Abstract
Rationale and objective
Relapse to drug use in humans can be induced by exposure to drug-associated cues. The ability of drug cues to provoke ‘relapse’ has been studied in laboratory animals using a reinstatement model in which resumption of drug seeking is assessed after extinction of drug-reinforced responding. In this model, there are no adverse consequences of drug-seeking behavior. However, in humans abstinence is often self-imposed, and relapse episodes likely involve making a choice between the desire for the drug and the negative consequences of pursuing it (a conflict situation). Here, we describe a conflict model of cue-induced relapse in rats that approximate the human condition.
Methods
Rats were trained to lever-press for cocaine; infusions were paired with a discrete light-cue. An ‘electric barrier’ was then introduced by electrifying the floor area near the levers. Responding decreased over days with increasing shock intensities, until the rats did not approach the levers for 3 days. Subsequently, the effect of intermittent non-contingent-light-cue presentations on resumption of lever responding (relapse) was assessed in extinction tests, with the electric barrier remaining activated; during testing lever-presses led to contingent light-cue presentations.
Results
Non-contingent cue exposure led to resumption of lever presses during the relapse tests in 14 of the 24 rats. Surprisingly, 24 h later, 11 of the 24 rats resumed lever responding in a subsequent post non-contingent cue test under similar extinction conditions. Large individual differences in responding were observed during both tests.
Conclusions
At its current stage of development, the conflict relapse model appears particularly suitable for studying individual differences in cue-induced relapse to cocaine seeking or factors that promote this relapse.
Keywords: Animal models, Drug cues, Cocaine self-administration, Reinstatement, Relapse
Cocaine addiction is characterized by high rates of relapse to drug use during abstinence (Mendelson and Mello 1996). In humans, cocaine relapse and craving can be induced by acute exposure to cues previously associated with cocaine use (O’Brien 2005). The ability of cocaine cues to provoke ‘relapse’ to drug use has been studied in laboratory animals using different variations of a reinstatement model (Shaham et al. 2003). Typically, in these studies laboratory animals are trained to lever-press for drug infusions in the presence of distinct cues (e.g., tone, light, specific odors); then, following extinction of lever responding in the absence of the drug cues, non-reinforced resumption of pressing on the drug-associated lever is induced by acute exposure to these cues (Crombag and Shaham 2002; Meil and See 1996; Spealman et al. 1999; Weiss et al. 2000).
The conditions under which relapse is studied using the reinstatement model, however, differ from those under which relapse occurs in humans in two important ways. First, abstinence in humans often occurs because the drug’s rewarding effects are outweighed by the aversive consequences of seeking or using them, whereas in studies using the reinstatement model, abstinence is achieved through experimenter imposed extinction procedures (Epstein and Preston 2003; Katz and Higgins 2003). Second, human drug-seeking episodes during abstinence often involve a conflict situation—a choice between experiencing the positive effects of a drug and the aversive consequences of pursuing it (Epstein and Preston 2003), whereas in the reinstatement model there are no adverse consequences of drug seeking. The degree to which the lack of homology between the reinstatement model and the human condition challenges the validity of the model is subject to an ongoing debate in the addiction field (Epstein et al. 2006; Katz and Higgins 2003; Marlatt 2002). Both proponents and opponents of the reinstatement model, however, agree that it is important to develop alternative (or complementary) models for the experimental study of relapse to drugs.
To have the greatest likelihood of construct validity, an animal model of relapse to drugs should ideally involve a procedure in which animals become abstinent due to delayed aversive consequences of drug taking. However, such a procedure would require training so complex that the model would be, at best, impractical. Therefore, we and others have begun to develop models that might be acceptable compromises. One approach has been to deliver a punishment immediately after drug-taking. Based on earlier work (Smith and Davis 1974), Panlilio et al. (2003; 2005) introduced a punishment-based relapse model in which lever pressing for drug infusions is suppressed by electric shock delivered immediately following drug delivery. They found that in rats that had ceased opiate-taking behavior under these punishment conditions, opiate seeking was reinstated by a priming injection of heroin, which models the human condition of relapse induced by acute re-exposure to heroin during abstinence (de Wit and Stewart 1983). The punishment model therefore may be suitable for modeling human conditions in which abstinence is achieved because the negative consequences of drug taking exceed the positive consequences (Panlilio et al. 2003; 2005). However, the punishment model still does not accurately mimic the human condition, since in humans the major aversive consequences of drug-taking behavior rarely coincide with acute drug intoxication.
