Abstract
The study of human genetic disorders and mutant mouse models has provided evidence that genome maintenance mechanisms, DNA damage signalling and metabolic regulation cooperate to drive the ageing process. In particular, age-associated telomere damage, diminution of telomere ‘capping’ function and associated p53 activation have emerged as prime instigators of a functional decline of tissue stem cells and of mitochondrial dysfunction that adversely affect renewal and bioenergetic support in diverse tissues. Constructing a model of how telomeres, stem cells and mitochondria interact with key molecules governing genome integrity, ‘stemness’ and metabolism provides a framework for how diverse factors contribute to ageing and age-related disorders.
Medical advances over the past century have produced a near doubling of human life expectancy in industrialized countries. This longevity boom — due primarily to improved neonatal care, to prevention and treatment of infections and to interventions for cardiovascular and endocrine diseases — is expected to lead to a population of 1.2 billion individuals aged 60 years or older by the year 2025. For medical technology to have a meaningful impact on the continued health and wellbeing of this ageing population, there is an increasingly urgent need for a more complete understanding of the molecular pathways and biological processes underlying ageing and age-related disorders.
Our progress in understanding the details of the ageing process has been hampered by issues such as the gradual and heterogeneous onset of tissue and organismal decline, the complexity and diversity of phenotypic presentations, and the lack of adequate biomarkers capable of quantifying the ‘degree’ of ageing at the molecular and cellular levels. Further complicating matters, the pace of mammalian ageing is influenced by external factors such as dietary intake, intrinsic stresses such as reactive oxygen species (ROS) and telomere erosion, and germline variation in genetic elements governing DNA repair, among other processes1,2. Studies in organisms ranging from yeast to primates have highlighted the importance of metabolic flux through the phosphatidylinositol-3-OH kinase (PI(3)K) pathway, as well as the activity of the master metabolic regulators such as sirtuins, the preservation of genome integrity by means of an efficient DNA repair and oxidative defence apparatus, the levels of genotoxic stress resulting from eroded telomeres or excessive ROS, the activation of the p53 and p16 tumour suppressor pathways, the robustness of mitochondrial reserve or function, and the integrity of a normal extracellular milieu and cytokine profile2–4. However, how these pathways and processes act together either to promote or to retard the ageing process is not entirely clear.
Increasing evidence points to telomeres and p53-mediated DNA damage signalling being core components that drive the senescent or apoptotic depletion of tissue stem-cell reserves and age-related tissue degeneration. Although such cellular checkpoint mechanisms contribute to the functional decline of highly proliferative tissues, how they would adversely affect the more quiescent tissues that are equally ravaged by the ageing process (such as heart, brain and liver) has been more difficult to rationalize. Here, we put forth a speculative model that posits a connection linking telomere damage and p53 activation with stem-cell and mitochondrial dysfunction. This model offers a unifying explanation of how telomeres influence the health of the ageing organism across diverse tissues with wide-ranging proliferative profiles.
The ageing phenotype
Regardless of the precise underlying molecular mechanisms, the fundamental defining manifestation of ageing is an overall decline in the functional capacity of various organs to maintain baseline tissue homeostasis and to respond adequately to physiological needs under stress3,5. In many aged tissues, these declines are gradual and modest in middle years but accelerate rapidly late in life and become particularly apparent under conditions that challenge the organism to overcome various stressors with a robust physiological or regenerative response. At the anatomical and physiological levels, diminished tissue cellularity and inadequate regenerative responses seem to be intimately linked to many of the classic age-associated medical maladies, such as muscle atrophy, anaemia, feeble immune responses and impaired wound healing (Box 1).
Box 1. Organ-specific changes associated with advancing age.
The anatomical and physiological changes associated with advancing age emerge with variable onset, pace and severity in individuals, and they affect organs and tissue types both with highly mitotic profiles and with quiescent profiles. The hallmarks of ageing include loss of muscle mass (sarcopenia), decreased musculoskeletal mobility, reduction in bone mass (osteoporosis), and thinning and reduced elasticity of skin (wrinkling). The ageing haematopoietic system exhibits progressive normocytic anaemia and altered immune-cell profiles such as increasing CD8+ /CD4+ T-cell ratios and accumulating myeloid cells in the bone marrow. Other tissues, such as the brain, liver and kidneys, experience a low degree of cellular loss and overall reduction in mass. These age-progressive tissue cytoarchitectural changes are intimately linked to a functional (physiological) decline at baseline, a marked inability to mount responses to various stresses and an increased risk of disease development. This functional decline is most prominent in the musculoskeletal system, where there are decreased activity levels, strength and range of motion owing to stiffness and development of arthropathies. Additionally, healing and proliferative stress responses are universally blunted in the aged, as evidenced by impaired wound repair on injury or surgery and by feeble immune-cell proliferative responses in the face of infection. Aged individuals also show a decreased capacity of the liver and kidneys to metabolize drugs and a diminished cardiovascular reserve driven by declines in maximal heart rates, cardiac contractility and lung capacity91.
In accordance with this functional decline, age is the major risk factor for the development of chronic medical conditions. In the United States, nearly 50% of individuals over the age of 65 develop cardiovascular disease; 35% develop arthropathies; 15% develop type 2 diabetes; and 10% develop pulmonary disease. Some of these conditions are particularly debilitating and extract a significant social and economic toll. Stroke and dementia, the most common causes of long-term institutionalization of individuals over 65, cost the US health-care system alone $21 billion per year — a cost projected to rise by 14%, to $24 billion, in 2010 (ref. 6). Advancing age is also the major risk factor for the development of cancer. Specifically, between ages 40 and 80, there is a rapid increase in cancer incidence that produces an overall lifetime cancer risk of nearly one in two individuals in industrialized nations7. Thus, diseases of the aged collectively constitute the major causes of human suffering and consume the bulk of our health-care resources. From this perspective, the need to understand the nature of ageing and how its consequences can be managed, and possibly reversed, has never been greater.
Tissue stem cells and age-related disorders
Our bodies possess a remarkable ability for extensive and sustained tissue renewal throughout a lifetime. This continuous self-renewal capacity is maintained by reservoirs of somatic tissue stem cells8,9. These tissue stem cells have garnered increasing attention in ageing and regenerative research given accumulating evidence that age-associated physiological decline, particularly in highly proliferative organs, parallels blunted proliferative responses and misdirected differentiation of resident tissue stem cells (Fig. 1). At the same time, these long-lived renewable reservoirs may also negatively affect the health of aged individuals by providing a preferred cellular compartment for malignancy 9.
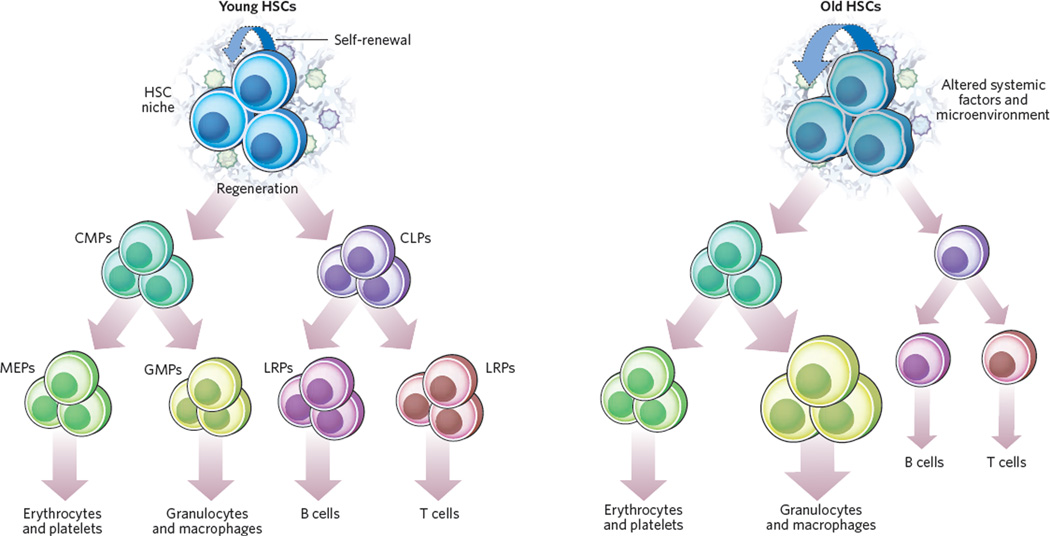
Figure 1. Haematopoietic stem cells experience functional decline with ageing.
