Abstract
The developing auditory cortex is highly plastic. As such, the cortex is both primed to mature normally and at risk for re-organizing abnormally, depending upon numerous factors that determine central maturation. From a clinical perspective, at least two major components of development can be manipulated: 1) input to the cortex and 2) the timing of cortical input. Children with sensorineural hearing loss (SNHL) and auditory neuropathy spectrum disorder (ANSD) have provided a model of early deprivation of sensory input to the cortex, and demonstrated the resulting plasticity and development that can occur upon introduction of stimulation. In this article, we review several fundamental principles of cortical development and plasticity and discuss the clinical applications in children with SNHL and ANSD who receive intervention with hearing aids and/or cochlear implants.
Keywords: Auditory neuropathy spectrum disorder, central auditory maturation, cortical auditory evoked potential, cochlear implants, hearing aids, experience-dependent plasticity, cochlear nerve deficiency
The central auditory system is highly plastic in early childhood. During periods of increased cortical plasticity, sensory deprivation (e.g., hearing loss) can lead to developmental abnormalities. However, the fact that the cortex is highly plastic also means that intervention can produce positive effects. Because the time during which the cortex is most responsive to intervention is finite, proper timing of intervention is vital. Thus, at least two factors may be important for taking advantage of the brain’s plasticity for management of a sensory disorder such as hearing loss: 1) input or stimulation and 2) timing. These two factors have proven to be critical to the plasticity of the auditory cortex in children with sensorineural hearing loss (SNHL). On the other hand, relatively little is known about cortical development and plasticity in children with Auditory Neuropathy Spectrum Disorder (ANSD). In this review, we summarize principles of cortical plasticity in SNHL and more recent work aimed at characterizing central auditory maturation and plasticity in children with ANSD.
I. GENERAL PRINCIPLES OF CENTRAL MATURATION AND PLASTICITY
Neuroplasticity, or the ability of neuronal groups to adjust function based upon input, is present throughout a human’s lifetime. The potential for neuroplasticity appears to be greatest at the level of the cortex, with development being more dependent upon environmental input rather than innate mechanisms (see Buonomano and Merzenich, 1998, for a review; Hartmann et al, et al, 1997; Kral, 2007). At the cortical level, various sensory and cognitive systems interact and adjust functional properties based upon experience and learning. At no time is human cortical neuroplasticity greatest than during the first years of life. During this developmental period, cortical neurons cultivate in abundance, creating a framework upon which various cortical systems build and begin to mature. Thus, input to the cortex provided by experience and environment is key during early development when neuroplasticity is at peak levels (Sur et al, 1988, Pallas, 2001, Eggermont, 2008).
In early development, neuroplasticity reaches its highest levels due to developmental physiologic changes. Neurons at the level of the cortex proliferate rapidly in the first 3.5 years of life, in areas including visual, auditory, and pre-frontal cortices (Huttenlocher and de Courten, 1987; Huttenlocher and Dabholkar, 1997). This growth results in an overabundance of synapses, or connections between neurons. Rapid myelination of axons occurs as well during this time. By one year of age, the cortex demonstrates the presence of all six layers (Moore and Guan, 2001). After approximately 4 years of age, cortical neurons in the visual, auditory, and pre-frontal cortices undergo a pruning phase, during which extraneous neurons and their synapses are eliminated from the respective sensory system if not functionally necessary (Huttenlocher and de Courten, 1987; Huttenlocher and Dabholkar, 1997).
The mechanisms that drive pruning and refinement of cortical neural circuits are still largely under debate. However, several components of the process have been identified. For example, as early as 1947, Hebb and colleagues introduced the principle of coincidental activity. That is, neural circuits that exhibit both the requisite levels and patterns of concurrent activity are stabilized throughout development, while the brain eliminates those with insufficient levels or abnormal patterns of synchronized activity (i.e., ‘neurons that fire together, wire together) (Goodman et al, 1993; Katz et al, 1996). More recent findings have supported these notions and expanded upon them. For instance, it appears that neurotrophins, a category of proteins found in both the peripheral and central nervous system (CNS), are instrumental in plasticity (see McAllister et al, 1999 for a review). That is, these activity-dependent molecular factors may be responsible, to a large degree, for both molecular changes within neurons (i.e., modulation of levels of membrane excitability) and neuronal morphology and connectivity, such as axonal branching, dendritic modification, and mediation of number of synapses. It also seems that there is a limited amount of neurotrophins available in the cortex, especially in layer 4, the target layer of thalamo-cortical projections (Riddle et al, 1994). Coincident activity appears to alter the function and structure of connected neurons, at least in part by triggering release of restricted neurotrophins. These constraints set up a true competition between neurons for establishing and maintaining activity-dependent and neuroptrophin mediated connections.
Where excitatory activity (i.e., long-term potentiation and depression; LTP and LTD, respectively) was once thought to be the primary mediator of development and plasticity, it now appears that inhibitory activity in the CNS may be instrumental in the opening and closing of critical periods, or finite periods of time during which the brain is most plastic. Strong evidence has been provided for the notion that sensory experience initiates systematically increasing inhibitory activity in the cortex (Foeller et al, 2004). Variations in the levels of inhibitory activity appear to mark both the beginning and end of critical periods (Fagiolini et al, 2000; Hensch, 2005). In all, it seems that a proper balance of excitatory and inhibitory activity is requisite for typical cortical maturation, especially in terms of critical period regulation. This has been specifically shown to be the case in the auditory cortex (Kral et al, 2001; Takesian et al, 2009).
While activity-dependent circuits are a major focus of cortical maturation, both intrinsic and extrinsic factors drive cortical development. Intrinsic factors include genetic encoding, maturation specific to designated regions of the cortex, and ongoing specific cortical developmental patterns occurring without external influence. Extrinsic factors include molecular input arising from afferent pathways and external input to the cortex from the environment (Pallas, 2001). Lower levels of central development tend to be driven intrinsically. For example, animal data have shown that basic auditory inputs into auditory cortex are present in congenitally deaf cats (Hartmann et al, 1997; see Kral and Eggermont , 2007 for a review). Such development is clearly intrinsic, as the primary central auditory development was not dependent upon successful auditory stimulation. Similar maturation has been identified for the central visual pathways in animals (Moore and Linthicum, 2007).
The mere existence of intrinsically developed pathways does not guarantee normal function or transmission of sensory information in auditory deprivation, even if stimulation such as auditory input is later introduced (Kral et al, 2000). This discrepancy is likely due to the significance of extrinsic factors in cortical development. Sur and colleagues (1988) demonstrated the importance of extrinsic stimulation. These investigators changed the pattern of stimulation to the auditory cortex, without changing the anatomy of the thalamo-cortical projections, by surgically inserting visual inputs to the thalamus into auditory thalamic areas in animals. Results from this study revealed that auditory cortex began to exhibit similarities to visual cortex (i.e., in their structure and function). Thus, it seems that the pattern, in addition to the presence, of extrinsic stimulation is a key factor in cortical development.
Auditory input, specifically, has been shown to drive the development of both lower- and higher-level cortical connections. For instance, both reduced and delayed activity in superficial cortical layers have been observed in adult congenitally deaf cats stimulated via cochlear implants. Additionally, even less activation was present in deep cortical layers due to a lack of maturation in top-down projections from the superficial layers (Kral et al, 2000; Kral and Eggermont, 2007). Thus, though intrinsic factors contribute to anatomical development of the central auditory pathways, the absence of auditory stimulation is reflected in the lack of maturation of cortical layers.
II. CENTRAL AUDITORY MATURATION AND PLASTICITY IN CHILDREN WITH SENSORINEURAL HEARING LOSS
SNHL in children has allowed for a naturally occurring model of deprivation of cortical input and plasticity resulting from appropriate intervention. As such, the general principles of cortical maturation and plasticity described above can be applied in children with SNHL.
From investigations performed in both humans and animals, one can see that auditory neuroplasticity flourishes in the first few years of life, decreasing thereafter. It appears that intrinsic factors set up the cortical framework upon which extrinsic factors inform further development, such as the formation and strengthening of synapses (Holtmaat et al, 2006; Fu and Zuo, in press). If the extrinsic auditory input is not delivered to the auditory cortex normally during optimal levels of plasticity, deficits are observed upon introduction of auditory stimulation (Ponton and Eggermont, 2001; Sharma and Dorman, 2006, Gordon et al., 2003, 2005). These assertions are based on large-scale studies of auditory cortical maturation in both congenitally deaf animals and human children who receive cochlear implants at various ages. For instance, Kral and colleagues (2005) reported several differences in cortical development between congenitally deaf cats and controls. One example of these differences is generation of functional synapses in the auditory cortices of congenitally deaf cats was delayed, as compared to young control cats, even after the introduction of hearing via a cochlear implant. Thus, while cortical neurons may have been in place, important connections were not formed in a normal manner in congenitally deaf cats. This synaptic deficit highlights one of the consequences of sensory deprivation and the significance of extrinsic stimulation to cortical development.
Evidence that insufficient stimulation of the cortex during optimal periods of plasticity can cause abnormalities has also been shown in humans. Using the P1 cortical auditory evoked potential (CAEP) which is considered a biomarker of auditory cortical maturation, research by Sharma and colleagues (Sharma et al, 1997, 2002a, 2002b, 2002c, 2005a, 2005b; Sharma and Dorman, 2006; Sharma et al, 2007, 2009) has demonstrated a sensitive period, or timeframe of optimal neuroplasticity, during which sound may be introduced to auditory cortex and promote normal, age-appropriate development. It should be noted that the term ‘sensitive period’ significantly differs from ‘critical period’ in that a critical period of neuroplasticity denotes a sudden cut-off after which a neural system is unable to adapt. In other words, neuroplasticity is absent outside of the critical period. In contrast, neuroplasticity reaches its height during a sensitive period, with reduced levels of plasticity still present as the period ends (Harrison et al, 2005; Tomblin et al, 2007). For instance, Sharma and colleagues (Sharma et al, 2002a, 2002b, 2002c; Sharma and Dorman, 2006) studied 245 children with congenital deafness, and showed that the latency of the P1 CAEP biomarker response decreases to within normal limits in children who receive a cochlear implant by 3.5 years, while children implanted after the age of 7 years demonstrate abnormal P1 CAEP responses. These abnormal responses persist even after years of experience with implant use. Children implanted between the ages of 3.5 and 7 years show mixed auditory cortical development, with some children demonstrating P1 CAEP responses within normal limits, and others never reaching normal central auditory maturational status. These findings are consistent with both positron emission tomography (PET) imaging studies performed pre- and post-implantation in children, as well as intra-cortical studies in congenitally deaf cats (Hartmann et al, 1997; Kral et al, 2000, 2001; Lee et al, 2001; Kral et al, 2002; Oh et al, 2003; Kral et al, 2005, 2006; Lee et al, 2007). Thus, 3.5 years of age has been described as the terminus of the sensitive period for cochlear implantation in congenitally deaf children; approximately the same period of time during which exponential increases in synaptic density are observed and thereafter decrease (Huttenlocher and Dabholkar, 1997). After 7 years of age, neuroplasticity in the central auditory system is significantly reduced, resulting in an inability of auditory cortex to process auditory information normally if sound is introduced at this time (Sharma et al, 2005a). Furthermore, studies describing developmental outcomes of speech and language skills in children implanted at various ages indicate significantly improved outcomes with younger implantation age (Oh et al, 2003; Geers, 2006; Geers et al, 2009), especially for development of oral spoken language (Holt and Svirsky, 2008).
