Abstract
Peroxisome proliferator-activated receptor-gamma co-activator 1α (PGC-1α) and PTEN-induced putative kinase 1 (PINK1) are powerful regulators of mitochondrial function. Here, we report that a previously unrecognized, novel 35 kDa PGC-1α isoform localizes to the mitochondrial inner membrane and matrix in brain as determined by protease protection and carbonate extraction assays, as well as by immunoelectron microscopy. Immunoelectron microscopy and import experiments in vitro revealed that 35 kDa PGC-1α colocalizes and interacts with the voltage-dependent anion channel (VDAC), and that its import depends on VDAC. Valinomycin treatment which depolarizes the membrane potential, abolished mitochondrial localization of the 35 kDa PGC-1α. Using blue native-PAGE, co-immunoprecipitation, and immunoelectron microscopy analyses, we found that the 35 kDa PGC-1α binds and colocalizes with PINK1 in brain mitochondria. This is the first report regarding mitochondrial localization of a novel 35 kDa PGC-1α isoform and its association with PINK1, suggesting possible regulatory roles for mitochondrial function in the brain.
Keywords: mitochondria, PGC-1α, PINK1, brain, VDAC
1. Introduction
PGC-1α and PINK1 have recently emerged as key molecules for the maintenance of mitochondrial integrity potentially linking metabolic abnormality and neurodegeneration. Recent studies have indicated an important role of PGC-1α in neurodegenerative diseases and diabetes [1–4]. The PGC- 1α null mouse displays severe systemic mitochondrial and metabolic abnormalities, and spongiform neurodegeneration [5]. Consistent with this observation, PGC-1α overexpression protects neurons against oxidative stress by inducing antioxidant enzymes [6]. Down-regulation of PGC-1α is associated with mitochondrial dysfunction and impaired lipid metabolism in obesity and diabetes [4], both of which increases the risk of neurodegenerative diseases.
PINK1 dysfunction is implicated in both neurodegenerative diseases and diabetes. Loss-of-function mutations in PINK1 have been linked to PD [7]. Polymorphisms in PINK1 are associated with altered plasma fatty acid concentration and oxidative energy metabolism in diabetes [8]. PINK1 transcription is suppressed in diabetes and associated with its severity [9]. PINK1 depletion causes mitochondrial dysfunction, increased oxidative stress, and neuronal inactivity, while its overexpression protects cells from these abnormalities [10–11].
Here, we show that a novel 35 kDa PGC-1α isoform localizes to the mitochondrial inner membrane and matrix, and associates with PINK1 within brain mitochondria. This result suggests a possible role of mitochondrial 35 kDa PGC-1α and PINK1 for regulating mitochondrial function in brain.
2. Materials and Methods
2.1. Animals
All animal procedures were approved by the Institutional Animal Care and Use committee at the University of Maryland School of Medicine and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The PINK1 knockout mouse was provided by Dr. Shen Jie at Havard Medical School [11].
2.2. siRNA Transfection
For depletion of VDAC, or PGC-1α in human embryonic kidney cells (HEK) cells, the following siRNAs were tested: VDAC siRNA-1, 5′-GAAUACCGACAAUACACUATT-3′ (Ambion, s14768); VDAC siRNA-2, 5′-GCGCTTCGGAATAGCAGCCAA-3′ (Qiagen, S103220399); PGC-1α siRNA-1, 5′-GAGAAUUCAUGGAGCAAUA-3′ (Dharmacon); PGC-1α siRNA-2 (sc-38884, Santa Cruz Biotechnology). Scrambled siRNAs with no known mammalian homology (non-targeting siRNA #1 (Ambion) and #2 (Santa Cruz Biotechnology)) were used as negative controls. HEK cells were transfected with the siRNAs using the Mirus TransIT-TKO® reagent (Mirus, Madison, WI) according to the manufacture’s manual. Untransfected cells were used as controls for all the experiments.
