Abstract
Objective
Systemic lupus erythematosus (SLE) and lupus nephritis (LN) disproportionately affect racial/ethnic minorities and lower socioeconomic status (SES) individuals. We investigated the epidemiology and sociodemographics of SLE and LN in the low-income U.S. Medicaid population.
Methods
We utilized Medicaid Analytic eXtract data, with billing claims from 47 states and Washington, D.C. for 23.9 million individuals, aged 18–65 years, enrolled in Medicaid for >3 months, 2000–2004. Individuals with SLE (> 3 visits, ICD-9 code 710.0, >30 days apart) and with LN (>2 ICD-9 codes for glomerulonephritis, proteinuria or renal failure) were identified. We calculated SLE and LN prevalence and incidence, stratified by sociodemographic categories, and adjusted for number of American College of Rheumatology (ACR) member rheumatologists and SES using a validated composite of U.S. Census variables.
Results
We identified 34,339 individuals with SLE (prevalence = 143.7/100,000) and 7,388 (21.5%) with LN (prevalence = 30.9/100,000). SLE prevalence was 6 times higher among women, nearly double in African American compared to White women, and highest in the U.S. South. LN prevalence was higher among all racial/ethnic groups compared to Whites. The lowest SES areas had the highest prevalence; areas with the fewest ACR rheumatologists had the lowest. SLE incidence was 23.2/100,000 person-years, and LN incidence was 6.9/100,000 person-years, with similar sociodemographic trends.
Conclusions
In this nationwide Medicaid population, there was sociodemographic variation in SLE and LN prevalence and incidence. Understanding the increased burden of SLE and its complications in this low-income population has implications for resource allocation and access to subspecialty care.
Keywords: Systemic lupus erythematosus, lupus nephritis, epidemiology, socioeconomic factors, disparities
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with substantial variation by sex, race, ethnicity and socioeconomic status (1–4). Past estimates of SLE prevalence in the adult U.S. population range from 24 to 150 per 100,000 (5, 6) and incidence from 2.2 to 5.6 per 100,000 (7, 8) (Table 1). Despite the wide variation in these estimates, rates are consistently higher in women compared to men and in African Americans compared to Caucasians. Prior studies also suggest increased prevalence among Asians, Hispanics and Native Americans (2, 9–11). To date, however, there are no U.S. nationwide administrative database examinations of the sociodemographics of adult SLE prevalence or incidence. A number of studies suggest that lupus nephritis (LN), one of the most severe manifestations of SLE, is both more common and more severe in racial and ethnic minorities, and progression to end-stage renal disease is higher in minority, uninsured, and low socioeconomic status (SES) groups (3, 11–18). Currently, no studies are available that investigate the prevalence or incidence of LN in a large, low-income U.S. population.
Table 1.
Previous Estimates of the Prevalence and Incidence of Systemic Lupus Erythematosus (SLE) in the U.S. per 100,000 Adults
| Author; Year of Publication | Study Method; Location | Prevalence Estimate (per 100,000 adults) |
Incidence Estimate (per 100,000 adults) |
|---|---|---|---|
| Fessel et al; 1974 | HMO inpatient and outpatient records; CA |
44 (White adults) 100 (White women) 400 (AA women)* |
--- |
| Hochberg et al; 1985 |
First hospitalization discharge diagnosis, age and race adjusted; MD |
--- |
2.2 (White adults) 0.4 (White men) 3.9 (White women) 7.2 (AA adults) 2.5 (AA men) 11.4 (AA women) |
| McCarty et al; 1995 |
Medical record review, 3 source capture-recapture technique; PA |
--- |
2.0 (White adults) 0.4 (White men) 3.5 (White women) 5.3 (AA adults) 0.7 (AA men) 9.2 (AA women) |
| Maskarinec et al;1995 |
Population-based medical records and patient support group; HI |
55 (White and Japanese adults) 100 (Chinese adults) |
--- |
| Jacobson et al.; 1997 |
Pooled from 23 prior studies; North America and Europe |
23.8 (All adults) | --- |
| Uramoto et al; 1999 |
Medical record review, age and sex-adjusted; MN |
130 (All adults) | 5.56 (Overall) |
| Balluz et al; 2001 | Interviews, examination, serology; AZ |
103 (Hispanic women) | --- |
| Ward et al; 2004 | Self-reported diagnoses and prescriptions, NHANES III; U.S. |
53.6 (All adults) 100 (Adult women) |
--- |
| Naleway et al; 2005 |
Medical record review; WI |
--- |
5.1 (Overall) 1.9 (Adult men) 8.2 (Adult women) |
| Karlson et al; 2007 | Medical record review; MA |
256 (AA women) | --- |
| Chakravarty et al; 2007 |
Frequency of hospitalization, discharge diagnoses, chart review; CA, PA |
107.6 (All adults, CA) 406.3 (AA women, CA) 149.5 (All adults, PA) 693.7 (All women, PA) |
--- |
| Helmick et al; 2008 | City-based SLE prevalence and 2005 U.S. Census population estimates; U.S. |
54.3 (Definite SLE) † 108.6 (Suspected SLE) |
--- |
AA=African American
This study differentiated between “definite SLE” and “suspected SLE.” Suspected SLE were cases that did not meet ACR SLE criteria but thought to be likely to at some point meet criteria.
In this study we used nationwide Medicaid claims data to investigate sociodemographic differences in the incidence and prevalence of SLE and LN among U.S. adults. Medicaid is a U.S. federal-state jointly run insurance program that provides health and long-term care coverage to eligible low-income individuals (19). Within this population, we investigated whether county-level SES and the number of rheumatologists per state (approximated using American College of Rheumatology (ACR) members), were related to differences in incidence and prevalence of SLE and LN, and the degree to which these variables could explain variation by race and ethnicity. Our goal was to provide a better understanding of the burden of SLE and LN among low-income, high-risk U.S. adults, which will encourage the necessary allocation of resources for early detection and essential treatment. We hypothesized that there would be significant variation in incidence and prevalence of SLE and LN by sociodemographic group, but that SES and the number of rheumatologists would modify these differences.
Methods
Study Population
The Medicaid Analytic eXtract (MAX) administrative data system contains billing claims and demographics for all Medicaid enrollees from 47 states and the District of Columbia. Arizona, Tennessee and Maine do not contribute data to MAX. We included all adults, aged 18 to 65 years, who were enrolled for at least three months, from January 1, 2000 to December 31, 2004.
Outcomes
We defined individuals with SLE as having ≥ 3 International Classification of Diseases, ninth revision (ICD-9) codes for SLE (710.0), each at least 30 days apart, from hospital discharge diagnoses or physician visit claims. We required three billing codes to eliminate “rule-out” SLE cases. Among individuals with SLE, we identified those with lupus nephritis (LN), defined as having ≥ 2 ICD-9 hospital discharge diagnoses or physician billing claims for nephritis, proteinuria and/or renal failure, on or after the SLE diagnosis, and at least 30 days apart. This algorithm has been demonstrated to have a positive predictive value of 80 percent for the identification of adults with LN in a Medicaid population (20). We also performed a sensitivity analysis for LN that used >2 SLE claims with the aforementioned >2 LN-related claims.
