Abstract
A high prevalence of obesity exists among National Football League (NFL) players as determined by body mass index (BMI). It is not established whether elevated BMI is associated with a greater prevalence of CV risk factors or coronary atherosclerosis in former NFL players as in non-athletes. This study compared cardiovascular (CV) risk factors and coronary atherosclerosis among retired NFL players and two groups of community controls, the population-based Dallas Heart Study and the preventive medicine cohort, the Aerobics Center Longitudinal Study. Retired NFL players (n=201) were matched for ethnicity, age and BMI (Aerobics Center Longitudinal Study, age only). CV risk factors were assessed by survey and screening visit. Coronary atherosclerosis was measured by computed tomography as coronary artery calcium (CAC). Compared to population-based controls, retired NFL players had a significantly lower prevalence of diabetes, hypertension, sedentary lifestyle and the metabolic syndrome, yet a higher prevalence of impaired fasting glucose and hyperlipidemia. However, there was no significant difference in the prevalence of detectable CAC (46 v 48.3%, p=0.69) or distribution of CAC (0-10, 10-100, 100-400, 400+, p=0.11). Comparing retired NFL players to the physically active preventive medicine controls, there was no difference in the amount of CAC. Among retired NFL players, age and hyperlipidemia, not body size, were the most significant predictors of CAC. In conclusion, despite their large body size, retired NFL players do not have a greater prevalence of CV risk factors or amount of CAC than community controls.
Keywords: obesity, cardiovascular risk factors, coronary artery disease
The large body size of professional football players is increasingly a concern given the established risks of obesity and recent cardiovascular (CV) deaths of young players like Thomas Herrion.1 Over 50% of players meet body mass index (BMI) criteria for overweight and obesity in the National Football League (NFL).2 Of greater concern is the growing size of high school athletes with more than 60% of linemen, generally the largest players, characterized as being overweight and obese.3 It is well-established that obesity, as classified by BMI criteria, increases the risk for CV disease and death from all causes.4,5 In large muscular athletes, population BMI cut-offs may overestimate body fat.6 Therefore, it is not known whether the CV risks associated with higher BMI in non-athletes are relevant for the majority of professional football players, even after retirement. This study was designed to compare CV risk factors and coronary atherosclerosis among retired NFL players and community controls. Coronary atherosclerosis was assessed non-invasively with computed tomography to measure coronary artery calcium (CAC) - a very sensitive, validated surrogate biomarker for CV disease that can predict risk for events.7-9 The primary hypothesis tested was whether retired NFL players would have more CV risk factors and coronary calcium than controls matched by BMI and age. Two control groups were selected for this study: (1) a multi-ethnic, population-based sample from the Dallas Heart Study; and (2) a physically active, preventive medicine clinic sample from the Aerobics Center Longitudinal Study.
Methods
The Living Heart Foundation is a nonprofit organization conducting health screening for retired NFL players since 2004. The data for this study were obtained from screening visits at Miami, Dallas, Atlanta and San Francisco in 2007. Retired NFL players living around the screening city were invited to participate through direct mailing or local NFL Players Association chapter meetings. The study was approved by a centralized Institutional Review Board organized through the Pennsylvania State University. Players provided informed consent before undergoing screening. Prior to imaging, each participant provided informed consent approved by the local Institutional Review Board.
Participants provided demographic, medical and professional career information through a self-administered health survey. Players categorized as linemen reported playing on the offensive or defensive line. At the screening visit, height, weight, and cuff blood pressure readings using the CardioVision automated cuff system were measured. Two consecutive seated blood pressure readings were obtained and averaged.
The first control group was selected from the Dallas Heart Study (DHS), a probability-based cohort of Dallas County adults, oversampled for African Americans (n = 6,101). Details of the DHS design, characteristics and data collection have been previously described.10 The study protocol was approved by the University of Texas Southwestern Medical Center Institutional Review Board, and all participants provided written informed consent prior to participation in the study. For each retired NFL player, one male participant was randomly selected from those individuals of the same self-identified ethnic group, age ± 2 years and BMI ± 2 kg/m2.
The second control group was selected from a 5322 subset of the 17,967 participants from the Aerobics Center Longitudinal Study who underwent a complete medical history, physical examination and measurement of CAC. The Institutional Review Board at The Cooper Institute approves the study annually, and all participants provided informed consent prior to enrolling in the study. For each retired NFL player, 2 male participants were randomly selected with an age ± 3 years.