Major differences between young (left) and old (right) haematopoietic stem cells (HSCs) are shown, exemplifying general mechanisms that may occur with age in stem cells, including decreased regenerative potential and dysregulated differentiation. Cell-extrinsic and cell-intrinsic factors contribute to the overall functional decline of ageing HSCs. Although the self-renewal capacity might be increased in aged HSCs, there is decreased functional regenerative capacity, particularly under stress conditions. Importantly, aged HSCs have an altered differentiation programme with reduced output of common lymphoid progenitors (CLPs), whereas common myeloid progenitors (CMPs) are produced at the same rate as by young HSCs. The decrease in numbers of CLPs and mature B and T cells (‘immunosenescence’) is in contrast to the increased frequency of common granulocyte–macrophage progenitors (GMPs) and, consequently, granulocytes and macrophages. Numbers of megakaryocyte–erythrocyte progenitors (MEPs) are not altered. Possible cell-extrinsic alterations relevant for HSC function include altered stromal compositions and altered cytokine profiles that favour specific differentiation programmes such as decreased lymphopoiesis and increased myelopoiesis. Other age-related changes could affect osteoblast and endothelial cells that have been shown to modulate HSC function. The relevance of systemic factors in modulating stem-cell function has been shown in parabiosis studies: the regenerative capacity of muscle satellite cells in aged mice was increased by exposure to the circulatory system of young mice through the restoration of Delta–Notch signalling20. LRPs, lineage-restricted progenitors.
Tissues have widely varying levels of baseline proliferative activity and regenerative potential. In high-turnover tissues, resident stem cells have been documented to generate large numbers of specialized cell progeny and thereby maintain tissue cellularity and functionality over a lifetime. In tissues with lower proliferative or regenerative capacity, such as the heart, the identification of stem cells has been more difficult, although their existence in some low-proliferative-capacity organs such as the brain has been documented10. Intuitively, it would seem reasonable to posit that preserving an adequate pool of tissue stem cells with robust potential for renewal would be vital to maintaining organ function with advancing age. Such a view is supported by the premature ageing phenotypes observed in mice with conditional deletion of the genes encoding ATR (ataxia telangiectasia and Rad3 related), FOXO transcription factors or ATM (ataxia telangiectasia mutated); these mice exhibit tissue stem-cell defects or diminished oxidative defence and ROS-induced stem-cell depletion (Box 2). Although these genetic models are suggestive, a major question remains about whether numerical or functional decline in tissue stem cells contributes to declining health in the elderly and underlies at least some aspects of ageing. In this regard, an agerelated analysis of well-defined stem-cell types in three organ systems — haematopoietic, neural and muscular — with varying regenerative profiles has proven particularly instructive.
Box 2. Genetic models of stem-cell compromise and ageing.
Adult mice lacking ATR show an initial pronounced depletion of tissue-specific stem and progenitor cells in the skin, intestine and bone marrow, among other systems, resulting in widespread tissue atrophy. Within months, rare ATR-proficient stem cells restore sufficient tissue function, but the animals still experience diverse ageing phenotypes, consistent with the possibility that intensive regenerative demands can precipitate premature ageing; however, perturbations of the stem-cell niche cannot be ruled out92. FOXO proteins, which are regulators of lifespan in less-evolved organisms, can regulate expression of oxidative defence genes that detoxify intracellular ROS21,22. Their deletion in the mouse germline causes progressive depletion of haematopoietic stem cells (HSCs) and neural stem cells (NSCs). The FOXO-deficient HSCs show poor transplant potential, and the FOXO-deficient NSCs show poor renewal potential in vitro and decreased ability to support the production of new neurons in the brain21,22. Similarly, mice deficient in ATM show progressive bone marrow failure in adulthood. HSC failure in both the ATM and FOXO mutant models is shown to be mediated by increased concentrations of ROS, as treatment with antioxidative agents rescues HSC function and bone marrow failure22,93.
Haematopoietic system
The ageing human and murine haematopoietic systems experience a well-documented age-dependent functional decline of stem cells due to cell-autonomous and microenvironmental (niche) factors9. These haematopoietic system changes coincide with alterations across all blood effector lineages. There is decreased competence in the innate immune system (decreased natural-killer activity, decreased phagocytic ability of neutrophils and macrophages, and a pro-inflammatory state) and the adaptive immune system (decreased numbers of naive B and T cells, and increased numbers of memory B and T cells), as well as expansion of the myeloid lineage and mild to moderate normocytic anaemia11. Most notably, retrospective analysis of bone marrow transplantations in patients has established that donor age is the only parameter significantly associated with survival of the transplant recipients, and this evidence is consistent with a cell-autonomous compromise in the function of haematopoietic stem cells (HSCs) from aged donors12. Importantly, these observations in ageing humans are supported by studies in ageing mice that clearly document a decrease in the number of functionally competent HSCs, as well as a preferred differentiation towards myeloid lineages over lymphoid lineages through a cell-autonomous process9.
Neural system
In the human and mouse brain, continued neurogenesis has been documented throughout adulthood. These new neurons derive from neural stem cells (NSCs) residing in the subventricular zone and in the subgranular zone of the hippocampal dentate gyrus13. Progeny of stem cells located in the subventricular zone travel through the rostral migratory stream into the olfactory bulb to differentiate into interneurons, whereas progeny from the subgranular zone of the dentate gyrus migrate to the granular layer, where they integrate with its resident cells13. Although a few studies have demonstrated that the human brain experiences a decline in neurogenesis with ageing, much of our understanding of age-associated changes in neurogenesis comes from rodent studies. Old mice (>2 years of age) experience a twofold reduction in the number of NSCs relative to young mice, a loss that mirrors the respective numbers of neurospheres derived from young and old mouse brains14,15. Accompanying this age-associated decline is an overall drop in the number of newly generated neurons, a drop that has apparent functional consequences. For example, in the ageing mouse brain, the decline in neurogenesis is associated with an impairment of olfactory discrimination due to the presence of fewer NSCs in the subventricular zone and, thus, a diminished ability to replenish the olfactory epithelium continually16.
Muscular system
Skeletal muscle also experiences a decline in regenerative potential with ageing, which is manifested by decreased generation of myogenic fibres and their replacement with fibrous tissue in humans, as well as in invertebrate species17. Diminished regeneration is most apparent after injury, with a protracted and incomplete recovery in aged humans18. The regenerative impairment is thought to result from compromised resident stem cells, the so-called satellite cells. With ageing, there are documented decreases in proliferative and differentiation capacity, as well as possible decreases in numbers of satellite stem cells in both mice and humans17,19. Age-associated changes in satellite cells seem to be driven predominantly by an ‘aged microenvironment’ because the functional capacity of satellite cells from aged muscles can be fully restored if exposed to the milieu of young muscles in parabiotic (surgically conjoined) mice20.