We propose an alternative approach suggested by the possibility that, in humans, some aversive events related to drug use might be those that occur during drug seeking, for instance those associated with obtaining money to purchase an illegal drug, and fear of being caught by family members or the police. It could also be argued that the anticipation of delayed negative consequences of drug relapse is itself an aversive event. To mimic the aversive components of the drug seeking period, we have begun to develop a conflict model of relapse. The basic concept of this model is similar to that of the ‘Columbia Obstruction Box’ method, which has been used many years ago to assess rats’ motivation to obtain rewards under different deprivation conditions in the presence of an ‘electric barrier’ (Jenkins et al. 1926; Olds and Olds 1958; Warden 1931). Our model was also inspired by results of more recent studies in which motivation to seek and take drugs was assessed in the presence of aversive stimuli (Deroche-Gamonet et al. 2004; Vanderschuren and Everitt 2004; Wolffgramm and Heyne 1995). The authors of these studies reported that a proportion of rats with an extensive history of exposure to drugs will continue to engage in drug-taking behavior under adverse conditions that normally would suppress drug- or food-taking responding.
In the present experiment, we trained rats to self-administer intravenous cocaine; cocaine infusions were paired with a 20-sec light cue. Subsequently, we introduced an electrical barrier over daily sessions by administering shock of increasing intensities to the floor near the cocaine-associated lever, until the rats did not approach the cocaine-paired lever for 3 d. After these 3 d of ‘self-imposed’ abstinence, we used extinction tests to assess resumption of cocaine seeking (relapse) induced by non-contingent exposure to the light cue, while the ‘electrical barrier’ remained activated at the previously established intensity. During the extinction tests lever-presses led to contingent light-cue presentations, but not cocaine. The term ‘cue-induced relapse’ in the present study refers to the resumption of lever responding after exposure to the non-contingent cue in the presence of the electric barrier.
Materials and methods
Subjects
Subjects were 30 male Sprague Dawley rats (Harlan, Israel, 250–350 g) housed in an animal facility (temperature 21–22°C) under a reverse 12-h light-dark cycle (lights off at 8 am). Food and water were freely available in the rats’ home cage. The experimental procedures were approved by the local animal care and use committee and were conducted in accordance with NIH guidelines. Six rats were excluded because of loss of catheter patency or poor health.
Intravenous surgery
The rats were anesthetized with Ketamine (170 mg/kg, i.p.; KEPRO, Holland) + Promace (Acepromazine 1.7 mg/kg, i.p.; Fort Dodge, lowa), and silastic catheters were inserted into the jugular vein using established procedures (Shaham et al. 1996; Shalev et al. 2001a; Shalev et al. 2001b). The catheters were passed subcutaneously to the top of the skull and were then attached to a modified 22-gauge cannula (Plastics One, Roanoke, VA), which was mounted to the rat’s skull with dental cement. Baytril (Enrofloxacin 2.5 mg/2 ml saline, i.p.) was given after surgery and the rats were allowed to recover for 4–7 days. Catheters were flushed every 24–48 h with sterile saline containing gentamicin (0.08 mg/ml).
Apparatus
The experiments were performed in standard Med-Associates (Georgia, VT) self-administration chambers that were equipped with two levers located 9 cm above the grid floor on one side, a 7.5-mA white cue light located above the cocaine-paired lever, and a red houselight. The setup of the ‘electric barrier’ included constant-voltage stimulators that were connected to ~2/3 of the floor adjacent to the active and inactive levers (13 out of 19 metal bars of the chambers). The stimulators produced a constant voltage and the rats closed the electric circuit by touching any two grids with opposite charge. The remaining ~1/3 of the chamber, to which electricity was not applied, was the rat’s no-shock area.