Other Characteristics
We extracted demographic data for all Medicaid enrollees including age, sex, and race/ethnicity. Race/ethnicity is categorized by Medicaid based on self-report, as White, Black or African American, American Indian or Alaskan Native, Asian, Hispanic or Latino, Native Hawaiian or other Pacific Islander, Hispanic or Latino and one or more races (starting in May 2000), more than one race (starting in May 2000), or unknown. Due to small numbers, our analysis utilized the following previously defined, combined categories: White, Black or African American, Hispanic or Latino (including Hispanic or Latino and one or more races), Asian (including Native Hawaiian or other Pacific Islander), Native American (including American Indian or Alaskan Native), and Other (including unknown) (21). We determined location of residence by ZIP code and U.S. Census region (Northeast, Midwest, South or West).
From the 2000 U.S. Census (22), we identified seven socioeconomic indicators at the ZIP code level: median household income, proportion with income below 200% of the federal poverty level, median home value, median monthly rent, mean education level, proportion of people age >25 who were college graduates, and proportion of employed persons with a professional occupation (23).
At our request, the ACR provided the number of ACR member rheumatologists practicing per year in each ZIP code in the US between 2000 and 2004. We first aggregated the number of ACR member rheumatologists per ZIP code to the number per county. We found that 80% of counties had no ACR member rheumatologists, 6.8% had only one ACR member rheumatologist and 13.2 % had greater than one. We thus aggregated these data to the average number of ACR member rheumatologists per year per state.
Statistical Analysis
We calculated the prevalence of SLE and of LN per 100,000 Medicaid-enrolled adults, aged 18 to 65 years, between 2000 and 2004, with 95% confidence intervals (CIs). Overall prevalence was calculated, stratified by sex, age, race/ethnicity, and U.S. region, and then cross-classified by sex and racial/ethnic group. We used Poisson regression techniques to estimate prevalence rate ratios and corresponding 95% CIs.
To calculate annual incidence rates and 95% CIs for SLE and LN, individuals with newly diagnosed SLE and LN, identified by the above method, were included if they had a minimum of 24 months of Medicaid enrollment without any SLE or LN claims. SLE onset was defined as the date of the first SLE or LN claim. Individuals contributed to the person-months denominator once they had 24 months of Medicaid enrollment with no SLE-related claims. Once an individual met the billing claim definition for SLE or LN, they were censored from the cohort denominator at the time of the first SLE or LN claim. Average annual incidence rates (per 12 months enrollment) for 2002 through 2004 were calculated for the total cohort and then stratified by sex, racial/ethnic group, and region. Incidence rate ratios with 95% CIs were calculated using Poisson regression techniques.
As Medicaid patients may have discontinuous coverage, we conducted sensitivity analyses for incidence rate calculations, restricted to adults with 24 months of continuous Medicaid enrollment with no prior SLE-related claims. We also performed sensitivity analyses requiring 36 months of continuous and non-continuous enrollment with no prior SLE-related claims to reduce misclassification of prevalent cases.
We defined area-level SES using a previously validated composite score of the aforementioned U.S. Census variables by ZIP code (23). We aggregated ZIP code level SES data to the county level and then divided it into quartiles (< −1.62, >1.62- −0.74, >−0.74 – 0.26 and >0.26). We adjusted SLE and LN prevalence, prevalence rate ratios, incidence and incidence rate ratios by these SES quartiles using generalized linear models, and cross-classified by SES and sex, race/ethnicity and geographic region. We adjusted prevalence in each SES strata by age, sex and race/ethnicity. We then performed the Cochran-Armitage trend test to assess for a linear trend across quartiles. We also adjusted SLE and LN prevalence, prevalence rate ratios, incidence and incidence rate ratios by the number of ACR member rheumatologists per state, categorized by quartiles (<13.6, >13.6 – 33.1, >33.1 – 75 and >75), and assessed for prevalence and incidence rate ratios, and for trend, as above.
All analyses were conducted in SAS, (Version 9.2, Cary, NC). Data were obtained from the Center for Medicare and Medicaid Services (CMS) through an approved data use agreement and are presented in accordance with their policies. The Institutional Review Board of Partners Healthcare waived human subjects’ approval for this study.
Results
