Abstract
Transforming growth factor-β (TGFβ) plays a critical role in pancreatic development and cell proliferation. Binding of TGFβ to its membrane receptor kinases activates the Smad signaling proteins, allowing them to translocate to the nucleus and participate in the transcriptional control of TGFβ target genes. In addition, there is an increasing number of cellular mechanisms affecting the final response of a cell to TGFβ. This includes crosstalk with other signaling pathways and the induction of TGFβ early response genes, such as the TGFβ-inducible early response gene (TIEG) family of transcription factors. Like the Smads, TIEGs behave as downstream effector proteins in TGFβ-mediated pancreatic growth control. The discovery of the Smads and TIEGs has provided new insights into TGFβ-regulated functions. Their significance in pancreatic development and cancer is discussed in this review.
The transforming growth factor-β (TGFβ) family consists of multifunctional cytokines that regulate a broad spectrum of biologic functions including wound healing, cellular differentiation, and deposition of extracellular matrix proteins [1]. Among the most dramatic effects of TGFβ, however, are those associated with cell differentiation during development and inhibition of cell proliferation, mediated largely by the induction of cell cycle arrest and apoptosis [2••,3–5]. TGFβ exerts its biologic function mainly by its effects on regulation of gene expression. Elucidating its signal transduction pathway has become a subject of intense investigation in recent years [6]. This has resulted in the identification of a family of membrane receptor protein kinases, TGFβ receptor kinase I (TβRI) and TGFβ receptor kinase II (TβRII), and the subsequent discovery of their intracellular mediators, the Smads [7,8]. Although Smad signaling appears to be involved in most actions of TGFβ, recent evidence suggests the existence of additional TGFβ downstream mediators. For example, TGFβ has been shown to rapidly activate the mitogen-activated protein kinases (MAPK) extracellular regulated kinase (Erk), p38 and JNK, which regulate gene expression by activating specific downstream transcription factors [9•,10]. In the same line of evidence, we have previously reported the identification of the TGFβ-inducible early response gene (TIEG) family of TGFβ-inducible transcription factors, the expression of which is enriched in the exocrine pancreas. Like the Smads, TIEG proteins are TGFβ-regulated effector proteins that significantly affect homeostasis of the pancreas. Expression of both TIEG1 and TIEG2 is rapidly upregulated by TGFβ stimulation, leading to the control of pancreatic cell growth through inhibition of cell proliferation and induction of apoptosis [11,12].
Deregulation of TGFβ function is a common feature of pancreatic cancer and is frequently associated with genetic disturbances of either the TGFβ receptor kinases (TβRs) or the Smads [13]. During carcinogenesis, many tumor cells harbor mutations in TGFβ signaling, rendering them unresponsive to TGFβ-stimulated cell growth inhibition.
In this review we focus on the significance of TGFβ and its downstream mediators in pancreas development, and we particularly emphasize its important functional role in the early and late stages of pancreatic cancer.