Clearly, it is crucial to determine candidacy for cochlear implantation in a timely manner, due to a sensitive period of neuroplasticity in the auditory cortex and resulting speech and language development. Because the P1 CAEP is a biomarker for central auditory maturation, measurement of this electrophysiologic response provides a non-invasive means of direct assessment of the developmental status of auditory cortex in individual patients. The human CAEP contains several peak components that evolve across the lifespan, providing information indicative of various stages of cortical auditory maturation (Naatanen and Picton, 1987; Gilley et al, 2005; Wunderlich and Cone-Wesson, 2006; Sussman et al, 2008). For example, in normal-hearing children under the age of 7 years, the P1 peak (occurring between 150–250 ms post-stimulus) decreases exponentially in latency values as the central auditory system forms efficient cortico-cortical circuits and as normal neuronal development increases processing speed of auditory information (Liegeois-Chauvel et al, 1994; Ceponiene et al, 1998). The P1 is generated in primary auditory cortex and secondary auditory cortices, arising from thalamo-cortical and cortico-cortical connections, or a ‘flow’ of information between thalamus, and primary and secondary auditory cortices (Erwin and Buchwald, 1987; Liegeois-Chauvel et al, 1994; McGee and Kraus, 1996; Eggermont and Ponton, 2002). Because of the decrease in P1 peak latency with increases in age, measurement of this component can provide valuable information concerning central auditory development in children with hearing loss, including cochlear implant candidates and recipients (Sharma et al, 2002a, 2002b, 2002c). Between the ages of 7 and 11 years, the N1 component (occurring around 100 ms post-stimulus) begins to reliably appear, separating the P1 and P2 peak components (Ponton et al, 2000; Gilley et al, 2005). This component arises from several cortical generators, and may be indicative of normal development for higher-level auditory processing (Naatanen and Picton, 1987; Eggermont and Ponton, 2003). The P2 and N2 peak components of the CAEP response are not as well understood in terms of generator site or as neurophysiologic correlates of language processing, though research is ongoing in examining the underlying neurophysiology of these components in relation to speech and language (Vidal et al, 2005; Gomot et al, 2007; Tong et al, 2009).
Analysis of the CAEP components allows for observation of the state and organization of the cortex as a result of appropriate intervention being provided within or outside of the sensitive period. For instance, if auditory deprivation is not ameliorated within the sensitive period, animal studies suggest that cortical ‘de-coupling’ may take place. De-coupling occurs when cortico-cortical loops between primary and secondary auditory cortices fail to develop properly because of the lack of auditory input. A lack of these connections results in higher-order auditory cortex de-coupling from primary auditory cortex, prohibiting the top-down modulatory input from higher-order auditory cortex to primary auditory cortex, necessary for speech and oral language development (Kral et al, 2005; Kral, 2007; Kral and Eggermont, 2007). In humans, the absence of the N1 component, (which reflects activity of cortico-cortical loops between higher-order auditory cortex and primary auditory cortex) in late implanted children suggests that some degree of partial or complete de-coupling may occur in long-term congenital deafness (Eggermont and Ponton, 2003; Sharma et al., 2007; Kral, 2007; Kral and Eggermont, 2007). Such de-coupling likely leaves higher auditory cortices available for possible recruitment by other sensory modalities, such as somatosensory, visual or multi-modal input (cross-modal re-organization) (Finney et al, 2001, 2003; Sharma et al, 2007; Gilley et al, 2008).
III. CROSS MODAL RECRUITMENT IN AUDITORY DEPRIVATION
Evidence for cross-modal re-organization due to auditory deprivation has been demonstrated in several studies. Sharma et al (2007) showed evidence of somatosensory activation of auditory cortex in long-term congenital deafness, while Finney et al (2001, 2003) demonstrated auditory cortical activation by visual motion stimuli in congenitally deaf adults. Thus, cross-modal plasticity appears to be a compensatory plasticity that, at the same time, may negatively affect auditory processing capability upon introduction of sound (e.g. a cochlear implant). Such recruitment also appears to be correlated with poor speech perception skills in cochlear-implanted adults and children (Doucet et al, 2006; Giraud and Lee 2007; Buckley and Tobey, 2010). For example, Doucet and colleagues (2006) presented visual motion stimuli to normal-hearing adults, adult cochlear implant users with good speech perception, and adult cochlear implant users with poor speech perception. Upon analysis, these researchers found a correlation between visual activation of temporal cortices (auditory cortical area) and speech perception scores. In other words, adult cochlear implant users who demonstrated less cortical activation in temporal cortices in response to visual stimuli also tended to show better speech performance, and adult cochlear implant users with greater cortical activation in temporal cortices in response to visual stimuli showed poorer speech performance. These results have been corroborated by Buckley and Tobey (2010), who found a correlation between the strength of cortical activation in response to visual stimuli in the right temporal cortex and poor speech perception scores in adult cochlear implant users. Similarly, Giraud and Lee (2007) reported that congenitally deaf children who showed greater than normal activation in visual areas on PET scans showed poor outcomes after cochlear implantation.
In addition to cross-modal recruitment of auditory areas by somatosensory and visual systems, there exists evidence of ‘re-routing’ of introduced auditory information outside of auditory cortex to multi-modal cortical areas in children implanted over 7 years of age. Gilley et al (2008) recorded cortical responses to auditory stimulation for children who received cochlear implants under 3.5 years of age and over 3.5 years of age. Children implanted under 3.5 years of age demonstrated auditory cortical activation relegated to the temporal cortex (auditory cortical area), whereas children implanted after 3.5 years showed the greatest cortical response in the parietal region, an area involved in multi-sensory or multi-modal processing. This finding suggests that cross-modal re-organization appears to render higher-order auditory cortex unavailable for auditory processing, likely affecting oral language acquisition in late-implanted children.
Functionally, cross-modal re-organization appears to result in enhanced processing of the recruiting modality. For example, visual processing abilities such as enhanced visual localization, visual attention and motion detection have been reported to be enhanced in congenital deafness (Neville et al, 1983; Neville and Lawson, 1987; Bavelier et al, 2000, 2001; Dye and Bavelier, 2010; Lomber et al, 2010). As visual performance becomes enhanced, it is likely that auditory performance suffers. Such results have been observed in studies measuring auditory-visual integration. Children implanted after 3.5 to 4 years of age show deficits in auditory-visual integration performance (Bergeson et al, 2005; Gilley et al, 2010). When visual and auditory information conflict, many implanted children tend to rely on visual information in comparison to normal-hearing children, but show greater capability and success in the blending of auditory-visual information if implanted at a younger age (Schorr et al, 2005).
Overall, the results of auditory deprivation at the level of the cortex beyond the sensitive period may be seen as a sequence of events. First, due to a lack of auditory input there are deficits in the development of top-down modulatory connections between higher-order auditory cortex and primary auditory cortex, therefore, higher-order auditory cortex may de-couple either partially or totally from primary auditory cortex, becoming available for recruitment by other sensory modalities (Kral and Eggermont, 2007). Second, recruitment of higher-order auditory cortex by visual and somatosensory systems may take place (Finney et al, 2001, 2003; Sharma et al, 2007). Third, if auditory input is introduced after the sensitive period and cross-modal re-organization has occurred, it appears to be re-routed to multi-sensory cortices for higher-order processing (Gilley et al, 2008). Finally, functional consequences of this re-organization appear as increases in performance of the recruiter modality (e.g., increases in visual performance) and deficits in auditory performance, including sensory integration (e.g., auditory-visual fusion) (Bergeson et al, 2005; Schorr et al, 2005; Gilley et al, 2010). However, auditory cortical organization may be maintained if extrinsic auditory input is introduced in a timely fashion, resulting in normal maturation of the central auditory pathways and optimal outcomes in speech and language performance, as well as multi-sensory processing (Sharma et al, 2002a, 2002b, 2002c; Holt and Svirsky, 2008; Gilley et al, 2010).
To summarize this portion of the review, we find that studies of animals and children with cochlear implants have provided a reasonable model of neuroplasticity in deafness with clear clinical implications for early intervention with appropriate auditory prostheses.
IV. CENTRAL AUDITORY MATURATION AND PLASTICITY IN CHILDREN WITH AUDITORY NEUROPATHY SPECTRUM DISORDER
A recently documented form of hearing loss, auditory neuropathy spectrum disorder (ANSD) appears to affect at least 10% of children with SNHL (Uus & Bamford, 2006; Kirkim et al, 2008; Talaat et al, 2009; Berlin et al, 2010; Maris et al, 2011). Due to the relatively recent documentation of this disorder, only a handful of investigations have examined cortical development in this population (Starr et al, 1996; Kraus et al, 2000; Rance et al, 2002; Cone 2008; Narne and Vanaja, 2008; Pearce, 2008; Michalewski et al, 2009; Cardon & Sharma, 2011; Sharma et al, 2011). This may mean that about one in ten children who are seen clinically for SNHL actually have ANSD and could be at risk for developmental abnormalities about which there is very little information to date.
ANSD was first described in the 1990’s (Starr et al, 1991, 1996). The classic clinical findings of ANSD, leading to a diagnosis, include present otoacoustic emissions (OAEs), absent or grossly abnormal auditory brainstem response (ABR) with a robust cochlear microphonic (CM) that inverts when the polarity of the stimulus is reversed, speech perception that is unduly poor in relation to behavioral auditory thresholds and that is further degraded in the presence of background noise, and absent or elevated acoustic reflexes (ARs) (Starr et al, 1996; Berlin et al, 1998; Sininger, 2001; Berlin et al, 2003, 2005). Patients with ANSD can present with any configuration or degree of hearing loss (Sininger, 2001; Lingyan et al, 2011). In addition, unilateral ANSD is possible (Hood, 1998).
Several researchers have proposed three sites of lesion for ANSD. These include the inner hair cells of the cochlea (IHC), the synapse between the IHC and the VIII (vestibulocochlear) cranial nerve, and the VIII nerve itself, or any combination thereof (Starr et al, 1996; Harrison, 1998; Yasunaga et al, 1999; Varga et al, 2003; Schwander et al, 2007; Shapiro & Popelka, 2011). A disorder at any of these sites of lesion, or an amalgamation of them, leads to dys-synchrony of the auditory nerve and brainstem (Berlin et al, 1998; Kraus, 2000; Starr et al, 2001). Dys-synchrony, in turn, has been shown to cause primarily temporal processing deficits in patients with ANSD (e.g., Zeng et al, 1999, 2001, 2004; Rance et al, 2004, 2005; Michaelewski et al, 2005; Zeng et al, 2006; Rance et al, 2008; Hassan, 2011), as opposed to spectral deficits that are the hallmark of SNHL. In other words, in order for the peripheral and central pathways to encode the fine-grained temporal acoustic information from the environment, these structures must be able to first transduce acoustic signals into neural impulses and then convey the rapid acoustic changes that are characteristic of these signals (e.g., formant transition, rate of speech) along the neural pathways in a precisely synchronous manner. Unfortunately, synchrony is degraded to differing degrees in patients with ANSD, leading to varying behavioral outcomes for speech understanding, especially in the presence of background noise (Kraus et al, 2000; Sininger, 2001).
While diagnosis of ANSD is relatively established, clinical decision-making beyond diagnosis can be challenging. That is, many of the widely accepted clinical procedures for children with SNHL are not as effective for children with ANSD. For instance, for a child with SNHL who is diagnosed early, ABR and ASSR thresholds can be used to provide early intervention in the form of amplification and/or cochlear implantation allowing relatively little time during which the child’s auditory system is deprived of stimulation. In contrast, an infant diagnosed with ANSD does not often follow the same time course for treatment, mainly because clinicians do not have ABR or ASSR results to guide intervention. Though stable behavioral thresholds may be obtained later in life for some patients, in other patients behavioral results may be variable or inconsistent. Given these and other clinical hurdles, which have the potential to compromise central auditory maturation in the ANSD population, innovative solutions that address cortical plasticity and development must be pursued.
The dys-synchronous activity of the auditory pathway in ANSD represents an abnormal pattern of extrinsically driven subcortical input to the cortex. Since the level and pattern of extrinsic stimulation coming into the cortex dictates, to a large degree, the developmental course of the latter (Sur et al, 1998; Pallas, 2001; Foeller et al, 2004), one might make at least two conjectures as these principles are applied to ANSD. First, given a reduced level of input (resulting from the hearing loss which is often co-existent with ANSD) cortical development might be affected negatively (Starr et al, 2001). Second, it is reasonable to believe that a dys-synchronous or abnormal pattern of extrinsic input would cause cortical developmental abnormalities (Kraus et al, 2000; Rance et al, 2002; Sharma et al, 2011). In this scenario, important experience-dependent synaptic connections may not be made, or may be altered, in the developing brains of children with ANSD.