From 2000 to 2004, a total of 23,893,092 individuals, aged 18 to 65 years were enrolled in Medicaid. We identified 34,339 individuals with SLE, for an overall prevalence of 143.7 per 100,000. The prevalence of SLE among females was 6 times higher compared to males; 38.5% of individuals with SLE were African American, 13.9% Hispanic, 4.2% Asian, 1.5% Native American, and 36.2% White. The highest SLE prevalence was in the South (163.5 per 100,000) and the lowest was in the Northeast (125.2 per 100,000). The prevalence of SLE stratified by sociodemographic group is shown in Table 2. African American women had the highest prevalence of SLE, 286.4 per 100,000; nearly twice as high as White women.
Table 2.
Prevalence of SLE and LN per 100,000 Medicaid-enrolled Adults, by Sociodemographic Group, 2000–2004
| SLE | Lupus Nephritis (LN) | ||||||
|---|---|---|---|---|---|---|---|
| Denominator | Cases (% of total) | Prevalence | 95% CI | Cases (% of total) | Prevalence | 95% CI | |
| Total | 23,893,092 | 34,339 (100) | 143.72 | 142.21–145.25 | 7,388 (100) | 30.92 | 30.22–31.63 |
| Sex | |||||||
| Female | 16,665,956 | 32,039 (93.3) | 192.24 | 190.15–194.36 | 6,652 (90.0) | 39.931 | 38.97–40.88 |
| Male | 7,227,136 | 2,300 (6.7) | 31.82 | 30.55–33.15 | 736 (10.0) | 10.18 | 9.47–10.95 |
| Age (years)* | |||||||
| 18–29 | 12,366,982 | 9,507 (27.7) | 76.87 | 75.45–78.43 | 3,082 (41.7) | 24.92 | 24.06–25.82 |
| 30–49 | 9,907,523 | 19,848 (57.8) | 200.33 | 197.56–203.14 | 3,703 (50.1) | 37.38 | 36.19–38.60 |
| 50–64 | 3,187,974 | 9,320 (27.1) | 292.35 | 286.47–298.34 | 1,364 (18.5) | 42.79 | 40.57–45.12 |
| Race/Ethnicity | |||||||
| African-American | 5,923,775 | 13,236 (38.5) | 223.44 | 219.66–227.28 | 3,536 (47.9) | 59.69 | 57.76–61.69 |
| Female | 4,334,630 | 12,413 (36.1) | 286.37 | 281.37–291.45 | 3,233 (43.8) | 74.59 | 72.06–77.20 |
| Male | 1,589,145 | 823 (2.4) | 51.79 | 48.37–55.45 | 303 (4.1) | 19.07 | 17.04–21.34 |
| Hispanic | 3,767,002 | 4,767 (13.9) | 126.55 | 123.00–130.19 | 1,124 (15.2) | 29.84 | 28.14–31.63 |
| Female | 2,739,030 | 4,461 (13.0) | 162.87 | 158.16–167.72 | 1,011 (13.7) | 36.91 | 34.70–39.26 |
| Male | 1,027,972 | 306 (0.9) | 29.77 | 26.61–33.30 | 113 (1.5) | 10.99 | 9.14–13.22 |
| White | 11,146,710 | 12,436 (36.2) | 111.57 | 109.62–113.54 | 1,765 (23.9) | 15.83 | 15.11–16.59 |
| Female | 7,692,686 | 11,546 (33.6) | 150.09 | 147.38–152.85 | 1,547 (20.9) | 20.11 | 19.13–21.14 |
| Male | 3,454,024 | 890 (2.6) | 25.77 | 24.13–27.52 | 218 (3.0) | 6.31 | 5.53–7.21 |
| Asian | 829,209 | 1,452 (4.2) | 175.11 | 166.33–184.35 | 469 (6.3) | 56.56 | 51.67–61.92 |
| Female | 527,669 | 1,347 (3.9) | 255.27 | 242.00–269.28 | 426 (5.8) | 80.73 | 73.42–88.77 |
| Male | 301,540 | 105 (0.3) | 34.82 | 28.76–42.16 | 43 (0.6) | 14.26 | 10.58–19.23 |
| Native American | 310,736 | 515 (1.5) | 165.74 | 152.02–180.69 | 113 (1.5) | 36.37 | 30.24–43.73 |
| Female | 220,836 | 471 (1.4) | 213.28 | 194.86–233.44 | 99 (1.3) | 44.83 | 36.81–54.59 |
| Male | 89,900 | 44 (0.1) | 48.94 | 36.42–65.77 | 14 (0.2) | 15.57 | 9.22–26.29 |
| Other | 1,915,660 | 1,933 (5.6) | 100.91 | 96.51–105.51 | 381 (5.2) | 19.89 | 17.99–21.99 |
| Female | 1,151,105 | 1,801 (5.2) | 156.46 | 149.40–163.85 | 336 (4.5) | 29.19 | 26.23–32.48 |
| Male | 764,555 | 132 (0.4) | 17.26 | 14.56–20.48 | 45 (0.6) | 5.89 | 4.39–7.88 |
| Geographic Region | |||||||
| South | 7,999,877 | 13,078 (38.1) | 163.48 | 160.79–166.30 | 2,877 (38.9) | 35.96 | 34.67–37.30 |
| Northeast | 5,843,279 | 7,318 (21.3) | 125.24 | 122.40–128.14 | 1,564 (21.2) | 26.77 | 25.47–28.13 |
| Midwest | 4,708,856 | 6,430 (18.7) | 136.55 | 133.25–139.93 | 1,442 (19.5) | 30.62 | 29.08–32.25 |
| West | 5,341,080 | 7,513 (21.9) | 140.66 | 137.52–143.88 | 1,505 (20.4) | 28.18 | 26.79–29.64 |
Age-range group cases and denominators exceed the total in order to include individuals, 18–64, who changed age groups between 2000–2004.
We identified 7,388 individuals with LN, (Table 2) with a prevalence of 30.9 per 100,000. The prevalence of LN was four times higher among females compared to males and nearly four times higher among African Americans compared to Whites. The prevalence of LN was also highest in the South, and among African American women (74.6 per 100,000) and Asian women (80.7 per 100,000). Our sensitivity analysis, which required >2 SLE claims, rather than the original >3, resulted in an overall LN prevalence of 34.1 per 100,000, or 8,158 cases.
