Abstract
Objective:
To compare the effect of Brāhmī Ghṛta with piracetam (a reference standard chemical) in amnesia.
Materials and Methods:
Brāhmī Ghṛta contained Brāhmī (Bacopa monneri), Vacā (Acorus calamus), Kuṣṭha (Sassurea lappa), Śaṅkhapuṣpī (Convolvulos pluricalis), and Purāṇa Ghṛta, prepared as per snehapāka process. Antiamnesic activity of Brāhmī Ghṛta (400 and 800 mg/kg, p.o.) was evaluated in scopolamine (1 mg/kg, s.c.) induced amnesia in Charles Foster rats using elevated plus maze, passive avoidance, and active avoidance tests. Piracetam (500 mg/kg, p.o.) was used as standard drug. This effect was compared with standard chemical used in experimental study.
Results:
Brāhmī Ghṛta - (in both doses) and piracetam-treated rats significantly reversed the effect of scopolamine in modified elevated plus maze, passive avoidance, and active avoidance tests. But there were no significant differences observed in antiamnesic activity of Brāhmī Ghṛta and standard drug.
Conclusion:
Brāhmī Ghṛta and piracetam produced significant beneficial effect on scopolamine-induced amnesic effect, but no significant difference was observed in between them.
KEY WORDS: Brāhmī Ghṛta, modified elevated plus maze test, scopolamine
INTRODUCTION
The complexities of the central nervous system make diagnoses, treatment, and amelioration of debilitating illnesses like anxiety and depression exceptionally difficult. Advancement in diagnosis and treatment areas would be invaluable contributions in the effort to reduce the global impact of anxiety-based conditions. The universality of herbal remedies in many cultures makes them an appropriate treatment to explore. Ācārya Caraka, Suśruta, Vāgbhaṭa often emphasized consideration of more serious form of mental imbalances and distress - Unmāda (insanity). Exercise has long been recognized as a successful alternative/supplement to drug therapy for depression. Brāhmī is tridoṣaghna and sāttvic in nature, Vacā increases the pitta and enhances the clarity of perception and used in Kaphaja depression. Śaṅkhapuṣpī nurses the mind as well as the central nervous system.[1] Brāhmī has an antifertility[2] effect by affecting Śukra Dhātu and Kuṣṭha have a special property of Śukra Śhodhana[3] and also have anti-inflammatory activity.[4] Ghee formulations are used in mental disorders.[5] Since alternative remedies will be used by about half of the consumers, it is essential that this information be widely disseminated, so that consumers and families affected by mental illness and providers of mental health care can properly discuss and evaluate these alternatives as part of the treatment dialogue. In the present study, effect of Brāhmī Ghṛta and piracetam (standard drug) was evaluated in respect to learning and memory activity in amnesic rats.
MATERIALS AND METHODS
Animals
Charles Foster rats of either sex weighing between 160 and 180 g were used for experimental study. The animals were obtained from the Central Animal House, Institute of Medical Sciences, Banaras Hindu University, Varanasi. The animals were housed in polypropylene cages at an ambient temperature of 25°C ± 1°C and 45-55% relative humidity, with a 12:12 h light/dark cycle. Animals were provided with commercial food pellets and water ad libitum unless stated otherwise. They were acclimatized to laboratory conditions for at least 1 week before using them for the experiments. Principles of laboratory animal care (NIH publication number # 85-23, revised in 1985) guidelines were always followed and prior approval of Institutional Animal Ethical Committee (No. Dean/10-11/150) of Banaras Hindu University was obtained before commencing.