Transforming growth factor-β signaling through the Smad proteins
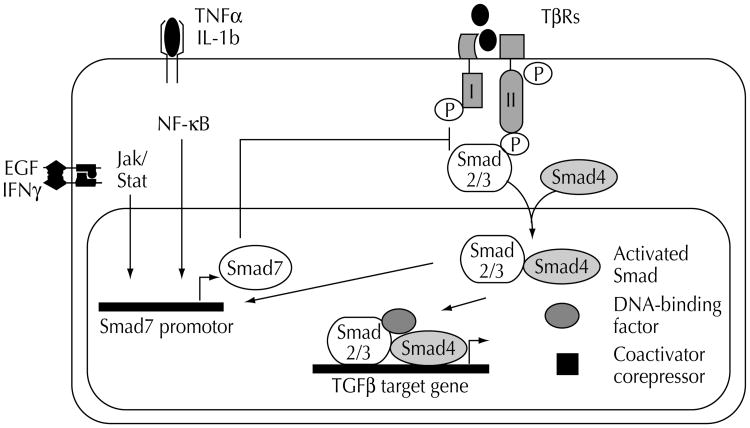
The TGFβ exhibits its antiproliferative functions by activating a signaling pathway that mediates cell cycle arrest and induction of apoptosis. Signaling is initiated by binding of TGFβ to the TβRII cell surface receptor. This, in turn, recruits the TβRI kinase, which phosphorylates the receptor R-Smads proteins, Smad2 and Smad3 (Fig. 1) [14••]. Activated R-Smads form a complex with the Co-Smad, Smad4, which shuttles directly to the nucleus. Here, the complex can either directly bind to DNA, interact with other DNA-binding proteins, or recruit other transcription factors such as AP1 to regulate the transcription of target genes involved in cell growth control (eg, p15, p21, c-myc). These partner proteins are crucial for the selection of Smad-regulated target genes and therefore have an enormous impact on the biologic outcome of TGFβ stimulation [14••]. TGFβ signaling is further controlled by a third class of Smads, the inhibitory Smad6 and Smad7 proteins, which negatively regulate R-Smad activation. Smad7 inhibits signaling from the serine/threonine kinase receptors by binding the TβRI, thereby preventing the phosphorylation of Smad2 and Smad3 [15–19]. Evidence demonstrates distinct crosstalk and feedback between the Smads and other signaling pathways, resulting in either stimulation or inhibition of Smad signaling [20–25]. The signaling network of the cell at large also modulates the expression and activity of the Smads' nuclear partner proteins, thereby fine-tuning the final selection of target genes [26••].
Figure 1. Schematic diagram of transforming growth factor-β (TGFβ) signaling and regulation.
The signaling pathway includes two types of membrane Ser/Thr receptor kinases (TβR-I and TβR-II), the receptor Smads2/3, the co-Smad4, the inhibitory Smad7 and nuclear partner proteins (eg, DNA-binding factors). The Smad signaling is negatively regulated by Smad7, which itself can be induced by several crosstalks, including epidermal growth factor signaling and NFkB.
Together, the current data describe a simple TGFβ signal transduction pathway through the Smad proteins, under the tight control of a complex web of regulator proteins, the ultimate effect on gene expression being determined by intranuclear coproteins. In addition to this well-defined pathway, growing evidence suggests the existence of Smad-independent TGFβ effector pathways.
Transforming growth factor-β-inducible early response genes: new players in transforming growth factor-β-induced cell growth inhibition in the pancreas
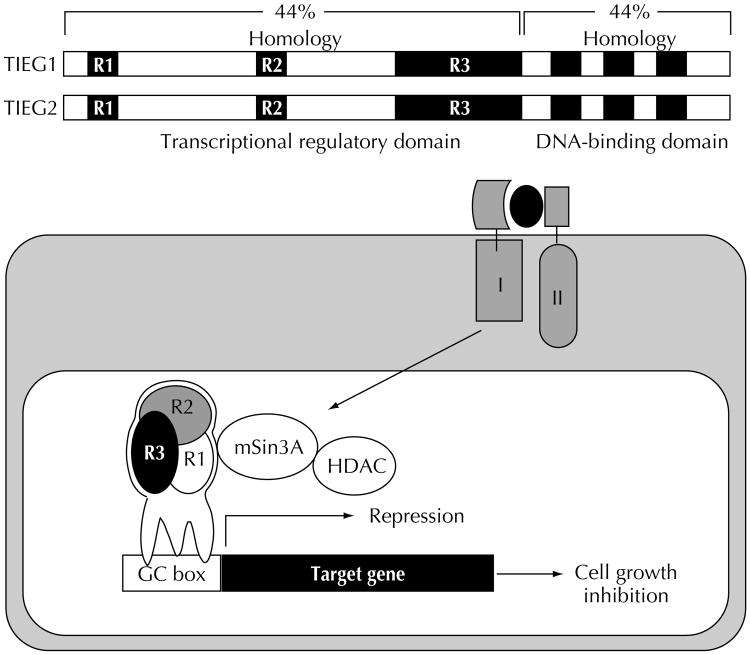
The antiproliferative response of a cell to TGFβ requires the transcriptional regulation of a set of target genes, including TGFβ signal transduction components, cell cycle regulators (p15, p21, and p27), and transcription factors (eg, c-myc). The identification and characterization of TGFβ early response genes involved in growth control of exocrine pancreatic cell populations is a focus of our laboratory. We recently reported the cloning of TIEG1 and TIEG2 (TGFβ-inducible early response genes) from a rat pancreas cDNA library [12,27]. TIEG1 and TIEG2 comprise a novel family of pancreasenriched Sp1-like transcription factors and are early-response genes for TGFβ that inhibit epithelial cell proliferation [28•]. TIEG1 and TIEG2 share overall structural homology within both DNA-binding and transcriptional regulatory domains and, similarly to other Sp1-like proteins, bind GC-rich promotor sequences found in a large number of genes. Many of these target genes are involved in the control of cell proliferation, including cell cycle regulators (p15, p21, p27), MAPK, mitogenic GTPases (H-ras), and DNA synthesis proteins [11]. In contrast to most Sp1-like proteins, the TIEG