From 2002 to 2004, the average annual incidence rate of SLE in this Medicaid population was 23.17 per 100,000 person-years. The average incidence rate of LN was 6.85 per 100,000 person-years. Incidence rates, by sociodemographic group, are presented in Table 3. The incidence of SLE was higher both in the 30–49 year and the 50–64 year age groups, compared to the youngest stratum. Incidence of SLE was highest among African American females (38.62 per 100,000 person-years), and among Native American females (37.31 per 100,000 person-years). Similar to prevalence, the South had the highest incidence of SLE compared to other geographic regions. The incidence of LN was also highest in the older age groups, in females, and in African Americans. The results of our three sensitivity analyses for SLE and LN with varying times of enrollment, both continuous and non-continuous, yielded incidence rates comparable to our main analyses (Supplemental Tables 1 and 2).
Table 3.
Average Incidence Rates of SLE and LN among Medicaid-enrolled Adults, per 100,000 person-years, by Sociodemographic Group, 2002–2004
| SLE | Lupus Nephritis (LN) | ||||||
|---|---|---|---|---|---|---|---|
| Denominator (Person-Years) |
Cases (% of total) | Incidence Rate | 95% CI | Cases (% of total) | Incidence Rate | 95% CI | |
| Total | 15,064,937 | 3,490 (100) | 23.17 | 22.41–23.95 | 1,034 (100) | 6.85 | 6.44–7.28 |
| Sex | |||||||
| Female | 10,774,905 | 3,282 (94.0) | 30.46 | 29.44–31.52 | 971 (93.9) | 8.98 | 8.43–9.56 |
| Male | 4,290,031 | 208 (6.0) | 4.85 | 4.23–5.55 | 63 (6.1) | 1.47 | 1.15–1.88 |
| Age (years) | |||||||
| 18–29 | 5,650,736 | 807 (23.1) | 14.28 | 13.33–15.30 | 291 (28.1) | 5.14 | 4.59–5.77 |
| 30–49 | 6,426,860 | 1,795 (51.4) | 27.93 | 26.67–29.25 | 485 (46.9) | 7.52 | 6.88–8.22 |
| 50–64 | 2,987,340 | 888 (25.4) | 29.73 | 27.83–31.75 | 258 (25.0) | 8.60 | 7.61–9.72 |
| Race/Ethnicity | |||||||
| African-American | 4,501,676 | 1,404 (40.2) | 31.19 | 29.60–32.86 | 481 (46.5) | 10.65 | 9.74–11.65 |
| Female | 3,428,004 | 1,324 (37.9) | 38.62 | 36.60–40.76 | 462 (44.7) | 13.43 | 12.26–14.71 |
| Male | 1,073,672 | 80 (2.3) | 7.45 | 5.98–9.28 | 19 (1.8) | 1.77 | 1.13–2.77 |
| Hispanic | 2,278,325 | 506 (14.5) | 22.21 | 20.36–24.23 | 157 (15.2) | 6.88 | 5.88–8.04 |
| Female | 1,703,102 | 485 (13.9) | 28.48 | 26.05–31.13 | 151 (14.6) | 8.84 | 7.54–10.37 |
| Male | 575,224 | 21 (0.6) | 3.65 | 2.38–5.60 | NR* | NR* | NR* |
| White | 6,524,850 | 1,172 (33.6) | 17.96 | 16.96–19.02 | 266 (25.7) | 4.07 | 3.61–4.59 |
| Female | 4,514,842 | 1,101 (31.5) | 24.39 | 22.99–25.87 | 239 (23.1) | 5.28 | 4.65–5.99 |
| Male | 2,010,008 | 71 (2.0) | 3.53 | 2.80–4.46 | 27 (2.6) | 1.34 | 0.92–1.96 |
| Asian | 713,474 | 119 (3.4) | 16.68 | 13.94–19.96 | 39 (3.8) | 5.46 | 3.99–7.47 |
| Female | 449,005 | 108 (3.1) | 24.05 | 19.92–29.05 | 34 (3.3) | 7.56 | 5.40–10.57 |
| Male | 264,469 | 11 (0.3) | 4.16 | 2.30–7.51 | NR* | NR* | NR* |
| Native American | 156,639 | 47 (1.3) | 30.01 | 22.54–39.94 | 11 (1.1) | 7.00 | 3.88–12.64 |
| Female | 115,240 | 43 (1.2) | 37.31 | 27.67–50.31 | 11 (1.1) | 9.51 | 5.26–17.17 |
| Male | NR* | NR* | NR* | NR* | NR* | NR* | NR* |
| Other | 889,974 | 242 (6.9) | 27.19 | 23.97–30.84 | 80 (7.7) | 8.95 | 7.19–11.15 |
| Female | 564,714 | 221 (6.3) | 39.13 | 34.30–44.65 | 74 (7.2) | 13.03 | 10.37–16.36 |
| Male | 325,260 | 21 (0.6) | 6.46 | 4.21–9.90 | NR* | NR* | NR* |
| Geographic Region | |||||||
| South | 3,872,032 | 1,077 (30.9) | 27.81 | 26.20–29.53 | 353 (34.1) | 9.09 | 8.19–10.09 |
| Northeast | 4,243,303 | 875 (25.1) | 20.62 | 19.30–22.03 | 230 (22.2) | 5.41 | 4.75–6.16 |
| Midwest | 2,987,033 | 611 (17.5) | 20.46 | 18.90–22.14 | 198 (19.1) | 6.61 | 5.75–7.60 |
| West | 3,962,569 | 927 (26.6) | 23.39 | 21.94–24.95 | 253 (22.7) | 6.37 | 5.63–7.20 |
Data on 10 or fewer individuals were not reported (NR) to protect privacy
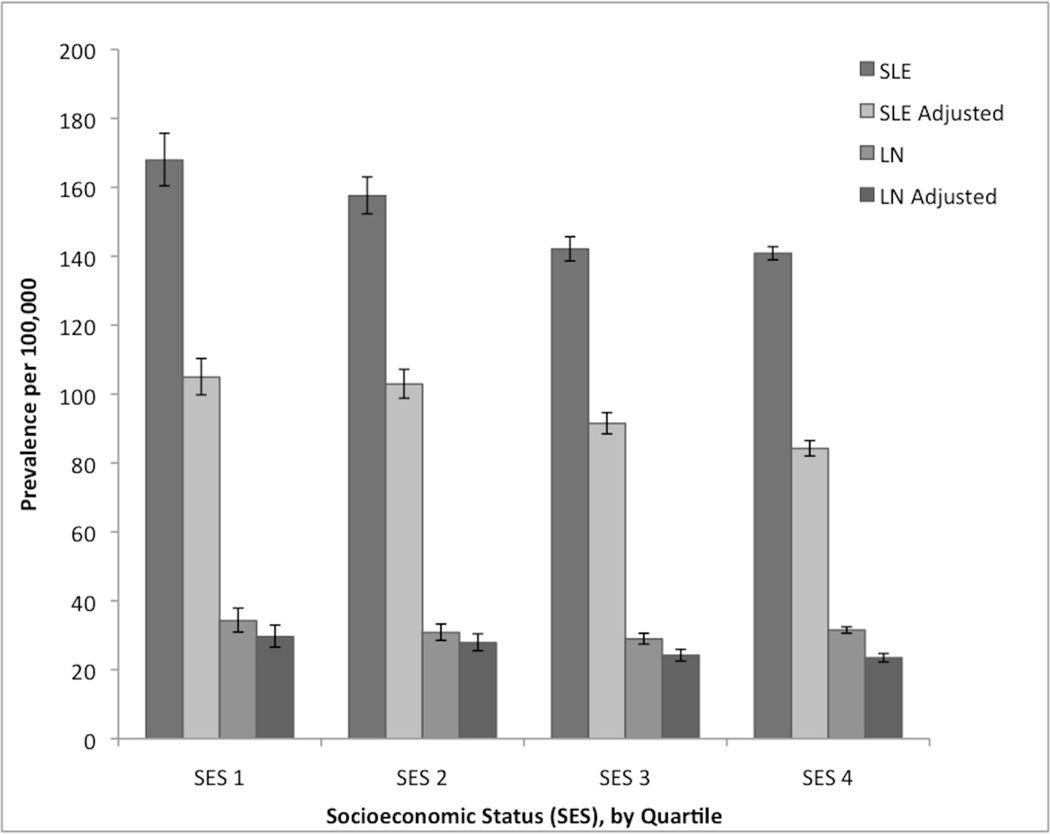
We investigated the overall prevalence and incidence of SLE and LN by quartiles of county-level SES and number of ACR member rheumatologists per state, both crude and adjusted for age, sex, and race/ethnicity. Stratified SLE and LN prevalence estimates by SES are displayed graphically in Figure 1; incidence did not vary significantly by SES quartile. We found statistically significant differences between the lowest SES group, which had the highest SLE prevalence (167.9 per 100,000, 95% CI 160.4–175.7) and the two highest SES quartiles, which had the lowest SLE prevalence. We observed a similar pattern after adjusting for age, sex and race/ethnicity with the highest prevalence in the lowest SES group (104.9 per 100,000, 95% CI 99.8–110.3). The difference in prevalence between the two lowest and the two highest SES strata was small, however we noted a trend of decreased SLE prevalence with increased SES (Cochran-Armitage test for linear trend, p=0.001). The trend of LN prevalence did not differ significantly across SES quartiles (p=0.98).
Figure 1.
Prevalence of systemic lupus erythematosus (SLE) and lupus nephritis (LN) per 100,000, stratified by socioeconomic status (SES) quartile (SES 1 (lowest): ≤ −1.62, SES 2: >1.62 and ≤−0.72, SES 3: >0.72 and ≤0.26, SES 4 (highest): >0.26), crude and adjusted by age group, sex and race/ethnicity. Bars represent 95% confidence intervals.