Procedure
The experimental procedure included 3 phases: cocaine self-administration (10–13 d), ‘electric barrier’ application that resulted in the elimination of the cocaine-reinforced behavior (4–9 d), and relapse tests (2–3 d) during which the rats were exposed or not exposed to non-contingent cocaine cue. All phases were conducted during the rats’ dark phase.
Cocaine self-administration
The rats were initially trained for 3 h/d to lever-press for intravenous cocaine (0.5 mg/kg/infusion; 0.13 ml/infusion over 3.3 sec) for 10–13 d; cocaine-HCl (RTI, Raleigh, NC) was dissolved in sterile saline. Prior to the daily training sessions, the modified cannula on the rat’s skull was connected to a liquid swivel with PE-50 tubing; the tubing was protected by a metal spring and was connected to the infusion pump’s syringe. For the first 7–10 d, lever responding was reinforced under a fixed-ratio-1 (FR-1) 20-sec timeout reinforcement schedule, until the rats met an acquisition criterion of at least 20 infusions/session during 3 consecutive days; during the 20-sec-timeout the cue light was turned on. After the rats achieved the acquisition criterion, they were given 3 additional training sessions under an FR-2 20-sec timeout reinforcement schedule during 2-hr daily sessions. The training sessions began with the illumination of the houselight and the insertion of the active lever. Responding on the ‘active’ lever activated the infusion pump, while responding on the ‘inactive’ lever was recorded, but had no programmed consequences. To prevent overdose, the number of infusions was limited to 35 per session.
‘Electric barrier’ application
During this phase, cocaine self-administration behavior was initially reduced and then completely eliminated by gradually increasing the electrical intensity of the electric barrier over successive sessions. Cocaine was available under an FR-2 20-sec timeout reinforcement schedule for 30 min/d. The rats were placed in the ‘no-shock zone’ of the chamber and the electrical barrier was turned on 5 min before the start of the self-administration sessions. The daily sessions began with illumination of the houselight. The duration of the daily sessions was reduced to 30 min, because in pilot studies we found that it took numerous daily sessions to achieve complete ‘abstinence’ (no approaches to the active lever) when the daily sessions were for 2 h. Thus, the feasibility of completing the experiments due to catheter failure became a concern.
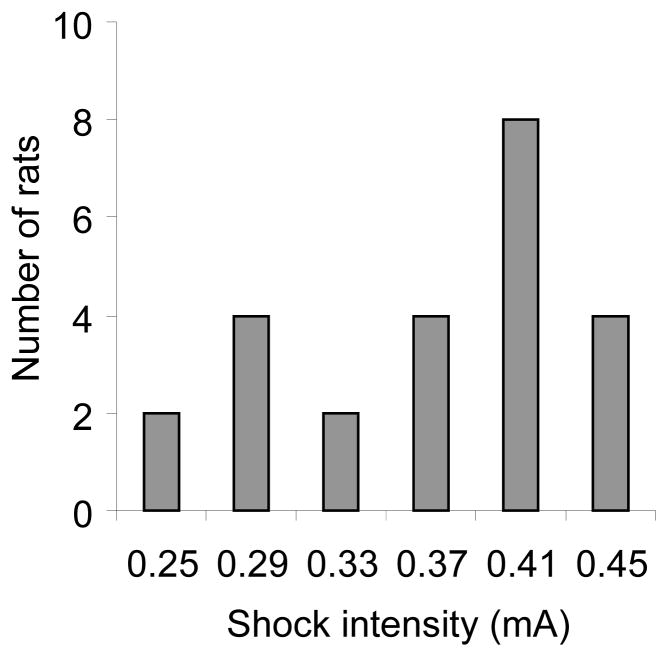
On the first day of the electric barrier application, the current was set at 0.25 mA and current intensity was gradually increased every day by 0.04 mA, until the rats did not lever press during the 30-min session for 3 consecutive daily sessions. The final shock intensity for individual rats that led to 3 d of self-imposed abstinence was between 0.25 to 0.45 mA (Fig. 1). Tests for cue-induced relapse started after the rats had 3 d of ‘abstinence’ (zero infusions).