proteins behave as transcriptional repressors. The TIEGs transcriptional-regulatory region contains three domains, R1, R2, and R3 [29], separated by linker regions, which may be targets for different signaling pathways. In fact, the most recent data from our laboratory demonstrates that epidermal growth factor (EGF) signaling through the proliferative Ras-MEK-ERK-MAPkinase pathway antagonizes the transcriptional activity of TIEG2 by phosphorylation of sites in the linker region between R1 and R2 (unpublished data). Such inhibition of TIEG2 activity is likely to interfere with its antiproliferative function. Our data support a model in which TGFβ-induced expression of TIEGs stimulates binding to GC-rich promotor sequences, where they regulate the transcription of genes necessary for epithelial cell growth control (Fig. 2). We have shown that overexpression of TIEGs inhibits the proliferation of TGFβ-sensitive pancreatic cells and induces apoptosis in a variety of epithelial cell systems following the same mechanistic pattern as TGFβ, characterized by the formation of reactive oxygen species and loss of mitochondrial membrane potential [12,27,30]. The significant role TIEGs play in pancreatic growth control is further supported by data from our laboratory using a transgenic mouse model. Targeted overexpression of TIEG2 in the acinar cells of the exocrine organ resulted in increased apoptosis associated with significant weight loss of the pancreas (unpublished data).
Figure 2. Schematic representation of transforming growth factor-β (TGF-β)-inducible early response gene (TIEG) structure and function.
TIEG1 and TIEG2 share a structural homology of 44% within the proline-rich transcription domain (N terminus) and a 91% similarity within the three Sp1-like zinc finger motifs of the C-terminal domain, which is responsible for DNA binding. Biochemical analysis of the transcription domain revealed the presence of three highly conserved domains that behave as potent transcriptional repression domains (R1, R2, and R3). TGFβ signaling rapidly induces TIEG1 and TIEG2 expression in exocrine pancreatic cells. TIEGs then regulate the transcription of their target genes through recruiting the Sin3A corepressor complex, which contains histone deacetylase activity.
Together, these data strongly suggest that in addition to the Smad proteins, the TIEGs function as downstream mediators of TGFβ, involved in the regulation of pancreatic growth. It is not yet clear whether the TIEG proteins work with or independently of the Smads in TGFβ-mediated pancreatic cell growth control.
Transforming growth factor-β in pancreas development
In recent years, several converging lines of evidence have indicated a crucial role for TGFβ in regulating pancreatic morphogenesis. Interestingly, TGFβ affects the development of both the endocrine and the exocrine pancreas and also has an impact on the extracellular composition of the organ. Evidence suggests that TGFβ plays a significant role in the balance between the exocrine and endocrine composition of the developing pancreas [31••,32].
The TGFβ effectively controls cell proliferation of the exocrine organ by imposing a strong antiproliferative signal on acinar cells. This is strikingly seen on supraphysiologic stimulation of cultured embryonic pancreatic buds with TGFβ, resulting in increased apoptosis of acinar cells and reflecting significant regression of the acinar compartment in the developing pancreas [32]. Also, recent in vivo work by Sanvito et al. [31••] reported dramatic morphologic alterations in the exocrine pancreas of transgenic mice overexpressing TGFβ. The architecture and composition of the exocrine portion of the pancreatic gland was heavily changed in TGFβ overexpressing mice, characterized by loss of acinar cells and replacement of acini by fibrotic tissue.