Plant material and preparation of Brāhmī formulation
First of all Mūrcchanā of Purāṇa Ghṛta was done by kalka made up of Āmalakī, Harītakī, Vibhītakī, Haridrā, Nāgakesara, and Mātuluṅga Svarasa.[6] After this process Brāhmī Ghṛta was prepared by this Mūrcchanā Ghṛta, Brāhmī, Vacā, Kuṣṭha and Saṅkhapuṣpī. During the process, Mūrcchita Ghṛta was heated on mild heat, when Ghṛta was slightly warm then Brāhmī Swarasa was added into it and mixed thoroughly, during mixing of Swarasa heating process was continued. Then Kalka dravya made up of Brāhmī, Vacā, Kuṣṭha,, and Śaṅkhapuṣpī was added, after adding the Kalka dravya continuous stirring of whole material was done. Initially, above material was heated up to boiling for 2 hr. On the second day, heating process was restarted and heated for 5 hr after that, heating process was again stopped. On the 3rd day heating process again started and continued up to obtaining Snehasiddhilakaṣana like varti-vat Sneha kalka (wick-like shape), agniniḳsipto (does not produce crackling sound on fire), etc. When Snehasiddhilakaṣana[7] was obtained, then Ghrita was filtered with the help of cotton cloth. This filtered Ghrita was known as Brāhmī Ghṛta. Thus, Brāhmī Ghṛta was prepared in 3 days of discontinuous heating.[8] In this way, three samples of Brāhmī Ghṛta were prepared.
Drug treatment
For the present study total 30 animals were used. These animals were divided in to five groups including control group i.e., 6 animals in each groups. In the control group (first groups), no drug was given and only diet and water were provided. In the second group, only single dose of scopolamine, i.e., (1 mg/kg body weight) was administered by intra peritoneal injection after training. In the third group, Brāhmī Ghṛta in dose of 400 mg/kg body weight was administered once a day along with scopolamine in dose of 1 mg/kg body weight. In the fourth group, Brāhmī Ghṛta in dose of 800 mg/kg body weight was administered once a day along with scopolamine in dose of 1 mg/kg body weight. In the fifth group, piracetam in dose of 500 mg/kg body weight was administered once a day along with scopolamine in dose of 1 mg/kg body weight. Brāhmī drug and piracetam were administered orally to rats once a day.
Antiamnesic study
Scopolamine-induced amnesia
A total of 30 animals were taken for this study and learning training was given to all animals. After that, scopolamine hydrobromide (1 mg/kg, i.p.) was administered immediately after the learning trial on day 1 to 24 animals; these animals were divided into four groups and rest of the 6 animals (in which scopolamine was not given) were allotted to the control group. In two groups, Brāhmī Ghṛta was given in dose of 400 and 800 mg/kg body weight, and rats of third group received piracetam in the dose of 500 mg/kg body weight of rats.
(i) Transfer latency on elevated plus maze: This test was used to assess the retention of learning and memory.[9] The plus maze consisted of two opposite open arms, 50 × 10 cm, crossed with two enclosed arms of the same dimensions with walls 40 cm high. The arms were connected with a central square (10 × 10 cm) to give the apparatus a plus sign appearance. The maze was kept in a dimly lit room elevated 50 cm above the floor level. On day 1, a rat was placed on the far end of one of the open arms, facing away from the center, and the time taken by the animal to enter one of the closed arms (transfer latency on day 1) was recorded with the help of a stopwatch. The rat was left in the enclosed arm for 10-15 sec and returned to its home cage. On day 2, same procedure was repeated and similarly after an interval of 1 week, on day 9, the transfer latency was again recorded.