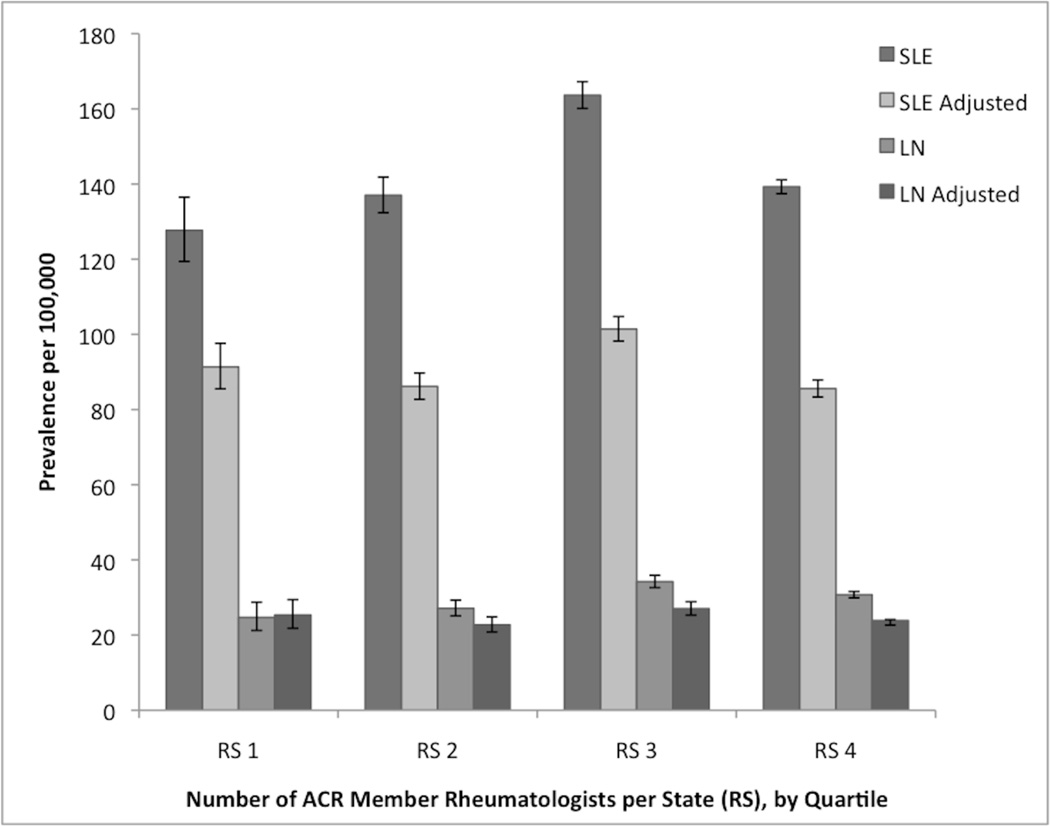
When stratified by the average number of ACR member rheumatologists per state in quartiles, areas with the fewest rheumatologists had the lowest prevalence of both SLE (127.7 per 100,000, 95% CI 119.4–136.5) and LN (24.7 per 100,000, 95% CI 21.2–28.8) (Figure 2). The test for linear trend across quartiles of rheumatologists per state was significant for SLE (p=0.02), and for LN (p=0.03). After adjusting both SLE and LN estimates for age, sex and race/ethnicity, this trend was no longer observed. Incidence estimates stratified by number of rheumatologists were not statistically significant.
Figure 2.
Prevalence of systemic lupus erythematosus (SLE) and lupus nephritis (LN) stratified by number of American College of Rheumatology Member Rheumatologists per state (RS) by quartile (RS 1 (lowest): ≤ 13.6, RS 2: >13.6 and ≤ 33.1, RS 3: >33.1 and ≤75, RS 4 (highest): >75), crude and adjusted by age group, sex and race/ethnicity. Bars represent 95% confidence intervals.
We calculated prevalence rate ratios and incidence rate ratios, with 95% CIs both for SLE and for LN, to compare rates between sociodemographic groups (Table 4). The crude rate ratios demonstrated the significantly increased prevalence and incidence of SLE and LN among females compared to males and African Americans compared to Whites. Table 4 also shows prevalence and incidence rate ratios adjusted by SES and rheumatologist per state quartiles, first separately, then combined. There were no statistically significant differences in the rate ratios by sex, race/ethnicity or region after adjusting by SES, rheumatologists or both.
Table 4.
Prevalence Rate Ratios (PRR) and Incidence Rate Ratios (IRR), Crude and Adjusted by Socioeconomic Status (SES) and Number of American College of Rheumatology Member Rheumatologists per State (Rheum State)
| Prevalence Rate Ratios (95% CI) | Incidence Rate Ratios (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | SES* | Rheum State † | SES + Rheum State | Crude | SES | Rheum State | SES + Rheum State | ||
| SLE | Gender | ||||||||
| Male | Reference | ||||||||
| Female | 6.04 (5.79–6.30) |
6.04 (5.79–6.30) |
6.03 (5.78–6.29) |
6.03 (5.78–6.29) |
6.28 (5.25–7.23) |
6.29 (5.47–7.24) |
6.26 (5.44–7.21) |
6.27 (5.45–7.21) |
|
| Race/Ethnicity | |||||||||
| White | Reference | ||||||||
| African American |
2.00 (1.95–2.05) |
2.03 (1.99–2.09) |
2.01 (1.96–2.06) |
2.03 (1.98–2.08) |
1.74 (1.61–1.88) |
1.75 (1.62–1.90) |
1.76 (1.62–1.90) |
1.76 (1.63–1.90) |
|
| Hispanic | 1.13 (1.10–1.17) |
1.18 (1.14–1.22) |
1.17 (1.13–1.21) |
1.19 (1.15–1.23) |
1.24 (1.11–1.37) |
1.24 (1.11–1.38) |
1.28 (1.15–1.43) |
1.27 (1.14–1.42) |
|
| Asian | 1.57 (1.49–1.66) |
1.64 (1.56–1.74) |
1.61 (1.52–1.70) |
1.66 (1.57–1.76) |
0.93 (0.77–1.12) |
0.93 (0.77–1.13) |
0.95 (0.78–1.15) |
0.94 (0.77–1.13) |
|
| Native American |
1.49 (1.36–1.62) |
1.46 (1.34–1.59) |
1.47 (1.35–1.61) |
1.46 (1.33–1.60) |
1.67 (1.25–2.24) |
1.67 (1.23–2.23) |
1.59 (1.19–2.14) |
1.60 (1.19–2.15) |
|
| Region | |||||||||
| Northeast | Reference | ||||||||
| South | 1.30 (1.27–1.34) |
1.34 (1.29–1.38) |
1.29 (1.25–1.32) |
1.33 (1.29–1.37) |
1.35 (1.23–1.47) |
1.49 (1.34–1.65) |
1.32 (1.20–1.45) |
1.45 (1.30–1.62) |
|
| West | 1.12 (1.09–1.16) |
1.17 (1.12–1.21) |
1.13 (1.10–1.17) |
1.18 (1.14–1.22) |
1.13 (1.03–1.24) |
1.17 (1.05–1.30) |
1.14 (1.03–1.25) |
1.17 (1.05–1.30) |
|
| Midwest | 1.09 (1.05–1.13) |
1.12 (1.08–1.16) |
1.07 (1.03–1.11) |
1.10 (1.07–1.15) |
0.99 (0.89–1.10) |
1.07 (0.96–1.20) |
0.97 (0.88–1.08) |
1.05 (0.93–1.18) |
|
| LN | Gender | ||||||||
| Male | Reference | ||||||||
| Female | 3.92 (3.63–4.23) |
3.92 (3.64–4.23) |
3.92 (3.62–4.23) |
3.92 (3.63–4.23) |
6.12 (4.74–7.89) |
6.15 (4.77–7.93) |
6.07 (4.70–7.83) |
6.09 (4.72–7.86 |
|
| Race/Ethnicity | |||||||||
| White | Reference | ||||||||
| African American |
3.77 (3.56–3.99) |
3.84 (3.63–4.07) |
3.77 (3.56–4.00) |
3.82 (3.61–4.05) |
2.61 (2.25–3.04) |
2.62 (2.26–3.05) |
2.63 (2.26–3.06) |
2.61 (2.24–3.04) |
|
| Hispanic | 1.88 (1.75–2.03) |
1.96 (1.81–2.11) |
1.93 (1.79–2.09) |
1.98 (1.83–2.14) |
1.69 (1.39–2.06) |
1.70 (1.39–2.08) |
1.80 (1.47–2.01) |
1.77 (1.45–2.18) |
|
| Asian | 3.57 (3.23–3.95) |
3.74 (3.37–4.16) |
3.65 (3.29–4.04) |
3.78 (3.40–4.20) |
1.34 (0.96–1.88) |
1.33 (0.95–1.88) |
1.41 (1.01–1.99) |
1.37 (0.97–1.92) |
|
| Native American |
2.30 (1.90–2.78) |
2.25 (1.86–2.72) |
2.29 (1.89–2.77) |
2.26 (1.87–2.74) |
1.72 (0.94–3.14) |
1.67 (0.92–3.06) |
1.66 (0.90–2.05) |
1.62 (0.88–2.98) |
|
| Region | |||||||||
| Northeast | Reference | ||||||||
| South | 1.34 (1.26–1.43) |
1.41 (1.32–1.51) |
1.36 (1.28–1.45) |
1.43 (1.33–1.53) |
1.68 (1.42–1.98) |
1.80 (1.49–2.18) |
1.65 (1.39–1.97) |
1.73 (1.42–2.11) |
|
| West | 1.05 (0.98–1.13) |
1.04 (0.97–1.12) |
1.08 (1.00–1.15) |
1.07 (0.99–1.16) |
1.18 (0.99–1.41) |
1.08 (0.89–1.33) |
1.20 (1.00–1.43) |
1.09 (0.89–1.33) |
|
| Midwest | 1.14 (1.07–1.23) |
1.20 (1.11–1.30) |
1.15 (1.07–1.23) |
1.20 (1.11–1.30) |
1.22 (1.01–1.48) |
1.25 (1.02–1.54) |
1.19 (0.98–1.44) |
1.20 (0.97–1.48) |
|
SES=Socioeconomic status, by quartile
Rheum State = Average number of American College of Rheumatology member rheumatologists per state, by quartile
Discussion
Our study employed nationwide Medicaid claims data to investigate sociodemographic variation in SLE and LN, and the impact of SES and rheumatologist number on disease prevalence and incidence. We found the overall prevalence of SLE to be 143.7 per 100,000 adults, which is slightly greater than prior U.S.-based estimates (Table 1), but in line with the high-risk population included in this study. The prevalence of LN was 30.9 per 100,000, or 21.5% of SLE cases, with higher rates among all racial/ethnic groups compared to Whites. This LN prevalence, particularly among African Americans, (26.7% of SLE cases), is lower than prior estimates (16). This may be a reflection of the short follow-up period of this study, or of under-diagnosis among low-income patients with public insurance, previously shown to have limited access to subspecialty care (15). We found the incidence of SLE to be 23 per 100,000 person-years, notably higher than prior U.S.-based estimates, but consistent in multiple sensitivity analyses. Both prevalence and incidence of SLE and of LN varied considerably between demographically, socioeconomically and geographically defined subgroups.