2.3. Isolation of mitochondria from mouse brain and cells
Hippocampal tissue of mouse brain was homogenized in isolation medium (225 mmol mannitol, 75 mmol sucrose, 5 mmol HEPES, 1 mmol EGTA, pH 7.4). The homogenate was centrifuged at 1,300 × g for 3 min, and the pellet will be re-suspended and centrifuged again at 1,300 × g for 3 min. The pooled supernatants will be centrifuged at 21,200 × g for 10 min, the crude mitochondrial pellet was re-suspended in 15% Percoll, and layered on a pre-formed gradient of 40% and 24% Percoll. After centrifugation at 31,700 × g for 8 min, the mitochondria were collected from the interface of the lower two layers, diluted with isolation medium and centrifuged at 16,700 × g for 10 min. The Percoll-purified mitochondrial pellet was used for co-immunoprecipitation assays and blue native (BN)-PAGE analyses. For the separation of mitochondria and other organelles, SH-SY5Y cells were homogenized and fractionated using mitochondria/cytosol fractionation kit (BioVision, CA) according to the manufacturer’s protocol.
2.4. Two-dimensional blue-native/SDS gel electrophoresis
Purified mitochondrial proteins (100 μg) were solubilized with 150 μl of buffer (50 mM NaCl, 50 mM imidazole/HCl, 2 mM 6-aminohexanoic acid, 1 mM EDTA, pH 7.0) containing a digitonin to a final concentration of 5 g per g protein. After incubation for 30 min on ice, samples were centrifuged at 20,000 × g for 20 min and were supplemented with 7 μl of 5% (w/v) Coomassie-Blue G-250 solution in 750 mM aminocaproic acid. Dye-treated protein samples were loaded onto 4–12% native gels followed by second-dimensional 4–20% SDS-PAGE. The gels were subjected to Western blot analysis.
2.5. Protease protection assay
Reaction mixtures contained 50 μg of isolated mitochondria in 50 μl of mitochondrial isolation buffer (225 mmol mannitol, 75 mmol sucrose, 5 mmol HEPES, 1 mmol EGTA, pH 7.4). Where indicated, samples were treated with 200 μg/ml proteinase K (for 25 min at 0°C) before stopping the reactions with 2 mM phenylmethylsulfonyl fluoride (PMSF). Trypsin was added to an estimated protein/enzyme ratio of approximately 50:1. The reaction mixture was incubated overnight at 4°C, and then the protease was inactivated with 2 mM PMSF. Samples were washed three times with 1× PBS and centrifuged, and the pellets were resuspended in protein extraction buffer, followed by Western blot analysis.
2.6. Carbonate extraction assay
The mitoplast was prepared followed by carbonate extraction assay as previously described [12]. Mitochondria were resuspended in buffer A (10 mM phosphate buffer, pH 7.4, 1 mM ethylene glycol-bis (β-aminoethyl ether)N,N,N,N-tetraacetic acid (EGTA), 1 mM PMSF, using a Dounce homogenizer, further diluted to a protein concentration of approximately 0.1 mg/ml and kept on ice for 20 min. Mitoplasts were resuspended in buffer A containing 8.6% (w/v) sucrose and recovered from the 33%/47% interphase, washed and resuspended in buffer B (20 mM 3-(N-morpholino) propanesulphonic acid, pH 7.2, 1 mM EGTA, 1 mM PMSF) and broken by freeze/thaw cycles followed by sonication. The inner membrane and matrix fractions of the mitoplasts were separated by centrifugation at 100,000×g for 30 min. The inner membrane fraction was further subjected to alkaline treatment (0.1 M Na2CO3, pH 11.5 for 30 min) and then centrifuged at 144,000 × g at 4°C to obtain integral inner membrane proteins.
2.7. Co-immunoprecipitation assay
Purified mitochondria were incubated with protease K and mitochondrial pellet was lysed in a buffer containing 50 mM HEPES (pH 7.4), 100 mM NaCl, 1% NP-40, and a mixture of protease inhibitors (Roche Molecular Biochemicals) and phosphatase inhibitors (Sigma). After homogenizing with 20 strokes by using a Dounce homogenizer (Bellco Glass), mitochondrial lysates were centrifuged at 20,000 × g, and the supernatants were used for co-immunoprecipitation assay using the Pierce co-immunoprecipitation kit. Co-immunoprecipitates were collected and used for Western blot analysis.