(ii) Passive avoidance test: This test uses normal behavior of rats and was developed by Kings and Glasser (1970).[10] The step through passive avoidance behavior was evaluated by using the light-dark apparatus, which had two walls of wood and the remaining two walls of transparent plexiglass. It was divided into two equal compartments (30 × 25 × 30 cm) by a plexiglass with a 10 × 10 cm. opening in the center. A guillotine door between the two compartments controlled the opening. The light compartment was painted white and a 15 W lamp illuminated it. The interior of the dark chamber was painted black and had a ceiling. Each compartment had a copper grid floor. To ensure electrical separation, there was a 1.5-cm gap between the two floors in the light-dark box, at the opening between the two chambers. In all the four groups excluding control group (details of grouping has been mentioned in drug treatment section), drug was given for 1 week before starting the experiment. On day 1, a rat was placed in the white box and time taken to enter into the dark box was noted. As soon as the rat entered the dark box, the guillotine door was closed and foot electric shock (0.5 mA, 3 sec) was delivered. The rat was then returned to its home cage. On the following day (24-hr retention interval) each rat was again placed in the white box and was given a 5-min inhibition period. Latency to step through to the dark chamber was recorded. Electric shock was not delivered on day 2. If the animal remained in the white box for a 5-min test period, the maximum score of 300 sec was assigned. On day 9 (after a gap of 1 week), latency to step through was again recorded to test the retention of the passive avoidance learning.
(iii) Active avoidance test: Active avoidance learning acquisition and its retention were tested by the method of Spignoli, et al. (1986).[11] The apparatus used was the conventional shuttle avoidance box (Techno, India), which consisted of two grid-floor compartments (29 × 29 × 25 cm each) separated by a plexiglass transparent partition with a single opening (14 × 17 cm) and a buzzer. The rats were placed individually on the right compartment of a shuttle box and allowed to adapt for 15 sec. Thereafter, the rats were exposed to a 15-sec acoustic buzzer stimulus (conditioned stimulus, CS) followed by both the acoustic stimulus and electric shock (unconditioned stimulus, UCS; 1.5 mA, 50 Hz) through the grid floor of the right for 30 sec. Jumping to the unelectrified adjacent (safe) left compartment during CS was designated as conditioned response (CR1), whereas jumping to the safe left chamber during the initial 15-sec adaptation period was designated as anticipatory conditioned response (CR2). The number of trials required by the animal to reach the criterion of two consecutive correct responses represents the learning rate. A 60-min inter-trial interval period was maintained. For statistical analysis, rats not reaching criterion within eight trials was arbitrarily assigned a score of 9. All the rats were subjected to this training schedule and were retested 24 hr later and at day 9 (after a gap of 1 week) for retention of the learned task. Besides CR1, CR2, and trial scores, the total time taken and the total number of shocks received to reach criterion were also recorded.
Statistical analysis
The data, expressed as mean ± SD, were subjected to Kruskal–Wallis one way analysis of variance (ANOVA). Intergroup comparisons were made by Mann–Whitney U test (two tailed) for only those responses that yielded significant treatment effects in the ANOVA test. P < 0.05 was considered statistically significant.
RESULTS
Elevated plus maze test
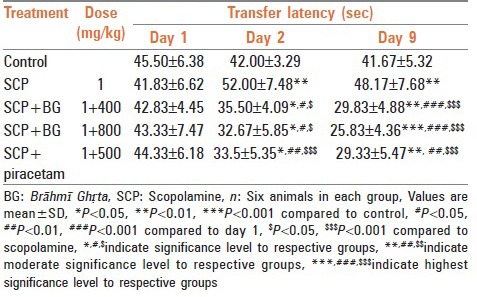
In elevated plus maze model, rats treated with scopolamine showed significant increase in transfer latency on 2nd and 9th day when compared with control group rats. Treatment with Brāhmī Ghṛta and piracetam significantly reversed the amnesia induced by scopolamine. Results have been summarized in Table 1.
Table 1.
Effect of Brāhmī Ghṛta on transfer latency in elevated plus maze test against scopolamine induced amnesia
Passive avoidance test
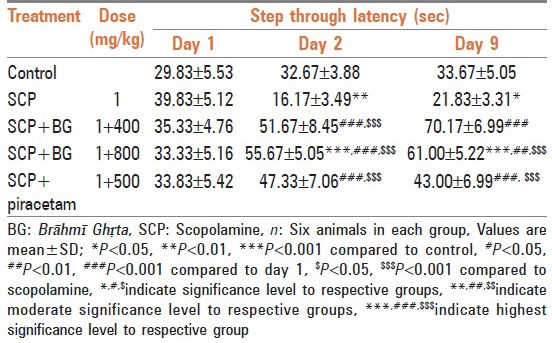
Rats treated with scopolamine showed decrease in step through latency on 2nd and 9th day in comparison to control group rats which indicated amnesia. Rats treated with Brāhmī Ghṛta (in both dose) and piracetam after inducing amnesia significantly reversed the effect of scopolamine, an antiamnesic agent. Results have been summarized in Table 2.