Figure 1. Frequency distribution of the electric barrier shock intensity that led to 3 d of self-imposed abstinence.
On the first day of the electric barrier application, the current was set at 0.25 mA, and the current intensity was gradually increased every day by 0.04 mA until the rats did not initiate lever pressing during three 30-min sessions.
Relapse tests
The experimental conditions during the tests for cue-induced relapse to cocaine seeking were the same to those of the last 3 sessions during which the rats did not approach the levers, with the exception that active lever responding led to saline infusions, but not cocaine. During the relapse tests, the rats were connected to the swivels, the electric barrier was turned on (at the shock intensity that led to 3 d of abstinence) 5 min before the start of the sessions, and lever presses led to response-contingent light-cue presentations under the FR-2 20-sec timeout reinforcement schedule.
Cocaine was not available during the relapse tests because we were interested in the effect of the experimental manipulations on drug seeking rather than drug taking. In this regard, extinction procedures have been used in many studies to assess mechanisms underlying the motivational effects of cues previously paired with drug or non-drug rewards (Balleine and Dickinson 1998; Di Ciano and Everitt 2004; Everitt et al. 1999; Lu et al. 2004b; Lu et al. 2005; Shalev et al. 2002). In addition, testing for lever responding under extinction conditions allows us to at least make some qualitative comparisons between results form the new conflict relapse model and results from studies using the established reinstatement model in which lever presses are not reinforced with drug during testing (Shalev et al. 2002).
The cue manipulation consisted of intermittent non-contingent exposure to the discrete light cue previously paired with cocaine injections during training. This manipulation was designed to mimic the human condition in which during abstinence initial exposure to drug cues that provoke relapse is typically not contingent on behavior. The light cue was presented for 20 sec every 5 min during the 30-min sessions. During testing, responding on the active lever led to contingent light-cue exposure in order to maximize our ability to provoke cue-induced relapse under our experimental conditions.
After 3 d of electric barrier-induced abstinence, we tested 24 rats in within-subjects counterbalanced design. Twelve rats were first exposed to the non-contingent-light-cue as described above and then, 1 d later, they were tested under the same extinction conditions without being exposed to the non-contingent cue manipulation; for the other 12 rats, the order of testing was reversed. Five rats in the former group also resumed lever responding in the test without being exposed to the non-contingent cue manipulation (termed herein ‘post non-contingent cue’). Therefore, the rats in the latter group were also tested 24 h after the non-contingent cue test in the post non-contingent cue condition.
Statistical analyses
The data from the relapse tests were analyzed for active and inactive lever responses. As mentioned above, unexpectedly 5 of the initial 12 rats tested resumed lever responding in the presence of the electric barrier in the post non-contingent cue condition, indicating that our original within-subjects counterbalanced design is confounded by an order effect. Therefore, the data were analyzed in two different ways. The first is a between-subjects analysis of lever responding during the first test day in the presence (n=12) or absence (n=12) of the non-contingent cue manipulation. The second is a within-subjects analysis (n=24) that included a pre non-contingent cue condition, the non-contingent cue condition, and the post non-contingent cue condition. For 12 rats, the pre non-contingent cue condition was the test session in the absence of the non-contingent cue manipulation after 3 d of abstinence. For the other 12 rats, the pre non-contingent cue condition was the third day of abstinence in which they did not approach the levers. These data provide a good approximation of the rats’ behavior in the presence of the final shock intensity that led to abstinence, because only 1 of the 12 rats from the other group resumed lever responding (7 responses/30 min) under extinction condition in the absence of non-contingent cue exposure (a group mean of 0.6 responses/30 min). Data were analyzed with the statistical program SPSS (GLM procedure) and significant effects (p < 0.05) were followed by Fisher PLSD post-hoc tests.