As previously mentioned, CAEPs have proven effective in the measurement of the developmental status of the auditory cortex. Until relatively recently, most investigations using CAEPs to measure cortical maturation focused on children with normal hearing and SNHL. However, several reports in the literature now illustrate the effective use of CAEP measures in children with ANSD (Rance et al, 2002; Campbell et al, 2011; Cardon & Sharma, 2011; Sharma et al, 2011). This may seem counterintuitive given that other evoked potentials from lower centers of the central auditory system are typically absent or unreliable in children with ANSD (e.g., ABR, ASSR; Rance et al, 2005). Kraus and colleagues (2000) assert that CAEPs may be measured despite absent ABR responses due to the fact that while the ABR measures action potentials in the auditory nerve and brainstem, CAEPs measure dendritic activity in the cortex. Thus, ABRs are much higher in frequency and more biphasic than CAEPs, which tend to be broader in shape and low frequency in comparison. Given these characteristics, when ABR sweeps that are out of synchrony by even a small degree (i.e., out of phase) are averaged, they are most often cancelled out. In contrast, because CAEP components typically comprise several tens of milliseconds (i.e., from the beginning to the end of a peak), some jitter in the timing of the evoked potential sweeps can still lead to an averaged waveform with observable, even normal, CAEP components. The fact that CAEPs can be measured in patients with ANSD, when traditional clinical tests provide limited information, positions this measure as a promising clinical tool to assess cortical development, plasticity, and function in patients with ANSD.
Recently, several studies have focused on central auditory maturation in children with ANSD using CAEPs. For example, Rance et al, (2002) reported being able to reliably measure CAEPs in approximately 50% of children with ANSD. The children that presented with robust responses were also those who benefitted from amplification and performed well on measures of speech perception. These findings seem at first to suggest that children with ANSD differ in the level of cortical development, with some progressing relatively well and others not maturing typically. The former group also appears to receive some benefit from hearing aids. In addition, Rance et al. (2002) suggest that the extent to which a given child’s auditory cortex has developed is important in predicting behavioral outcome.
The findings of Rance and colleagues were corroborated by recent findings from our lab (Sharma et al, 2011). Differences in cortical maturation were found in 21 children with ANSD who were fitted with hearing aids. CAEP measures of cortical maturation divided the 21 subjects into three discrete groups: 1) Those with robust and replicable P1 responses of normal latency and amplitude; 2) Children with replicable P1 responses of delayed latency and decreased amplitude; and 3) Participants with absent or abnormal P1 responses. Additionally, a significant difference was found between the groups on a measure of auditory skill development (IT-MAIS; Zimmerman-Phillips et al, 2002), such that the children with normal P1 responses performed considerably better than the other two groups. Interestingly, the children with normal P1 responses were also fitted with hearing aids significantly earlier than the other two groups (i.e., on average at less than one year of age). Finally, it is significant that behavioral auditory threshold was not significantly different between any of the groups suggesting that, unlike SNHL, in ANSD pure tone hearing thresholds may not be correlated with cortical development or behavioral outcome. In all, results showed that there were distinct differences in cortical maturation throughout the group of children with ANSD, which may be attributable to differing degrees of dys-synchrony, but not auditory threshold. In addition, these data suggest that the developmental status of the cortex is highly correlated with behavioral outcome. Findings surrounding hearing aid fit age also seem to support the idea that timing of normal or improved input is important for typical cortical maturation. These conclusions may be of interest clinically as they provide a means to offer prognoses and have the potential to help guide management decisions.
Cochlear Nerve Deficiency
A particularly challenging form of ANSD is found in children diagnosed with cochlear nerve deficiency (CND). CND reflects hypoplasia (abnormally small VIII nerve, absence of many neural fibers) or aplasia (absence of VIII nerve), identified via magnetic resonance imaging (MRI) (Buchman et al, 2006; McClay et al, 2008; Huang et al, 2010; Roche et al, 2010; Maris et al, 2011). These children commonly present with a comorbid diagnosis of CND and ANSD, likely due to the poor conductivity of neural firing as a result of few functional neural fibers (Buchman et al, 2006; Walton et al, 2008). Recent results from our laboratory (Roland et al., 2011) report that CAEP measurements in these children were useful in verification of the presence or absence of the VIII nerve. For instance, we reported that three out of four children diagnosed with CND demonstrated robust P1 peak responses, indicating at least partial stimulation of the central auditory pathways via the VIII nerve. In two of these three patients, the CAEP was present despite abnormal ABR responses. Children with robust P1 responses who met all other candidacy criteria were fit with cochlear implants. Thus, the P1 peak response was useful in determining cochlear implant candidacy, including VIII nerve viability, for children diagnosed with CND.
While these and other studies provide initial evidence that cortical maturation is adversely affected by neural dys-synchrony and/or dys-function in ANSD (and co-existing CND), more work needs to be done to elucidate the details of central auditory maturation and plasticity in pediatric ANSD and to develop cortical biomarkers for assisting in clinical decisions. To this end, we discuss four cases of children with ANSD. Each of these cases illustrates different principles of cortical development and plasticity in the ANSD population.
V. CASE REPORTS
Case 1
SC (participant #8 in Sharma et al, 2011) was born at full term and relatively healthy. While he required supplemental oxygen for approximately one minute at the time of birth, he quickly gained the ability to breathe and was found to be healthy otherwise. SC failed initial newborn hearing screening, though parents reported no family history of hearing loss. Further diagnostic testing revealed abnormal ABR responses (i.e., absent wave V) with present and robust CMs bilaterally. In addition, SC’s distortion product otoacoustic emissions (DPOAEs) were absent bilaterally. When SC was a little older, behavioral pure tone audiometry yielded pure tone averages (PTAs) in the severe hearing loss range bilaterally (i.e., 85 dB HL). At this point, SC’s audiologists suggested a trial with binaural hearing aids. SC’s parents agreed to the trial, but noted that they had observed SC hearing and responding to environmental sounds on many occasions prior to hearing aid fitting. SC was first fit with hearing aids at 0.68 years. He also began to receive weekly speech and language therapy at this time. SC received a score of 39/40 on IT-MAIS testing performed following significant experience with hearing aids, indicating promising auditory skill development.
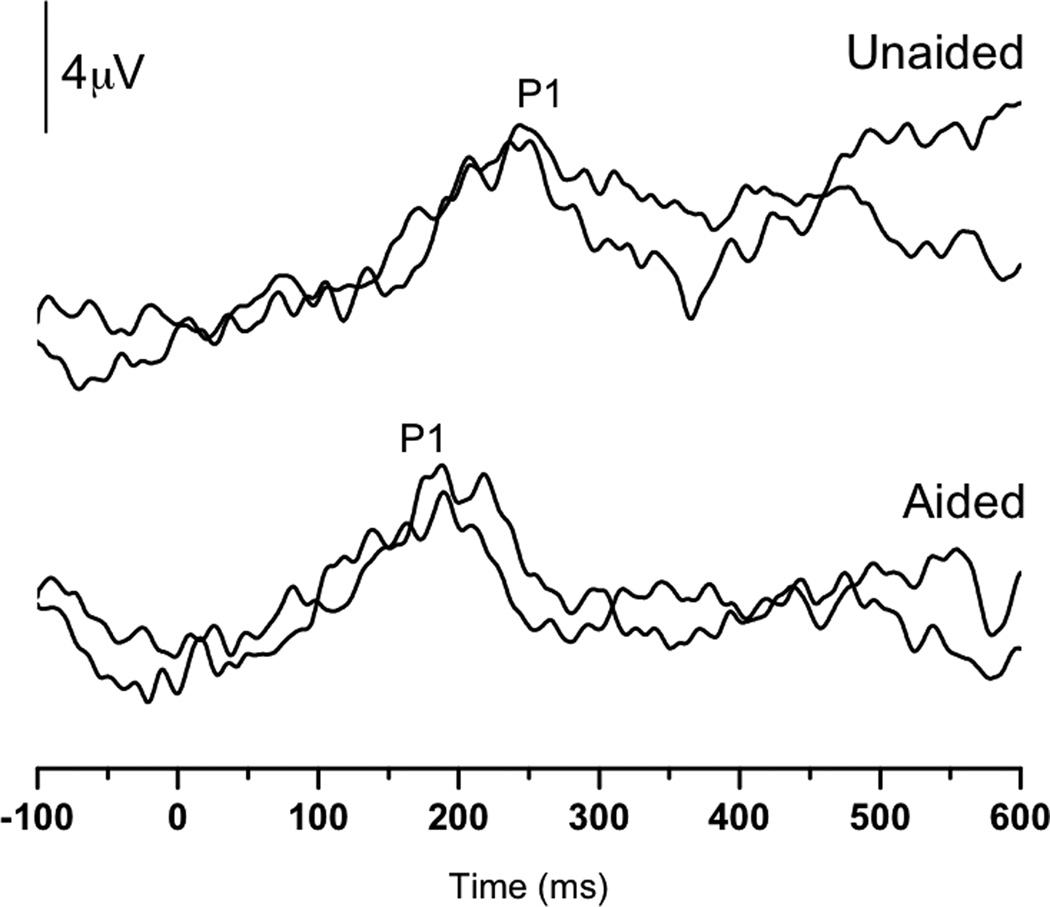
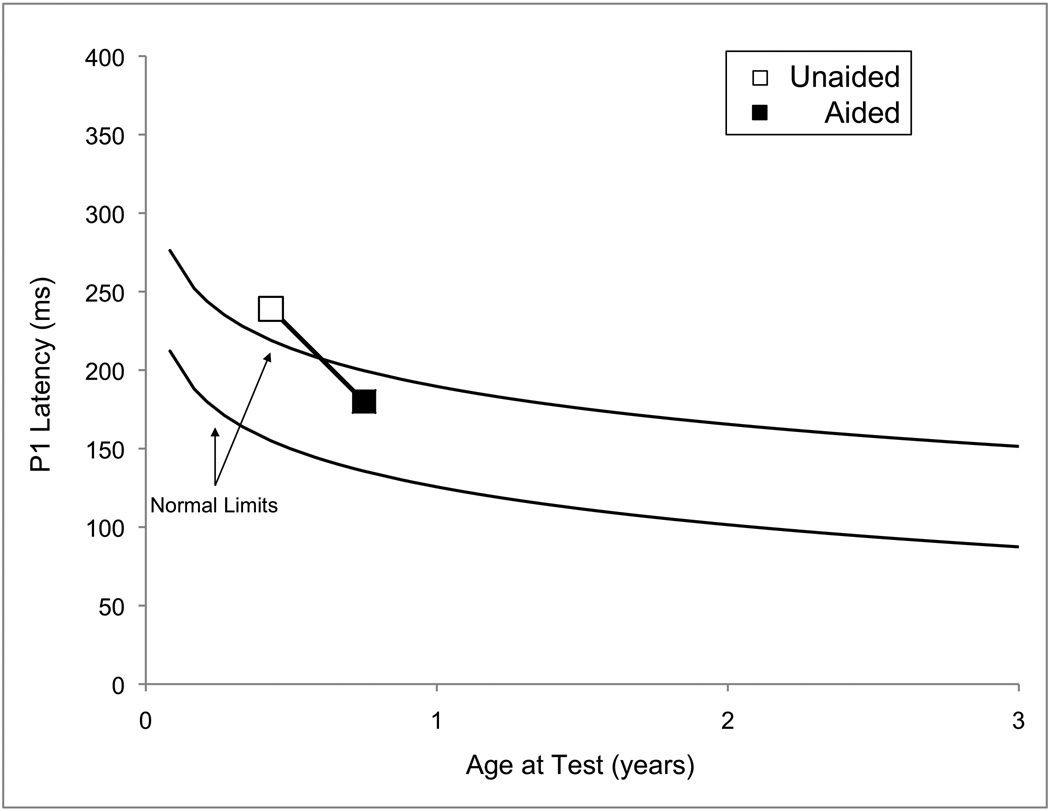
As a means to monitor the progress of SC’s central auditory maturation, P1 testing was performed both pre- and post-hearing aid fitting at 0.43 and 0.75 years, respectively. The pre-hearing aid fitting results showed replicable, albeit delayed, P1 responses, suggesting delayed maturation of the central auditory pathways. That is, the latency of the P1 peak was outside of the normal limits (i.e., 95% confidence intervals) for normal P1 latency development for SC’s age (Sharma et al, 2002c). In contrast, after some experience with amplification, replicable P1 responses of normal latency for his age were elicited. These findings suggest normal development of the auditory cortex. Both waveform and latency trajectory results can be seen in Figure 1A and B.
Figure 1.
(Panel A): P1 CAEP waveforms (with replications) for both unaided (top) and aided (bottom) P1 testing sessions performed with Case 1 (SC). (Panel B): Unaided (open square) and aided (filled square) P1 latencies plotted by age at test and compared to the 95% confidence intervals for normal P1 latency development (Sharma et al., 2002c). The unaided P1 latency was delayed, while the latency of the aided results was within normal limits.