Mice overexpressing TGFβ also exhibit severe morphologic changes in the endocrine pancreas, indicating a significant role of TGFβ in both acinar and islet formation. Multifocal fibrosis was observed throughout the organ, with centered clusters of endocrine cells and invading exocrine tissue. Additionally, the morphology of the islets of Langerhans themselves was affected. Although the islet cells were small and appeared fragmented, the number and distribution of insulin, glucagon, somatostatin, and pancreatic polypeptide–positive cells were normal, and the transgenic mice showed neither hyperglycemia nor changes in viability and overall health [31••]. As shown by Miralles et al. [33], TGFβ strongly upregulates the expression and activation of matrix metalloproteinases. This crucial step during the development of pancreatic islets enables activated matrix metalloproteinases (eg, matrix metalloproteinase-2) to degrade extracellular matrix components and thereby allows endocrine cells to migrate into the surrounding mesenchyme to form mature islets of Langerhans. Application of specific TGFβ-neutralizing antibodies abolished the islet morphogenesis without affecting endocrine cell differentiation.
The significance of TGFβ in pancreas development is further emphasized by recent reports describing disorders of the endocrine pancreas related to Smad mutations. For instance, hypoplasia of the β-cell, hypoinsulinemia, impaired glucose tolerance, and significant insulin resistance were observed in mice with heterozygous Smad2 mutations [34••]. Although recent studies provide increasing evidence for a critical role of TGFβ in multiple steps of pancreatic development, many effects of TGFβ in pancreatic development are still unknown and require further study to better understand the role of TGFβ signaling in this process.
Transforming growth factor-β in carcinogenesis
The TGFβ plays a dual role in carcinogenesis, acting early as a tumor suppressor but promoting tumor progression in later stages [35]. Its antiproliferative functions allow TGFβ to act as a strong tumor suppressor in early phases of tumorigenesis [36]. During tumor development, however, many tumor cells lose their growth inhibitory responses to TGFβ [37••]. This results from low expression levels of TGFβ receptors, mutations of Smad proteins, or the induction of TGFβ resistance by oncogenes. Additionally, genetic alterations within cellcycle regulators and activating mutations within proliferative crosstalk signaling pathways have been reported in association with reduced TGFβ cell growth inhibition [37••,38•].
Alterations of the transforming growth factor-β signaling pathway in pancreatic cancer
A loss of sensitivity to the antiproliferative effects of TGFβ is frequently associated with a reduced expression or inactivation of TGFβ receptors [39]. TβRII is a frequent locus of inactivating mutations and is particularly common in colon and gastric carcinomas, along with microsatellite instability as a result of defects in the DNA mismatch repair system. By contrast, there are only sporadic reports of mutations or deletions in TβRI in gastrointestinal tumors. A missense mutation in the kinase domain of TβRI has been identified in breast cancers [40], and deletions of the TβRI occur at a very low frequency in pancreatic cancer [41].
Inactivating mutations of the Smad4 tumor suppressor gene, located on 18q 21.1, have been described in more than 40% of pancreatic cancers. This is usually associated with complete loss of the remaining Smad4 allele and is strongly correlated with loss of growth inhibition [42]. Biallelic loss of Smad4 is also common in metastatic colon cancer (30%) and is occasionally described in other tumors of the gastrointestinal tract. Interestingly, functionally inactivating germline mutations of Smad4 have been reported in patients with familial juvenile polyposis, associated with an increased risk of gastrointestinal cancer. Like Smad4, the Smad2 and Smad7 genes are located on chromosome 18q21.1, the allelic loss of which is relatively common in gastrointestinal cancers. However, mutational analyses reveals that unlike Smad4, the remaining allele of Smad2 is rarely mutated in colorectal tumors and has not been described in pancreatic cancer [26••,37••]. Although increased expression of the candidate Smad7 oncogene has recently been reported in pancreatic cancer, no activating mutations or amplifications of the Smad7 gene have been identified in pancreatic cancers [43]. Nonetheless, increased expression of Smad7 was associated with a more malignant phenotype in pancreatic cancer, suggesting its role in tumorigenesis.
