Abstract
Background:
Non-alcoholic steatohepatitis (NASH) is a clinicopathological entity that is being recognized more frequently in recent years. This study aimed to evaluate the effects of Metformin, with and without a probiotic supplement on liver aminotransferases in patients with NASH.
Methods:
Sixty four patients 18-75 years with NASH confirmed by biopsy and histological assessment were enrolled to study. Patients were randomized to one of the following treatments for 6 months: Group I, probiotic (Protexin two tablets per day) plus Metformin 500 mg two tablets per day (Met/Pro), or group II, Metformin 500 mg two tablets per day plus two placebo tablet (Met/P). After 6 month alanine aminotransferase (ALT), aspartate aminotransferase, and ultrasound grading of NASH were assessed.
Results:
In group I, serum alanine aminotransferase (ALT: 133.7 ± 70 vs. 45.2 ± 32.5; P < 0.00), and aspartate aminotransferase activity (AST: 123.1 ± 72 vs. 44.2 ± 33.9; P < 0.001), and ultrasound grading of NASH (P < 0.001) all decreased significantly by the end of the treatment period. In group II, while serum alanine aminotransferase (ALT) was not significantly reduced (118.4 ± 67.9 vs. 112.5 ± 68.7; P < 0.064), aspartate aminotransferase activity (AST: 125.3 ± 71 vs. 113.4 ± 71; P < 0.001), and ultrasound grading of NASH did fall significantly (P < 0.01). Body mass index (BMI), fasting blood sugar (FBS), cholesterol, and triglyceride fell significantly in both groups.
Conclusions:
Probiotic combination with Metformin improves liver aminotransferases better than metformin alone in patients with NASH.
Keywords: Aminotransferase, insulin resistance, nonalcoholic steatohepatitis, non-alcoholic fatty liver disease, probiotic, randomized clinical trial
INTRODUCTION
Non-alcoholic steatohepatitis (NASH) is a progressive form of non-alcoholic fatty liver disease (NAFLD). NASH is characterized by hepatic steatosis and periportal and lobular inflammation.[1] In developed countries, it is estimated that 20-30% of the population has NAFLD;[2,3] whereas, the prevalence of NASH in the same population is estimated at 2-3%.[3,4] The growing incidence of NAFLD, and subsequently NASH, will almost certainly be reflected in future increases in cirrhosis and hepatocellular carcinoma.[5]
There is no proven effective therapy for NASH; modification of risk factors, such as obesity, hyperlipidemia, and strict diabetic control is, however, generally recommended. In developing countries, the trend for an increasing prevalence of obesity, diabetes, and metabolic syndrome are likely to lead to an increased prevalence of chronic liver disease.[6,7]
Probiotics are live microbes that can be found in many different types of product, including foods, drugs, and dietary supplements. Probiotics suppression of growth pathogenic bacterial by modulation of immune system.[7]
Probiotics may play an important role in treatment of NASH, as the bacterial overgrowth increase of pro-inflammatory cytokines that might lead to NASH by altering small intestinal permeability and, thereby, increasing absorption of endotoxin that induced liver damage.[8] Patients with non-alcoholic steatohepatitis have a higher prevalence of small intestinal bacterial overgrowth.[9] Several observations have suggested that small intestinal bacterial overgrowth (SIBO) may play a role in the pathogenesis of NASH.[10,11] The main benefits of probiotics might occur through preventing the production and/or absorption of lipopolysaccharides in the gut, and, therefore, reducing of inflammation and improvement histological finding of the liver parenchyma.[8]
Mechanisms for the benefits of probiotic are incompletely understood. However, this might be of major importance in modulation of immune system, suppression of pathogenic bacterial growth.[12]
The hypothesis we wished to test was whether metformin with and without a probiotic supplement would improve liver aminotransferases and ultrasound appearance of patients with NASH.
METHODS
Patients between 18 and 75 years of age, attending the Hepatology clinic of Al Zahra University Hospital (A central and referral hospital in Isfahan, Iran) with NASH were recruited to this randomized, double blind study between December 2010 and April 2012. All patients gave written informed consent and the study was approved by Ethics Committee of Medical University of Isfahan, Iran.