Venous blood was collected in standard blood collection tubes containing ethylenediamine-tetraacetic acid for plasma and in serum separator tubes for serum. Samples were centrifuged on site and transferred to Quest Laboratories the same day for analysis. High-sensitive C Reactive Protein assay was performed using the nephelometric method, utilizing latex particles coated with C Reactive Protein monoclonal antibodies [analytical sensitivity of 1.9 nmol/L (0.2 mg/L)]. All other assays were performed by spectrophotometry.
In the DHS, standard serum chemistries were performed.10 Plasma total and lipoprotein cholesterol concentrations were determined using commercial enzymatic reagents as previously described.11 C Reactive Protein was measured using a latex-enhanced immunoturbidimetric method as previously described.12 In the Aerobics Center Longitudinal Study, serum samples were analyzed by automated techniques in a laboratory on site that participates in the Centers for Disease Control and Prevention Lipid Standardization Program.4
Computed tomography imaging was performed within one week of the screening visit. Each site used a modified Agatston protocol, and CAC scores were expressed in Agatston units.13 There was one designated reader at the Dallas site. The majority of the images were read by one reader at the San Francisco, Miami and Atlanta sites. Both the Dallas and San Francisco sites used a GE Lightspeed V Computed Tomography scanner with 2.5mm slices (Dallas, 100 kV and San Francisco, 120kV). In Atlanta, a Siemens Biograph 64 was employed with 3mm slices at 120 kV. In Miami, a Brilliance 64 was used with 2.5 mm slices at 120kV. At each site, a torso phantom (from Image Analysis, Columbia, Ky) was imaged over a calibration phantom using the same protocols.
In the DHS, 3-mm slices were obtained with an Imatron C-150XP Electron Beam Computed Tomography scanner (Imatron Inc., San Bruno, California), as previously described.14 A mean CAC score was calculated if 2 consecutive scans were obtained. A score > 10 was selected as a data-derived threshold to define the presence of detectable calcium to maximize the signal-to-noise ratio and reproducibility.14 For the Aerobics Center Longitudinal Study, images were obtained using a Siemens Evolution Electron Beam Computed Tomography scanner (C-150XP) with 3mm slices and 2mm table increments to allow slice overlap. This overlap increased confidence in a zero score. Reproducibility of the CAC measurements was verified as previously described.8
BMI was defined as weight (in kilograms)/height2 (meters). Waist circumference was measured at the top of the umbilicus in the retired NFL players and Aerobics Center Longitudinal Study controls and 1 cm above the iliac crest in the DHS. Hip circumference was measured at the area of the greater trochanters. Hypertension was defined using a combination of survey information, systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg or the use of antihypertensive medication. In the DHS, the last 3 of 5 sequential blood pressure readings at visit 1 were averaged.15 The Aerobics Center Longitudinal Study definition used any one blood pressure measurement (by auscultatory methods with a mercury sphygmomanometer).4 Diabetes was defined from the survey, a fasting serum glucose concentration ≥ 126 mg/dl or the use of any glucose-lowering medication. Impaired fasting glucose was identified only in individuals without diabetes who had a glucose ≥ 100 mg/dl.16 Hyperlipidemia was defined as calculated low-density lipoprotein cholesterol ≥ 160 mg/dl on a fasting sample, from the medical history or the use of statin medication.17 The National Heart, Lung, and Blood Institute/American Heart Association consensus statement criteria for metabolic syndrome were used with the updated impaired fasting glucose definition.16 Positive smoking status was defined as current or past smoking history. Sedentary activity status was defined as less than 30 min of physical activity three times per week, corresponding to less than 405 MET*min*week in the DHS.
Statistical analyses were performed using SAS Version 9.1, (Cary, NC). Differences in baseline characteristics were analyzed with a t-test for normally distributed variables and Wilcoxon rank sum tests of significance for variables with non-normal distributions. Chi-square analysis was employed for categorical variables and grouping of CAC scores (0, 0-100, 100-400 and over 400). For comparisons of ethnic differences and linemen versus non-linemen group differences, the Cochran-Mantel-Haenszel chi-square statistic and the Breslow-Day test of homogeneity were performed. Multiple linear regression analysis was performed with log-transformed CAC as the dependent variable, and independent variables were selected with a combination of stepwise and known variables that influence CAC score. Logistic regression was also performed with the dependent variable of detectable CAC and the same independent variables. With 200 retired NFL players and 400 matching controls, this study had 95% power (alpha = 0.05) to detect a 15% difference in the prevalence of detectable CAC between groups.