Table 2.
Effect of Brāhmī Ghṛta on step through latency in passive avoidance test against scopolamine amnesia
Active avoidance test
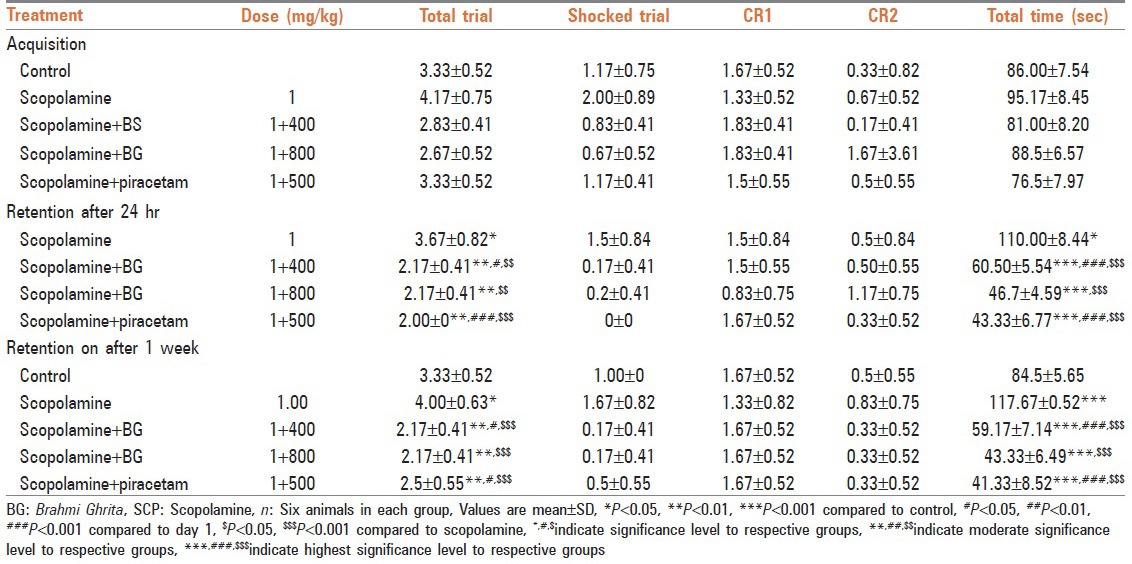
In active avoidance model, rats treated with Brāhmī Ghṛta took significantly less number of total trials, shock trials, and total time for jumping into safe compartment on 2nd and 9th day as compared with scopolamine challenged rats. Treatment with Brāhmī Ghṛta and piracetam significantly reversed the amnesia induced by scopolamine. Results have been summarized in Table 3.
Table 3.