Finally, there is no evidence that either Smad3 or Smad6 is the locus of homozygous deletions, functionally inactivating mutations, or amplifications in any given human malignancy.
Altered expression of transforming growth factor-β effector proteins by mutational alterations of crosstalk proteins
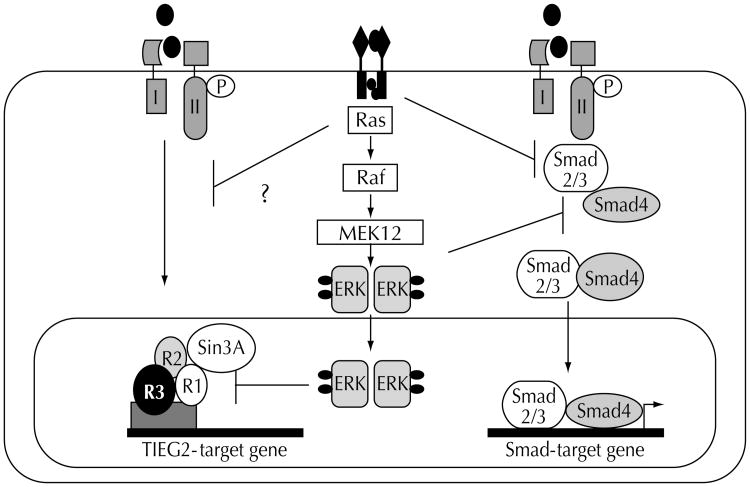
Many tumor cells lacking known mutations of the Smad signaling pathway become resistant to TGFβ-induced growth inhibition during carcinogenesis. Recent data demonstrate that altered expression of crosstalk signaling proteins or downstream Smad partner proteins may have a significant impact on TGFβ-induced growth inhibition [44•]. It has been shown that epithelial cells harboring oncogenic Ras mutations frequently lose TGFβ antimitogenic responses. Oncogenic Ras and its downstream mediator, ERK-MAPkinase, compromise TGFβ signaling in mammary epithelial cells by inhibiting TGFβ-induced nuclear accumulation of Smad2/Smad3, thereby antagonizing TGFβ-mediated growth inhibition [20]. This suggests a mechanism whereby hyperactive Ras silences antimitogenic TGFβ functions in cancer cells. Similarly, in the above-described work, we showed that EGF signaling antagonizes the transcriptional repression activity of TIEG2 in pancreatic cancer cells. These findings support a model wherein oncogenic Ras mutations antagonize the antiproliferative effects of TGFβ through inhibiting the Smad and the TIEG families of TGFβ-effector proteins (Fig. 3). This may be of particular relevance in pancreatic cancer, because activating k-Ras mutations and loss of TGFβ growth inhibition are both found in the majority of pancreatic cancer cells, even without inactivating mutations of the TGFβ pathway.
Figure 3. Crosstalk between the epidermal growth factor (EGF)-Ras- extracellular regulated kinase (Erk)-mitogen-activated protein (MAP) kinase pathway and both the transforming growth factor-β-inducible early response gene TIEG and Smad signaling pathway in cancer.
Hypersensitive Ras and its downstream mediator kinase Erk block transforming growth factor-β (TGFβ) signaling through phosphorylation of the receptor Smads S2/S3 and thereby inhibit the complex formation with Smad4. In addition, EGF-activated Erk MAPkinase phosphorylates TIEG2 and thereby antagonizes its transcriptional repression activity through interfering with the binding of the Sin3A corepressor complex.
Transforming growth factor-β in tumor progression
Reduced TGFβ growth inhibition in pancreatic cancer cells is often associated with disease progression and accompanied by increased expression of TGFβ itself and its type II receptor [45,46]. In fact, increased TGFβ secretion has been shown to promote tumor progression both through direct effects on the tumor cells themselves and effects on accessory cells. For example, TGFβ can stimulate angiogenesis, repress immune surveillance, and induce desmoplasia—all characteristics of pancreatic cancer [46–48]. Its direct effects on the tumor cells include reduction of cell-cell contacts, upregulation and activation of matrix-degrading proteinases, and induction of epithelial-mesenchymal transdifferentiation, leading to increased tumor cell invasion and metastasis [49–51]. The tumor-promoting effects of TGFβ on the tumor cells themselves are observed particularly in cells possessing activating Ras mutations in which the TGFβ signaling pathways remain functional despite loss of growth control by TGFβ [52••,53].