Molecular pathways in ageing
Mechanistic analyses of stem cells from these three representative tissues have linked several molecular pathways to age-associated changes. First is the PI(3)K pathway, which can profoundly alter both the numbers and activity of stem cells and is strongly linked to ageing and lifespan regulation. For example, disruption of FOXO proteins, which are key downstream components of the PI(3)K pathway, results in the ROS-induced depletion of tissue stem cells21,22. Similarly, deletion of another downstream pathway component, TSC1 (a negative regulator of mammalian target of rapamycin (mTOR)), causes profound HSC failure in association with increased ROS concentrations and inappropriate mobilization from the bone marrow niche23. The importance of this TSC1–mTOR signalling axis has been validated at the pharmacological and genetic levels: the hyperproliferative HSC phenotype and consequent bone marrow failure in Pten knockout mice can be reversed by treatment with rapamycin, an mTOR inhibitor24, and the deletion in mice of the gene encoding ribosomal protein S6 kinase 1 (Rps6kb1), a downstream mediator of mTOR activity, can extend lifespan with decreased age-related pathologies25.
Second are the DNA repair pathways. Specifically, mice that are defective in DNA repair capacity age prematurely and experience neuro-degeneration and loss of various stem-cell compartments, including HSCs, although the precise nature of the affected DNA repair pathway dictates the outcome. For example, nucleotide excision repair defects lead to progeroid syndromes, whereas mismatch repair defects increase the cancer risk but do not produce accelerated ageing. DNA damage in the form of telomere dysfunction has also been shown to precipitate premature ageing and diminish lifespan, causing widespread tissue atrophy with depletion of almost all tissue stem-cell reserves examined. This loss of stem cells is due largely to p53-mediated proliferative arrest, senescence and/or apoptotic elimination, as discussed in detail below26,27.The relevance of p53 activation in ageing is reinforced by florid premature ageing phenotypes and diminished stem-cell function in certain strains of mice engineered with germline alleles of hyperactive mutant p53 (the gene encoding p53, which is also known as Trp53)28,29.
Third are cellular mortality pathway molecules such as the p16 tumour suppressor, which, judging from increased p16 expression in aged human and mouse tissues, including in HSCs, NSCs and islet β-cells in mice30, seems to be involved in the ageing process. Accordingly, mice lacking p16 show enhanced HSC, NSC and islet β-cell function with age30. Last are the mitochondrial pathways. There is increasing recognition that intact mitochondrial function is crucial for the maintenance of stem cells, as evidenced by compromised HSC function, profound anaemia and lymphopenia in mice expressing a mutant form of mitochondrial DNA polymerase-γ that is associated with increased mitochondrial DNA mutations31. Recent evidence also indicates that loss of BMI1, a negative regulator of the Ink4a/Arf locus (also known as Cdkn2a), is associated with decreased mitochondrial function manifesting as decreased electron transport flux, resulting in increased ROS concentrations and stem-cell compromise32. ROS have also been shown to accelerate telomere shortening and activate the p53–p21 axis, as well as modulate signalling pathways essential for maintaining HSC and NSC quiescence, as revealed by loss of oxidative defence in the setting of FOXO deficiency 21,33,34.
In summary, studies derived mainly from animal models but also from corroborating human correlative studies have demonstrated that stem cells of different organs show compromised proliferative and differentiation potential with advancing age and that this may not be reflected in a decrease in their numbers. This functional decline parallels compromised organ function, inadequate physiological responsiveness under stress and increased incidence of disease. Mechanistically, a wide range of genetic factors and pathways seem to contribute to the ageing process through their regulation of stem-cell and mitochondrial function. It is particularly notable that many of these genetic factors connect one way or another to genotoxic stress and the response to such DNA damage, as we discuss below.
Telomeres and ageing
Telomeres are specialized structures that adorn the ends of human chromosomes. Early studies showed the essential role of telomeres in the integrity of chromosomes35–37. These nucleoprotein caps are maintained by the enzyme telomerase38–40. The importance of ad equate telomerase activity and maintenance of telomere length for both replicative potential in culture and ageing in organisms was initially inferred from studies of primary human fibroblasts. In culture, division of fibro-blasts results in progressive telomere attrition, culminating in a state of proliferative arrest — or cellular senescence — after a finite number of cell divisions, a barrier known as the Hayflick limit41. Proliferation beyond this limit drives further telomere erosion, ultimately triggering rampant chromosomal instability driven by chromosome breakage–fusion–breakage events42.
In a seminal series of decisive studies, enforced expression of TERT, the catalytic subunit of telomerase, in cultured human fibroblasts stabilized telomere length and endowed the cells with unlimited replicative potential without engendering malignant properties43,44. The remarkable capacity of experimentally induced telomerase activity to circumvent senescence and allow indefinite growth has been documented in many other human cell types. These compelling cell culture studies and complementary studies in telomerase knockout mice (see below) have since inspired significant efforts to determine whether telomere dynamics bear relevance to the processes of ageing and/or various degenerative diseases in humans.
Human population studies have correlated decreased telomere length in peripheral blood leukocytes with higher mortality rates in individuals who are more than 60 years old; however, a recent large cohort study found no such correlation but did report a positive link between telomere length and years of healthy life45,46. By contrast, a recent study in centenarians and their offspring found a positive link between telomere length and longevity; in particular, those with longer telomeres had an overall improved health profile (with decreased age-associated disease and better cognitive function and lipid profiles) relative to controls47. Corroborating these observations in humans are recent studies showing evidence, albeit preliminary, that telomeres are also relevant to normal ageing in mice (Box 3).
Box 3. Relevance of telomeres to normal ageing in wild-type mice.
In Tert transgenic mice on a cancer-resistant background (a mouse model made by introducing extra copies of wild-type p53 and the Ink4a/Arf gene locus), it was shown that TERT overexpression can result in an increase in median lifespan, accompanied by a more youthful profile characterized by increased epidermal thickness, reduced dermatitis, reduced gastritis and enteritis with improved intestinal and epidermal barrier function in vivo, as well as enhanced clonogenic activity of epidermal stem cells in vitro94. Although telomere dysfunction is presumably not present in this model, it is reasonable to speculate that TERT overexpression operates to stabilize telomeres in epidermal stem cells, suggesting a role for telomere length in stem-cell activity in wild-type mice. Indeed, recent studies in wild-type mice have accumulated evidence, albeit preliminary evidence, supporting the thesis that telomere shortening is important for ageing of these mice. These studies have shown that telomere shortening does occur in mice of both the Mus spretus (with short telomeres) and the Mus musculus C57BL/6 (with longer telomeres) strains across different tissues, to variable degrees95,96. In the M. musculus C57BL/6 strain, telomere shortening occurs precipitously when mice reach the age of two years and affects differentiated, as well as progenitor-cell, populations, leading to decreased functional capacity96. Furthermore, telomerase overexpression in mice with long telomeres prevents age-related telomere shortening and delays associated functional decline in epidermal and intestinal stem cells, although a telomere-length-independent mechanism of TERT overexpression has not been ruled out68,94. Finally, with increased telomere shortening, CAST/EiJ mice with naturally occurring short telomeres (∼15 kilobases) that are either haploinsufficient or intact for telomerase succumb to degenerative decline in highly regenerative organs, including the intestine (villous atrophy) and testes (hypocellularity) and in the haematopoietic system (pancytopenia)97,98.