Results
Of the 240 participants who attended the Miami, Dallas, Atlanta and San Francisco Living Heart Foundation screenings, 201 consented to additional CAC imaging. There were only 6 participants who did not self-identify as Caucasian or African-American (2 bi-racial - African-American and American Indian/Alaska native or Hispanic, 1 American Indian/Alaska native, 2 Hispanic and 1 missing). Therefore, we restricted to Caucasians and African-Americans for the DHS analysis (n=195). Matching by ethnicity, age, and BMI, 150 DHS participants were matched with 150 retired NFL players. Forty-one retired NFL players exceeded the upper age limit of the DHS. Three retired players with a BMI > 40 and 1 player with missing BMI could not be matched in the DHS. The second ACLS control group included the entire age range of retired NFL players and excluded only 1 person with a missing BMI value (n=200).
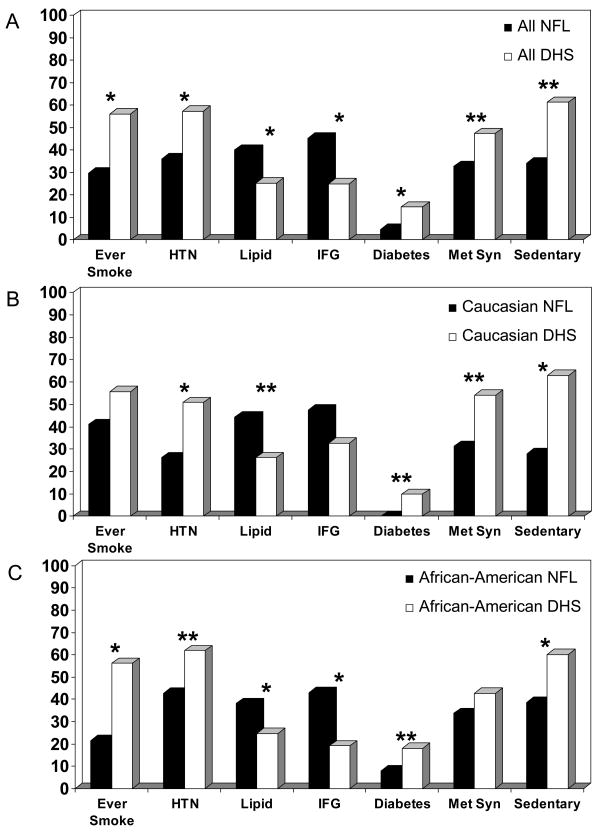
Baseline characteristics are shown in Table 1 for the retired NFL players matched to DHS controls for ethnicity, age and BMI. Retired NFL players had significantly smaller waist size, lower blood pressure, triglycerides, glucose and C Reactive Protein, and higher high density lipoprotein cholesterol than DHS controls. Only the total cholesterol and low density lipoprotein concentrations were higher in the retired NFL players. Looking at the overall profile of CV risk factors (Figure 1A), there was a significantly lower percentage of retired NFL players who had ever smoked, were sedentary, had diabetes, hypertension or the metabolic syndrome. The only CV risk factors that were significantly more prevalent among the retired NFL players were the diagnoses of hyperlipidemia (treated and untreated) and impaired fasting glucose. The greater prevalence of hyperlipidemia could not be attributed to any significant differences in statin use (14.3% retired NFL players and 16.6% among the DHS controls, p=0.60).
Table 1. Baseline characteristics for retired National Football League (NFL) players and Dallas Heart Study (DHS) controls*.