A complex interplay of genetic, hormonal, environmental, and socioeconomic factors likely contributes to the incidence and prevalence of SLE, and to variations by gender, race/ethnicity and income level (24, 25). Genetic differences by race have been invoked to explain the younger age of onset of SLE among African Americans, and a higher prevalence of serologic abnormalities in African Americans compared to Whites (4, 7, 26). In addition to genetics however, occupational and environmental exposures may relate to the risk of SLE development (24, 25, 27–29). Low SES neighborhoods are often in closer proximity to hazardous wastes and experience more air pollution (30) and workplace exposures, particularly to silica dust, are more likely from manual labor jobs. Risk of SLE is likely higher among current cigarette smokers, and there are higher rates of smoking in poorer populations (31, 32). A relationship between psychosocial stress and the development of autoimmune diseases like SLE has been suggested (33) and there are known associations between residence in a high poverty neighborhood and increased stress (34).
The U.S. Medicaid population is a high poverty group, with significant racial and ethnic minority representation, and a considerable burden of chronic diseases. Prior studies have demonstrated that living in a low SES area confers increased risk of progressive chronic illnesses and an additional mortality risk (35–38). After stratifying Medicaid enrollees by SES quartile and adjusting for age, sex, race and ethnicity, we found that the quartile with the lowest county-level SES had the highest prevalence of both SLE and LN. Although there are no past studies of prevalence of SLE according to SES, a relationship between poverty and increased SLE severity and mortality, independent of race and ethnicity, has been demonstrated (2, 39–41). Chronic disease and poor health likely also contribute to lower SES because of disability, missed work, and decreased earning potential (42). The trend of decreasing prevalence with increasing SES was statistically significant for SLE but not for LN. This suggests that genetics may be a more important determinant in the development of LN compared to SLE. It is also possible that once individuals enter into care for their SLE, socioeconomic factors contribute less to disease complications.
Both SLE and LN incidence were not strongly related to county-level SES. Similarly, prevalence and incidence rate ratios were not significantly different after stratifying by sociodemographic group and adjusting by SES. The Medicaid population is enriched for lower SES compared to the overall U.S. population and therefore, variation by degree of poverty may be less pronounced. It is also plausible that the relatively small sample size of individuals in the stratified groups reduced the possibility of detecting subtle differences.
We also demonstrated a relationship between the average number of ACR member rheumatologists per state and the prevalence of SLE and LN, suggesting that access to subspecialty care may play a role in both diagnosis and management. Lower income, even when adjusted for health insurance status, has been associated with decreased access to rheumatologic care (43). A prior study showed that individuals who were older and poorer were significantly less likely to identify a rheumatologist as their primary SLE provider (43). African Americans, and women in particular, were also shown to have significantly fewer visits to a rheumatologist compared to White men (44). We found the lowest prevalence of SLE and LN in the areas with the fewest rheumatologists and a statistically significant trend of higher disease prevalence in areas with more rheumatologists. However, when adjusted for age, sex, race and ethnicity, this trend was no longer observed. Our analysis parallels a recent study that suggested that subspecialty care might modify the relationship between race and receipt of appropriate medications for individuals with rheumatoid arthritis (45).
In keeping with prior studies, our SLE prevalence and incidence estimates were significantly higher in women compared to men. The incidence of LN paralleled that of SLE; both six times higher in women than men; whereas the prevalence of LN was only three times higher in women than men. Male patients with lupus nephritis have more significant renal dysfunction and laboratory abnormalities than female patients (46, 47). Suspicion for SLE among health care providers may be higher for women, and they therefore may be diagnosed earlier with less severe disease. It is also possible that the stringent definition of LN we used restricted inclusion to the most severe cases and shifted the observed female to male ratio downward.
There are other limitations to administrative database analyses. A prior Canadian-based study utilized two SLE billing diagnoses at least two months apart to determine prevalence and incidence of SLE and demonstrated a sensitivity of 98.2% and a specificity of 72.5% for this method (48). In our study, we raised this to three billing diagnoses in an attempt to increase our specificity, to account for the inability to differentiate between provider specialties in this Medicaid database, and to exclude individuals who were seen for one “rule-out” SLE visit and once in follow-up. Given our more stringent definition of SLE, it is plausible that our higher prevalence of SLE compared to prior studies is a reflection of the increased disease burden in this high-risk population.
A validation study using adult Medicaid enrollees had an 80% positive predictive value for correctly identifying LN cases with the ICD-9 diagnostic codes we utilized (20). Both diagnoses of SLE and renal disease were required for classification as LN, although these diagnoses could occur simultaneously. Thus, it is possible, that cases were excluded, as they did not receive the combination of ICD-9 codes for both SLE and renal disease. While the prevalence of LN did not vary substantially when fewer diagnoses of SLE were required in a sensitivity analysis, some cases may have been missed given the duration of Medicaid enrollment necessary to accumulate the required codes.