NASH was identified in patients by a persistent elevation of serum alanine aminotransferase (ALT) levels of at least 1.5 times the upper limit of normal for at least 6 months, in the presence of a weekly alcohol consumption of <20 g in men and <10 g in women, and NASH was confirmed histologically following a liver biopsy (METAVIR score-at least grade 1). All of the liver biopsies were read by a single pathologist.
Exclusion criteria included: Autoimmune hepatitis, Wilson's disease, metabolic liver disease, cholestatic liver disease: Primary biliary cirrhosis/primary sclerosing cholangiatis (PSC), insulin dependent diabetes mellitus, pregnancy or lactation, impaired renal function (serum creatinine > 1.5 mg/dL), heart failure, hepatocellular carcinoma, treatment with drugs associated with fatty liver (methotrexate, amiodarone, tamoxifen, valproate), the requirement to take antibiotics more than 1 week during the study period or before recruitment to the study, weight loss of greater than 10% of baseline body weight during the study period.
Study design
Based on the primary outcome, a power calculation indicated that 32 patients were required in each group (power 80% and α = 5%). To allow for a 15% drop-out rate, we aimed to enroll 70 patients.
Randomization was stratified according to age (18-40, 40-60, ≥60) and gender (male, female).
Group I were treated with Metformin 500 mg (Arya Pharmaceutical Co, Tehran - Iran) two tablets per day plus Protexin two tablet per day and group II were treated with Metformin 500 mg two tablets per day plus two placebo tablets (120 mg of starch) per day for the duration of 6 months.
The probiotic supplement, Protexin (made by Science and nature in balance Co, UK) contained: Lactobacillus acidophilus 1 × 108 CFU, Lactobacillus casei 5 × 108 CFU, Lactobacillus rhamnosus 7.5 × 107 CFU, Lactobacillus bulgaricus 1.5 × 108 CFU, Bifidobacterium breve 5 × 107 CFU, Bifidobacterium longum 2.5 × 107 CFU, Streptococcus thermophilus 5 × 107 CFU, fructooligosaccharides 350 mg.
Placebo tablets were similar in shape and appearance as Protexin, and were prepared by the School of Pharmacy (Isfahan, Iran).
All patients were advised to have a low-fat diet, regular exercise and to continue with their diet and exercise during the study period, but no other specific dietary measures were instituted.
For ruling out any other chronic liver disease and the rest of the baseline data, additional laboratory tests were conducted, including hepatitis B surface antigen (Hbs Ag), hepatitis C antibody (HCV), hepatitis A antibody (HAV), anti nuclear antibody (ANA), anti-mitochondrial antibody, anti smooth muscle antibody (ASMA), anti liver-kidney microsome antibody (LKM), anti-endomysial antibody, albumin, globulin, protein electrophoresis, cell blood count (CBC), fasting blood sugar (FBS), total cholesterol, triglycerides, low-density cholesterol (LDL-C) and high-density cholesterol (HDL-C), serum ceruloplasmin, and α1AT phenotype.
Height and weight of patients before and after the intervention were measured and recorded. Before and after the intervention period, ultrasound was performed to determine NASH grade by a single radiologist using the National Health and Nutrition Examination Survey (NHANES) III criteria: Grade 0 normal, grade 1 mild, grade 2 moderate, grade 3 severe.
The radiological evaluation of hepatic steatosis was assessed using five main criteria: Parenchymal brightness, liver to kidney contrast, deep beam attenuation, bright vessel walls, and gallbladder wall definition. Based on the presence or absence of these five criteria, a main finding was recorded.
The primary outcome measures were a decrease in ALT, aspartate aminotransferase (AST), and ultrasound grade of NASH. Secondary outcome were decreased BMI (body mass Index), FBS, triglyceride, and cholesterol. Normal values for aminotransferases in serum were <30 U/L for men and <19 U/L for women and treatment response were ALT and AST levels below two times the upper limit.
Statistical analysis
Data analysis was performed by Statistical Package for the Social Sciences (SPSS) 18 software. P < 0.05 considered significant. Chi- square was used for comparison of quantitative variables. Paired t-test was used for comparison median of qualitative variables before and after treatment in each group. Independent t-test as used for comparison median of qualitative variables between two groups. Wilcoxon was used for comparison of rating variable before and after treatment in each group. Mann-Whitney was used for comparison of rating variable between two groups.