| Characteristic | Retired NFL (n=150) | DHS (n=150) | P value |
|---|---|---|---|
| Age (years) | 51.2 ± 9.7 | 51.1 ± 9.5 | - |
| Time since retirement (years) | 21.8 ± 10.1 | - | |
| African-American | 89 (59.3 %) | 89 (59.3 %) | - |
| Linemen | 44 (29.3 %) | - | - |
| Weight (kg) | 111.3 ± 18.4 | 96.3 ± 14.5 | < 0.001 |
| Height (cm) | 187.4 ± 7.1 | 175.1 ± 7.6 | < 0.001 |
| Body mass index (kg/m2) | 31.5 ± 4.2 | 31.4 ± 4.0 | - |
| Waist (cm) | 103.8 ± 11.5 | 107.4 ± 10.9 | 0.007 |
| Waist/hip ratio | 1.08 ± 0.85 | 0.98 ± 0.05 | < 0.001 |
| Systolic blood pressure (mmHg) | 127.6 ± 16.7 | 135.6 ± 17.0 | < 0.001 |
| Diastolic blood pressure, (mmHg) | 77.3 ± 11.2 | 82.5 ± 10.4 | < 0.001 |
| Total cholesterol (mg/dL)‡ | 197.8 ± 42.1 | 176.8 ± 40.1 | < 0.001 |
| Low density lipoprotein (mg/dL)‡ | 128.5 ± 36.0 | 107.7 ± 37.5 | < 0.001 |
| High density lipoprotein (mg/dL)‡ | 50.8 ± 16.8 | 43.7 ± 10.9 | < 0.001 |
| Triglyceride (mg/dL)† ‡ | 81 [61, 115] | 111 [74, 160] | < 0.001 |
| Glucose (mg/dL)‡ | 101.4 ± 14.1 | 110.4 ± 47.7 | 0.03 |
| High sensitive C Reactive Protein (mg/L) † ‡ | 0.8 [0.4, 1.8] | 2.4 [1.4, 5.2] | < 0.001 |
presented as means ± SD, except where otherwise noted
presented as median [interquartile range]
SI conversion factors: To convert low-density and high-density lipoprotein and total cholesterol to mmol/L, multiply by 0.0259. To convert triglycerides to mmol/L, multiply by 0.0113. To convert glucose to mmol/L, multiply by 0.0555. To convert C Reactive Protein to nmol/L, multiply by 9.524.
Figure 1.
CV risk factors among A) all NFL v. DHS; B) Caucasians v matched DHS controls; C) African-Americans v. matched DHS controls. HTN: hypertension; Lipid: hyperlipidemia; IFG: impaired fasting glucose; Met Syn: metabolic syndrome. *p < 0.01 **p < 0.05.
Stratified by ethnic group, the differences in CV risk factor profiles between retired NFL players and their DHS controls were similar in Caucasians and African-Americans (Figure 1B and 1C). When stratified by former linemen status, differences in CV risk factor profiles between former linemen and their matched controls and non-linemen and their controls were similar with the exception of the prevalence of hypertension (Breslow-Day test for homogeneity, p = 0.03; chi-square, p=0.83).
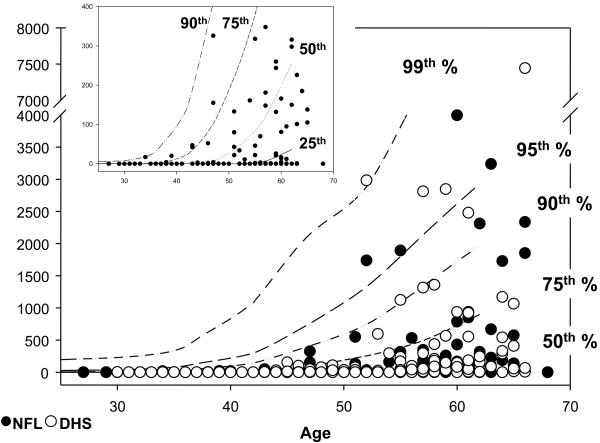
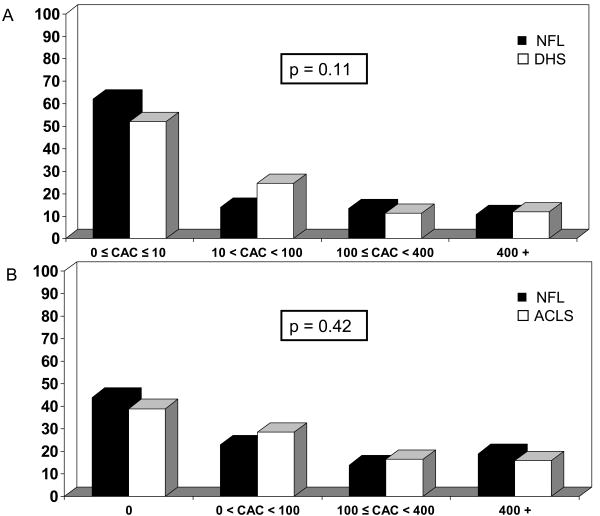
The distribution of individual CAC scores among the retired NFL players and DHS controls are plotted against percentiles for age derived from the Aerobics Center Longitudinal Study database (Figure 2). The majority of the retired NFL players (81.0%) had CAC scores below 400, with more than 2/3 below the 50th percentile for their age group (Figure 2 inset). Interestingly, despite the lower prevalence of many CV risk factors, there was no difference in the prevalence of detectable CAC between the retired NFL players and DHS controls (46% and 48.3% respectively, p=0.69). Based on previous studies evaluating the use of CAC score ranges to predict CV events, CAC score ranges of 0-10, 10-100 and 100-400 and over 400 were compared between groups (Figure 3A).8,9 There was no statistically significant difference across these CAC score ranges (Figure 3A).