Our incidence rates may be higher than in prior studies as only 5 years of billing claims data were available. Sensitivity analyses utilizing 24 to 36 months of prior disease-free continuous and non-continuous enrollment yielded similar incidence rates. Given fluctuations in Medicaid coverage however, it is possible that a proportion of these incident cases may be misclassified prevalent cases. These incident rates therefore reflect the number of new SLE cases without a prior diagnosis while enrolled in Medicaid during the study period, which is not necessarily generalizable to the incidence of SLE in the U.S. population.
Our study was also constrained by Medicaid’s pre-specified categories for race and ethnicity, which we had to further combine in related groups to allow for a sample size amenable to analysis. However, prior studies have shown different risks between individuals categorized within the same racial/ethnic group, for example, between African Americans of varying degrees of European ancestry, and among Hispanics with higher Native American ancestry (49). Crossover between the groups may have reduced even more significant variations.
In addition, we utilized data from the ACR to approximate the number of practicing rheumatologists per state. A prior study used a combination of American Medical Association records and ACR records and determined that in 2005, there were 5,164 total U.S. rheumatologists. In 2005, 4,146 (80.3%) were ACR members (50). To our knowledge, there were no significant temporal changes between 2000 and 2004 that would contribute to a different percentage of ACR membership compared to 2005. We do note however, that the percentage of rheumatologists who do not choose to be ACR members may not be evenly distributed geographically and this is a limitation of this study.
In summary, we have examined the incidence and prevalence of SLE and LN in a nationwide Medicaid database with information on nearly 24 million racially, ethnically and geographically diverse individuals. Patients from the lowest SES areas had the highest prevalence of disease. Areas with the fewest ACR member rheumatologists had the lowest prevalence, suggesting under-diagnosis and likely unequal access to treatment. Medicaid enrollees are among the poorest subset of the U.S. population. It is clear that increased resources need to be allocated to this group to target this heightened burden of chronic diseases.
Supplementary Material
Acknowledgements
Drs. Feldman and Costenbader had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Feldman, Hiraki, Costenbader
Acquisition of data: Feldman, Costenbader
Analysis and interpretation of data: Feldman, Hiraki, Liu, Winkelmayer, Alarcón, Solomon, Fischer, Costenbader
Drafting of the manuscript and critical revision for important intellectual content: Feldman, Hiraki, Liu, Fischer, Alarcón, Solomon, Winkelmayer and Costenbader.
Statistical analysis: Liu, Winkelmayer, Feldman, Costenbader
Study supervision: Costenbader
Supported by a grant from the National Institutes of Health (RO1 AR057327) awarded to Dr. Costenbader. The funding source played no role in the design, conduct, interpretation or report of these analyses.
Footnotes
Previous Presentation: This research was presented in part at the American College of Rheumatology Conference, November 2011.
Role of the sponsor: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number RO1 AR057327 (Dr. Costenbader). Dr. Feldman is currently funded by the Clinical Orthopedic and Musculoskeletal Education and Training Program, 5 T32 AR055885 (NIH/NIAMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. These funding sources played no role in the design, conduct, interpretation or report of these analyses. Dr. Costenbader obtained a Data Use Agreement from the Centers for Medicare and Medicaid Studies for these MAX data and results are presented in accordance with CMS Policy. CMS had no role in the design, conduct or interpretation of these analyses.
Disclosures: Drs. Feldman, Hiraki, Fischer, Liu, Alarcón, Winkelmayer and Costenbader have no disclosures. Dr. Solomon receives research grants from Amgen and Lilly, consults for CORRONA, and serves in unpaid roles in two Pfizer trials.
References
- 1.Fessel WJ. Systemic lupus erythematosus in the community. Incidence, prevalence, outcome, and first symptoms; the high prevalence in black women. Arch Intern Med. 1974;134(6):1027–1035. [PubMed] [Google Scholar]
- 2.Fernandez M, Alarcon GS, Calvo-Alen J, Andrade R, McGwin G, Jr, Vila LM, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum. 2007;57(4):576–584. doi: 10.1002/art.22672. [DOI] [PubMed] [Google Scholar]
- 3.Iseki K, Miyasato F, Oura T, Uehara H, Nishime K, Fukiyama K. An epidemiologic analysis of end-stage lupus nephritis. Am J Kidney Dis. 1994;23(4):547–554. doi: 10.1016/s0272-6386(12)80377-5. [DOI] [PubMed] [Google Scholar]
- 4.McCarty DJ, Manzi S, Medsger TA, Jr, Ramsey-Goldman R, LaPorte RE, Kwoh CK. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995;38(9):1260–1270. doi: 10.1002/art.1780380914. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56(6):2092–2094. doi: 10.1002/art.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochberg MC. The incidence of systemic lupus erythematosus in Baltimore, Maryland, 1970–1977. Arthritis Rheum. 1985;28(1):80–86. doi: 10.1002/art.1780280113. [DOI] [PubMed] [Google Scholar]
- 8.Uramoto KM, Michet CJ, Jr, Thumboo J, Sunku J, O'Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum. 1999;42(1):46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Maskarinec G, Katz AR. Prevalence of systemic lupus erythematosus in Hawaii: is there a difference between ethnic groups? Hawaii Med J. 1995;54(2):406–409. [PubMed] [Google Scholar]
- 10.Balluz L, Philen R, Ortega L, Rosales C, Brock J, Barr D, et al. Investigation of systemic lupus erythematosus in Nogales, Arizona. Am J Epidemiol. 2001;154(11):1029–1036. doi: 10.1093/aje/154.11.1029. [DOI] [PubMed] [Google Scholar]
- 11.Peschken CA, Esdaile JM. Systemic lupus erythematosus in North American Indians: a population based study. J Rheumatol. 2000;27(8):1884–1891. [PubMed] [Google Scholar]
- 12.Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology (Oxford) 2006;45(9):1144–1147. doi: 10.1093/rheumatology/kel039. [DOI] [PubMed] [Google Scholar]
- 13.Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int. 1997;51(4):1188–1195. doi: 10.1038/ki.1997.162. [DOI] [PubMed] [Google Scholar]
- 14.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63(6):1681–1688. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 2010;37(6):1158–1163. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastian HM, Roseman JM, McGwin G, Jr, Alarcon GS, Friedman AW, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11(3):152–160. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 17.Huong DL, Papo T, Beaufils H, Wechsler B, Bletry O, Baumelou A, et al. Renal involvement in systemic lupus erythematosus. A study of 180 patients from a single center. Medicine (Baltimore) 1999;78(3):148–166. doi: 10.1097/00005792-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Carreno L, Lopez-Longo FJ, Monteagudo I, Rodriguez-Mahou M, Bascones M, Gonzalez CM, et al. Immunological and clinical differences between juvenile and adult onset of systemic lupus erythematosus. Lupus. 1999;8(4):287–292. doi: 10.1191/096120399678847786. [DOI] [PubMed] [Google Scholar]
- 19.Foundation THJKF. State Health Facts. 2011 [Google Scholar]
- 20.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010;19(6):741–743. doi: 10.1177/0961203309356289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chronic Condition Data Warehouse: Medicaid Enrollment by Race. [Accessed October 2012]; http://www.ccwdata.org/summary-statistics/demographics/index.htm.