Future biochemical and functional studies investigating the biologic relevance of crosstalk between TGFβ and other signaling cascades in normal and neoplastic epithelial cells are required for a better understanding of the mechanims of TGFβ-induced cell growth inhibition, the loss of antiproliferation, and the switch to tumor progression in late stages of pancreatic cancer.
Conclusions
Current information about TGFβ signaling and transcriptional regulation in the developing and transformed pancreatic cell populations is discussed here. In summary, TGFβ functions mainly through a relatively simple signal transduction pathway, composed of the Smad proteins. The Smads transduce the TGFβ signal from the cell membrane to the nucleus to regulate the transcription of target genes. It is now apparent, however, that this simple signaling system is tightly controlled by an increasing number of cytoplasmic (eg, Smad7) and nuclear (eg, coproteins) mechanisms that integrate the TGFβ signal within a regulatory network of the cell. Furthermore, TGFβ activates a distinct pattern of signaling pathways (eg, PI3K, JNK) and transcription factors such as the TIEG proteins, which may work concurrently or independently of the Smads in TGFβ-mediated pancreatic growth control. Loss of growth responsiveness to TGFβ is a common feature in advanced stages of pancreatic cancer and can result from inactivating mutations within the TGFβ pathway or from genetic alterations of crosstalk pathways (eg, Ras), leading to profound changes in TGFβ signal transduction.
Thus, we are optimistic that this knowledge will provide the theoretical framework for future studies aimed at developing effective therapeutic strategies to treat this dismal disease.
Acknowledgments
The authors thank Dr. Umraan Ahmad for his invaluable contributions toourwriting, Dr. Abigail Conley for her very helpful editorial assistance, and Dr. Vijay Shah for critical reading of the manuscript.
Abbreviations
- TGFβ
transforming growth factor-β
- TIEG
TGFβ-inducible early response gene
- MAPK
mitogen-activated protein kinase
- EGF
epidermal growth factor
- Erk
extracellular regulated kinase
- TβRI/II
TGFβ receptor kinases I/II
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• Of special interest
•• Of outstanding interest
- 1.Moses HL, Serra R. Regulation of differentiation by TGFβ. Curr Opin Genet Dev. 1996;6:581–586. doi: 10.1016/s0959-437x(96)80087-6. [DOI] [PubMed] [Google Scholar]
- 2••.Edlund H. Pancreas: how to get there from the gut? Curr Opin Cell Biol. 1999;11:663–668. doi: 10.1016/s0955-0674(99)00033-2. This excellent review provides a clear outline of pancreatic development, in particular the molecules that might influence pancreatic morphogenesis during the initiation, cell-type specification, and progression of development. [DOI] [PubMed] [Google Scholar]
- 3.Wells JM, Melton D. Vertebrate endoderm development. Ann Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Whitman M. The role of transcription factors involved in TGFβ superfamily signaling during development. Cell Mol Biol. 1999;45:537–543. [PubMed] [Google Scholar]
- 5.Zimmerman CM, Padgett RW. Transforming growth factor beta signaling mediators and modulators. Gene. 2000;16:17–30. doi: 10.1016/s0378-1119(00)00162-1. [DOI] [PubMed] [Google Scholar]
- 6.Massague J. TGFβ signal transduction. Ann Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 7.Piek E, Heldin CH, Ten Dijke P. Specificity, diversity, and regulation in TGFβ superfamily signaling. FASEBJ. 1999;13:2105–2124. [PubMed] [Google Scholar]
- 8.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 9••.Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. This paper is the first report describing the regulation of an endogenous gene by TGFβ in a Smad4-independent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai JL, Schutte M, Bansal RK, et al. Transforming growth factor-beta responsiveness in DPC4/SMAD4-null cancer cells. Mol Carcinog. 1999;26:37–43. doi: 10.1002/(sici)1098-2744(199909)26:1<37::aid-mc5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Cook T, Gebelein B, Urrutia R. Sp1 and its likes: biochemical and functional predictions for a growing family of zinc finger transcription factors. Ann NY Acad Sci. 1999;880:94–102. doi: 10.1111/j.1749-6632.1999.tb09513.x. [DOI] [PubMed] [Google Scholar]