SC’s case illustrates several points related to plasticity and maturation of the central auditory pathways in ANSD. For instance, prior to amplification, SC’s central auditory maturation was delayed. This suggests that the input provided to SC’s auditory system without the aid of amplification was insufficient to drive normal cortical development. In contrast, the addition of hearing aids to the system allowed SC’s auditory cortex to mature in a more typical manner.
Several researchers have argued that hearing aids likely do not provide adequate benefit for children with ANSD (e.g., Hood, 1998; Berlin et al, 1999). While it is probably true that many do not profit sufficiently from such devices, it appears that some do, at least in terms of cortical maturation. The latter point is supported by the current case, the studies previously discussed, and others (Rance et al, 2002; Pearce et al, 2007; Berlin et al, 2010; Cardon & Sharma, 2011; Sharma et al, 2011). Rance et al (2002) asserted that hearing aids might provide benefit for children with ANSD for several reasons. First, hearing aids serve to make speech elements more salient to their user, whether the patient has SNHL or ANSD. In addition, others (e.g., Javel, 1986) have noted that increasing the intensity of input to the cochlea, in turn, may cause at least two phenomena: 1) increased phase locking and synchronous discharge of IHCs; and 2) an overall increase of IHCs and VIII nerve fiber activity. Because ANSD may arise from disordered phase locking (i.e., dys-synchrony) and/or fewer than normal functional IHCs or VIII nerve fibers, it is reasonable to believe that traditional amplification might help certain patients with ANSD, albeit a minority. Sharma et al (2011) conjectured that the children who exhibited normal CAEP responses with hearing aids likely had a milder form of ANSD (i.e., less severe dys-synchrony) than those who either had delayed or abnormal/absent P1 responses. If this is true, then merely amplifying incoming stimuli may prove to provide adequate benefit for those children with the mildest forms of ANSD, at least in terms of allowing normal central auditory maturation to progress.
It is also interesting to note that SC received his hearing aids at a relatively early age (0.68 years). His hearing aid fit age was certainly within the sensitive period that appears to be important for treatment of children with SNHL (i.e., 3.5 years; see Sharma et al, 2002a, 2002b, 2005b), which may also be critical in the optimal treatment of children with ANSD. The concept of a sensitive period for treatment in ANSD is further supported by recent findings (Sharma et al, 2011), in which children with ANSD who were fit under approximately one year of age had normal P1 responses and good behavioral outcome compared with children who were fit at later ages. These results are in keeping with animal studies which have demonstrated the existence of sensitive periods in development for animals reared in degraded listening environments (Chang and Merzenich, 2003; Villers-Sidani et al., 2007) which may have some parallels with congenital ANSD. Overall, this case corroborates the critical nature of the timing aspect of plasticity in clinical intervention.
Case 2
JF (participant #6 in Sharma et al, 2011) was born at 31 weeks gestation after a complicated pregnancy and delivery via cesarean section. Following birth, JF stayed in the NICU for 7 weeks, during which he was treated for jaundice with a blood transfusion and required mechanical ventilation. In addition, JF presented with early feeding problems, which later resolved. JF failed his newborn hearing screening. Subsequently, diagnostic ABR testing performed at approximately three months of age revealed present CM bilaterally. During the same testing session, present transient evoked otoacoustic emissions (TEOAEs) and DPOAEs were observed bilaterally. JF was fitted with binaural hearing aids at a very early age (0.36 years). Later, it was determined via behavioral audiometric testing that JF had unaided PTAs of 38 and 70 dB HL in the right and left ears, respectively. In addition, aided testing yielded PTAs of 38 dB HL in the right ear and 41 dB HL in JF’s left ear.
The Receptive-Expressive Emergent Language Scale – 3 (REEL-3; Bzoch et al, 2003) was performed with JF’s mother by his speech/language pathologist when JF was three months old. The REEL-3 is a parent interview regarding receptive and expressive language that is commonly used in children up to three years of age. This measure indicated that while JF scored in the normal range for his corrected age in the expressive language category, his scores for receptive language were delayed for his age. At 1.67 years of age, JF’s speech/language therapist performed a MacArthur Communicative Developmental Inventory (Fenson et al, 1993) with his mother. This assessment focuses on a child’s developmental status on several categories of development by polling a parent or guardian as to the child’s behavioral developmental performance. In the developmental category of Words and Gestures, JF scored severely below age-level expectations. Furthermore, JF showed a severe delay in use of vocabulary for his age on the Words and Sentences developmental category (<5th percentile). These findings lead JF’s family to pursue cochlear implantation for him. A pre-implant MRI revealed normal VIII nerves. At 1.76 years of age, JF received a cochlear implant in the left ear.
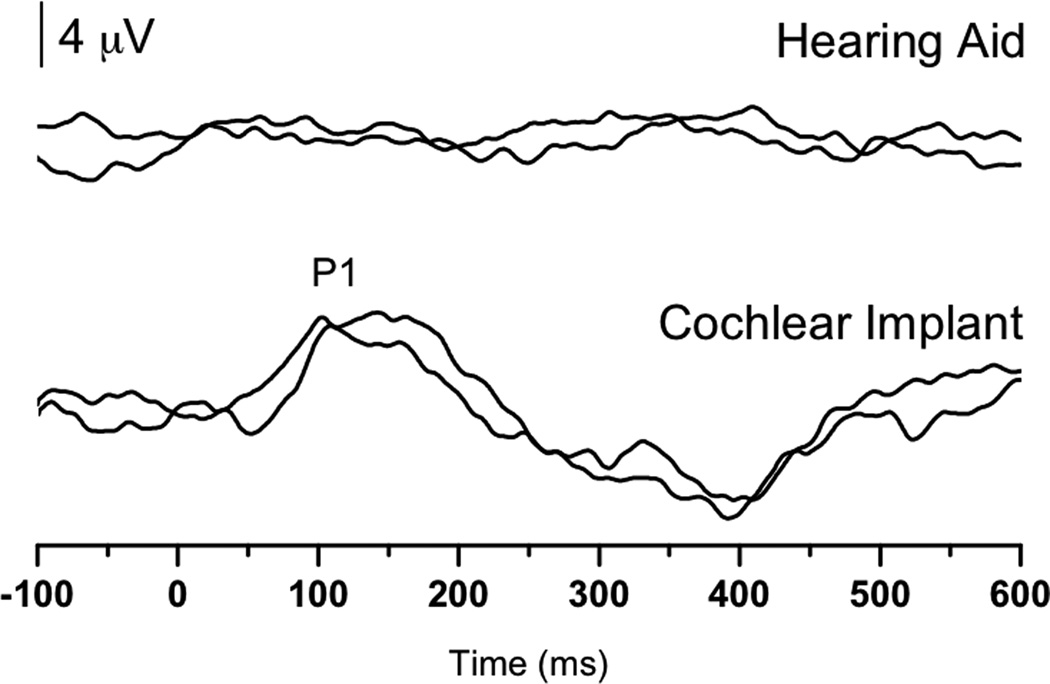
P1 testing was performed several times throughout JF’s life and progression through his various treatments (Figure 2A). Initially, even with significant experience with his hearing aids (from 0.67 up to 1.36 years), no replicable P1 response could be identified. In contrast, replicable, but delayed, P1 responses were observed during testing that was done when JF had recently received his cochlear implant. Later results, however, revealed that JF’s P1 latency systematically decreased until it finally entered the normal limits for P1 latency development when JF was 2.18 years of age (0.44 years of experience with the device) (see Figure 2B).
Figure 2.
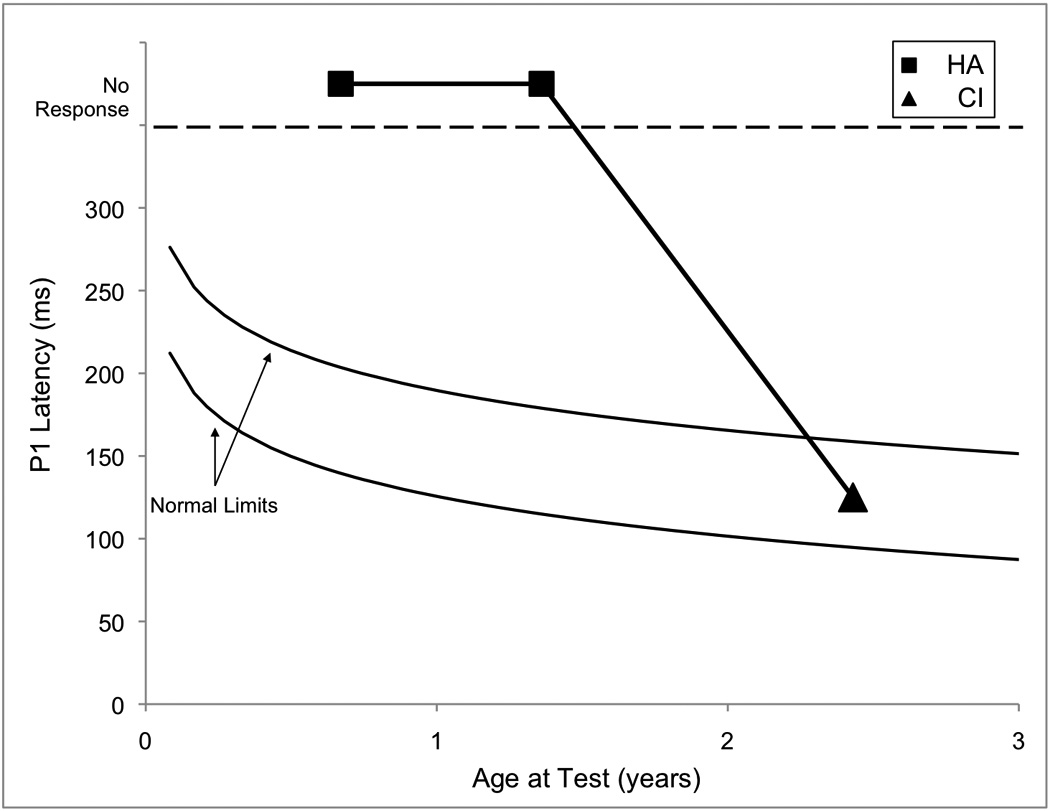
(Panel A): P1 CAEP waveforms (with replications) for both unaided (top) and aided (bottom) P1 testing sessions performed with Case 2 (JF). (Panel B): P1 latencies for testing done with hearing aids (closed squares) and a cochlear implant (closed triangle) plotted by age and compared with the 95% confidence intervals for normal P1 latency development (Sharma et al., 2002c). Results from hearing aid testing are plotted in the no response region at two different ages. Cochlear implant results showed P1 latencies that were within normal limits.
Given that JF’s pre-cochlear implant P1 responses were absent and that hearing aids did not provide adequate stimulation to drive normal central auditory maturation, it is reasonable to argue that he had a more severe case of dys-synchrony than SC (Case 1). Since cochlear implantation caused a systematic decrease in P1 latency, such that by the latest recorded data point, JF’s P1 results were normal, we might conclude that electric stimulation improved input sufficiently to promote normal cortical development. It should also be noted here that JF received his cochlear implant before the end of the sensitive period for treatment of children with SNHL (i.e., 3.5 years). Thus, not only was the input to his auditory system improved, the timing also seemed optimal. CAEPs were instrumental in both determining candidacy and verifying treatment decisions in JF’s case.
Several investigators have produced reports supporting the use of cochlear implants for treatment of children with ANSD (Miyamoto et al, 1999; Shallop et al, 2001; Trautwein et al, 2001; Rance et al, 2009; Berlin et al, 2010; Teagle et al, 2010; Fulmer et al, 2011). However, several of these reports caution that while cochlear implantation seems to produce positive results in many children with ANSD, that it is not optimal for all (see Case 3 below). That is, children with ANSD represent a heterogeneous group. As such, very few clinical decisions regarding the management and treatment of children with ANSD should be automatic. For example, Walton et al (2008) found that children with ANSD who had associated CND did not benefit as much from having received cochlear implants as those without CND. In fact, it is possible that the site of lesion of a given child’s ANSD may be correlated with his or her success with the device. That is, if the site of lesion is cochlear or synaptic, a cochlear implant is likely to bypass the problem areas and stimulate the VIII nerve directly, thereby restoring synchrony and resulting in good outcome. On the other hand, if the site of lesion is post-synaptic, in some cases a cochlear implant may not be able to overcome the anatomical and physiologic phenomena that lead to dys-synchrony, such as demyelination. Several investigators are attempting to devise methods of determining the site of lesion and the characteristics of pre- and post-synaptic forms of ANSD (e.g., Santarelli and Arslan, 2002; Varga et al, 2003; Santarelli et al, 2008, 2009; Dimitrijevic et al, 2011; Santarelli, 2011). In addition, ongoing investigations in our lab are focusing on determining the characteristics of successful cochlear implant use in children with ANSD.