Figure 2.
Distribution of CAC scores among retired NFL players (●) and DHS controls (○) along age-derived percentiles from the ACLS database. Inset: Distribution of retired NFL player CAC scores < 400 show the majority below the 50th percentile.
Figure 3.
Distribution of CAC scores among the retired NFL players and (A) DHS controls; (B) ACLS controls. A CAC cut-off of 10 represents the data-derived definition of a negative score in the DHS comparison.
Controlling for ethnicity, there was no difference in detectable CAC between retired NFL players and DHS controls (Caucasians: 67.2% NFL v. 57.4% DHS; African-Americans: 31.5% NFL v. 42.1% DHS; Breslow-Day test for homogeneity, p = 0.07; Cochran-Mantel-Haenszel statistic, p=0.69). Controlling for lineman position, the result was also the same (former linemen detectable CAC, 40% and non-linemen detectable CAC, 48.6%, Breslow-Day Test, p = 0.79, Cochran-Mantel-Haenszel statistic, p=0.68).
To compare the entire age range of retired NFL players to physically-active community controls of a similar socioeconomic demographic, a second control group from the Aerobics Center Longitudinal Study database was obtained. Baseline characteristics are shown in Table 2. When matched on age only, BMI and all other measurements of body size were significantly greater in the retired NFL players. Conversely, diastolic blood pressure, total cholesterol and triglycerides were lower in retired NFL players than the controls, and there was no difference in the proportion of smokers or individuals who were sedentary, had diabetes, hypertension, hyperlipidemia or impaired fasting glucose. Only the prevalence of the metabolic syndrome was greater among retired NFL players compared to Aerobics Center Longitudinal Study controls (39.5% v. 23.0%, p < 0.001). Again, there was no difference in the prevalence of detectable CAC (56.0% NFL v. 61.0% ACLS, p = 0.24) or the distribution of CAC scores of the retired NFL players compared to this more active control group (Figure 3B).
Table 2. Baseline characteristics for retired National Football League (NFL) players and Aerobics Center Longitudinal Study (ACLS) controls*.
| Characteristic | Retired NFL (n=200) | ACLS (n=400) | P Value |
|---|---|---|---|
| Age (years) | 55.2 ± 11.9 | 52.5 ± 11.9 | 0.01 |
| Time since retirement (years) | 24.8 ± 12.2 | - | - |
| Linemen | 115 (57.5 %) | - | - |
| Body mass index (kg/m2) | 31.7 ± 4.7 | 28.6 ± 3.1 | < 0.001 |
| Weight (kg) | 110.6 ± 19.6 | 94.6 ± 14.7 | < 0.001 |
| Height (cm) | 186.2 ± 6.9 | 179.8 ± 7.4 | < 0.001 |
| Waist (cm) | 105.7 ± 12.7 | 98.4 ± 8.9 | < 0.001 |
| Waist/hip ratio | 1.06 ± 0.73 | 0.93 ± 0.05 | < 0.001 |
| Systolic blood pressure (mmHg) | 129.2 ± 17.0 | 129.0 ± 16.0 | 0.70 |
| Diastolic blood pressure (mmHg) | 77.5 ± 11.1 | 85.0 ± 9.8 | < 0.001 |
| Total cholesterol, (mg/dL)‡ | 192.9 ± 41.9 | 204.0 ± 41.6 | < 0.001 |
| Low density lipoprotein, (mg/dL)‡ | 126 ± 36.2 | 124.7 ± 37.2 | 0.37 |
| High density lipoprotein, (mg/dL)‡ | 49.4 ± 17.0 | 46.4 ± 11.5 | 0.35 |
| Triglyceride, (mg/dL)† ‡ | 83.5 [61, 122] | 127.5 [92, 177] | < 0.001 |
| Glucose, (mg/dL)‡ | 102.3 ± 17.0 | 104.5 ± 20.5 | 0.08 |
| High sensitive C Reactive Protein, (mg/L) † ‡ | 4.2 [0,196.5] | 11.0 [0, 186] | 0.69 |
presented as means ± SD, except where otherwise noted
presented as median [interquartile range]
SI conversion factors: To convert low-density and high-density lipoprotein and total cholesterol
To evaluate predictors of CAC among the retired NFL players, multivariable linear and logistic regression models were evaluated using log-transformed CAC and detectable CAC, respectively. Adjusted for study site and BMI, age and hyperlipidemia were the most significant predictors of CAC (Table 3).