2.8. Western blotting
Cells were rinsed in cold PBS and collected in 200 μl RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate in 50 mM Tris, pH 8.0) and protease inhibitors (Roche Molecular Biochemicals) for Western blot analysis as previously described [13]. Protein content was determined using bovine serum albumin as a standard (Pierce Biotechnology). Proteins were separated by SDS-PAGE electrophoresis (25 μg/lane) and transferred to nitrocellulose membranes (Bio-Rad Laboratories). Primary antibodies used were included rabbit PGC-1α (P-120 and H-300) [13–14], goat anti-PGC-1α (K-15, Santa Cruz Biotechnology, sc-5816), anti-actin (Millipore, MAB1501R), anti-HSP 60 (Cell Signaling, 4870), anti-Histone H3 (AbCam, ab1791), anti-GAPDH (Cell Signaling, 2118), anti-VDAC (AbCam, ab14734), anti-Tom 40 (Santa Cruz Biotechnology, sc-11025), anti-NDUFV2 (Sigma, HPA003404), anti-Grp 75 (Cell Signaling, 2816), anti-PINK1 (LSBio, LS-B3384). Horseradish peroxidase-conjugated secondary antibodies were purchased from Pierce Biotechnology. Antibody binding was detected by using the SuperSignal chemiluminescence kit (Pierce Biotechnology) and an Alpha Innotech imaging system.
2.9. Immunoelectron microscopy
Mice were perfused with saline followed by 4% paraformaldehyde in 0.1 mol/L of phosphate buffer. Mouse hippocampus tissue was dissected under an operating microscope, mounted in the slot of a small screw, and snap frozen in liquid nitrogen. Ultra-thin sections (70–100 nm) from mouse hippocampus tissue were incubated concurrently with primary antibodies against PGC-1α, VDAC, or PINK1 for 1 h and followed by incubation with appropriate 5 nm or 10 nm immunoGold conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, PA) for 1 h. Sections were fixed with 2.5% glutaraldehyde, counter-stained with 4% neutral uranyl acetate, embedded in 1.25% methyl cellulose, then observed using a JEOL JEM 1210 electron microscope (JEOL USA, Inc., MA) at 80 kV.
3. Results
3.1. Validation of the PGC-1α antibodies used for detection of the 35 kDa PGC-1α isoform protein
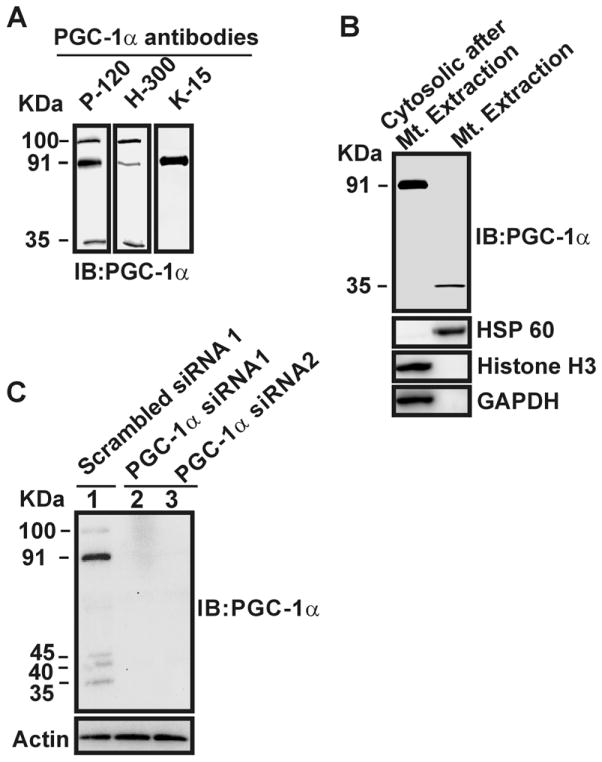
In this study, we utilized different antibodies raised against PGC-1α to ensure accuracy in the detection of the 35-kDa PGC-1α; rabbit polyclonal PGC-1α (P-120) antibody raised against amino acids 1–120 [13], rabbit polyclonal PGC-1α (H-300) antibody [14] raised against amino acids 1–300, and goat polyclonal PGC-1α (K-15) antibody raised against amino acids near the C-terminus. The results show that a band at 91 kDa was detected by the three P-120, H-300, and K-15 antibodies in total lystates of SH-SY5Y cells (Fig. 1A). However, both the 100 and 35 kDa protein bands were detected only by P-120 and H-300, but not K-15 (Fig. 1A). Western blot analysis of PGC-1α on purified mitochondria from SH-SY5Y cells with P-120 antibody shows that 35 kDa protein band was detected in the mitochondrial fraction, whereas 100 and 91 kDa bands were detected in the remaining cytosolic fractions devoid of mitochondria (Fig. 1B).