Case 3
RB was born full-term without complications. The family reports no family history of hearing loss. RB initially failed his newborn hearing screening in only the left ear. Upon diagnostic follow-up testing, his audiologist found absent TEOAEs bilaterally and limited presence of DPOAEs, despite normal middle ear function. In addition, RB presented with abnormal ABR morphology (i.e., no wave V), with present CMs bilaterally. These findings, taken together, ultimately lead to a diagnosis of ANSD.
Following several behavioral audiometry sessions, which consistently revealed profound rising to severe bilateral hearing loss, RB was fit with binaural hearing aids (approximately one year of age). Subsequently, aided audiometry showed a mild sloping to severe hearing loss in the 0.5–4 kHz frequency range. Though at some frequencies RB’s functional gain with the devices had improved significantly, the managing team ultimately decided that traditional amplification was insufficient for RB. An MRI confirmed the presence of normal anatomy including VIII nerves bilaterally, cochleae, and internal auditory meatuses. Subsequently, RB received a cochlear implant in the right ear at 1.64 years of age. Additional treatment included weekly home intervention for hearing, oral speech and language, and sign language from the age of six months until RB was three years old. RB now attends a center-based school that specializes in serving children with hearing loss.
Initial audiology and parent reports conveyed that RB was doing well with his implant. For instance, parents mentioned on many occasions that RB did not like when the implant was off and would ask for it upon waking in the morning or after naps. However, as time passed, it became clear to the managing team (i.e., home intervention provider, speech/language therapist, audiologist) and family that RB had limited interest in sound and had very inconsistent responses of questionable reliability during behavioral audiometric testing. Parents and therapists also reported, on several occasions, significant variability in RB’s everyday performance (i.e., ‘good and bad hearing days’). Behavioral audiometry with the cochlear implant in place was inconsistent at best. However, the best thresholds that were obtained showed moderate hearing loss rising to normal auditory thresholds and speech awareness thresholds (SAT) between 20–30 dB HL.
Serial P1 testing was performed with RB to monitor progress with his cochlear implant (2.53, 3.61, and 4.19 years of age; See Figure 3A). Initial testing revealed a replicable P1 of normal latency for RB’s age. In contrast, the second testing session yielded P1 waveforms that were not replicable, despite several CAEP recording runs. Finally, during the most recent P1 appointment, RB produced P1 waveforms that exhibited a delayed P1 response that was only marginally replicable (Figure 3B). IT-MAIS testing performed with RB at 3.61 and 4.19 years of age yielded scores of 14/40 and 20/40, respectively. These seem to correlate well with RB’s P1 results suggesting that CAEP morphology and latency are correlated with behavioral outcome in ANSD (Rance et al., 2002; Sharma et al., 2011). It should be noted that the IT-MAIS is specifically designed to be administered to infants and toddlers. The fact that this measure has been used in older children with ANSD, may be indicative of the severity of their auditory impairment.
Figure 3.
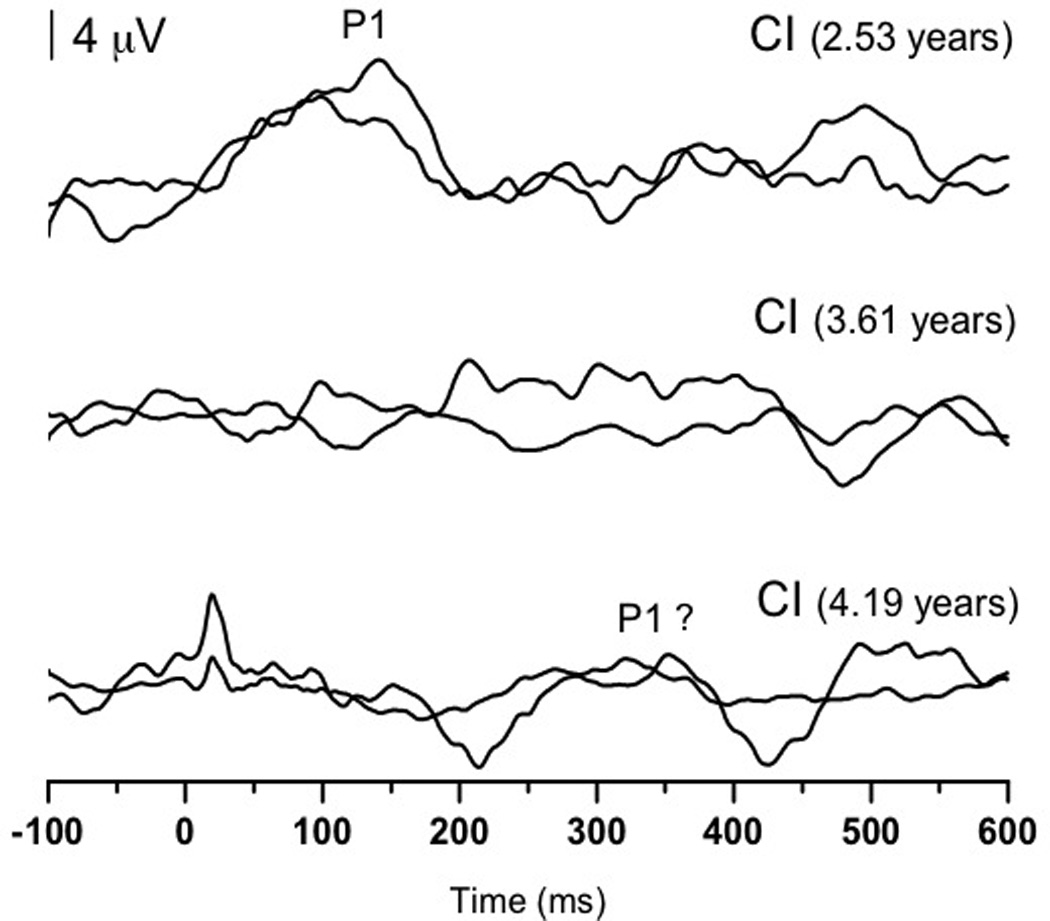
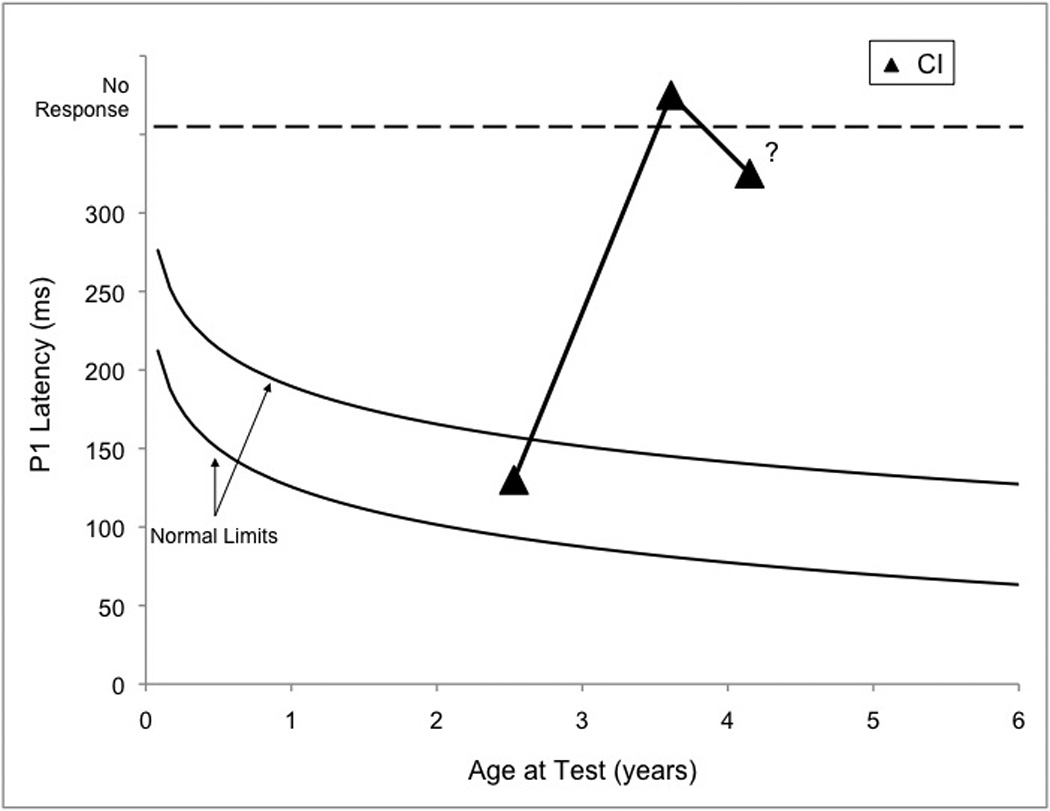
(Panel A): P1 CAEP waveforms (with replications) for three testing sessions (2.53, 3.61, and 4.19 years of age) performed with cochlear implants for Case 3 (RB). Testing at: 2.53 years (top) shows replicable and robust P1 responses; at 3.61 years (middle) show no replicable response; at 4.19 years (bottom) show a somewhat replicable response. (Panel B): P1 latencies from three testing sessions (closed triangles) plotted by age and compared to the 95% confidence intervals for normal P1 latency development (Sharma et al., 2002c). Testing at 2.53 years of age revealed responses with normal latencies, while testing at 3.61 and 4.19 years of age yielded no response and a marginally replicable P1 response that was delayed in latency, respectively.
Case 3 illustrates that even with optimal timing and input, the negative effects of a neural dys-synchrony cannot always be ameliorated by a cochlear implant. Though RB’s case is a difficult one, several assertions and conclusions can be made from studying it. For example, ANSD is a disorder that likely cannot be fully explained in every case with means and averages. While some patterns have been established in patients with ANSD (e.g., diagnostic criteria), it seems that there are many exceptions to proposed clinical rules in this population. For example, classically, OAEs are found to be present in patients with ANSD, however, this is not always the case (e.g., Berlin et al, 2010). Variability in patients with ANSD may be due to various factors such as the genetic, anatomical, and physiological heterogeneity of those with the diagnosis of ANSD, diversity of etiology, and differences in site of lesion (Rapin & Gravel, 2003; Hood, 2011). In addition, it appears that there is a wide spectrum of the severity of dys-synchrony between patients with ANSD (Sharma et al, 2011). As such, care must be taken to consider each patient as an individual (Hood, 2011).
Furthermore, it is interesting to note that both RB’s parents and the professionals with whom he works have reported variability in RB’s listening capability from day to day. A number of accounts from the literature surrounding several neurologic disorders other than ANSD have reported similar intra-individual variability in performance (see MacDonald et al, 2006 for a review). For example, this phenomenon appears to be a hallmark of age-related cognitive decline, schizophrenia, attention deficit hyperactivity disorder (ADHD), dementia, and head injury. It seems, as well, that intra-individual variability can be a sign of various neural problems, such as degeneration and dis-connectivity. Thus, it is not surprising that a variable pattern might be found in some with ANSD. In fact, some investigators have found variation in performance on various measures driven by changes in body temperature in children with ANSD (Starr et al, 1998; Varga et al, 2006). This case illustrates that CAEPs may provide an objective means to assess intra-individual variability in children with ANSD. We have noted that while some cases of children with ANSD benefit from cochlear implantation, others, such as RB, do not appear to share these results. Additional research is needed to elucidate the details of important factors in cochlear implantation in children with ANSD.
