone J, Circelli P, Costantino P, Vittorioso P. 2010. The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1 . The Plant Journal 61, 312–323 [DOI] [PubMed] [Google Scholar]
- Gilman EF. 2004. Effects of amendments, soil additives, and irrigation on tree survival and growth. Journal of Arboriculture 30, 301–310 [Google Scholar]
- Gou J, Ma C, Kadmiel M, Gai Y, Strauss S, Jiang X, Busov V. 2011. Tissue-specific expression of Populus C19 GA 2-oxidases differentially regulate above- and below-ground biomass growth through control of bioactive GA concentrations. New Phytologist 192, 626–639 [DOI] [PubMed] [Google Scholar]
- Gou JQ, Strauss SH, Tsai CJ, Fang K, Chen YR, Jiang XN, Busov VB. 2010. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. The Plant Cell 22, 623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, Thomas SG. 2006. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis . The Plant Cell 18, 3399–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissig BE. 1972. Meristematic activity during adventitious root primordium development: influences of endogenous auxin and applied gibberellic acid. Plant Physiology 49, 886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KM, Dharmawardhana P, Arias RS, Ma C, Busov V, Strauss SH. 2011. Gibberellin-associated cisgenes modify growth, stature and wood properties in Populus . Plant Biotechnology Journal 9, 162–178 [DOI] [PubMed] [Google Scholar]
- Harberd NP, Belfield E, Yasumura Y. 2009. The angiosperm gibberellin–GID1–DELLA growth regulatory mechanism: how an ‘inhibitor of an inhibitor’ enables flexible response to fluctuating environments. The Plant Cell 21, 1328–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M, de Waele D, Depicker A, Messens E, van Montagu M, Schell J. 1978. Transfection and transformation of Agrobacterium tumefaciens . Molecular and General Genetics 163, 181–187 [DOI] [PubMed] [Google Scholar]
- Ikezaki M, Kojima M, Sakakibara H, Kojima S, Ueno Y, Machida C, Machida Y. 2010. Genetic networks regulated by ASYMMETRIC LEAVES1 (AS1) and AS2 in leaf development in Arabidopsis thaliana: KNOX genes control five morphological events. The Plant Journal 61, 70–82 [DOI] [PubMed] [Google Scholar]
- Israelsson M, Sundberg B, Moritz T. 2005. Tissue-specific localization of gibberellins and expression of gibberellin-biosynthetic and signaling genes in wood-forming tissues in aspen. The Plant Journal 44, 494–504 [DOI] [PubMed] [Google Scholar]
- Jacobs M, Gilbert SF. 1983. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science 220, 1297–1300 [DOI] [PubMed] [Google Scholar]
- Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M. 2005. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Current Biology 15, 1560–1565 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Zeevaart JA. 2005. Molecular cloning of GA 2-oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris . Plant Physiology 138, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SF, Yang SY, Chen KT, Hsing YI, Zeevaart JA, Chen LJ, Yu SM. 2008. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. The Plant Cell 20, 2603–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriat M, Moritz T. 2009. Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. The Plant Journal 58, 989–1003 [DOI] [PubMed] [Google Scholar]
- Mauriat M, Sandberg LG, Moritz T. 2011. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. The Plant Journal 67, 805–816 [DOI] [PubMed] [Google Scholar]
- Moubayidin L, Perilli S, Dello IR, Di, Mambro R, Costantino P, Sabatini S. 2010. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Current Biology 20, 1138–1143 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497 [Google Scholar]
- Nadeau CD, Ozga JA, Kurepin LV, Jin A, Pharis RP, Reinecke DM. 2011. Tissue-specific regulation of gibberellin biosynthesis in developing pea seeds. Plant Physiology 156, 897–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama A, Park S, Zheng-Jun X, Nakajima M, Yamaguchi I. 2002. Immunohistochemistry of active gibberellins and gibberellin-inducible alpha-amylase in developing seeds of morning glory. Plant Physiology 129, 1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Hong F, Chory J. 2006. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126, 467–475 [DOI] [PubMed] [Google Scholar]
- Osmont KS, Sibout R, Hardtke CS. 2007. Hidden branches: developments in root system architecture. Annual Review of Plant Biology 58, 93–113 [DOI] [PubMed] [Google Scholar]