RESULT
Figure 1 shows a flow chart of patients who entered the study. Of the 70 patients randomized (36 in the Metformin/placebo group and 34 in the Metformin/probiotic group), 63 completed the study. Table 1 shows baseline patient characteristic. There was no significant difference between two groups regarding age, sex, and BMI at baseline. In the Metformin/probiotic group, patients (15 men and 17 women) the mean age was 41.5 ± 12.7 years and the Metformin/placebo (17 men and 14 women) had a mean age of 38.7 ± 11.9 years. Means of ALT, AST were similar in the two groups at baseline [Table 1]. Baseline liver ultrasound was also similar for the two groups. The mean serum level cholesterol and triglyceride were not significantly difference between two groups.
Figure 1.
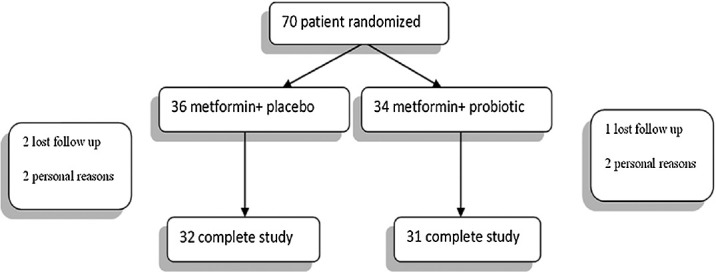
Participation flow chart
Table 1.
Effect of 6 months treatment on aminotransferase
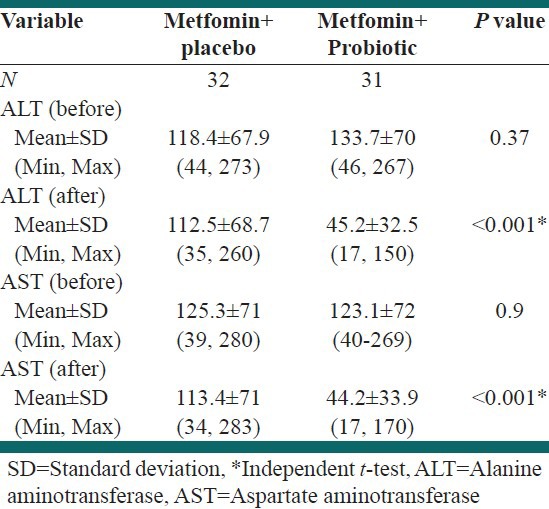
There was a significant decrease in mean serum ALT levels in Metformin/probiotic (45.2 ± 32.5; P < 0.001) than in Metformin/placebo (112.5 ± 68.7). The mean AST reduction was also significant in Metformin/Probiotic compared with Metformin/placebo (44.2 ± 33.9 vs. 113.4 ± 71; P < 0.001) [Table 1].
Figures 2 and 3 show the proportion of patients with normal liver ultrasound at 6 month, 38.7% patients treated with Metformin/Probiotic had normal grade of ultrasound compared with 6.3% of patients that received Metformin/placebo. The ultrasound grade NASH significantly decreased in the Metformin/Probiotic group (P < 0.001). Wilcoxon ranks test showed ultrasound grade NASH was reduced in both groups after the 6 month treatment (P < 0.01).
Figure 2.
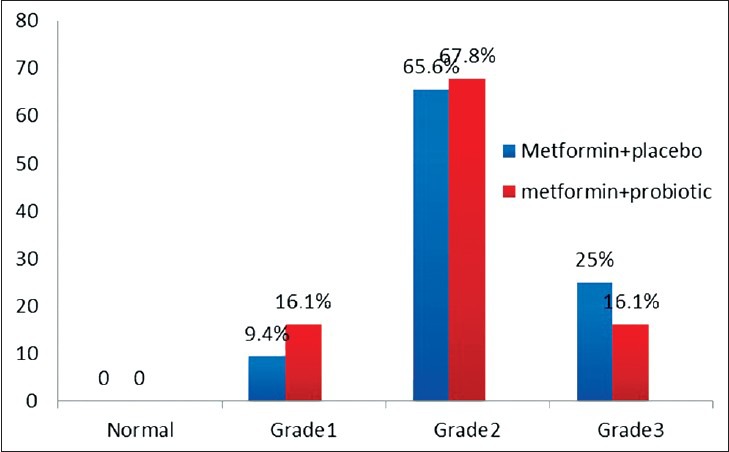
Ultrasound grade steatosis before intervention (M0). None of patients had normal grade ultrasound
Figure 3.