Fig. 1.
Detection of a 35 kDa PGC-1α isoform by different antibodies, and down-regulation of the protein by PGC-1α siRNAs. A) Total protein extracts of SH-SY5Y cells were analyzed by Western blotting with anti-PGC-1α antibodies. B) Purified mitochondria and the remaining cytosolic fraction including nucleus were analyzed by Western blotting with P-120, anti-HSP60, anti-Histone H3, and anti-GAPDH antibodies. C) HEK cells were transiently transfected with scrambled siRNA (lane 1) or two separate siRNAs against PGC-1α (lanes 2 and 3), followed by Western blot analysis with P-120 and actin antibodies.
We verified the purity of the mitochondrial and the remaining cytosolic fractions using Western blot analysis with anti-HSP 60, anti-Histone H3, and anti-GAPDH antibodies. We further checked the specificity of the P-120 antibody. The results show that in the total extracts of HEK cells, five protein isoforms (100, 91, 45, 40, and 35 kDa) were detected by the P-120, and all these protein bands were completely down-regulated by transiently transfecting HEK cells with siRNA against PGC-1α, but not with a scrambled siRNA 1 (Fig. 1C) or a scrambled siRNA 2 (data not shown). These experiments clearly document the specificity and the capability of P-120 antibody for detecting all potential isoforms of PGC-1α protein. Therefore, we have utilized this antibody to further investigate the mitochondrial localization of PGC-1α in brain and to identify its binding partner in the following studies.
3.2. 35 kDa PGC-1α protein localizes in the mitochondrial inner membrane and the matrix in the brain
To further confirm the mitochondrial localization of 35 kDa PGC-1α, we performed a protease protection assay, a carbonate extraction assay, and immunoelectron microscopy analysis. Purified mitochondria from mouse hippocampus were treated with protease K or trypsin followed by a Western blot analysis (Fig. 2A). Results showed that the PGC-1α detected in the mitochondria is only the 35 kDa isoform (Fig. 2A). The 35 kDa PGC-1α isoform and NDUFV2, an inner mitochondrial membrane protein, were unaffected by proteinase K treatment, as evidenced by the presence of a single 35 or 24 kDa protein band, respectively. In contrast, VDAC, the integral outer mitochondrial membrane protein, was degraded by this treatment, resulting in multiple VDACs with lower molecular mass (25 and 18 kDa) (Fig. 2A, lane 1). Upon treatment of the mitochondria with trypsin under stringent conditions (overnight incubation at 4°C), both VDAC and NDUFV2 were completely degraded, as evidenced by a shift of 30 kDa and 24 kDa proteins to lower mass, respectively (Fig. 2A, lane 2). By contrast, 35 kDa PGC-1α was only partially fragmented. As expected, without protease K or trypsin treatment, all three proteins, 35 kDa PGC-1α, VDAC, and NDUFV2 remained intact (Fig. 2A, lane 3).
Fig. 2.

35 kDa PGC-1α localizes to the mitochondrial inner membrane and the matrix. A) Purified mitochondria from the mouse hippocampus were treated with protease K (lane 1), treated with trypsin (lane 2), or left untreated (lane 3). The amount of PGC-1α, VDAC, NDUFV2, Histone H3, or GAPDH was determined by Western blotting. B) Western blot analysis of mitochondrial inner membrane (Mem) and matrix (Sol.) probed with antibodies directed against P-120, NDUFV2, Grp 75, Histone H3 (a nuclear marker), and GAPDH (a cytosolic marker). Immunoelectron microscopy of mouse hippocampus tissue with P-120 antibody (C), followed by OsO4 treatment (D), or without P-120 antibody (E). Arrows indicate PGC-1α Immunogold labeling.