Effect of Brāhmī Ghṛta on learning, acquisition, and retention in active avoidance test against scopolamine induced amnesia
DISCUSSION
Depending on the nature of the diseased, variety of drug dosage forms, modes of administrations, diet, and lifestyle modifications are advised in Āyurvedic. Āyurvedic medicated Ghee is one such dosage form that has been widely used in mental disorders. This dosage form is particularly used in the treatment of psychological disorders (Mānasa Vikāra) and also in disorders that affect nervous system. As we take a review of the chikitsa of Mānasa Vikāra in Āyurveda, we notice that most of the important Medhya aushadhi (psychotropic drugs) have been used in the form of Ghṛta or ghee.[12] The best examples being Unmāda, Apasmāra and certain syndromes involving central nervous system (CNS) such as Bālagraha, Skanda, and Skandāpasmāra. The main clinical features of these diseases being altered sensorium, behavioral changes, seizure manifestations, and partial or total loss of higher functions of brain. The extensive use of ghee in such conditions in Āyurveda compels us to think why this dosage form in particular was selected. The distribution of drug in blood is chiefly influenced by its lipid solubility, ionization, differences in the regional blood flow, etc., A water-soluble drug is usually distributed in the extracellular spaces and it may not readily diffuse in to cerebro spinal fluid (CSF) and other body cavities, whereas the lipid soluble drugs are rapidly distributed throughout the intra- and extracellular spaces. The drugs that are rapidly absorbed from the gut due to their lipid solubility are known to readily diffuse into the CSF and the brain. The drugs given in the form of Ghee (Ghṛta), a form of lipid, is likely to be rapidly absorbed and distributed in the target areas of the body such as the nervous system in this case. The main reason behind this is the molecular structure of the blood brain barrier. The membrane separating the CNS tissue and the circulating blood is lipophilic in nature. Thus, it selectively allows the passage of lipids and lipid soluble drugs across it. Therefore, any drug given in the form of ghee will not only be digested and absorbed fast but will also be able to reach some of the most distant areas of the body such as the CNS. Ghee Kalpas are therefore one of the most effective drug dosage forms used in Āyurvedic medicine. Ghee is also prescribed for anxiety, depression, dementia, insanity, epilepsy, and other disorders of consciousness. Ghee older than 1 year is considered especially good for healing the mind. Its bitter property enables it to remove blockages in the mind's subtle channels. It is also considered auspicious and is given in mental disorders with no clear physical cause, along with Sanskrit mantras. The effectiveness of such ghee formulations in mental disorders has been researched and shown to be effective.[13] One study does look at ghee's inherent properties for treating Alzheimer's via its insertion in the nose.[14] Such Nasya therapy is part of Pańchakarma and is a natural route for delivering rejuvenating substances to the brain. Beyond treating serious mental disorders ghee can be used to pacify mild anxiety (due to aggravated Vāta). Āyurveda claims that several plants have Medhya (intellect promoting) properties such as Convolvulus microphyllus (C. pluricaulis), Centell aasiatica, Bacopa monnieri, Acorus calamus, Zingiber officinale, and Celastrus paniculatus.[15] All these drugs also influence cholinergic function by increasing high affinity choline uptake facilitating acetylcholine production and turnover with varying actions at both muscarinic and nicotinic receptors.[16] The name Brāhmī is derived from Brahman, the sanskrit name of God, whose nature is Sat-Chit-Ānanda or reality nondual consciousness-bills: Because it is tridoshic as well as Sāttvic, it is used in appropriate combination with others herbs in nearly all psychiatric treatments. It imparts to the mind a quality of peaceful improvement and rejuvenates the nervous system. Significant reversal of scopolamine (amnesic agent) induced amnesia by Brāhmī Ghṛta, indicates an underlying cholinergic mechanism as scopolamine impairs spatial cognition by blocking not only the postsynaptic M1 receptor but also the presynaptic M2 receptor.