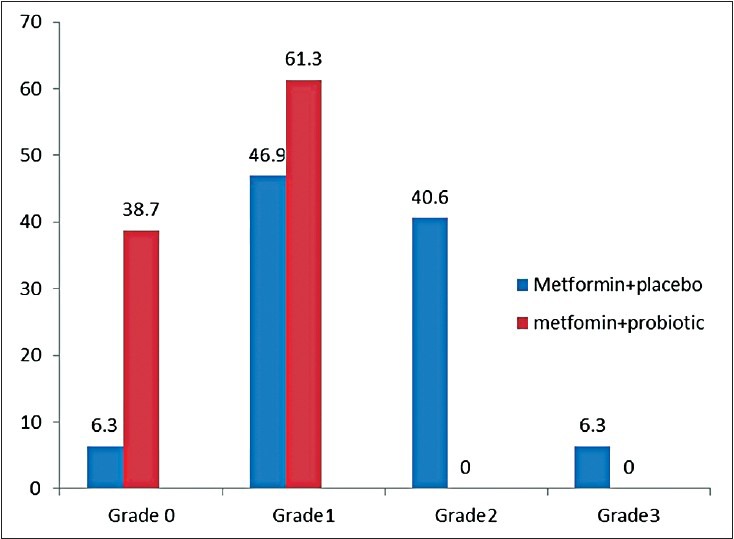
Ultrasound grade steatosis after six month treatment.38.7% patients had normal grade ultrasound in metformin/probiotic group and 61.3% patients had grade 1 and none of patient had grade 2, 3. Wilcoxon signed ranks test showed significant decrease in ultrasound grade steatosis (P = 0.01) in groups
Paired samples test showed that mean ALT and AST in Metformin/Probiotic group were lower than at baseline (P < 0.001, P < 0.001). ALT and AST values in the Metformin/placebo group were also lower than at baseline but this did not attain statistical significance (P = 0.067, P = 0.064).
Table 2 shows that the mean BMI decreased significantly in the Metformin/Probiotic (P < 0.001) vs. Metformin/placebo. Assessments of total cholesterol and triglyceride after 6 month of treatment with Metformin/Probiotic demonstrated a significant decrease in these patients (P = 0.01, P = 0.02). There was no significant difference between groups in mean FBS [Table 2].
Table 2.
Effect of 6 months treatment on clinical and biochemical parameters
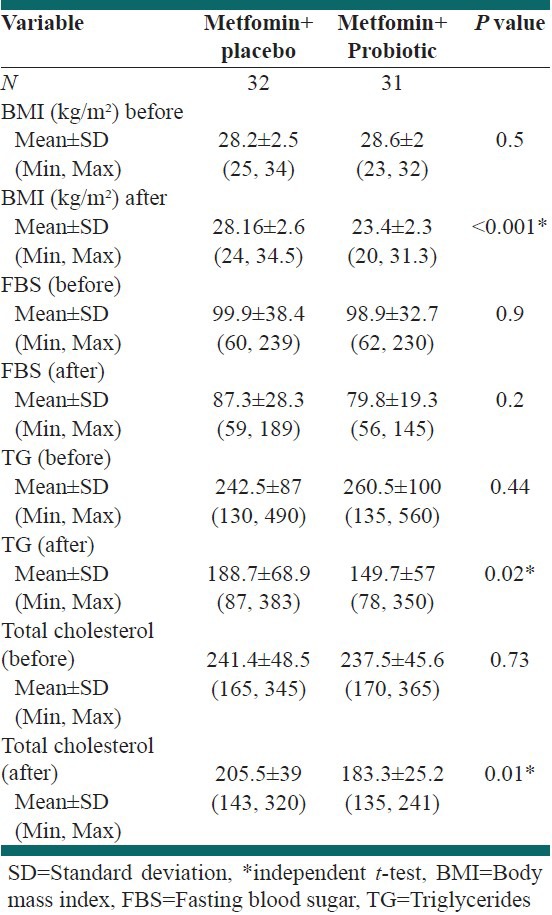
The results showed mean of BMI decrease in two groups after 6 month treatment (Metformin/placebo P = 0.02, Metformin/Probiotic P < 0.001) and decrease mean of FBS, total cholesterol and triglyceride (P < 0.001 bout groups).