With respect to the role of telomeres in disease, a remarkable twist comes from the observations that telomere reserves parallel the levels of psychological stress and risk for development of psychiatric disease48–50. In women aged 20–50 years, those with the highest levels of psychological stress had the shortest telomeres and the lowest telomerase activity in peripheral blood leukocytes, and showed the highest levels of oxidative stress49. The connection is particularly intriguing because individuals subject to chronic psychological stress show a shortened lifespan and more rapid onset of diseases typically associated with ageing, such as cardiovascular disease and accelerated ageing of the immune system50. The activation of autonomic and neuroendocrine systems, and the subsequent glucocorticoid-driven increase in ROS, might underlie accelerated telomere erosion and damage telomeres directly51,52. More speculatively, such damaged telomeres may not be repaired efficiently, owing to low levels of telomerase activity and an inherent shielding of telomeres from DNA repair machinery. As such, damaged telomeres may provide a reservoir of persistent DNA damage signals and consequent sustained p53 activation with senescent sequelae.
The importance of telomeres in the maintenance of a healthy human lifespan is also inferred from the study of a variety of inherited degenerative disorders. For instance, patients with autosomal dominant dyskeratosis congenita are now known to carry mutations in either the catalytic, TERT, component of telomerase or in the gene, TERC, that encodes its RNA template53. These patients have shortened telomeres and reduced lifespan, and they show signs of accelerated ageing and bone marrow failure with increased risk of life-threatening infections53. Additional evidence of the relevance of telomeres to the ageing process comes from the analysis of patients, and derivative cells, afflicted with premature-ageing conditions such as Werner syndrome and ataxia telangiectasia (Box 4). However, TERC or TERT mutations have been linked to more organ-restricted dysfunction as well, for instance to idiopathic pulmonary fibrosis (a progressive fatal lung disease with excessive scarring)54 and bone marrow failure syndromes55, highlighting the complex and tissue-specific manifestation of telomere length maintenance defects. Beyond inherited genetic disorders, telomeres also seem to be relevant to ‘acquired’ degenerative conditions associated with chronically elevated tissue turnover. The most notable example is liver cirrhosis, the seventh most common cause of death by disease in humans worldwide56, which shows a progressive decline in telomere reserves with increased heaptocyte turnover, leading to sustained hepatocyte proliferative arrest and apoptosis culminating in liver failure.
Box 4. Premature ageing (progeroid) syndromes associated with telomere dysfunction.
A number of single-gene mutations have been shown to induce premature ageing to variable degrees. These genetic syndromes can be classified into two categories on the basis of whether they affect multiple organs and tissues (segmental progeroid syndromes) or predominantly a single organ (unimodal progeroid syndromes). Segmental progeroid syndromes include Werner syndrome, ataxia telangiectasia, dyskeratosis congenita, Hutchinson–Gilford progeria syndrome and Bloom syndrome. Unimodal progeroid syndromes include familial Alzheimer's disease, familial Parkinson's disease and attenuated familial polyposis99.
Werner syndrome is a rare autosomal recessive disorder that affects 1 in 100,000 individuals and results from a deficiency of the WRN protein, which is a RecQ DNA helicase involved in DNA repair, DNA recombination and telomere maintenance. The hyper-recombination and multiple chromosomal aberrations in Werner syndrome cells suggest that accelerated ageing and increased cancer susceptibility stem from the failure to suppress illegitimate recombination events and global genome instability. Importantly, Werner syndrome fibroblasts show accelerated telomere attrition and undergo premature senescence that can be rescued by enforced TERT expression. These patients develop normally until puberty, at which time they stop growing and begin to manifest multiple progressive premature ageing pathologies, including senile cataracts, osteoporosis, skin atrophy, hair greying, myocardial infarction and cancer. These patients have a markedly decreased life expectancy (with a median of 47–48 years) mainly owing to development of myocardial infarction and cancer99.
Ataxia telangiectasia is an autosomal recessive disorder that causes the development of progressive cerebellar degeneration, skin abnormalities (telangiectasia, atrophy, pigmentary abnormalities and hair greying), immunodeficiency and a wide range of malignant neoplasms. The disease results from various crippling mutations in the ATM gene, which has key roles in DNA damage signalling, DNA repair and telomere maintenance. Ataxia telangiectasia cells that experience marked telomere attrition show increased chromosomal instability and premature senescence that can be rescued by enforced TERT expression99.
Strikingly, the Wrn-null and Atm-null mouse models both fail to show the classic degenerative pathologies observed in the human conditions. However, once WRN-deficient or ATM-deficient mice are placed on a telomerase-deficient background with limiting telomeres, these mice exhibit a host of degenerative pathologies associated with accelerated ageing. Such observations emphasize the fact that limiting telomeres are prominent rate-limiting pathogenetic elements governing the onset of age-related phenotypes in these genetic disorders62.
Taken together, evidence from the study of a wide range of human degenerative diseases, both inherited and acquired, points to limiting telomeres as key pathogenetic elements driving degenerative pathologies, increasing cancer risk and shortening lifespan. In this light, the sizes of telomere reserves and their level of persistent damage or, better, measurements of their capping status may prove useful as biomarkers of disease progression and may offer new opportunities for proactive therapeutic interventions involving transient somatic activation of endogenous telomerase to replenish or repair telomeres.
The telomerase knockout mouse
The in vivo relevance and specific roles of telomeres in the processes of ageing, degenerative diseases and cancer development were initially defined by the generation and characterization of laboratory mice null for either the Terc or Tert gene. Remarkably, mice null for either Terc or Tert seemed healthy and phenotypically unaffected, establishing that telomerase activity is dispensable for life57,58. This notable lack of a phenotype was postulated to be due to generous telomere reserves that retain capping function in first-generation (G1) Terc−/− mice57. Indeed, successive generations of Terc−/− mice had critically short telomeres and chromosomal end-to-end fusions. Coincident with cytogenetic evidence of telomere dysfunction (signal-free ends and end-to-end fusions), late-generation (G3 and beyond) Terc−/− mice had a shortened lifespan, overall frailty, decreased fecundity and tissue atrophy with impaired organ function59 (Fig. 2). The widespread tissue degenerative phenotypes included anaemia and lymphopenia, kyphosis and osteoporosis, mild glucose intolerance and other classic age-related phenotypes26. The severity of these degenerative phenotypes parallels the degree of telomere dysfunction in successive Terc−/− generations, as measured by the number of chromosomal fusions and anaphase bridges and, in a more recent study, the number of DNA damage foci at telomeres60,61. Moreover, the relevance of telomeres to the ageing process was reinforced by the strikingly muted ageing phenotypes of mice engineered with classic human degenerative-disease mutations (for example WRN and AT M), unless such mutant mice were rendered telomerase deficient and possessed short, limiting telomeres62,63.
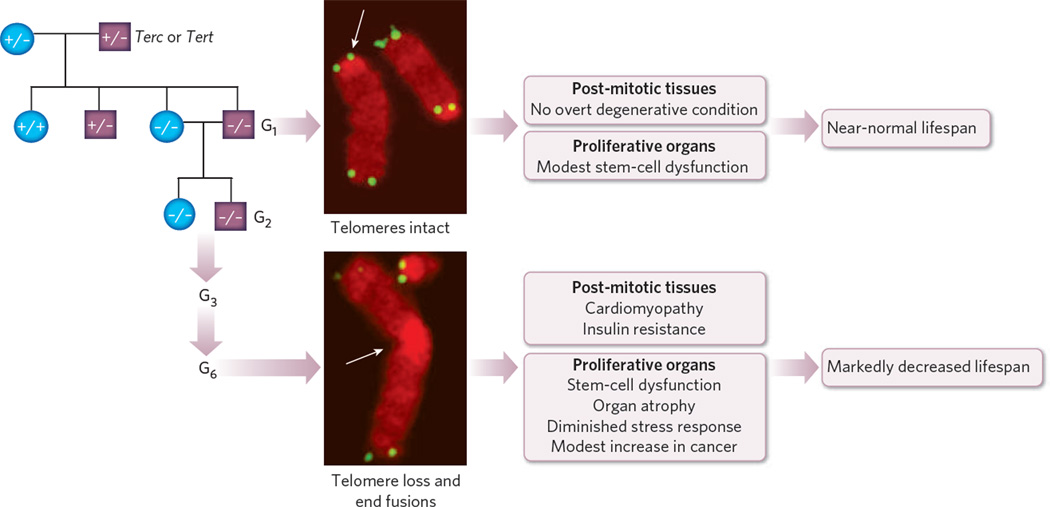
Figure 2. Telomerase knockout mice with dysfunctional telomeres develop premature ageing.