[17] The result obtained in our study showed that Brāhmī Ghṛta significantly antagonises action of scopolamine. So Brāhmī Ghṛta might act through augmenting cholinergic functions in brain. Treatment with scopolamine caused prolongation of step through latency, which was significantly reversed by Brāhmī Ghṛta and piracetam. Brāhmī Ghṛta and piracetam significantly attenuated the effect of scopolamine-induced amnesia. Brāhmī might have reversed the scopolamine-induced amnesia significantly mainly by improving calmodulin and by partially attenuating protein kinase C and pCREB (cyclic AMP responce-element binding protein).[18]
CONCLUSION
On the basis of the present study we conclude that Brāhmī Ghṛta and piracetam significantly antagonise the effect of scopolamine in all experimental models used in the present study. It was also observed that there was no significant difference in learning and memory activity between Brāhmī Ghṛta-treated rats (in both doses) and standard reference drug (piracetam). So that Brāhmī Ghṛta may be used as herbal medicine in learning and memory disorders.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Buhrman S. Ayurvedic psychology and psychiatric approaches to the treatment of common affective disorders. Prot J Bot Med. 1997;1:1–8. [Google Scholar]
- 2.Singh A, Singh SK. Evaluation of anti fertility potential of Brahmi in male mouse. Contraception. 2009;79:71–9. doi: 10.1016/j.contraception.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Sharma PV. Varanasi: Chaukhambha Bharti Academy; 2004. Dravyaguna Vigyanam; pp. 573–575. [Google Scholar]
- 4.Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398:399–407. doi: 10.1016/s0014-2999(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 5.Chandre R, Narasimha Murthy VS, Singh RH. Evaluation of the efficacy of Kushmanda Ghrita in the management of depressive illness. Aryavaidyan. 2004;2:87–90. [Google Scholar]
- 6.Shastri A. Varanasi, Jwaradhikara: Chaukhambha Prakashana; 2008. Bhaishajya Ratnawali; pp. 1285–7. [Google Scholar]
- 7.Shrivastava S. Patana: Baidhyanath Ayurveda Bhawan; 2008. Sharangdhar. Saranghdhar Samhita; pp. 12–3. [Google Scholar]
- 8.Mishra SM. Varanasi: Chaukhambha Surbhrtiya Prakashan; 2004. Bhaishjya Kalpana Vigyan; pp. 229–231. [Google Scholar]
- 9.Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for the evaluation of memory in mice: Effect of nootropic, scopolamine and electroconvulsive shock. Psychopharmacology (Berl) 1990;101:27–33. doi: 10.1007/BF02253713. [DOI] [PubMed] [Google Scholar]
- 10.Kings RA, Glasser RL. Duration of electroconvulsive shock-induced retrograde amnesia in rats. Physiol Behav. 1970;5:335–9. doi: 10.1016/0031-9384(70)90107-1. [DOI] [PubMed] [Google Scholar]
- 11.Spignoli G, Pepeu G. Oxiracetam prevents electroshock-induced decrease in brain acetylcholine and amnesia. Eur J Pharmacol. 1986;126:253–7. doi: 10.1016/0014-2999(86)90055-5. [DOI] [PubMed] [Google Scholar]
- 12. [Last cited on 2012 Sept 15]. Available from: http://www.selfgrowth.com/articles .
- 13.Achliya GS, Wadodkar SG, Dorle AK. Evaluation of sedative and anticonvulsant activities of Unmadnashak Ghrita. J Ethnopharmacol. 2004;94:77–83. doi: 10.1016/j.jep.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Madhavikutty P. The role of nasya and dhoopa in dementia and Alzheimer's disease. Aryavaidyan. 2000;4:228–33. [Google Scholar]
- 15.Joshi H, Parle M. Zingiber officinale: Evaluation of its nootropic effect in mice. Afr J Tradit Complement Altern Med. 2006;3:64–7. [Google Scholar]
- 16.Balaraman R, Shingala J. Molecules of the millennium. Indian J Pharmacol. 2002;34:439–40. [Google Scholar]
- 17.Mishima K, Iwasaki K, Tisukikawa H, Matsumoto Y, Egashira N, Abe K, et al. The scopolamine-induced impairment of spatial cognition parallels the acetylcholine release in ventral hippocampus in rats. Jpn J Pharmacol. 2000;84:163–73. doi: 10.1254/jjp.84.163. [DOI] [PubMed] [Google Scholar]
- 18.Anand A, Saraf M, Prabhakar S. Scopolamine induced amnesia reversed by Bacopa monniera through participation of kinase-CREB pathway. Neurochem Res. 2009;35:1172–8. doi: 10.1007/s11064-009-0051-4. [DOI] [PubMed] [Google Scholar]