There were −60.43% reduction mean of ALT and −64.9% reduction of AST in Metformin/Probiotic group, 6 months after treatment whiles there were only 11.44% reduction of ALT and 8.61% reduction of AST in Metformin/placebo group, 6 months after treatment.
Mean FBS, TG, total cholesterol change relative to baseline were −19.86%, −39.8%, −23.64% at 6 month in Metformin/Probiotic group compared with −12.13%, −25.89%, −23.64% respectively, in the Metformin/placebo group.
Multivariate analysis showed no relationship between increasing age with high baseline ALT, AST, and increase ultrasound grade. Furthermore, there was no significant relationship between gender and high baseline ALT, AST, and increase ultrasound grade were treatment after 6 months in groups.
There were no death and important complication during the study. Flatulence was reported to occur more frequently in Metformin/Probiotic than in the Metformin/placebo group.
DISCUSSION
The combination of probiotic and Metformin significantly reduced ALT, AST, and ultrasound grade in patients with histologically proven NASH in this randomized double-blind clinical trial. ALT and AST levels were normal in 19.3%, 16.1% Metformin/Probiotic-treated patients (<30 U/L for men and <19 U/L for women) and treatment response in 45% (below two times the upper limit). None of the patients in the Metformin/placebo group had normal ALT and AST levels and treatment response was observed in in 21.8% and 9.3%, respectively.
Probiotics has been shown in animal studies to reduce methionine-choline deficient diet (MCDD)-induced steatohepatitis in rats[13] and treatment with vs. L#3 or anti-TNF antibodies improved NAFLD histology and reduced serum ALT levels in mice.[14] Carmela et al., have shown that the Probiotic vs. L#3 improved cytokine concentrations (TNF-α, IL-6, and IL-10) in alcoholic liver cirrhosis patients.[15] Aller et al., showed that 3 month treatment with a tablet containing 500 million of Lactobacillus bulgaricus and Streptococcus thermophilus in patients with NAFLD was associated with alanine aminotransferase, aspartate aminotransferase, and gamma glutamine transferase improvement but anthropometric parameters remained unchanged.[16]
Data about the efficacy of probiotic treatment in NASH are limited. Wong et al., showed that hepatic triglyceride content significant decreased in the probiotics group vs. usual care group and ALT and AST levels decreased but this was not statistically significant. In the latter study, the sample size was small (10 person in usual care group, 14 person in probiotic group).[17] In this study the aminotransferase levels significantly decreased probiotic/Metformin group and after six month treatment, 38.17% of patient had normal grade ultrasound.
It has been reported that Metformin treatment can improve aminotransferase levels and also decreased glucose, BMI, and cholesterol.[18] In a 12 month study of Metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease, Bugianesi et al., reported that Metformin improved the aminotransferase levels to a greater degree than other groups.[19] This drug reduced mean transaminase concentrations, insulin resistance, and liver volume.
Haukeland et al., showed no significant differences between treatment with metformin or placebo were observed for changes in liver steatosis or on markers of insulin resistance or inflammation. In contrast, beneficial effects of metformin were observed on changes in body-weight (P < 0.001), serum levels of cholesterol (P = 0.004), LDL-cholesterol (P < 0.001), glucose (P = 0.032), and on HbA1c (P = 0.020).[20] Pioglitazone therapy over a 12-month period in non-diabetic patients with NASH resulted in improvements in metabolic and histologic parameters, most notably liver injury and fibrosis.[21] Our study showed Metformin alone decreased aminotransferase levels but not significant, whereas, BMI, FBS, triglyceride, and cholesterol significantly decreased.