Results
Effect of electric barrier application on drug-taking behavior
The rats that were subsequently tested in the different relapse conditions demonstrated reliable cocaine self-administration behavior at the end of the training phase. The mean±sem number of infusions during the last 3 training days was 29.0±1.1, 30.6±1.3 and 31.0±1.3 per 2 h, respectively. The mean±sem number of active and inactive lever responses during the last 3 training days was 71.0±3.8 and 11.0±6.1; 79.5±5.4 and 13.2±9.8; 79.4±5.7 and 9.7±6.1 per 2 h, respectively. Subsequent application of gradually increasing electric barrier shock intensity from 0.25 to 0.45 mA eliminated cocaine self-administration. Fig. 1 shows the frequency distribution of the final shock intensity values that led to 3 d of self-imposed abstinence in the 24 rats. The mean±sem final electric barrier shock intensity was 0.37±0.02 mA. The mean±sem number of days to achieve 3 days of abstinence was 7.4±1.2 days.
Relapse tests
Between-subjects analysis
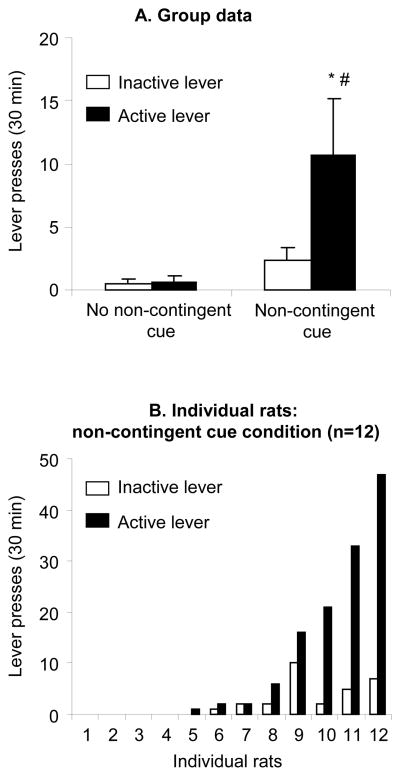
The effect of non-contingent exposure to the cocaine light cue (previously paired with cocaine infusions during training) on resumption of lever responding (relapse) after 3 d in which the rats did not approach the cocaine-paired lever in the presence of the electric barrier is shown in Fig. 2. Exposure to the non-contingent cue led to resumption of active lever responding during testing; the non-contingent cue manipulation also modestly increased inactive lever responding. In the between-subjects analysis, we assessed in two groups of rats (n=12 per group) lever responding during the first test day in the presence or absence of the non-contingent cue manipulation. The mixed ANOVA included the between-subjects factor of Non-contingent Cue (no, yes) and the within-subjects factor of Lever (active, inactive). This analysis revealed significant effects of Non-contingent Cue (F1,22=5.1, p<0.05), Lever (F1,23=4.6, p<0.05), and Non-contingent Cue by Lever interaction (F1,23=4.4, p<0.05). The significant interaction indicates that the effect of the non-contingent cue manipulation was more pronounced on active lever responding than on inactive lever responding. Post-hoc differences are indicated in Fig. 2.
Figure 2. Relapse to cocaine seeking induced by exposure to non-contingent cocaine cues: between-subjects analysis.
(A) Mean±sem number of lever presses on the previously active lever and on the inactive lever in two groups of rats that were either exposed (n=12) or not exposed (n=12) to non-contingent cue presentations during a single test session after 3 d of self-imposed abstinence induced by exposure to the electric barrier. (B) Mean number of active and inactive lever responses of individual rats in the non-contingent cue condition. In the non-contingent cue session, the rats were exposed to the light cue previously paired with cocaine injections during the 30-min sessions every 5 min for 20 sec. During testing, the rats had to cross the electric barrier in order to gain access to the levers. Lever responding during testing was conducted under extinction conditions and resulted in contingent presentation of the discrete light cue under a fixed-ratio-2 20 sec timeout reinforcement schedule, but not cocaine. * Different from the no non-contingent cue control condition, p<0.05; # Different from the inactive lever, p<0.05.