To further investigate the sub-mitochondrial localization of 35 kDa PGC-1α, the isolated mitoplasts were disrupted and purified inner membrane fraction was subjected to carbonate extraction to obtain intrinsic proteins embedded in the inner membrane. Western blot analysis revealed a strong signal at 35 kDa in the both soluble and pellet fractions (Fig. 2B). We verified the purity of the inner membrane fraction and the matrix fraction using Western blot analysis with anti-Grp 75 (a mitochondrial matrix marker), anti-NDUFV2 (a mitochondrial inner membrane marker), anti-Histone H3, and anti-GAPDH antibodies. This result indicates that 35 kDa PGC-1α was associated with both the mitochondrial inner membrane fraction and matrix fraction (Fig. 2B).
Immunoelectron microscopy analysis of mouse hippocampal tissue with anti-PGC-1 α antibody and post-OsO4 treatment showed apparent localization of PGC-1α protein in the mitochondrial outer and inner membranes, and in association with cristae (Fig. 2C and 2D). Quantification of the distribution of PGC-1α inside mitochondria showed that ≈92% of the gold particles were detected in cristae and matrix, and 8% associated with the outer membrane (342 gold particles from 30 mitochondria were counted total). No gold particles were detected in the controls when the anti-PGC-1α antibody was omitted (Fig. 2E), confirming the specificity of our observations. Taken together, these experiments demonstrate that 35 kDa PGC-1α isoform localizes to the mitochondrial inner membrane, as well as in the mitochondrial matrix.
3.3. 35 kDa PGC-1α transport is dependent on the VDAC and mitochondrial membrane potential
PGC-1α contains no classical mitochondrial import signal, thus we suspect that the protein might be transported via non-traditional protein import machinery. To explore this concept, three independent studies were performed: immunoelectron microscopy, BN-PAGE, and siRNA-mediated knockdown of the mitochondrial import machinery. Double immunogold-labeling electron microscopy of mouse hippocampal tissue with antibodies against PGC-1α and VDAC revealed a colocalization of these proteins on the surface of the outer mitochondrial membrane (Fig. 3A). No gold particles were detectable in the control, when the primary anti-PGC-1α or anti-VDAC antibody was omitted (data not shown). We next performed BN-PAGE analysis that allows separating native multi-protein complexes. Immunoblot analysis of the second-dimension SDS-PAGE gels followed by BN-PAGE analysis of purified hippocampal mitochondria revealed that both 35 kDa PGC-1α and 30 kDa VDAC proteins co-migrated with a molecular mass of 170 kDa (Fig. 3B). Interestingly, a ~335-kDa band that is immunoreactive to VDAC but not 35-kDa PGC-1α was detected (Fig. 3B), likely representing additional protein complexes containing VDAC. Western blot analysis of the BN-PAGE gels (10 – 1,236 kDa) with anti-Histone H3 and anti-GAPDH antibodies verified the purity of the mitochondrial fraction (Fig. 3B). Next, we examined the effect of siRNA-mediated VDAC down-regulation on the mitochondrial localization of 35 kDa PGC-1α in HEK cells. Results showed that VDAC siRNA completely down-regulated VDAC and 35 kDa PGC-1α expression within mitochondria, whereas control siRNA did not (Fig. 3C). Tom 40 protein, a major component of outer mitochondrial membrane transporter machinery, had little effect, indicating the specificity of siRNA against VDAC. To determine whether 35 kDa PGC-1α import was dependent on the mitochondrial membrane potential, HEK cells were treated with valinomycin, a potassium ionophore that depolarizes the mitochondrial membranes. Valinomycin treatment completely inhibited mitochondrial localization of the 35 kDa PGC-1α isoform (Fig. 3C).
Fig. 3.