An important finding of this study was the significant decrease in BMI, triglyceride and cholesterol in the Metformin/Probiotic compared to the Metformin/placebo group. Previous smaller study testing 3 months treatment with probiotic did not show a fall in BMI, triglyceride and cholesterol.[16]
There were no relationships between increasing age with high baseline ALT, AST, and increase ultrasound grade in current study. Rogha et al., showed significantly correlation between ALT level and age.[22] Sample size of current study was less than Rogha study (64 vs. 2030). In this study, unlike previous studies, ultrasound was used for follow-up treatment after 6 months.
CONCLUSIONS
Probiotic decrease liver aminotransferases level in patients with NASH and improve liver function. The benefits reported with probiotic in this study support the start of larger studies with histological end point in patients with NASH.
ACKNOWLEDGMENT
The authors wish to thank the following practitioner and other staff involved in this study; Professor Peyman Adibi, Vice Chancellery for Research Isfahan University of medical science, Akram Assali and Akbar Hassanzadeh for statistical expertise.
Footnotes
Source of Support: Isfahan University of Medical Science Vice Chancellery for Research
Conflict of Interest: None declared
REFERENCES
- 1.Medina J, Fern’andez-Salazar JI, Garc′ia-Buey L, Moreno-Oteror Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057–66. doi: 10.2337/diacare.27.8.2057. [DOI] [PubMed] [Google Scholar]
- 2.Ruhl CE, Everhart JE. Epidemiology of nonalcoholic fatty liver. Clin Liver Dis. 2004;8:501–19. doi: 10.1016/j.cld.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Preiss D, Sattar N. Non-alcoholic fatty liver disease: An overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci. 2008;115:141–50. doi: 10.1042/CS20070402. [DOI] [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri B, Caldwell S. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 5.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–98. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 6.Heidari K, Sajjadi SA, Hadian R, Hadi S, Hosseinkhani R, Amini S, et al. Establishment of health clinics as mass screening and referral systems for chronic non-communicable diseases in primary health care. Int J Prev Med. 2012;3:173–80. [PMC free article] [PubMed] [Google Scholar]
- 7.Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, et al. World gastroenterology organisation global guidelines: Probiotics and prebiotics October 2011. J Clin Gastroenterol. 2012;46:468–81. doi: 10.1097/MCG.0b013e3182549092. [DOI] [PubMed] [Google Scholar]
- 8.Lata J, Jurankova J, Kopacova M, Vitek P. Probiotics in hepatology. World J Gastroenterol. 2011;17:2890–6. doi: 10.3748/wjg.v17.i24.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor á in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–11. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 11.Shanab AA, Scully P, Crosbie O, Buckley M, O’Mahony L, Shanahan F, et al. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: Association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56:1524–34. doi: 10.1007/s10620-010-1447-3. [DOI] [PubMed] [Google Scholar]
- 12.Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: A special focus on liver diseases. World J Gastroenterol. 2010;16:403–10. doi: 10.3748/wjg.v16.i4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karahan N, Işler M, Koyu A, Karahan AG, Başyığıt Kiliç G, Cırış IM, et al. Effects of probiotics on methionine cholinedeficient diet-induced steatohepatitis in rats. Turk J Gastroenterol. 2012;23:110–21. doi: 10.4318/tjg.2012.0330. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, et al. Probiotics and Antibodies to TNF Inhibit Inflammatory Activity and Improve Nonalcoholic Fatty Liver Disease. Hepatology. 2003;37:343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 15.Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, et al. Beneficial effects of a probiotic VS. L#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 16.Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–5. [PubMed] [Google Scholar]
- 17.Wong VW, Wong GL, Tse CH, Chan HL. The International Liver Congress 2011. Berlin, Germany: 2011. Mar 30, Treatment of nonalcoholic steatohepatitis with probiotics-A proof-of-concept study with serial gut microbiota analysis by ultra-deep sequencing. [Google Scholar]
- 18.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 20.Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, et al. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–60. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 21.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic Steatohepatitis. Gastroenterology. 2008;135:1176–84. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 22.Rogha M, Najafi N, Azari A, Kaji M, Pourmoghad Z, Rezaee M. Non-alcoholic Steatohepatitis in a sample of Iranian adult population: Age is a Risk Factor. Int J Prev Med. 2011;2:24–7. [PMC free article] [PubMed] [Google Scholar]