Table 3. Multivariable logistic regression model of positive (>0) coronary artery calcium score in retired National Football League Players.
| Variables* | Odds Ratio (95% Confidence Intervals) | P value |
|---|---|---|
| Age | 1.12 (1.08, 1.17) | <.001 |
| Hyperlipidemia | 1.40 (1.06, 1.84) | 0.02 |
| Body Mass Index | 1.02 (0.94, 1.10) | 0.69 |
Model also adjusted for study site to mmol/L, multiply by 0.0259. To convert triglycerides to mmol/L, multiply by 0.0113. To convert glucose to mmol/L, multiply by 0.0555. To convert C Reactive Protein to nmol/L, multiply by 9.524.
Discussion
Compared to age and BMI-matched population-based controls, retired NFL players had significantly fewer CV risk factors. However, there was no difference in the presence or extent of coronary atherosclerosis, as measured by CAC, when matching the retired NFL players to either sedentary or physically active control groups. Retired NFL players do not have significantly greater or lesser amounts of coronary atherosclerosis than expected for their age. Nevertheless, the higher prevalence of hyperlipidemia and impaired fasting glucose in the younger retired NFL players demonstrates the need for early CV risk factor screening and maintenance of a physically active lifestyle.
This is the first study to systematically evaluate a measurement of coronary atherosclerosis in retired NFL players compared to matched controls. Importantly, there was no difference in the amount of detectable CAC or the distribution among clinically meaningful ranges of CAC scores (0, 0-100, 100-400, 400+) among retired NFL players and age-matched controls, even when the retired players had a greater BMI than the Aerobics Center Longitudinal Study controls. Furthermore, it is reassuring that 76 % of the retired NFL players fell below a CAC score of 100. CAC scores above 100 predict a risk for CV events several fold higher than a score below 100, above and beyond Framingham scores and the number of CV risk factors.9 Therefore, although previous reports have raised concern about the large body size of football players, retired NFL players do not have a greater prevalence of CV risk factors or an excessive burden of detectable coronary atherosclerosis than attributable to their age.
This study is consistent with the overall findings of the National Institute of Occupational Safety and Health that former NFL players had a lower mortality rate than the general population. While their subgroup data suggested that overall CV disease risk may be 50% higher in former linemen, this risk is not apparent in our study based on CAC scores or CV risk factor burden.18 This analysis was not powered to detect smaller (less than 10%) differences in CV risk factors or CAC prevalence among linemen versus non-lineman. This study is an important follow-up to initial reports estimating a higher prevalence of the metabolic syndrome among linemen.19 The previous study could not assess whether linemen have a higher prevalence of the metabolic syndrome compared to a control group of similar body size. When matched on BMI, retired NFL players had a significantly lower prevalence of metabolic syndrome than population-based controls. It is even more striking that matched by BMI, retired NFL players were taller and heavier, yet still did not necessarily have higher blood pressure or more CAC. When matched by age only with ACLS controls, greater body size among the retired players corresponded to a higher prevalence of metabolic syndrome but no other difference among the individual CV risk factors or the amount of coronary calcium. Therefore, the metabolic syndrome categorization can overestimate CV risk in larger former professional athletes who may meet the larger waist or BMI criteria without necessarily having a higher prevalence of CV risk factors or coronary atherosclerosis. In these retired NFL players as in other studies,20,21 the metabolic syndrome per se does not necessarily independently predict CV disease or add information beyond traditional CV risk factor assessment.