Within-subjects analysis
The results of the within-subjects analysis are presented in Fig. 3. As mentioned above, the within-subjects analysis (n=24) included a pre non-contingent cue condition, the non-contingent-cue condition, and the post non-contingent cue condition. Resumption of active lever responding was observed during both the non-contingent cue session and the post non-contingent cue session; during these test days, inactive lever responding was also modestly increased. The repeated-measures ANOVA included the within-subjects factor of Non-contingent Cue (pre non-contingent cue, non-contingent cue, post non-contingent cue) and the within-subjects factor of Lever (active, inactive). This analysis revealed significant effects of Non-contingent Cue (F2,46=4.8, p<0.05), Lever (F1,23=8.0, p<0.01), and Non-contingent Cue by Lever interaction (F2,46=4.1, p<0.05). The significant interaction indicates that the effect of the non-contingent cue and the post non-contingent cue conditions were more pronounced on active lever responding than on inactive lever responding. Post-hoc differences are indicated in Fig. 3.
Figure 3. Relapse to cocaine seeking induced by non-contingent cocaine cue: within-subjects analysis.

(A) Mean±sem number of lever presses on the previously active lever and on the inactive lever in the session before, the session during, and the session after non-contingent cue exposure (n=24); the three conditions are termed ‘Pre non-contingent cue’, ‘Non-contingent cue’ and ‘Post non-contingent cue’ in the figure. (B–C) Mean number of active and inactive lever responses of individual rats in the non-contingent cue condition and the post non-contingent cue condition. In the non-contingent cue exposure session, the rats were exposed to the light cue previously paired with cocaine injections during the 30-min sessions every 5 min for 20 sec. During testing, the rats had to cross the electric barrier in order to gain access to the levers. Lever responding during the relapse tests was conducted under extinction conditions and resulted in contingent presentation of the discrete light cue under a fixed-ratio-2 20 sec timeout reinforcement schedule, but not cocaine. * Different from the no non-contingent cue condition, p<0.05; # Different from the inactive lever, p<0.05.
Correlational analyses
We also analyzed Pearson product-moment correlation coefficients among cocaine intake during training, final (abstinence) electric barrier shock intensity, and lever responding or onset of lever responding in the different relapse conditions. These data are not reported because the different correlations were not significant. However, we did find a significant correlation between active lever responding in the non-contingent cue condition and the post non-contingent cue condition (r=0.61, p<0.01, see Fig. 3bc).
Discussion
Our goal was to develop a cue-induced relapse model that would mimic the human condition in which abstinence often occurs not because drugs are unavailable, but because they become associated with aversive consequences that outweigh their rewarding effects. Under these circumstances, relapse can occur because the desire for the drug (induced by drug cues or other stimuli) momentarily outweigh the negative consequences of pursuing it. In our conflict relapse model, abstinence is achieved by introducing an electric barrier of increasing shock intensities that leads to cessation of cocaine-taking behavior, and then relapse induced by cocaine cues is determined in the presence of the electric barrier.
Under these conditions, we found that 14 of the 24 of the rats exposed to non-contingent cue presentations during testing resumed lever responding. Surprisingly, 11 of the 24 rats also resumed lever responding in the presence of the electric barrier on the subsequent day when the non-contingent cue was not presented. This finding is unique, because to our knowledge delayed post-cue effects have not been reported in studies using the reinstatement model (Bossert et al. 2005; See 2005; Weiss 2005). However, unlike the reinstatement model in which robust cue-induced reinstatement of cocaine seeking is observed in over 80% of the rats and the individual variability is relatively low (Bossert et al. 2004; Meil and See 1996; Weiss et al. 2000), in the conflict model a lower percentage of the rats (less than 60%) respond to the cue manipulation and the individual variability is high.
The more variable response (and the low overall mean number of active lever presses during testing) in response to the cue manipulation in the conflict relapse model than in the reinstatement model is to be expected, because there are no adverse consequences to the rat’s behavior during testing in the reinstatement model. The large individual differences in relapse to cocaine seeking in the conflict model is also to be expected based on results of studies in which electric shock was used to suppress cocaine-taking behavior (Deroche-Gamonet et al. 2004; Pelloux et al. 2007). In these studies only a relatively small proportion of the rats (less than 25%) continued to engage in drug-taking behavior in the presence of the aversive stimulus.