Transport of 35 kDa PGC-1α depends on the VDAC protein and the mitochondrial membrane potential. A) Immunoelectron microscopy showing colocalization of mitochondrial PGC-1α and VDAC. White and black arrows indicate VDAC (10 nm) and PGC-1α (5 nm) immunoGold labeling in mouse hippocampus tissue. B) Immunoblot analysis of the second-dimension SDS-PAGE gels followed by BN-PAGE analysis of native mitochondrial protein complexes with P-120, anti-Histone H3, and anti-GAPDH antibodies. C) HEK cells were transfected with scrambled control siRNA (lane 1), two separate VDAC siRNAs (lanes 2 and 3), or treated with valinomycin. Mitochondrial lysates were analyzed by Western blotting with P-120, anti-VDAC, and anti-Tom40 antibodies.
3.4. 35 kDa PGC-1α physically associates with PINK1 in the mitochondria
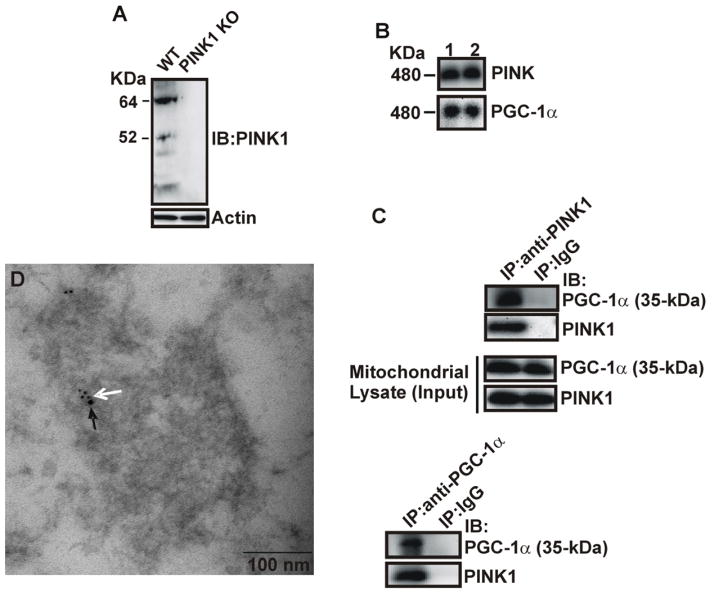
To assess the potential functional significance of the findings of the 35 kDa PGC-1α localized in the mitochondria, we sought to identify its protein binding partner inside mitochondria. Immunoblotting analysis of the BN-PAGE gels of purified mitochondria from mouse brain with various antibodies against well-known mitochondrial proteins revealed that both PGC-1α and PINK1 proteins are immunoreactive to the same protein band with a molecular mass of 480 kDa (Fig. 4B), indicating that the proteins form a native complex in the mitochondria of mouse brain. The specificity of the mouse monoclonal anti-PINK1 antibody used for this study was confirmed in the hippocampus tissue of PINK 1 knockout and control mice (Fig. 4A). Total mitochondrial lysates were subjected to co-immunoprecipitation with anti-PGC-1α, anti-PINK1, or anti-IgG (negative control) antibodies. Western blot analysis showed that PGC-1α co-immunoprecipitated with PINK1 in the mitochondria of mouse brain (Fig. 4C). Neither PGC-1α nor PINK1 immunoreactivity was detectable in samples immunoprecipitated with anti-IgG rabbit antibodies (Fig. 4C). Double labeling immunoelectron microscopy analysis with antibodies against PGC-1α and PINK1 revealed that these two proteins colocalize in the hippocampal mitochondria of mouse brain (Fig. 4D). The average number of gold particles per mitochondria was 7 ± 4.5 for PGC-1α and 2 ± 1.1 for PINK1. No gold particles were detected in the controls, when the anti-PGC-1α or anti-PINK1 antibody was omitted (data not shown). We did not observe their colocalization outside mitochondria. Together, these results provide strong evidence for an in vivo association of 35 kDa PGC-1α with PINK1 in brain mitochondria.
Fig. 4.