The observation that certain metabolic risks - hyperlipidemia and impaired fasting glucose - were greater in retired NFL players suggests that size does matter, particularly in the younger retired players. Even former elite athletes are susceptible to the associated metabolic risks of greater mass and accompanying fat. The prevalence of impaired fasting glucose was almost 3 fold > the NHANES estimates of 6 to 14% in the US or even twice what was observed in the DHS. This finding is in agreement with previous observations that BMI may still trump fitness in terms of risk for diabetes, especially at relatively younger ages.22 Interestingly, in contrast, the prevalence of diabetes was lower than population estimates of 8% or the DHS control group. While the development of metabolic risks associated with large body size may not be avoidable, fitness or physical activity may at least slow the progression of impaired fasting glucose to diabetes 23,24. Furthermore, with no difference in the extent of CAC, this study supports the concept that activity and/or fitness can attenuate the CV risks that accompany large body size.4, 25
Ultimately, BMI was not a significant predictor of coronary calcium in the retired NFL players. The most significant predictors of coronary atherosclerosis were the traditional risk factors of age and hyperlipidemia. Other studies have also found inconsistent associations of BMI with coronary atherosclerosis and/or events.26 Especially at larger BMI, other measurements of body size may serve as better predictors. In the DHS, waist-hip-ratio provided better discriminatory value for increasing prevalent CAC than BMI.27
The issue of different CT scanners at multiple sites has been previously addressed by large multisite cohorts. Despite significant variability among their scanners, adjusted scores obtained from calibration of all images to a phantom standard were highly correlated to unadjusted CAC scores, with only 84 of 9533 (0.9%) participants moving from a 0 to non-zero score and only 3.5% moving between clinical Agatston score categories after calibration.28 Therefore, site variability in this study is not likely to have affected categorization of negative or positive scans or score groupings. Variations in CAC scores were more significantly attributed to individual factors of age and hyperlipidemia than site factors (Table 3).
One of the strengths of this study is the ability to compare the retired NFL players to the DHS cohort which has a similar representation of African-Americans. Ethnic differences in lipids have been previously described29 and might explain the lower cholesterol among retired NFL players versus the Caucasian sample of Aerobic Center Longitudinal Study. This difference in lipids was not seen in the ethnically-matched DHS analysis. Controlling for ethnicity in the NFL-DHS analysis, there was no statistically significant difference in detectable CAC.
Since the retired NFL players presented for voluntary health screening, there may have existed a selection bias in either direction. It is also possible that the amount of coronary atherosclerosis may underestimate or overestimate the risk for future CV events in retired NFL players. Ongoing follow-up of the LHF cohort will be necessary to determine the correlation of these findings with risk for CV events. Whether the younger generation of retired players or current players may be at greater risk for CV events or mortality merits further study, especially given the larger body sizes now required to be competitive and the greater metabolic risks observed in the younger players.
Acknowledgments
Funding Sources: This work was supported by the Living Heart Foundation and educational grants from the National Football League Players Association, the National Football League Alliance, the Donald W. Reynolds Cardiovascular Clinical Research Center at Dallas, Texas, NIH grants AG06945 and HL62508, the Communities Foundation of Texas, on the recommendation of Nancy Ann and Ray L. Hunt, the S. Finley Ewing Jr. Chair for Wellness at Presbyterian Hospital of Dallas, Texas, the Harry S. Moss Heart Foundation, NIH grant UL1RR024982, titled, “North and Central Texas Clinical and Translational Science Initiative” from the National Center for Research Resource, and the American Heart Association (Dallas, Tx).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AP. Coroner says Herrion died of heart disease USA Today. Denver: Gannett Company; 2005. [Google Scholar]
- 2.Harp JB, Hecht L. Obesity in the National Football League. JAMA. 2005;293:1061–1062. doi: 10.1001/jama.293.9.1061-b. [DOI] [PubMed] [Google Scholar]
- 3.Laurson KR, Eisenmann JC. Prevalence of overweight among high school football linemen. JAMA. 2007;297:363–364. doi: 10.1001/jama.297.4.363. [DOI] [PubMed] [Google Scholar]