In the reinstatement model, non-contingent exposure to discrete cues (tone, light), after extinction of lever responding in their absence, is either ineffective (Grimm et al. 2000; Tran-Nguyen et al. 1998) or only modestly effective (de Wit and Stewart 1981; Deroche-Gamonet et al. 2002) in reinstating cocaine seeking. These discrete cues, however, are highly effective in reinstating lever responding when they are presented contingently (See 2005), presumably by functioning as conditioned reinforcers (Goldberg 1976; Robbins 1975). A conditioned reinforcer is defined as a previously neutral stimulus (e.g., tone, light) that acquired reinforcing effects through its prior association with a primary or unconditioned reinforcer (e.g., food, drug) (Catania 1992). In the conflict relapse model, non-contingent cue exposure resulted in significant increases in lever responding. However, under our experimental conditions lever responding resulted in contingent presentation of the same cue, and therefore, it is likely that both modes of cue presentation (non-contingent and contingent exposure) contributed to the resumption of cocaine seeking.
In the conflict relapse model, the discrete light cue may serve a dual function. First, when the rat is in the no-shock area, non-contingent exposure to the light cue likely functions as a Pavlovian conditioned incentive cue (Di Chiara 1999; Everitt and Wolf 2002; Robinson and Berridge 2003; Stewart et al. 1984) that motivated the rats to cross the electric barrier to gain access to cocaine after 3–4 daily sessions in which they did not cross this barrier to press the lever. Second, since lever presses in the relapse tests, performed under extinction conditions, resulted in contingent cue presentations, increased lever responding may be also due to the conditioned reinforcing effects of the light cue. The relative role of Pavlovian incentive processes and conditioned reinforcer processes in the effect of light cue exposure on resumption of cocaine seeking in the conflict relapse model is a subject for additional research.
An issue to consider in the interpretation of the present data is that the rats not only increased active lever responding in the non-contingent cue and post non-contingent cue conditions, but also modestly increased inactive lever responding (Fig. 2–3). Our relapse tests were conducted under extinction conditions in which increased inactive lever responding in response to drug cues (Fuchs et al. 1998; Lu et al. 2004a) and other manipulations that induce non-reinforced active lever responding such as stress (Shalev et al. 2001a) is often observed. Some investigators interpret drug-induced increases in inactive lever responding during testing to reflect a non-specific behavior effect of relapse-provoking manipulations. However, it is also possible that increased inactive lever responding in tests conducted under extinction conditions reflects, in part, response generalization (Shalev et al. 2002).
Finally, an important question is the suitability of the conflict relapse model to study the effects of other stimuli that provoke drug craving and relapse in humans such as acute exposure to drugs and stress (Jaffe et al. 1989; Sinha et al. 2007). In the reinstatement model, exposure to drug priming (de Wit and Stewart 1981; Self et al. 1996; Stretch et al. 1971), environmental stressors such as footshock (Erb et al. 1996; Shaham and Stewart 1995) and food deprivation (Carroll 1985; Shalev et al. 2001b), and the pharmacological stressor yohimbine (Le et al. 2005; Lee et al. 2004; Shepard et al. 2004) reliably reinstate drug seeking. However, in our preliminary experiments on the ability of cocaine priming, yohimbine and acute 1 day food deprivation, only food deprivation led to reliable and selective resumption of active lever responding in the conflict relapse model. Therefore, in the absence of additional parametric studies, the utility of the conflict model for studying drug-priming- and stress-induced relapse to cocaine seeking is unknown.