Telomerase knockout mice (G1) are viable, with intact chromosomes, and have minor physiological abnormalities in the case of long telomeres (top image; arrow); however, with advanced age, they develop degenerative symptoms sooner than do age-matched mice with wild-type Terc . Continuous interbreeding of telomerase knockout mice leads to subsequent generations of mice (G2, G3 and so on) with telomeres of decreasing length. Mice with dysfunctional telomeres have chromosomal abnormalities (bottom image; arrows point to loss of telomere signal, resulting in fused chromosomes), and they develop multiple ageing-associated degenerative disorders in highly proliferative organs, as well as in post-mitotic tissues. Highly proliferative organs such as the intestine, skin and testes are characterized by atrophic changes indicating stem-cell-based failure. Functional decline in post-mitotic tissues (such as cardiomyopathy) and age-associated metabolic changes (such as insulin resistance) have been noted in mice with dysfunctional telomeres. Such mice have a shortened lifespan and a modest increase in cancer, in line with the role of telomeres in preventing illegitimate recombination events.
What are the underlying mechanisms of these striking telomere-associated degenerative phenotypes? For highly proliferative organs that rely on resident tissue stem cells for organ renewal and homeostasis, there is clear evidence that reserves of stem and progenitor cells across various tissue types of the late-generation Terc−/− mice are depleted and/or functionally compromised through various processes such as increased apoptosis, senescence and impaired differentiation. For example, the degree of intestinal atrophy in aged, late-generation Terc−/− mice parallels high levels of p53-dependent apoptosis in the stem-cell-rich and progenitor-cell-rich crypt compartment26. Additionally, late-generation Terc−/− HSCs have been shown to be severely disadvantaged in competitive transplantation experiments and show pronounced differentiation towards the myeloid lineage, a profile similar to that in aged humans64,65. In the skin, late-generation Terc−/− epidermal stem cells are also impaired in their proliferative capacity and ability to move out of the hair follicle, defects that may underlie delayed wound healing, hair greying and hair loss in these mice66. Even the brain, an organ of relatively low proliferative activity, shows a compromise of NSC renewal and differentiation capacity in late-generation Terc−/− mice63,67. The molecular pathways that sense telomere dysfunction and activate cellular checkpoint responses leading to these stem-cell defects and tissue degeneration phenotypes are not completely understood, although p53 has been shown to have a prominent role (see below).
Telomerase and stem-cell homeostasis
Although much of our understanding of telomerase in degenerative conditions and cancers has been rooted in studies of telomerase knockout mice and focused on its telomere capping function, there is emerging evidence of a role for telomerase in stem-cell biology that does not involve its telomere maintenance function. In a series of transgenic studies, enforced TERT expression in the skin was shown to activate quiescent hair-follicle stem cells and stimulate hair growth. This TERT-induced stem-cell activation effect is not related to TERT’s classic telo mere synthesis activities, as it can occur on a telomerase-deficient background (Terc−/−) or with an enzymatically inactive TERT transgene68. These observations point to telomere-independent function of TERT in tissue stem-cell homeostasis, an area that gains added significance in light of the ability of human TERT to bind the RNA component (RMRP) of the ribonucleoprotein endoribonuclease (the RNase MRP complex)69. In the context of our model, this report is intriguing because the RNase MRP complex is involved in diverse cellular and mitochondrial functions; moreover, patients with mutations in RMRP develop cartilage–hair hypoplasia syndrome that is characterized by premature multi-organ failure mainly in highly proliferative organs, consistent with possible stem-cell failure. Thus, there is a growing appreciation that the activities of TERT beyond telomere maintenance may affect age-relevant processes linked to optimal mitochondrial and stem-cell function.
The p53 tumour suppressor and genome maintenance
The importance of genome integrity in ageing is apparent from genetic studies of patients with defects in the DNA repair machinery. Genetic studies in mice have reinforced the importance of DNA repair processes in ageing and degenerative diseases. The essential role of p53 signalling in response to DNA damage and consequent degenerative ageing phenotypes is underscored by the rescue — in fact the near-complete reversal — of these phenotypes on its deletion.
Genome maintenance
Syndromes with defective genome maintenance are often associated with accelerated ageing in humans. Mice engineered with analogous mutations demonstrate an essential role for intact DNA repair machinery in stem-cell maintenance and ageing processes. In young mice deficient in KU80 (also known as XRCC5) or XPD (also known as ERCC2), HSCs do not show significant DNA damage or functional decline; however, HSCs from the corresponding aged mice show an accumulation of DNA damage foci and are significantly compromised in their repopulation capacity in the transplantation setting64. The relevance of these experimental findings to normal ageing is not clear, although it is notable that greater numbers of DNA damage foci and increased functional impairment are observed in HSCs of aged wild-type mice64. Several other engineered mouse lines with defects in the DNA repair machinery, including mice with a hypomorphic ligase IV allele and mice in which Msh2 and Fancd1 (also known as Brca2) have been knocked out, also experience increased DNA damage and stem-cell decline8. Hyperactivation of the DNA damage response pathway, apparently in the absence of excess DNA damage, can also induce stem-cell attrition, as demonstrated in mice possessing a hypermorphic Rad50 allele; these mice experience bone marrow failure by four weeks of age as a result of increased apoptosis70. A recent study indicates that ionizing-radiation-induced DNA damage leads to melanocyte stem-cell attrition, although in this cell type the basis for depletion relates to accelerated exit from quiescence and enhanced differentiation rather than to apoptosis and senescence71. Furthermore, it is worth noting that some DNA repair proteins, including RAD50, KU70 or KU80, ATM and WRN, are essential for telomere maintenance. One notable protein that is essential for DNA stability and repair is SIRT6, a member of the sirtuin family of proteins that associates with telomeres. The deletion of the gene encoding SIRT6 induces telomere dysfunction with end-to-end chromosomal fusions, resulting in increased cellular senescence, pronounced acceleration of ageing phenotypes and a life expectancy of only three weeks72,73. Together, these engineered mouse models with defective DNA repair machinery provide convincing genetic evidence linking maintenance of genome integrity to the process of organismal ageing.
The p53 tumour suppressor
As ‘guardian’ of the genome, p53 is a major cellular stress sensor that is activated in response to DNA damage such as telomere dysfunction and to other adverse stimuli such as ROS, oncogene activation and hypoxia. The cellular consequences of p53 activation include growth arrest and repair or apoptosis and senescence, depending on the degree of activation74. In keeping with this role, germline deletion of p53 in mice with critically short telomeres results in a marked decrease in apoptosis and a significant increase in proliferation across many tissues27. The reversal of cellular attrition in the setting of p53 deficiency correlates with improved functionality in organs such as the testes, intestine and skin and in the haematopoietic system. In the skin of late-generation Terc−/− p53−/− mice, there is improved wound healing, hair growth and skin renewal, to levels comparable to those of wild-type mice, as well as increased epidermal stem-cell numbers and improved mobilization capacity75. Similarly, late-generation Terc−/− p53−/− HSCs show improved repopulation capacity in transplantation studies64. Collectively, these data indicate that the p53-dependent telomere checkpoint operates in diverse stem-cell types.