Case 4
KG was born at 26 weeks gestation after a complicated pregnancy. She spent the following 116 days in the NICU, where she received several blood transfusions, underwent heart surgery, and remained on mechanical ventilation until she went home. While in the NICU, KG failed newborn hearing screening three times. Hearing loss was confirmed at approximately five months of age, at which point a diagnosis of ANSD was made. Since diagnosis, behavioral auditory testing has revealed thresholds that have fluctuated between the moderate and severe hearing loss ranges bilaterally. KG was fitted with binaural amplification at approximately eight months of age. Aided behavioral audiometry confirmed aided auditory thresholds between 20 and 30 dBHL. In addition, from the time of hearing loss diagnosis, to the present time, KG’s family has received weekly visits from two home interventionists: one who focuses on oral speech and language development and another who teaches American Sign Language (ASL) to the family.
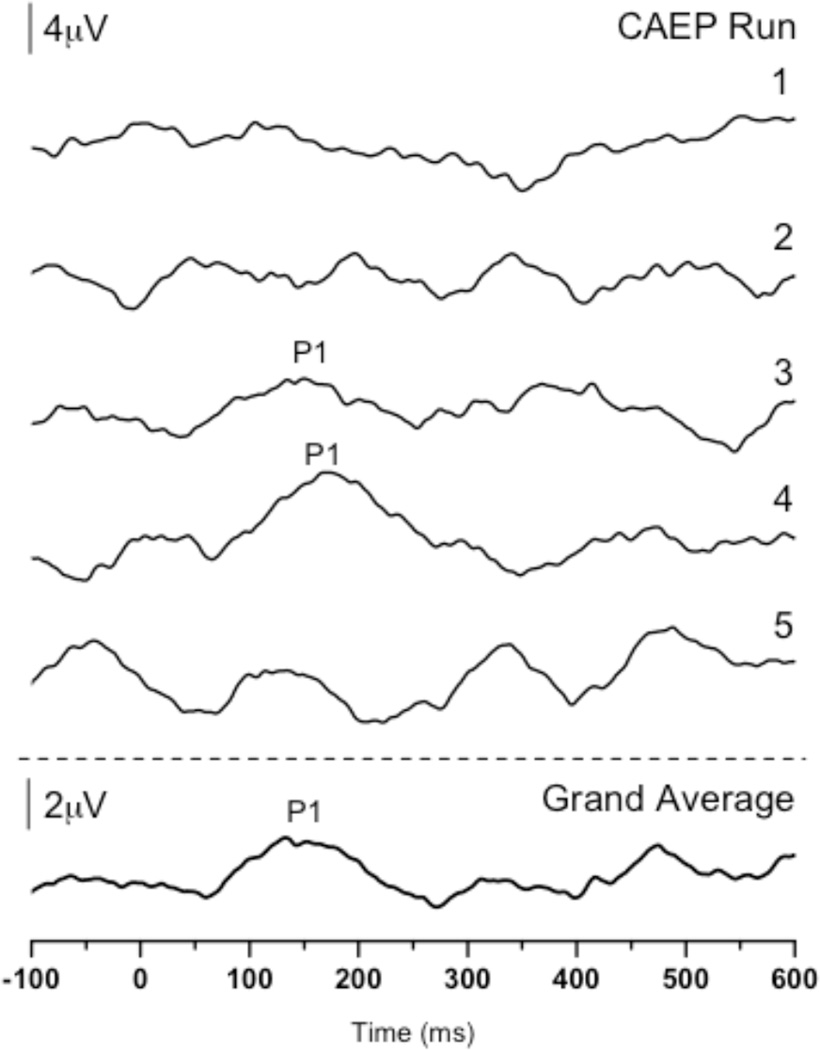
We performed P1 testing with KG at 1.48 years of age to evaluate the status of her central auditory maturation with the use of her hearing aids. Five CAEP recording runs—comprised of approximately 250 sweeps each (1251 sweeps total)—were executed with KG on the day of testing. When averaged together we saw a P1 of normal latency (see Figure 4, bottom trace). On the surface, this normal P1 response appeared to be inconsistent with KG’s lower than expected score of 28/40 on the IT-MAIS. However, upon closer inspection of each of the five recording runs, we noted that while two of the CAEP runs yielded waveforms that were replicable (runs 3 and 4), the remaining runs’ waveforms had atypical morphology and did not replicate (Figure 4, upper traces).
Figure 4.
P1 CAEP waveforms for individual CAEP recording runs and grand average performed in the aided condition for Case 4 (KG; 1.48 years of age). Upper traces: Waveforms from CAEP runs 1 (212 sweeps), 2 (256 sweeps), and 5 (252 sweeps) show no replicable P1 response, whereas waveforms from runs 3 (266 sweeps) and 4 (265 sweeps) show a robust and replicable P1 response of normal latency for KG’s age. Bottom trace: The waveform marked ‘Grand Average’ is the grand average of all waveforms from the five CAEP recording runs.
Case 4 further demonstrates the intra-individual variability inherent in some people with ANSD (Nash et al, in preparation; Starr et al, 1998; Uus, 2011). CAEP testing with KG showed neural inconsistency during an hour-long testing session. At times, the P1 response was normal in morphology and latency, indicating an appropriate and synchronous neural response for KG’s age. In contrast, during the other recording runs, the morphology of the CAEP was abnormal, such that no P1 could be identified, likely due to underlying neural dys-synchrony. In our experience, while possible in children with ANSD, this type of variability does not occur in CAEP recording in most children with normal hearing or children with SNHL, who presumably have appropriate and stable neural synchrony. Furthermore, it is interesting to note that the variability in CAEP recordings occurred after an early hearing aid fitting and more than nine months of hearing aid use. That is, despite appropriate aided auditory thresholds, timing, and use of the devices, a portion of KG’s cortical responses was still atypical. Ultimately, KG’s highly variable cortical responses were consistent with her lower than expected behavioral performance on the IT-MAIS. Thus, this case exhibits at least two important points: 1) hearing aids may not adequately improve neural synchrony in some patients with ANSD (consistent with Berlin et al, 1998, 2002, 2003; Hood, 2011; Rance, et al, 2002; Sharma et al, 2011); 2) even with a high degree of intra-individual variability, the auditory brain may be plastic enough to develop some degree of normal function, as indicated by the two runs exhibiting P1 responses of normal latency. In light of the results from Case 4, we suggest that multiple CAEP recordings of large sweep counts be performed in a given testing session to appropriately estimate the intra-individual variability in some patients with ANSD.
As reviewed in Cases 1–4, some of the commonly held principles of plasticity hold true in the ANSD population. However, much work remains to be done to fully characterize central auditory maturation and plasticity in children with ANSD. Both previously published data and two of the above cases suggest that providing appropriate input to the cortex and doing so early in life play an important role in the development of many children with ANSD. These reports also highlight CAEPs as a potentially useful tool that might be employed to determine candidacy for a given treatment and the efficacy of such intervention. However, it has also been shown that even treatment provided at a young age may not always ameliorate the negative effects of neural dys-synchrony on cortical maturation. Thus, the degree of severity of a given patient’s dys-synchrony may be predictive of outcome. In all, children with ANSD must be considered individually, although assessments of cortical maturation may provide clinicians and families with information that may positively guide management decisions.
VI. SUMMARY
The principles that govern cortical maturation and plasticity are numerous and complex. However, as we have discussed in this review, two major principles of neuroplasticity direct clinical outcomes: 1) adequate stimulation provided to the cortex and 2) appropriate timing of this stimulation. Early intervention with appropriate auditory prostheses (such as cochlear implants) results in high likelihood of normal auditory cortical development in children with congenital deafness. In addition, recent work regarding central auditory maturation and plasticity in children with ANSD suggests that plasticity can often be harnessed via amplification and/or electrical stimulation for positive clinical outcomes in this patient population. The initial reports of CAEP measures of central auditory maturation in children with SNHL and ANSD appear promising in their ability to help determine a given patient’s candidacy for treatment and in verifying the effectiveness of the audiologic intervention. A better understanding of the deleterious effects of absent, abnormal, and/or late auditory stimulation and the potential for plasticity via auditory prostheses in individual patients will allow us to better customize clinical management and rehabilitation for pediatric patients.
REVIEW QUESTIONS.
- Two fundamental principles of neural plasticity that can be manipulated clinically to maximize cortical maturation in children with hearing loss are:
- Neurotrophic factors and critical periods
- Input to the cortex and timing of stimulation
- Inhibitory function and sensitive periods
- Cortical re-organization and decoupling
- CAEPs can be reliably measured in ANSD, even when most in this population present with abnormal or absent ABR responses. Which one of the following reasons contributes to this phenomenon?
- CAEPs measure action potentials of the cortex, which tend to be lower in frequency than the brainstem dendritic activity recorded in the ABR
- CAEPs measure dendritic activity from the cortex and, therefore, are not affected by neural dys-synchrony
- CAEPs measure dendritic activity of the cortex, which tends to be lower in frequency than the brainstem action potentials recorded in the ABR
- CAEPs measure inhibitory activity of the cortex, which tends to be lower in frequency than the brainstem excitatory activity recorded in the ABR
- Recent studies have focused on cortical maturation in children with ANSD. What were their main findings?
- Differences in cortical maturation between groups of children with ANSD and a high correlation between CAEP results and behavioral outcome
- An effect of hearing aid fit age on behavioral outcome and a difference in auditory threshold between groups
- A correlation between present ABR wave V and normal CAEP results
- Differing levels of cortical maturation in children with ANSD which corresponded to behavioral auditory threshold
- What is the sensitive period for normal central auditory maturation in children with congenital deafness who receive cochlear implants?
- Over 7 years of age
- Between 3.5 and 7 years of age
- Under 3.5 years of age
- Between 3.5 and 6 years of age
- What critical event(s) contribute(s) to cortical re-organization in children implanted after the sensitive period?
- De-coupling of higher-order auditory cortex from primary auditory cortex
- Recruitment of higher-order auditory cortex by other sensory modalities such as vision or somatosensation
- Pruning of unnecessary neurons and synapses
- A and B
- B and C
ACKNOWLEDGEMENTS
This research was supported by NIH grant R01 DC006257 to A.S.