Co-localization and association of 35 kDa PGC-1α with PINK1. A) Total protein lystates from the hippocampus of PINK1 knockout and wild-type mice were analyzed by Western blotting with anti-PINK1 and actin antibodies. B) Purified mitochondria from mouse hippocampus were subjected to BN-PAGE analysis, followed by the second-dimension SDS-PAGE gels and immunoblotting with P-120 and anti-PINK1 antibodies. Lane 1&2 represent two different mice. C) Lystates from purified hippocampal mitochondria were co-immunoprecipitated with anti-PINK1, P-120, and anti-IgG antibodies, followed by immunoblotting using anti-PINK1 and P-120 antibodies. D) Immunoelectron microscopy of mitochondrial PGC-1α and PINK1 co-localization. White and black arrows indicate PGC-1α (5 nm) and PINK1 (10 nm) immunogold labeling.
4. Discussion
The present study demonstrates that a novel 35 kDa PGC-1α isoform localizes to mitochondria and associates with PINK1 in brain. We showed that the 35 kDa isoform is the only PGC-1α protein found in brain mitochondria. A recent report [3] showed that the human PPARGC1A locus, the gene encoding PGC-1α contains a novel promoter at the 583 kb upstream of exon 1. From the novel PPARGC1A promoter, several brain-specific transcripts are initiated and Western blot analysis of in vitro translation reactions of these abundant transcripts detects NT-PGC-1α with its molecular mass of ≈ 35 kDa. They showed that brain-specific NT-PGC-1α is detected in the cytoplasm, while full-length PGC- 1α localizes to the nucleus. Consistent with this report, we detected full-length 91 kDa PGC-1α in the remaining cytosolic fractions but not in the mitochondrial fraction. 91 kDa PGC-1α has long been considered a nuclear transcriptional co-activator. These suggest that a distinct 35 kDa or 91 kDa PGC-1α isoform may regulate mitochondrial function and nuclear transcription of mitochondrial proteins. To identify the molecular origin of brain mitochondrial 35 kDa PGC-1α protein, we attempted Edman sequencing of the purified mitochondrial 35 kDa PGC-1α protein. Unfortunately, despite repeated attempts, we were unable to sequence this 35 kDa PGC-1α species due to its low abundance or blocked N-terminal for Edman sequencing. Our findings show the presence of several PGC-1α protein bands (100, 91, 40, 45, and 35 kDa) in cells. The molecular basis for these various isoforms could be cell-specific differences in the usage of the reference promoter or the novel promoter of PPARGC1A, and/or post-transcriptional or post-translational modifications.
We suggest that 35 kDa PGC-1α is imported through a VDAC-mediated pore. A previous study suggested that VDAC exists as a multimer, instead of single polypeptide [15], which may provide a large enough pore to serve as a transport route for small proteins that do not contain a classical import signal, such as 35 kDa PGC-1α, to cross the outer mitochondrial membrane. We showed that valinomycin treatment results in an inhibition of mitochondrial localization of 35 kDa PGC-1α. This result indicates that import and integration of 35 kDa PGC-1α protein into the mitochondrial inner membrane requires a membrane potential.
Previous studies indicated that PINK1 is a putative mitochondrial kinase and that its loss results in mitochondrial dysfunction [10–11]. We demonstrated that 35 kDa PGC-1α associates with PINK1 in brain mitochondria. Therefore, it is conceivable that PINK1 may potentially phosphorylate 35 kDa PGC- 1α within brain mitochondria, which could alter its sub-mitochondrial localization, stability, or function. In supporting this possibility, we found that the 35 kDa PGC-1α isoform is localized on the mitochondrial inner and outer membranes, and is associated with cristae. The various sub-mitochondrial localizations of 35 kDa PGC-1α could suggest the presence of multiple signaling pathways regulated by the protein within brain mitochondria. Consistent with our findings, a previous report showed that PGC-1α forms a complex with mitochondrial transcription factor A or presents as free proteins within the mitochondrion, and exercise increases mitochondrial PGC-1α content [14]. It is important to note, however, that there is a lack of information regarding the exact molecular mass of mitochondrial PGC-1α, and skeletal muscle of mice was used in this study. We showed the mitochondrial localization of 35 kDa PGC-1α but not 91 kDa PGC-1α in tissue from mouse hippocampus. Thus, if there is a discrepancy in the finding of the molecular mass of mitochondrial PGC-1α, it could be due to the difference in tissues used between the previous and current studies.