Although p53 deletion improves stem-cell function in mice with short dysfunctional telomeres, it does not enhance their overall longevity, owing to an increased incidence of cancer. In contrast to lategeneration Terc−/− p53+/+ mice, late-generation Terc−/− p53−/− (or p53+/−) mice showed increased tumour incidence and, importantly, an altered tumour spectrum76. Instead of the typical lymphoma-dominated and sarcoma-dominated tumour spectrum in p53−/− mice with intact telomeres, telomere-dysfunctional Terc−/− p53−/− mice developed epithelial cancers of the skin, gastrointestinal tract and breast, a spectrum highly reminiscent of that seen in the aged population in humans76. The impact of p53 deficiency on ageing and cancer phenotypes of telomere-dys-functional mice is in contrast to the impact of deletion of Ink4a/Arf, which encodes the cyclin-dependent kinase inhibitor p16INK4A and the p53 activator p19ARF (ref. 30). Ink4a/Arf deletion did not attenuate the degenerative phenotypes elicited by telomere dysfunction nor lead to an increase in the number of epithelial tumours. Instead, the late-generation Terc−/− Ink4a/Arf−/− mice succumbed to the lymphoma and sarcoma spectrum expected for Ink4a/Arf−/− mice, albeit with a longer latency77. These contrasting studies not only underscore how the genetic context can dramatically dictate the role of telomeres in ageing and cancer but also pinpoint the importance of p53, as the preservation of DNA-damage-induced signalling and p53 activation may underlie the lack of a rescue with Ink4a/Arf deficiency77. Indeed, p53 activation and associated increased apoptosis has been documented in tissues of lategeneration Terc−/− Ink4a/Arf −/− mice that show testicular and intestinal atrophy, as well as impaired haematopoiesis77. Whether these defects are specifically associated with depletion of tissue stem cells is not yet known, but they warrant further investigation.
The complete lack of attenuation in the telomere-driven degenerative phenotypes in the setting of Ink4a/Arf deficiency was somewhat at odds with the reported role of the Bmi1–Ink4a/Arf axis in stem-cell maintenance30, as Ink4a/Arf deficiency has been shown to rescue the HSC–NSC depletion phenotype of Bmi1 deficiency and specific loss of p16INK4A through targeted mutation of Ink4a can enhance the regenerative potential of ageing HSCs, NSCs, lymphocytes and islet β-cells30. That said, the stem-cell depletion phenotype of Bmi1 deficiency has been linked not only to upregulated Ink4a/Arf expression (BMI1 is a repressor of the Ink4a/Arf locus) but also to increased mitochondrial dysfunction that results in increased ROS concentrations and subsequent activation of the DNA damage response pathway32. Thus, it is tempting to speculate that these increased ROS concentrations in the setting of Bmi1 deficiency may further drive telomere damage and erosion, and the consequent enhanced p53 activation, thereby overriding any ameliorative impact of Ink4a/Arf deficiency on the stem-cell and progenitor-cell compartments of late-generation Terc−/− Ink4a/Arf−/− mice. The molecular basis of how Bmi1–Ink4a/Arf regulates mitochondrial biology remains an important area for continued investigation.
Further evidence of a role for activated p53 in organismal ageing comes from two genetically engineered mouse alleles of hyperactive mutant p53: such mice experience premature ageing and are significantly cancer resistant28,29. As they age, different tissues derived from these hyper- p53 mice develop increased numbers of senescent cells; furthermore, their HSCs exhibit decreased activity in competitive transplants, as well as decreased engraftment capacity78. These observations are in line with the capacity of p53 deficiency to rescue many accelerated ageing phenotypes in various models engineered with deletion of Terc (as above), Brca1, Zmpste24 (a mouse model for Hutchinson–Gilford progeria syndrome, which is a human progeroid syndrome) or Ku80 (refs 79, 80); see Box 4 for other progeroid syndromes. However, transgenic mice carrying one extra copy of the intact (wild-type) p53 locus, the Ink4a/Arf locus or both are found to have a normal or extended lifespan (up to 16% longer), with delayed onset of the ageing phenotype, a result that has been attributed to lower ROS concentrations and ROS-related damage to proteins and lipids81. Further highlighting the complexity of p53 signalling, mice with a hypomorphic allele of Mdm2, the major negative regulator of p53, have not been reported to develop overt signs of premature ageing in the presence of enhanced p53 activity82. Finally, inactivation of p53 may not be sufficient to sustain viability in the setting of extremely severe telomere dysfunction and rampant chromosomal stability83,84.
The two apparently divergent aspects of p53 function — pro-ageing and anti-ageing — are probably non-exclusive and relate to the activity levels of p53, the kinetics of its activation and the cellular or genetic context. Although deletion of p53 is able to reverse many of these cellular and organismal ageing phenotypes, the beneficial impact of p53 inactivation on ageing and stem cells is offset by the increased risk of cancer. These results highlight the need for an improved understanding of the circuitry of the p53-mediated checkpoint network, as such insights may provide avenues for improved management of the ageing consequences while suppressing the development of cancer. Along these lines, it is notable that deletion of p21Cip, a p53 target and a negative cell-cycle regulator, attenuates tissue degeneration in a number of organs in late-generation Terc−/− mice without increasing the risk of cancer65. Thus, further systematic dissection of the network of p53 may illuminate novel therapeutic avenues for the optimal management of ageing and cancer risks.
Perspectives
Genotoxic stress — particularly from damaged telomeres — would seem, on the surface, less relevant as a basis for age-progressive functional decline in relatively quiescent organs, such as the heart and liver. However, cardiomyopathy is a prominent phenotype in the late-generation Terc−/− mouse model85. Thus, to describe these highly metabolic organs, we propose a model whereby mitochondrial dysfunction underlies an escalating cycle of genotoxic damage leading to, in turn, p53 activation, mitochondrial dysfunction, increased ROS concentrations and, consequently, further DNA damage2. In particular, decreasing mitochondrial reserves and function as documented in aged human and mouse tissues would result in increased ROS production, owing mainly to complex I and complex III dysfunction. This boost in ROS production would set in motion a detrimental cycle of increased genotoxic damage with rapid erosion of telomeres, followed by sustained activation of p53, further mitochondrial decline, more ROS generation and so on34. This downward spiral might also explain the cumulative and precipitative nature of ageing symptoms observed late in life.
We note that the cellular response to p53 activation during this cycle of damage and response might be dependent on threshold concentrations of ROS. Under conditions of low oxidative stress, p53 activation preferentially induces expression of antioxidant genes; however, when ROS production is high, p53 instead activates pro-oxidant genes86. This contrasting action of p53 might allow either cell-cycle arrest and repair under conditions of modest DNA damage or more robust cellular responses of senescence or apoptosis and/or mitochondrial dysfunction in cells with more substantial DNA damage, thus leading to tissue atrophy and functional decline74.
In this genotoxic stress model of ageing (Fig. 3), the core telomere–p53 axis integrates well with almost all genetic elements proven to be important in the ageing process. First, it accounts for the premature ageing phenotypes common to both telomere-dysfunctional mice and those with germline p53 hyperactivation28,29. Second, it explains why mice lacking SIRT1 or SIRT6 — proteins that attenuate p53 activity — develop premature ageing87. Third, it could account for the observed connections between mitochondria and key ageing factors such as PGC-1α, PGC-1β, FOXO proteins and BMI1; mice null for each of the genes encoding these proteins experience accelerated tissue degeneration and mitochondrial dysfunction.