- 4.Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS, Jr, Blair SN. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PWF, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and Obesity as Determinants of Cardiovascular Risk: The Framingham Experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 6.Ode JJ, Pivarnik JM, Reeves MJ, Knous JL. Body mass index as a predictor of percent fat in college athletes and nonathletes. Med Sci Sports Exerc. 2007;39:403–409. doi: 10.1249/01.mss.0000247008.19127.3e. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Jr, Stein JH, Tracy CM, Vogel RA, Wesley DJ. ACCF/AHA 2007 Clinical Expert Consensus Document on Coronary Artery Calcium Scoring By Computed Tomography in Global Cardiovascular Risk Assessment and in Evaluation of Patients With Chest Pain: A Report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Developed in Collaboration With the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YJ, Church TS, Kimball TE, Nichaman MZ, Levine BD, McGuire DK, Blair SN. Comparison of coronary artery calcium detected by electron beam tomography in patients with to those without symptomatic coronary heart disease. Am J Cardiol. 2003;92:498–503. doi: 10.1016/s0002-9149(03)00714-8. [DOI] [PubMed] [Google Scholar]
- 9.Church TS, Levine BD, McGuire DK, Lamonte MJ, Fitzgerald SJ, Cheng YJ, Kimball TE, Blair SN, Gibbons LW, Nichaman MZ. Coronary artery calcium score, risk factors, and incident coronary heart disease events. Atherosclerosis. 2007;190:224–231. doi: 10.1016/j.atherosclerosis.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. The American Journal of Cardiology. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 11.Mostaza JM, Schulz I, Vega GL, Grundy SM. Comparison of Pravastatin With Crystalline Nicotinic Acid Monotherapy in Treatment of Combined Hyperlipidemia. The American Journal of Cardiology. 1997;79:1298–1301. doi: 10.1016/s0002-9149(97)00109-4. [DOI] [PubMed] [Google Scholar]
- 12.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Jr, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Jain T, Peshock R, McGuire DK, Willett D, Yu Z, Vega GL, Guerra R, Hobbs HH, Grundy SM. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 15.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 17.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 18.Baron S, Rinsky R. In: NIOSH Mortality Study of NFL Football Players: 1959-1988. Center for Disease Control NIfOSaH, editor. Cincinnati: 1994. [Google Scholar]
- 19.Miller MA, Croft LB, Belanger AR, Romero-Corral A, Somers VK, Roberts AJ, Goldman ME. Prevalence of metabolic syndrome in retired national football league players. Am J Cardiol. 2008;101:1281–1284. doi: 10.1016/j.amjcard.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, Ford I, Forouhi NG, Freeman DJ, Jukema JW, Lennon L, Macfarlane PW, Murphy MB, Packard CJ, Stott DJ, Westendorp RG, Whincup PH, Shepherd J, Wannamethee SG. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927–1935. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- 21.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein AR, Sesso HD, Lee IM, Cook NR, Manson JE, Buring JE, Gaziano JM. Relationship of Physical Activity vs Body Mass Index With Type 2 Diabetes in Women. JAMA. 2004;292:1188–1194. doi: 10.1001/jama.292.10.1188. [DOI] [PubMed] [Google Scholar]
- 23.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The Association between Cardiorespiratory Fitness and Impaired Fasting Glucose and Type 2 Diabetes Mellitus in Men. Ann Intern Med. 1999;130:89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [DOI] [PubMed] [Google Scholar]
- 24.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical Activity of Moderate Intensity and Risk of Type 2 Diabetes: A systematic review. Diabetes Care. 2007;30:744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 25.Blair SN, Church TS. The Fitness, Obesity, and Health Equation: Is Physical Activity the Common Denominator? JAMA. 2004;292:1232–1234. doi: 10.1001/jama.292.10.1232. [DOI] [PubMed] [Google Scholar]
- 26.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 27.See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The Association of Differing Measures of Overweight and Obesity With Prevalent Atherosclerosis: The Dallas Heart Study. Journal of the American College of Cardiology. 2007;50:752–759. doi: 10.1016/j.jacc.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JC, Kronmal RA, Carr JJ, McNitt-Gray MF, Wong ND, Loria CM, Goldin JG, Williams OD, Detrano R. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005;235:403–414. doi: 10.1148/radiol.2352040515. [DOI] [PubMed] [Google Scholar]
- 29.Donahue RP, Jacobs DR, Jr, Sidney S, Wagenknecht LE, Albers JJ, Hulley SB. Distribution of lipoproteins and apolipoproteins in young adults The CARDIA Study. Arteriosclerosis. 1989;9:656–664. doi: 10.1161/01.atv.9.5.656. [DOI] [PubMed] [Google Scholar]