We demonstrate a new isoform of PGC-1α that co-localizes with PINK1. Further work is required to show that 35 kDa PGC-1α and PINK1 act together in mitochondrial degeneration. Given the potential importance of PGC-1α and PINK1 in pathophysiological conditions associated with mitochondrial dysfunction during neurodegenerative disease and diabetes, a possible interaction of 35 kDa PGC-1α and PINK1 in brain mitochondria would appear to be a promising therapeutic target for these disorders.
Highlights.
Novel 35 kDa PGC-1α localizes to mitochondrial inner membrane and matrix in brain
Mitochondrial localization of 35 kDa PGC-1α depends on VDAC protein.
Mitochondrial localization of 35 kDa PGC-1α depends on membrane potential.
The 35 kDa PGC-1α associates and colocalizes with PINK in brain mitochondria.
Acknowledgments
We thank Dr. Daniel Kelly and Ms. Theresa Leone for providing the PGC-1α antibody. This work was supported by Department of Veterans Affairs Biomedical Research Service, Office of Research and Development, Biomedical Laboratory Research and Development (NEUC-00508S - JWR), NIH RR024888 (JWR), VA Baltimore Research and Education Foundation (JC), and The Mid-Atlantic Nutrition Obesity Research Center (NIH P30 DK072488).
Footnotes
Competing Interests: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. 2012;120:419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soyal SM, Felder TK, Auer S, Hahne P, Oberkofler H, Witting A, Paulmichl M, Landwehrmeyer GB, Weydt P, Patsch W. A greatly extended PPARGC1A genomic locus encodes several new brain-specific isoforms and influences Huntington disease age of onset. Hum Mol Genet. 2012;21:3461–3473. doi: 10.1093/hmg/dds177. [DOI] [PubMed] [Google Scholar]
- 4.Benton CR, Holloway GP, Han X, Yoshida Y, Lally LA, Snook J, Glatz JF, Luiken JJ, Chabowski A, Bonen A. Increased levels of peroxisome proliferator-activated receptor gamma, coactivator 1 alpha improve lipid utilization, insulin signaling and glucose transport in skeletal muscle of lean and insulin-resistant obese Zucker rats. Diabetologia. 2010;53:2008–2019. doi: 10.1007/s00125-010-1773-1. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 8.Franks PW, Scheele C, Loos RJ, Nielsen AR, Finucane FM, Wahlestedt C, Pedersen BK, Wareham NJ, Timmons JA. Genomic variants at the PINK1 locus are associated with transcript abundance and plasma nonesterified fatty acid concentrations in European whites. FASEB J. 2008;22:3135–3145. doi: 10.1096/fj.08-107086. [DOI] [PubMed] [Google Scholar]
- 9.Scheele C, Nielsen AR, Walden TB, Sewell DA, Fischer CP, Brogan RJ, Petrovic N, Larsson O, Tesch PA, Wennmalm K, Hutchinson DS, Cannon B, Wahlestedt C, Pedersen BK, Timmons JA. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? FASEB J. 2007;21:3653–3665. doi: 10.1096/fj.07-8520com. [DOI] [PubMed] [Google Scholar]
- 10.Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci U S A. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto W, Spielewoy N, Bonnard G, Murata M, Wintz H. Mitochondrial localization of AtOXA1, an arabidopsis homologue of yeast Oxa1p involved in the insertion and assembly of protein complexes in mitochondrial inner membrane. Plant Cell Physiol. 2000;41:1157–1163. doi: 10.1093/pcp/pcd045. [DOI] [PubMed] [Google Scholar]
- 13.Cowell RM, Blake KR, Inoue T, Russell JW. Regulation of PGC-1alpha and PGC-1alpha-responsive genes with forskolin-induced Schwann cell differentiation. Neurosci Lett. 2008;439:269–274. doi: 10.1016/j.neulet.2008.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286:10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Zalk R, Israelson A, Garty ES, Azoulay-Zohar H, Shoshan-Barmatz V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem J. 2005;15:73–83. doi: 10.1042/BJ20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]