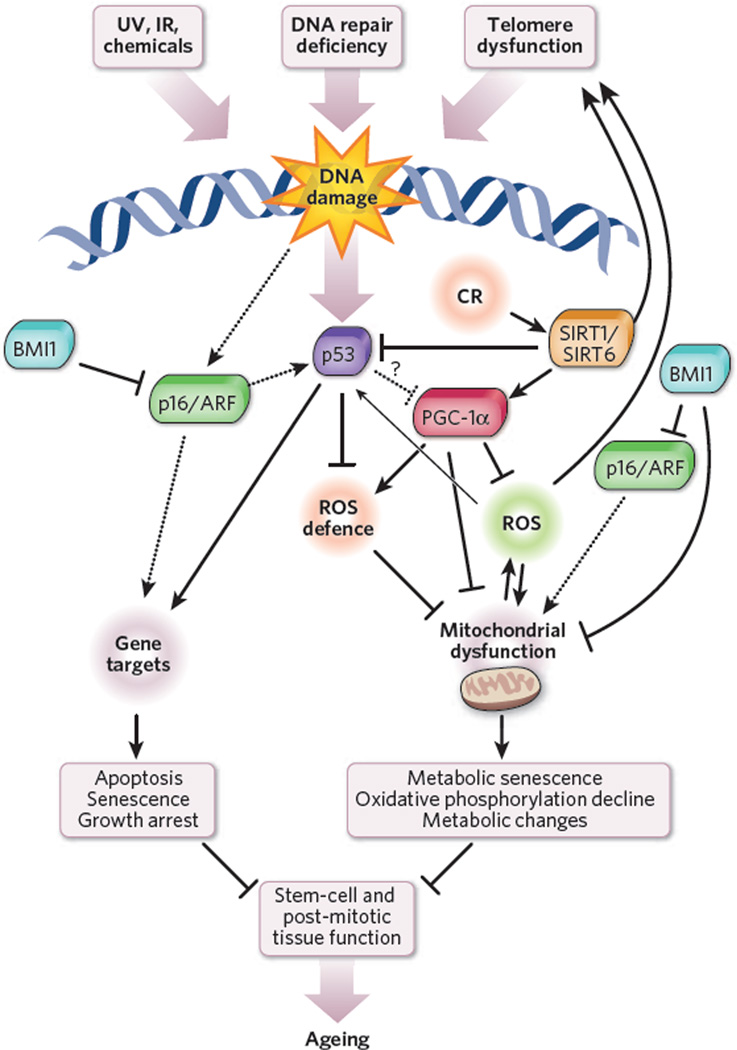
Figure 3. A model of interaction between DNA damage, p53 activation and mitochondrial dysfunction.
In this model, genotoxic stress brought about by telomere attrition, impaired DNA repair, ultraviolet (UV) radiation, ionizing radiation (IR), chemicals, ROS and other mechanisms activates p53 and induces cellular growth arrest (in proliferating compartments), senescence or apoptosis. We also propose that p53 can impair mitochondrial function either directly or indirectly (through regulation of ROS-detoxifying enzymes). This p53-mediated mitochondrial dysfunction triggers a cycle of DNA damage, p53 activation, mitochondrial compromise and increased ROS levels leading to additional DNA damage, and so on. The mitochondrial compromise could contribute to organ dysfunction through decreased ATP generation, as well as changes in mitochondrial metabolism. The interplay between p53 and other pathways implicated in ageing is also indicated. Caloric restriction (CR) activates SIRT1, which decreases p53 activity. Also, SIRT1 (and possibly SIRT6) activates PGC-1α and boosts mitochondrial biogenesis2. PGC-1α increases antioxidant defence through upregulation of antioxidants89, whereas p53 has been shown to increase or decrease the expression of antioxidants depending on cellular ROS concentrations86. BMI1 loss has been shown to induce mitochondrial dysfunction directly and induce upregulation of p16/ARF (refs 30, 32). ARF increases p53 activity through interaction with MDM2, the negative regulator of p53.
The integration of mitochondria into this core ‘axis of ageing’ is supported by the premature ageing conditions shared by telomere-dysfunctional or hyper-p53 mice, as well as mice that have excessive mitochondrial DNA mutation or are deficient in PGC-1α or PGC-1β, which are the master regulators of mitochondrial biogenesis and metabo-lism88–90, although the precise molecular basis for this commonality in premature ageing phenotypes remains to be elucidated. The marked decline in the function of largely post-mitotic organs in the telomer-ase knockout mouse prompts us to speculate that activated p53 may result in a decline in mitochondrial biogenesis and/or function through mechanisms that are as yet unknown. The identification of the specific molecular components and mechanisms linking p53 and mitochondrial dysfunction would provide a unifying basis for a central axis of ageing, linking genotoxic stress to stem-cell compromise, mitochondrial decline and, ultimately, organ atrophy, functional decline and the diminished energy production that typifies essentially all aspects of cellular and physiological decline in ageing organisms. Indeed, this mitochondrial perspective gains added appeal when one considers that a hallmark feature of ageing is generalized frailty, which may derive from a fundamental inability to produce adequate levels of cellular ATP. This genotoxic stress model of ageing emphasizes the need to develop a better understanding of the factors that influence telomere erosion, the genes that protect cells against ROS-induced damage, the signals that regulate p53 activity and the pathways that maintain mitochondrial reserves and function. It remains to be established how these various pathways are differentially activated during ageing and how they might be interconnected. Deciphering these networks could yield ageing biomarkers and advance therapeutic strategies (including stabilization of telomeres through transient telomerase reactivation, p53 modulation, improvement in mitochondrial function and biogenesis, and modulation of the mTOR and PI(3)K pathways) designed to rejuvenate both proliferating and quiescent tissues in the aged.
Acknowledgements
We thank L. Chin, N. Sharpless, S. Artandi, F. Muller, V. Walsh, S. Colla, M. Ugolotti, M. Jaskelioff, D. Liu and A.-J. Chen for discussions and critical reading of the manuscript. A. Protopopov and E. Ivanova kindly provided the chromosomal and telomere analysis in Fig. 2. We apologize to the members of the scientific community whose work could not be cited owing to space limitations. Grant support for our work was provided by National Institutes of Health grants RO1CA84628 and U01 CA84313, US Department of Defense grant W81XWH-08-1-0133, and the Ellison Medical Foundation. R.A.D. is an American Cancer Society Research Professor and is supported by the Robert A. and Renée E. Belfer Foundation Institute for Innovative Cancer Science. E.S. was supported by the Deutsche Forschungsgemeinschaft.
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Guarente L. Mitochondria — a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 4.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Arrighi HM, McLaughlin T, Leibman C. Prevalence and impact of dementia-related functional limitations in the United States, 2001 to 2005. Alzheimer Dis. Assoc. Disord. doi: 10.1097/WAD.0b013e3181a1a87d. (in the press) [DOI] [PubMed] [Google Scholar]
- 7.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 8.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nature Rev. Mol. Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 9.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 12.Kollman C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 13.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 14.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molofsky AV, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerletti M, Shadrach JL, Jurga S, Sherwood R, Wagers AJ. Regulation and function of skeletal muscle stem cells. Cold Spring Harb. Symp. Quant. Biol. 2008;73:317–322. doi: 10.1101/sqb.2008.73.054. [DOI] [PubMed] [Google Scholar]
- 18.Di Iorio A, et al. Sarcopenia: age-related skeletal muscle changes from determinants to physical disability. Int. J. Immunopathol. Pharmacol. 2006;19:703–719. doi: 10.1177/039463200601900401. [DOI] [PubMed] [Google Scholar]
- 19.Gopinath SD, Rando TA. Aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 20.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 21.Paik JH, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Gan B, et al. mTORC1-dependent and -independent regulation of stem cell renewal differentiation, and mobilization. Proc. Natl Acad. Sci. USA. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yilmaz OH, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 25.Selman C, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudolph KL, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. This paper describes the effect of critically short telomeres on lifespan and stress response in telomerase knockout mice. Mice with short telomeres have a reduced lifespan and a diminished regenerative capacity when stressed, such as by wound healing or haematopoietic ablation.