- 22.Bureau USC. [Accessed January 2011];United States Census. 2000 http://www.census.gov/main/www/cen2000.html.
- 23.Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J Rheumatol. 2007;34(10):2024–2027. [PubMed] [Google Scholar]
- 24.Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Parks CG, Gilkeson GS. Hormonal, environmental, and infectious risk factors for developing systemic lupus erythematosus. Arthritis Rheum. 1998;41(10):1714–1724. doi: 10.1002/1529-0131(199810)41:10<1714::AID-ART3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Zandman-Goddard G, Solomon M, Rosman Z, Peeva E, Shoenfeld Y. Environment and lupus-related diseases. Lupus. 2012;21(3):241–250. doi: 10.1177/0961203311426568. [DOI] [PubMed] [Google Scholar]
- 26.Ward MM, Studenski S. Clinical manifestations of systemic lupus erythematosus. Identification of racial and socioeconomic influences. Arch Intern Med. 1990;150(4):849–853. [PubMed] [Google Scholar]
- 27.Cooper GS, Wither J, Bernatsky S, Claudio JO, Clarke A, Rioux JD, et al. Occupational and environmental exposures and risk of systemic lupus erythematosus: silica, sunlight, solvents. Rheumatology (Oxford) 2010;49(11):2172–2180. doi: 10.1093/rheumatology/keq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlson EW, Watts J, Signorovitch J, Bonetti M, Wright E, Cooper GS, et al. Effect of glutathione S-transferase polymorphisms and proximity to hazardous waste sites on time to systemic lupus erythematosus diagnosis: results from the Roxbury lupus project. Arthritis Rheum. 2007;56(1):244–254. doi: 10.1002/art.22308. [DOI] [PubMed] [Google Scholar]
- 29.Green FH, Yoshida K, Fick G, Paul J, Hugh A, Green WF. Characterization of airborne mineral dusts associated with farming activities in rural Alberta, Canada. Int Arch Occup Environ Health. 1990;62(6):423–430. doi: 10.1007/BF00379058. [DOI] [PubMed] [Google Scholar]
- 30.Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- 31.Flint AJ, Novotny TE. Poverty status and cigarette smoking prevalence and cessation in the United States, 1983–1993: the independent risk of being poor. Tob Control. 1997;6(1):14–18. doi: 10.1136/tc.6.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costenbader KH, Karlson EW. Cigarette smoking and systemic lupus erythematosus: a smoking gun? Autoimmunity. 2005;38(7):541–547. doi: 10.1080/08916930500285758. [DOI] [PubMed] [Google Scholar]
- 33.Stojanovich L, Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmun Rev. 2008;7(3):209–213. doi: 10.1016/j.autrev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Schulz AJ, Israel BA, Zenk SN, Parker EA, Lichtenstein R, Shellman-Weir S, et al. Psychosocial stress and social support as mediators of relationships between income, length of residence and depressive symptoms among African American women on Detroit's eastside. Soc Sci Med. 2006;62(2):510–522. doi: 10.1016/j.socscimed.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Merkin SS, Coresh J, Diez Roux AV, Taylor HA, Powe NR. Area socioeconomic status and progressive CKD: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2005;46(2):203–213. doi: 10.1053/j.ajkd.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Winkleby MA, Cubbin C. Influence of individual and neighbourhood socioeconomic status on mortality among black, Mexican-American, and white women and men in the United States. J Epidemiol Community Health. 2003;57(6):444–452. doi: 10.1136/jech.57.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrell LN, Diez Roux AV, Rose K, Catellier D, Clark BL. Neighbourhood characteristics and mortality in the Atherosclerosis Risk in Communities Study. Int J Epidemiol. 2004;33(2):398–407. doi: 10.1093/ije/dyh063. [DOI] [PubMed] [Google Scholar]
- 38.Diez Roux AV, Borrell LN, Haan M, Jackson SA, Schultz R. Neighbourhood environments and mortality in an elderly cohort: results from the cardiovascular health study. J Epidemiol Community Health. 2004;58(11):917–923. doi: 10.1136/jech.2003.019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alarcon GS, McGwin G, Jr, Bastian HM, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum. 2001;45(2):191–202. doi: 10.1002/1529-0131(200104)45:2<191::AID-ANR173>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Duran S, Apte M, Alarcon GS. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc. 2007;99(10):1196–1198. [PMC free article] [PubMed] [Google Scholar]
- 41.Lotstein DS, Ward MM, Bush TM, Lambert RE, van Vollenhoven R, Neuwelt CM. Socioeconomic status and health in women with systemic lupus erythematosus. J Rheumatol. 1998;25(9):1720–1729. [PubMed] [Google Scholar]
- 42.Kington RS, Smith JP. Socioeconomic status and racial and ethnic differences in functional status associated with chronic diseases. Am J Public Health. 1997;87(5):805–810. doi: 10.2105/ajph.87.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yazdany J, Gillis JZ, Trupin L, Katz P, Panopalis P, Criswell LA, et al. Association of socioeconomic and demographic factors with utilization of rheumatology subspecialty care in systemic lupus erythematosus. Arthritis Rheum. 2007;57(4):593–600. doi: 10.1002/art.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odutola J, Ward MM. Ethnic and socioeconomic disparities in health among patients with rheumatic disease. Curr Opin Rheumatol. 2005;17(2):147–152. doi: 10.1097/01.bor.0000151403.18651.de. [DOI] [PubMed] [Google Scholar]
- 45.Solomon DH, Ayanian JZ, Yelin E, Shaykevich T, Brookhart MA, Katz JN. Use of disease-modifying medications for rheumatoid arthritis by race and ethnicity in the National Ambulatory Medical Care Survey. Arthritis Care Res (Hoboken) 2012;64(2):184–189. doi: 10.1002/acr.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soni SS, Gowrishankar S, Adikey GK, Raman A. Sex-based differences in lupus nephritis: a study of 235 Indian patients. J Nephrol. 2008;21(4):570–575. [PubMed] [Google Scholar]
- 47.de Carvalho JF, do Nascimento AP, Testagrossa LA, Barros RT, Bonfa E. Male gender results in more severe lupus nephritis. Rheumatol Int. 2010;30(10):1311–1315. doi: 10.1007/s00296-009-1151-9. [DOI] [PubMed] [Google Scholar]
- 48.Bernatsky S, Linehan T, Hanly JG. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J Rheumatol. 2011;38(8):1612–1616. doi: 10.3899/jrheum.101149. [DOI] [PubMed] [Google Scholar]
- 49.Wassel CL, Jacobs DR, Jr, Duprez DA, Bluemke DA, Sibley CT, Criqui MH, et al. Association of self-reported race/ethnicity and genetic ancestry with arterial elasticity: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Soc Hypertens. 2011;5(6):463–472. doi: 10.1016/j.jash.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deal CL, Hooker R, Harrington T, Birnbaum N, Hogan P, Bouchery E, et al. The United States rheumatology workforce: supply and demand, 2005–2025. Arthritis Rheum. 2007;56(3):722–729. doi: 10.1002/art.22437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.