- Rademacher W. 2000. Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annual Review of Plant Physiology and Plant Molecular Biology 51, 501–531 [DOI] [PubMed] [Google Scholar]
- Radi A, Lange T, Niki T, Koshioka M, Lange MJ. 2006. Ectopic expression of pumpkin gibberellin oxidases alters gibberellin biosynthesis and development of transgenic Arabidopsis plants. Plant Physiology 140, 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke CS. 2011. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. The Plant Cell 23, 1322–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Eriksson S, Powers SJ, et al. 2008. Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis . The Plant Cell 20, 2420–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M. 2001. KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes and Development 15, 581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani E, Yanai O, Ori N. 2006. The role of hormones in shoot apical meristem function. Current Opinion in Plant Biology 9, 484–489 [DOI] [PubMed] [Google Scholar]
- Smith DL, Fedoroff NV. 1995. LRP1, a gene expressed in lateral and adventitious root primordia of Arabidopsis . The Plant Cell 7, 735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Thorpe TA. 1975. Root initiation in cuttings of Pinus radiata seedlings. Journal of Experimental Botany 26, 193–202 [Google Scholar]
- Stavang JA, Lindgard B, Erntsen A, Lid SE, Moe R, Olsen JE. 2005. Thermoperiodic stem elongation involves transcriptional regulation of gibberellin deactivation in pea. Plant Physiology 138, 2344–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Imamura A, Watanabe A, et al. 2008. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiology 146, 1368–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Tomas S, Beemster GT, Bennett MJ. 2012. Hormonal regulation of root growth: integrating local activities into global behaviour. Trends in Plant Science 17, 326–331 [DOI] [PubMed] [Google Scholar]
- Ubeda-Tomas S, Federici F, Casimiro I, Beemster GT, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett MJ. 2009. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Current Biology 19, 1194–1199 [DOI] [PubMed] [Google Scholar]
- Ubeda-Tomas S, Swarup R, Coates J, Swarup K, Laplaze L, Beemster GT, Hedden P, Bhalerao R, Bennett MJ. 2008. Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nature Cell Biology 10, 625–628 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, et al. 2007. Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. The Plant Cell 19, 2140–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Ori N. 2007. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiology 144, 1240–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige BC, Ghosh S, Nill C, Zourelidou M, Dohmann EM, Maier A, Schwechheimer C. 2007. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis . The Plant Cell 19, 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige BC, Isono E, Richter R, Zourelidou M, Schwechheimer C. 2011. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana . The Plant Cell 23, 2184–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbang CM, Chandler PM, Smith JJ, Ross JJ. 2004. Auxin from the developing inflorescence is required for the biosynthesis of active gibberellins in barley stems. Plant Physiology 134, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbang CM, Ross JJ. 2001. Auxin promotes gibberellin biosynthesis in decapitated tobacco plants. Planta 214, 153–157 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. 2008. Gibberellin metabolism and its regulation. Annual Review of Plant Biology 59, 225–251 [DOI] [PubMed] [Google Scholar]
- Yamamoto YT, Taylor CG, Acedo GN, Cheng CL, Conkling MA. 1991. Characterization of cis-acting sequences regulating root-specific gene expression in tobacco. The Plant Cell 3, 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaxley JR, Ross JJ, Sherriff LJ, Reid JB. 2001. Gibberellin biosynthesis mutations and root development in pea. Plant Physiology 125, 627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Hanada A, Yamaguchi S, Kamiya Y, Beers EP. 2011. The Arabidopsis Myb genes MYR1 and MYR2 are redundant negative regulators of flowering time under decreased light intensity. The Plant Journal 66, 502–515 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.