- 27.Chin L, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. This paper shows that p53 mediates the cellular response to telomere dysfunction in both normal and neoplastic cells and that p53 deficiency ameliorates degenerative phenotypes.
- 28.Maier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyner SD, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. References 28 and 29 describe the pro-ageing phenotype in mice with hyperactive p53
- 30.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Chen ML, et al. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood. 2009;114:4045–4053. doi: 10.1182/blood-2008-08-169474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. This paper describes how in mice deficient in BMI1, mitochondrial dysfunction increases ROS levels and activates the DNA damage response, which can be partially rescued by antioxidant treatment.
- 33.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Passos JF Saretzki G, von Zglinicki T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 2007;35:7505–7513. doi: 10.1093/nar/gkm893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClintock B. The behavior in successive nuclear divisions of a chromosome broken at meiosis. Proc. Natl Acad. Sci. USA. 1939;25:405–416. doi: 10.1073/pnas.25.8.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29:245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 37.Shampay J, Szostak JW, Blackburn EH. DNA sequences of telomeres maintained in yeast. Nature. 1984;310:154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- 38.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 39.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. In this seminal paper, telomerase activity and its ability to lengthen a (TTGGGG)n oligonucleotide is identified in Tetrahymena cell extracts.
- 40.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 41.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 42.Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 43.Bodnar AG, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. This seminal paper showed that reintroduction of telomerase into human retinal epithelial cells and fibroblasts prevents senescence and immortalizes human cells
- 44.Counter CM, et al. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl Acad. Sci. USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 46.Njajou OT, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J. Gerontol. A. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atzmon, et al. GatGenetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl Acad. Sci. USA. 2010;107(suppl. 1):1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epel ES, et al. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epel ES, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Simon NM, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp. Gerontol. 2005;40:466–472. doi: 10.1016/j.exger.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Oexle K, Zwirner A. Advanced telomere shortening in respiratory chain disorders. Hum. Mol. Genet. 1997;6:905–908. doi: 10.1093/hmg/6.6.905. [DOI] [PubMed] [Google Scholar]
- 53.Kirwan M, Dokal I. Dyskeratosis congenita, stem cells and telomeres. Biochim. Biophys . Acta. 2009;1792:371–379. doi: 10.1016/j.bbadis.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armanios MY. et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 56.Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253–1258. doi: 10.1126/science.287.5456.1253. [DOI] [PubMed] [Google Scholar]
- 57.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. This paper follows from the McClintock hypotheses, providing experimental proof that telomerase activity has an essential role in maintaining telomeres and preventing end-to-end recombination, using telomerase knockout mice.
- 58.Farazi PA, Glickman J, Horner J, Depinho RA. Cooperative interactions of p53 mutation, telomere dysfunction, and chronic liver damage in hepatocellular carcinoma progression. Cancer Res. 2006;66:4766–4773. doi: 10.1158/0008-5472.CAN-05-4608. [DOI] [PubMed] [Google Scholar]
- 59.Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 60.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 61.Hande MP, Samper E, Lansdorp P, Blasco MA. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J. Cell Biol. 1999;144:589–601. doi: 10.1083/jcb.144.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang S, et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nature Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 63.Wong KK, et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature. 2003;421:643–648. doi: 10.1038/nature01385. This paper demonstrates that ATM deficiency and telomere dysfunction act together to impair stem-cell and progenitor-cell reserves and negatively affect cellular and whole-organism viability.
- 64.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. This paper shows that accumulated DNA damage in HSCs impairs their regenerative capacity, and it provides evidence that ageing-associated functional stem-cell decline is linked to DNA damage.
- 65.Choudhury AR, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nature Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 66.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 67.Ferron S, et al. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development. 2004;131:4059–4070. doi: 10.1242/dev.01215. [DOI] [PubMed] [Google Scholar]
- 68.Sarin KY, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maida Y, et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature. 2009;461:230–235. doi: 10.1038/nature08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morales M, et al. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev. 2005;19:3043–3054. doi: 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inomata K, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 72.Mostoslavsky R, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 73.Michishita E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vousden KH, Lane DP. p53 in health and disease. Nature Rev. Mol. Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 75.Flores I, Blasco MA. A p53-dependent response limits epidermal stem cell functionality and organismal size in mice with short telomeres. PLoS ONE. 2009;4:e4934. doi: 10.1371/journal.pone.0004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Artandi SE, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 77.Khoo CM, Carrasco DR, Bosenberg MW, Paik JH, Depinho RA. Ink4a/Arf tumor suppressor does not modulate the degenerative conditions or tumor spectrum of the telomerase-deficient mouse. Proc. Natl Acad. Sci. USA. 2007;104:3931–3936. doi: 10.1073/pnas.0700093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chambers SM, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donehower LA, Lozano G. 20 years studying p53 functions in genetically engineered mice. Nature Rev. Cancer. 2009;9:831–841. doi: 10.1038/nrc2731. [DOI] [PubMed] [Google Scholar]
- 80.Matheu A, Maraver A, Serrano M. The Arf/p53 pathway in cancer and aging. Cancer Res. 2008;68:6031–6034. doi: 10.1158/0008-5472.CAN-07-6851. [DOI] [PubMed] [Google Scholar]
- 81.Matheu A, et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 82.Mendrysa SM, et al. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 2006;20:16–21. doi: 10.1101/gad.1378506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nature Rev. Mol. Cell Biol. 2007;8:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- 84.Begus-Nahrmann Y, et al. p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nature Genet. 2009;41:1138–1143. doi: 10.1038/ng.426. [DOI] [PubMed] [Google Scholar]
- 85.Leri A, et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sablina AA, et al. The antioxidant function of the p53 tumor suppressor. Nature Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trifunovic A, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 89.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. In this paper, the authors demonstrate that PGC-1α is a strong positive regulator of the ROS defence system and that PGC-1α deficiency leads to ROS-induced neurodegeneration.
- 90.Vianna CR, et al. Hypomorphic mutation of PGC-1β causes mitochondrial dysfunction and liver insulin resistance. Cell Metab. 2006;4:453–464. doi: 10.1016/j.cmet.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vijg J. Aging of the Genome: The Dual Role of DNA in Life and Death. Oxford Univ. Press; 2007. [Google Scholar]
- 92.Ruzankina Y, et al. Deletion of the developmentally essential gene AT R in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ito K, et al. Regulation of oxidative stress by AT M is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 94.Tomas-Loba A, et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 95.Coviello-McLaughlin GM, Prowse KR. Telomere length regulation during postnatal development and ageing in Mus spretus. Nucleic Acids Res. 1997;25:3051–3058. doi: 10.1093/nar/25.15.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flores I, et al. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22:654–667. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Armanios M, et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am. J. Hum. Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hao LY, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 99.Martin GM. Genetic modulation of senescent phenotypes in Homo sapiens. Cell. 2005;120:523–532. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]