ABBREVIATIONS
- (ANSD)
Auditory neuropathy spectrum disorder
- (SNHL)
sensorineural hearing loss
- (AV)
audio-visual
- (A)
auditory-alone
- (V)
visual-alone
- (ABR)
auditory brainstem response
- (OAE)
otoacoustic emission
- (TEOAE)
transient evoked otoacoustic emission
- (DPOAE)
distortion product otoacoustic emission
- (CAEP)
cortical auditory evoked potential
- (CM)
cochlear microphonic
- (IHC)
inner hair cells
- (ASSR)
auditory steady state response
- (IT-MAIS)
Infant Toddler Meaningful Auditory Integration Scale
- (CND)
cochlear nerve deficiency
- (PTA)
pure tone average
- (REEL-3)
Receptive-Expressive Emergent Language Scale – 3
- (ASL)
American Sign Language
REFERENCES
- Bavelier D, Brozinsky C, Tomann A, Mitchell T, Neville H, Liu G. Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. J Neurosci. 2001;21(22):8931–8942. doi: 10.1523/JNEUROSCI.21-22-08931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Tomann A, Hutton C, Mitchell T, Corina D, Liu G, Neville H. Visual attention to the periphery is enhanced in congenitally deaf individuals. J Neurosci. 2000;20(17):RC93. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeson TR, Pisoni DB, Davis RA. Development of audiovisual comprehension skills in prelingually deaf children with cochlear implants. Ear Hear. 2005;26(2):149–164. doi: 10.1097/00003446-200504000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C, Bordelon J, St John P, Wilenski D, Annette K, Hood L. Reversing click polarity may uncover auditory neuropathy in infants. Ear Hear. 1998;19(1):37–47. doi: 10.1097/00003446-199802000-00002. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Li L, Hood LJ, Morlet T, Rose K, Brashears S. Auditory Neuropathy/Dys-Synchrony: After the Diagnosis, then What? Semin Hear. 2002;23(3):209–214. [Google Scholar]
- Berlin C, Li L, Hood L, Morlet T, Rose K, Brashears S. Auditory neuropathy/dys-synchrony: diagnosis and management. Ment Retard Dev Disabil Res Rev. 2003;9:225–231. doi: 10.1002/mrdd.10084. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, Wilensky D, St John P, Montgomery E, Thibodeaux M. Absent or elevated middle ear muscle reflexes in the presence of normal otoacoustic emissions: a universal finding in 136 cases of auditory neuropathy/dys-synchrony. J Am Acad Audiol. 2005;16(8):546–553. doi: 10.3766/jaaa.16.8.3. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, Wilensky D, Li L, Mattingly KR, Taylor-Jeanfreau J, Keats BJ, John PS, Montgomery E, Shallop JK, Russell BA, Frisch SA. Multi-site diagnosis and management of 260 patients with Auditory Neuropathy/Dys-synchrony (Auditory Neuropathy Spectrum Disorder) Int J Audiol. 2010;49(1):30–43. doi: 10.3109/14992020903160892. [DOI] [PubMed] [Google Scholar]
- Buchman CA, Roush PA, Teagle HF, Brown CJ, Zdanski CJ, Grose JH. Auditory neuropathy characteristics in children with cochlear nerve deficiency. Ear Hear. 2006;27(4):399–408. doi: 10.1097/01.aud.0000224100.30525.ab. [DOI] [PubMed] [Google Scholar]
- Buckley KA, Tobey EA. Cross-Modal Plasticity and Speech Perception in Pre- and Postlingually Deaf Cochlear Implant Users. Ear Hear. 2010;32(1):2–15. doi: 10.1097/AUD.0b013e3181e8534c. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Bzoch KR, League R, Brown VL. Receptive-expressive emergent language test—Third edition (REEL-3) Pearson: San Antonio; 2003. [Google Scholar]
- Campbell J, Cardon G, Sharma A. Clinical application of the P1 cortical auditory evoked potential biomarker in children with sensorineural hearing loss and auditory neuropathy spectrum disorder. Semin Hear. 2011;32(2):117–122. doi: 10.1055/s-0031-1277236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon G, Sharma A. Cortical auditory evoked potentials in auditory neuropathy spectrum disorder: clinical implications. Perspectives on Hearing and Hearing Disorders in Children. 2011;21:31–37. [Google Scholar]
- Ceponiene R, Cheour M, Naatanen R. Interstimulus interval and auditory event-related potentials in children: evidence for multiple generators. Electroencephalogr Clin Neurophysiol. 1998;108(4):345–354. doi: 10.1016/s0168-5597(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Cone B. The electrophysiology of auditory neuropathy spectrum disorder; Italy: Cernobbio. Paper presented at the Identification and Management of Infants and Young Children with Auditory Neuropathy Spectrum Disorder Guidelines Development conference.Jun, 2008. [Google Scholar]
- Doucet ME, Bergeron F, Lassonde M, Ferron P, Lepore F. Cross-modal reorganization and speech perception in cochlear implant users. Brain. 2006;129(Pt 12):3376–3383. doi: 10.1093/brain/awl264. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, Starr A, Bhatt S, Michalewski HJ, Zeng FG, Pratt H. Auditory cortical N100 in pre- and post-synaptic auditory neuropathy to frequency or intensity changes of continuous tones. Clin Neurophysiol. 2011;122(3):594–604. doi: 10.1016/j.clinph.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye MW, Bavelier D. Attentional enhancements and deficits in deaf populations: an integrative review. Restor Neurol Neurosci. 2010;28(2):181–192. doi: 10.3233/RNN-2010-0501. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. The role of sound in adult and developmental auditory cortical plasticity. Ear Hear. 2008;29(6):819–829. doi: 10.1097/AUD.0b013e3181853030. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW. The neurophysiology of auditory perception: from single units to evoked potentials. Audiol Neurootol. 2002;7(2):71–99. doi: 10.1159/000057656. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: correlations with changes in structure and speech perception. Acta Otolaryngol. 2003;123(2):249–252. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses in the human and the cat model. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:461–467. [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404(6774):183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP, Pethick S, Reilly JS. The MacArthur Communicative Development Inventories: User's Guide and Technical Manual. San Diego: Singular Publishing Group; 1993. [Google Scholar]
- Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4(12):1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Finney EM, Clementz BA, Hickok G, Dobkins KR. Visual stimuli activate auditory cortex in deaf subjects: evidence from MEG. Neuroreport. 2003;14(11):1425–1427. doi: 10.1097/00001756-200308060-00004. [DOI] [PubMed] [Google Scholar]
- Foeller E, Feldman DE. Synaptic basis for developmental plasticity in somatosensory cortex. Curr Opin Neurobiol. 2004;14(1):89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. doi: 10.1016/j.tins.2011.02.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulmer S, Runge C, Jensen J, Friedland D. Rate of neural recovery in implanted children with auditory neuropathy spectrum disorder. Otolaryngol Head Neck Surg. 2011;144(2):274–279. doi: 10.1177/0194599810391603. [DOI] [PubMed] [Google Scholar]
- Geers AE. Factors influencing spoken language outcomes in children following early cochlear implantation. Adv Otorhinolaryngol. 2006;64:50–65. doi: 10.1159/000094644. [DOI] [PubMed] [Google Scholar]
- Geers AE, Moog JS, Biedenstein J, Brenner C, Hayes H. Spoken language scores of children using cochlear implants compared to hearing age-mates at school entry. J Deaf Stud Deaf Educ. 2009;14(3):371–385. doi: 10.1093/deafed/enn046. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman MF. Cortical reorganization in children with cochlear implants. Brain Res. 2008;1239:56–65. doi: 10.1016/j.brainres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Martin K. Developmental changes in refractoriness of the cortical auditory evoked potential. Clin Neurophysiol. 2005;116(3):648–657. doi: 10.1016/j.clinph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Mitchell TV, Dorman MF. The influence of a sensitive period for auditory-visual integration in children with cochlear implants. Restor Neurol Neurosci. 2010;28(2):207–218. doi: 10.3233/RNN-2010-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Lee HJ. Predicting cochlear implant outcome from brain organisation in the deaf. Restor Neurol Neurosci. 2007;25(3–4):381–390. [PubMed] [Google Scholar]
- Gomot M, Bruneau N, Laurent JP, Barthelemy C, Saliba E. Left temporal impairment of auditory information processing in prematurely born 9-year-old children: an electrophysiological study. Int J Psychophysiol. 2007;64(2):123–129. doi: 10.1016/j.ijpsycho.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell Vol. 72/Neuron 10. 1993;(Suppl.):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC, Harrison RV. Activity-dependent developmental plasticity of the auditory brain stem in children who use cochlear implants. Ear Hear. 2003;24(6):485–500. doi: 10.1097/01.AUD.0000100203.65990.D4. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC, Harrison RV. Effects of cochlear implant use on the electrically evoked middle latency response in children. Hear Res. 2005;204(1–2):78–79. doi: 10.1016/j.heares.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Harrison RV. An animal model of auditory neuropathy. Ear Hear. 1998;19(5):355–361. doi: 10.1097/00003446-199810000-00002. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol. 2005;46(3):252–261. doi: 10.1002/dev.20052. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Shepherd RK, Heid S, Klinke R. Response of the primary auditory cortex to electrical stimulation of the auditory nerve in the congenitally deaf white cat. Hear Res. 1997;112(1–2):115–133. doi: 10.1016/s0378-5955(97)00114-7. [DOI] [PubMed] [Google Scholar]
- Hassan D. Perception of temporally modified speech in auditory neuropathy. Int J Audiol. 2011;50(1):41–49. doi: 10.3109/14992027.2010.520035. [DOI] [PubMed] [Google Scholar]
- Hebb DO. Spontaneous neurosis in chimpanzees; theoretical relations with clinical and experimental phenomena. Psychosom Med. 1947;9(1):3–19. [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear. 2008;29(4):492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441(7096):979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Hood LJ. A review of objective methods of evaluating auditory neural pathways. Laryngoscope. 1999;109(11):1745–1748. doi: 10.1097/00005537-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Hood LJ. Variation in auditory neuropathy spectrum disorder: implications for evaluation and management. Semin Hear. 2011;32(2):117–122. [Google Scholar]
- Huang BY, Roche JP, Buchman CA, Castillo M. Brain stem and inner ear abnormalities in children with auditory neuropathy spectrum disorder and cochlear nerve deficiency. Am J Neuroradiol. 2010;31(10):1972–1979. doi: 10.3174/ajnr.A2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6(1):1–9. [PubMed] [Google Scholar]
- Javel E. Basic response properties of auditory nerve fibers. In: Altschuler RPHRA, editor. Neurobiology of Hearing: The Cochlea. New York: Raven Press; 1986. pp. 213–245. [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kirkim G, Serbetcioglu B, Erdag T, Ceryan K. The frequency of auditory neuropathy detected by universal newborn hearing screening program. Int J Pediatr Otorhinolaryngol. 2008;72(10):1461–1469. doi: 10.1016/j.ijporl.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Kral A. Unimodal and cross-modal plasticity in the 'deaf' auditory cortex. Int J Audiol. 2007;46(9):479–493. doi: 10.1080/14992020701383027. [DOI] [PubMed] [Google Scholar]
- Kral A, Eggermont JJ. What's to lose and what's to learn: development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Res Rev. 2007;56(1):259–269. doi: 10.1016/j.brainresrev.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J. Brain plasticity under cochlear implant stimulation. Adv Otorhinolaryngol. 2006;64:89–108. doi: 10.1159/000094647. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cereb Cortex. 2000;10(7):714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Delayed maturation and sensitive periods in the auditory cortex. Audiol Neurootol. 2001;6(6):346–362. doi: 10.1159/000046845. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb Cortex. 2002;12(8):797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15(5):552–562. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham MA, Cunningham J, King CD, Koch DB, Nicol TG, McGee TJ, Stein LK, Wright BA. Consequences of a neural asynchrony: a case of auditory neuropathy. J Assoc Res Otolaryngol. 2000;1:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS. Cross-modal plasticity and cochlear implants. Nature. 2001;409(6817):149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Giraud AL, Kang E, Oh SH, Kang H, Kim CS, Lee DS. Cortical activity at rest predicts cochlear implantation outcome. Cereb Cortex. 2007;17(4):909–917. doi: 10.1093/cercor/bhl001. [DOI] [PubMed] [Google Scholar]
- Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol. 1994;92(3):204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 2010;13(11):1421–7. doi: 10.1038/nn.2653. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Nyberg L, Backman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29(8):474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Maris M, Venstermans C, Boudewyns AN. Auditory neuropathy/dyssynchrony as a cause of failed neonatal hearing screening. Int J Pediatr Otorhinolaryngol. 2011;75(7):973–975. doi: 10.1016/j.ijporl.2011.04.012. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17(6):1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McClay JE, Booth TN, Parry DA, Johnson R, Roland P. Evaluation of pediatric sensorineural hearing loss with magnetic resonance imaging. Arch Otolaryngol Head Neck Surg. 2008;134(9):945–952. doi: 10.1001/archotol.134.9.945. [DOI] [PubMed] [Google Scholar]