Conclusions
We described the first characterization of a novel conflict relapse model of cue-induced relapse to cocaine seeking. Our experimental manipulation of non-contingent cue exposure led to resumption of lever-presses during the relapse tests in 14 of the 24 rats, and surprisingly, 24 h later, 11 of the 24 rats also resumed lever responding in a subsequent post non-contingent cue test. In both tests, large individual differences in lever responding were observed. Thus, it appears that its current stage of development, the conflict relapse model appears suitable for studying individual differences in cue-induced relapse to cocaine seeking. The relatively low rate of responding and the large individual variations in the relapse tests limit the use of the conflict model for studying the effect of pharmacological and neuroanatomical manipulations on decreases in cue-induced relapse. However, the current version of the conflict relapse model may be useful for studying factors that promote cue-induced relapse. In this regard, a question for future research is whether the number of lever presses and the proportion of rats that cross the electric barrier during the relapse tests will be higher following longer self-administration training (several months) and/or longer daily training sessions (6–12 h). This is a likely possibility, because previous studies have shown that rats become less sensitive to adverse consequences associated with drug seeking and taking after prolonged drug exposure (Deroche-Gamonet et al. 2004; Vanderschuren and Everitt 2004; Wolffgramm et al. 2000), or when higher drug doses are available (Johanson 1975).
Acknowledgments
This research was supported by the by the Rosenzweig-Coopersmith Foundation (AZ), and the Intramural Research Program of the National Institute on Drug Abuse (YS). Dr. Zangen is an incumbent of the Reskin career development chair. We thank Emily Wentzel for editorial assistance, and Drs. Jane Stewart, Leigh Panlilio, Charles Schindler and David Epstein for helpful comments.
References
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Dependence. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Catania CA. Learning. 3. Prentice-Hall; Prentice-Hall: 1992. [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology. 1983;79:29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–70. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and drug-free periods. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharamacology. 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Stimuli associated with drug injections as events that control behavior. Pharmacol Rev. 1976;27:325–340. [PubMed] [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Jaffe JH, Cascell NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Jenkins TN, Warner LH, Warden CJ. Standard apparatus for the study of animal motivation. J Comp Psychol. 1926;6:361–382. [Google Scholar]
- Johanson CE. Pharmacological and environmental variables affecting drug preference in rhesus monkeys. Pharmacol Rev. 1975;27:343–355. [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004a;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. NatNeurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Do animal models provide a valid analogue for human drug lapse and relapse? Comment on Leri and Stewart (2002) Exp Clin Psychopharmacol. 2002;10:359–360. doi: 10.1037//1064-1297.10.4.359. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. N Engl J Med. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Olds J, Olds ME. Positive reinforcement produced by stimulating hypothalamus with iproniazid and other compounds. Science. 1958;127:1175–1176. doi: 10.1126/science.127.3307.1175. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology. 2003;168:229–235. doi: 10.1007/s00213-002-1193-0. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Lorazepam reinstates punishment-suppressed remifentanil self-administration in rats. Psychopharmacology. 2005;179:374–382. doi: 10.1007/s00213-004-2040-2. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Dickinson A, Everitt BJ. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007:79. doi: 10.1007/s00213-007-0805-0. in press. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The potentiation of conditioned reinforcement by psychomotor stimulant drugs: a test of Hill’s hypothesis. Psychopharmacologia. 1975;45:103–114. [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking under opioid maintenance: the effects of opioid withdrawal, heroin priming and stress. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin self-administration behavior in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001a;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shalev U, Yap J, Shaham Y. Leptin attenuates food deprivation-induced relapse to heroin seeking. J Neurosci. 2001b;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Smith SG, Davis WM. Punishment of amphetamine and morphine self-administration behavior. Psychol Rec. 1974;24:477–480. [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Stretch R, Gerber GJ, Wood SM. Factors affecting behavior maintained by response-contingent intravenous infusions of amphetamine in squirrel monkeys. Can J Physiol Pharmacol. 1971;49:581–589. doi: 10.1139/y71-075. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen TL, Fuchs RA, Coffey GP, O’Dell LE, Baker DA, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and dopamine overflow in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Warden CJ. Animal motivation: experimental studies on the albino rat. Columbia University Press, Columbia University Press; 1931. [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smit DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci (USA) 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J, Galli G, Thimm F, Heyne A. Animal models of addiction: models for therapeutic strategies? J Neural Transm. 2000;107:649–668. doi: 10.1007/s007020070067. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]