- McGee T, Kraus N. Auditory development reflected by middle latency response. Ear Hear. 1996;17(5):419–429. doi: 10.1097/00003446-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Starr A, Zeng FG, Dimitrijevic A. N100 cortical potentials accompanying disrupted auditory nerve activity in auditory neuropathy (AN): effects of signal intensity and continuous noise. Clin Neurophysiol. 2009;120(7):1352–1363. doi: 10.1016/j.clinph.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalewski H, Starr A, Nguyen T, Kong Y, Zeng F. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin Neurophysiol. 2005;116(3):669–680. doi: 10.1016/j.clinph.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Kirk KI, Renshaw J, Hussain D. Cochlear implantation in auditory neuropathy. Laryngoscope. 1999;109(2 Pt 1):181–185. doi: 10.1097/00005537-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Mo L, Yan F, Liu H, Han D, Zhang L. Audiological results in a group of children with auditory neuropathy spectrum disorder. ORL J Otorhinolaryngol Relat Spec. 2010;72(2):75–79. doi: 10.1159/000297572. [DOI] [PubMed] [Google Scholar]
- Moore JK, Guan YL. Cytoarchitectural and axonal maturation in human auditory cortex. J Assoc Res Otolaryngol. 2001;2(4):297–311. doi: 10.1007/s101620010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, Linthicum FH., Jr The human auditory system: a timeline of development. Int J Audiol. 2007;46(9):460–478. doi: 10.1080/14992020701383019. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Narne V, Vanaja C. Speech identification and cortical potentials in individuals with auditory neuropathy. Behav Brain Funct. 2008;4:15. doi: 10.1186/1744-9081-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A, Gilley P, Sharma A. Cortical organization and variability in unilateral auditory neuropathy spectrum disorder: a case study. in preparation. [Google Scholar]
- Neville HJ, Lawson D. Attention to central and peripheral visual space in a movement detection task: an event-related potential and behavioral study. II. Congenitally deaf adults. Brain Res. 1987;405(2):268–283. doi: 10.1016/0006-8993(87)90296-4. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Schmidt A, Kutas M. Altered visual-evoked potentials in congenitally deaf adults. Brain Res. 1983;266(1):127–132. doi: 10.1016/0006-8993(83)91314-8. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim CS, Kang EJ, Lee DS, Lee HJ, Chang SO, Ahn SH, Hwang CH, Park HJ, Koo JW. Speech perception after cochlear implantation over a 4-year time period. Acta Otolaryngol. 2003;123(2):148–153. doi: 10.1080/0036554021000028111. [DOI] [PubMed] [Google Scholar]
- Pallas SL. Intrinsic and extrinsic factors that shape neocortical specification. Trends Neurosci. 2001;24(7):417–423. doi: 10.1016/s0166-2236(00)01853-1. [DOI] [PubMed] [Google Scholar]
- Pearce W, Golding M, Dillon H. Cortical auditory evoked potentials in the assessment of auditory neuropathy: two cases. J Am Acad Audiol. 2007;18:380–390. doi: 10.3766/jaaa.18.5.3. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ. Of kittens and kids: altered cortical maturation following profound deafness and cochlear implant use. Audiol Neurootol. 2001;6(6):363–380. doi: 10.1159/000046846. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Rance G. Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends Amplif. 2005;9(1):1–43. doi: 10.1177/108471380500900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G, Roper R, Symons L, Moody LJ, Poulis C, Dourlay M, Kelly T. Hearing threshold estimation in infants using auditory steady-state responses. J Am Acad Audiol. 2005;16(5):291–300. doi: 10.3766/jaaa.16.5.4. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech perception in children with auditory neuropathy/dyssynchrony managed with either hearing aids or cochlear implants. Otol Neurotol. 2008;29(2):179–182. doi: 10.1097/mao.0b013e31815e92fd. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech and language outcomes in children with auditory neuropathy/dys-synchrony managed with either cochlear implants or hearing aids. Int J Audiol. 2009;48(6):313–320. doi: 10.1080/14992020802665959. [DOI] [PubMed] [Google Scholar]
- Rance G, McKay C, Grayden D. Perceptual characterization of children with auditory neuropathy. Ear Hear. 2004;25(1):34–46. doi: 10.1097/01.AUD.0000111259.59690.B8. [DOI] [PubMed] [Google Scholar]
- Rance G, Cone-Wesson B, Wunderlich J, Dowell R. Speech perception and cortical event related potentials in children with auditory neuropathy. Ear Hear. 2002;23(3):239–253. doi: 10.1097/00003446-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Rapin I, Gravel J. “Auditory neuropathy”: physiologic and pathologic evidence calls for more diagnostic specificity. Int J Pediatr Otorhinolaryngol. 2003;67(7):707–728. doi: 10.1016/s0165-5876(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Katz LC, Lo DC. Focal delivery of neurotrophins into the central nervous system using fluorescent latex microspheres. Biotechniques. 1997;23(5):928–934. doi: 10.2144/97235rr02. 936-927. [DOI] [PubMed] [Google Scholar]
- Roche J, Huang B, Castillo M, Bassim M, Adunka O, Buchman C. Imaging characteristics of children with auditory neuropathy spectrum disorder. Otology & Neurotology. 2010;31(5):780–788. doi: 10.1097/mao.0b013e3181d8d528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland P, Henion K, Booth T, Campbell JD, Sharma A. Assessment of cochlear implant candidacy in patients with cochlear nerve deficiency using the P1 CAEP biomarker. Cochlear Implants Int. doi: 10.1179/146701011X12962268235869. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli R, Arslan E. Electrocochleography in auditory neuropathy. Hear Res. 2002;170(1–2):32–47. doi: 10.1016/s0378-5955(02)00450-1. [DOI] [PubMed] [Google Scholar]
- Santarelli R, Starr A, Michalewski H, Arslan E. Neural and receptor cochlear potentials obtained by trasntympanic electrocochleaography in auditory neuropathy. Clinical Neurophysiology. 2008;119:1028–1041. doi: 10.1016/j.clinph.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Santarelli R, Del Castillo I, Rodriguez-Ballesteros M, Scimemi P, Cama E, Arslan E, Starr A. Abnormal cochlear potentials from deaf patients with mutations in the otoferlin gene. J Assoc Res Otolaryngol. 2009;10(4):545–556. doi: 10.1007/s10162-009-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli R. Information from cochlear potentials and genetic mutations helps localize the lesion site in auditory neuropathy. Genome Meidicine. 2011;2(12) doi: 10.1186/gm212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr EA, Fox NA, van Wassenhove V, Knudsen EI. Auditory-visual fusion in speech perception in children with cochlear implants. Proc Natl Acad Sci U S A. 2005;102(51):18748–18750. doi: 10.1073/pnas.0508862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Sczaniecka A, Grillet N, Bailey JS, Avenarius M, Najmabadi H, Steffy BM, Federe GC, Lagler EA, Banan R, Hice R, Grabowski-Boase L, Keithley EM, Ryan AF, Housley GD, Wiltshire T, Smith RJ, Tarantino LM, Müller U. A forward genetics screen in mice identifies recessive deafness traits and reveals that pejvakin is essential for outer hair cell function. J Neurosci. 2007;27(9):2163–2179. doi: 10.1523/JNEUROSCI.4975-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallop J, Peterson A, Facer G, Fabry L, Driscoll C. Cochlear implants in five cases of auditory neuropathy: postoperative findings and progress. Laryngoscope. 2001;111:555–562. doi: 10.1097/00005537-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Shapiro SM, Popelka GR. Auditory impairment in infants at risk for billrubin-induced neurologic dysfunction. Semin Perinatol. 2011;35(3):162–170. doi: 10.1053/j.semperi.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF. Central auditory development in children with cochlear implants: Clinical implications. Adv Otorhinolaryngol. 2006;64:66–88. doi: 10.1159/000094646. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005a;203(1–2):134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. Rapid development of cortical auditory evoked potentials after early cochlear implantation. Neuroreport. 2002b;13(10):1365–1368. doi: 10.1097/00001756-200207190-00030. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002c;23(6):532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Cardon G, Henion K, Roland P. Cortical maturation and behavioral outcomes in children with auditory neuropathy spectrum disorder. Int J Audiol. 2011;50(2):98–106. doi: 10.3109/14992027.2010.542492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Dorman M, Spahr A, Todd NW. Early cochlear implantation in children allows normal development of the central auditory pathways. Ann Otol Rhinol Laryngol Suppl. 2002a;189:38–41. doi: 10.1177/00034894021110s508. [DOI] [PubMed] [Google Scholar]
- Sharma A, Nash AA, Dorman M. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord. 2009;42(4):272–279. doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Gilley PM, Dorman MF, Baldwin R. Deprivation induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46(9):494–499. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalogr Clin Neurophysiol. 1997;104(6):540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Sharma A, Martin K, Roland P, Bauer P, Sweeney MH, Gilley P, Dorman M. P1 latency as a biomarker for central auditory development in children with hearing impairment. J Am Acad Audiol. 2005b;16(8):564–573. doi: 10.3766/jaaa.16.8.5. [DOI] [PubMed] [Google Scholar]
- Sininger Y, Oba S. Patients with auditory neuropathy: Who are they and what can they hear? In: Sininger Y, Starr A, editors. Auditory neuropathy: A new perspective on hearing disorders. San Diego: Singular; 2001. pp. 67–82. [Google Scholar]
- Starr A, Picton TW, Kim R. Pathophysiology of auditory neuropathy. In: Sininger Y, Starr A, editors. Auditory neuropathy: A new perspective on hearing disorders. San Diego: Singular; 2001. pp. 67–82. [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory Neuropathy. Brain. 1996;119(3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Winter M, Derebery M, Oba S, Michalewski H. Transient deafness due to temperature-sensitive auditory neuropathy. Ear Hear. 1998;19(3):169–179. doi: 10.1097/00003446-199806000-00001. [DOI] [PubMed] [Google Scholar]
- Starr A, McPherson D, Patterson J, Don M, Luxford W, Shannon R, Sininger Y, Tonakawa L, Waring M. Absence of both auditory evoked potentials and auditory percepts dependent on time cues. Brain. 1991;114(3):1157–1180. doi: 10.1093/brain/114.3.1157. [DOI] [PubMed] [Google Scholar]
- Sur M, Garraghty PE, Roe AW. Experimentally induced visual projections into auditory thalamus and cortex. Science. 1988;242(4884):1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- Sussman E, Steinschneider M, Gumenyuk V, Grushko J, Lawson K. The maturation of human evoked brain potentials to sounds presented at different stimulus rates. Hear Res. 2008;236(1–2):61–79. doi: 10.1016/j.heares.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurol. 2009;4(3):331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat HS, Kabel A, Samy H, Elbadry M. Prevalence of auditory neuropathy (AN) among infants and young children with severe o profound hearing loss. Int J Pediatr Otorhinolaryngol. 2009;73(7):937–939. doi: 10.1016/j.ijporl.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Teagle HF, Roush PA, Woodard JS, Hatch DR, Zdanski CJ, Buss E, Buchman CA. Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear Hear. 2010;31(3):325–335. doi: 10.1097/AUD.0b013e3181ce693b. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Barker BA, Hubbs S. Developmental constraints on language development in children with cochlear implants. Int J Audiol. 2007;46(9):512–523. doi: 10.1080/14992020701383043. [DOI] [PubMed] [Google Scholar]
- Tong Y, Melara RD, Rao A. P2 enhancement from auditory discrimination training is associated with improved reaction times. Brain Res. 2009;1297:80–88. doi: 10.1016/j.brainres.2009.07.089. [DOI] [PubMed] [Google Scholar]
- Trautwein P, Shallop J, Fabry L, Friedman R. Cochlear implantation of patients with auditory neuropathy. In: Sininger Y, Starr A, editors. Auditory neuropathy: A new perspective on hearing disorders. San Diego: Singular; 2001. pp. 67–82. [Google Scholar]
- Uus K, Bamford J. Effectiveness of population-based newborn hearing screening in England: ages if interventions and profile of cases. Pediatrics. 2006;117(5):e887–e893. doi: 10.1542/peds.2005-1064. [DOI] [PubMed] [Google Scholar]
- Varga R, Kelley PM, Keats BJ, Starr A, Leal SM, Cohn E, Kimberling WJ. Non-syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet. 2003;40(1):45–50. doi: 10.1136/jmg.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga R, Avenarius MR, Kelley PM, Keats BJ, Berlin CI, Hood LJ, Morlet TG, Brashears SM, Starr A, Cohn ES, Smith RJ, Kimberling WJ. OTOF mutations revealed by genetic analysis of hearing loss families including a potential temperature sensitive auditory neuropathy allele. J Med Genet. 2006;43(7):576–581. doi: 10.1136/jmg.2005.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J, Bonnet-Brilhault F, Roux S, Bruneau N. Auditory evoked potentials to tones and syllables in adults: evidence of specific influence on N250 wave. Neurosci Lett. 2005;378(3):145–149. doi: 10.1016/j.neulet.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Walton J, Gibson WP, Sanli H, Prelog K. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol Neurotol. 2008;29(3):302–309. doi: 10.1097/MAO.0b013e318164d0f6. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK, Shepherd R. Maturation of the cortical auditory evoked potential in infants and young children. Hear Res. 2006;212(1–2):185–202. doi: 10.1016/j.heares.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet. 1999;21(4):347–349. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res. 2006;49(2):367–380. doi: 10.1044/1092-4388(2006/029). [DOI] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93(6):3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- Zeng F, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuro Report. 1999;10(16):3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Nie K, Liu S, Stickney G, Del Rio E, Kong YY, Chen H. On the dichotomy in auditory perception between temporal envelope and fine structure cues. J Acoust Soc Am. 2004;116(3):1351–1354. doi: 10.1121/1.1777938. [DOI] [PubMed] [Google Scholar]
- Zimmerman-Phillips S, Robbins AM, Osberger MJ. Assessing cochlear implant benefit in very young children. Ann Otol Rhinol Laryngol Suppl. 2000;185:42–43. doi: 10.1177/0003489400109s1217. [DOI] [PubMed] [Google Scholar]