- 12.Cook T, Gebelein B, Mesa K, et al. Molecular cloning and characterization of TIEG2 reveals a new subfamily of transforming growth factor-beta-inducible Sp1-like zinc finger-encoding genes involved in the regulation of cell growth. J Biol Chem. 1998;273:25929–25936. doi: 10.1074/jbc.273.40.25929. [DOI] [PubMed] [Google Scholar]
- 13.Goggins M, Shekher M, Turnacioglu K, et al. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res. 1998;58:5329–5332. [PubMed] [Google Scholar]
- 14••.Massague J, Chen YG. Controlling TGFβ signaling. Genes Dev. 2000;14:627–644. A multitude of regulatory mechanisms control access of TGFβ signaling to their receptor, and regulate the activity of their receptor and the nuclear function of the transcriptional complexes. The regulatory mechanisms that are central for understanding TGFβ signaling are discussed in this review. [PubMed] [Google Scholar]
- 15.Brodin G, Ahgren A, ten Dijke P, et al. Efficient TGFβ induction of the Smad7 gene requires cooperation between AP-1, Sp1, and Smad proteins on the mouse Smad7 promoter. Biol Chem. 2000;275:29023–29030. doi: 10.1074/jbc.M002815200. [DOI] [PubMed] [Google Scholar]
- 16.Imamura T, Takase M, Nishihara A, et al. Smad6 inhibits signalling by the TGFβ superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 asso-ciates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 18.Nakao A, Afrakhte M, Moren A, et al. Identification of Smad7, a TGFβ-inducible antagonist of TGFβ signaling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 19.Heldin CH, Miyazono K, ten Dijke P. TGFβ signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 20.Kretzschmar M, Doody J, Timokhina I, et al. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Caestecker MP, Parks WT, Frank CJ, et al. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998;12:1587–1592. doi: 10.1101/gad.12.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JD, DiChiara MR, Anderson KR, et al. MEKK-1, a component of the stress (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells. J Biol Chem. 1999;274:8797–8805. doi: 10.1074/jbc.274.13.8797. [DOI] [PubMed] [Google Scholar]
- 23.Mulder KM. Role of Ras and MAPKs in TGFβ signaling. Cytokine Growth Factor Rev. 2000;11:23–35. doi: 10.1016/s1359-6101(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 24.Hartsough MT, Frey RS, Zipfel PA, et al. Altered transforming growth factor beta signaling in epithelial cells when ras activation is blocked. J Biol Chem. 1996;271:22368–22375. doi: 10.1074/jbc.271.37.22368. [DOI] [PubMed] [Google Scholar]
- 25.Engel ME, McDonnell MA, Law BK, et al. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 26••.Massague J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. This excellent review summarizes the most current knowledge in TGFβ signaling and its control by cellular events. It emphasizes the role of TGFβ family members in the control of cell growth and differentiation, and focuses on the significance of TGFβ pathway mutations in human disorders. [DOI] [PubMed] [Google Scholar]
- 27.Tachibana I, Imoto M, Adjei PN, et al. Overexpression of the TGFβ-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J Clin Invest. 1997;99:2365–2374. doi: 10.1172/JCI119418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Cook T, Urrutia R. TIEG proteins join the Smads as TGFβ-regulated transcription factors that control pancreatic cell growth. Am J Physiol Gastrointest Liver Physiol. 2000;278:513–521. doi: 10.1152/ajpgi.2000.278.4.G513. This is the first review describing the structure of the TIEG proteins and their role in the regulation of pancreatic cell growth. [DOI] [PubMed] [Google Scholar]
- 29.Cook T, Gebelein B, Belal M, et al. Three conserved transcriptional repressor domains are a defining feature of the TIEG subfamily of Sp1-like zinc finger proteins. J Biol Chem. 1999;274:29500–29504. doi: 10.1074/jbc.274.41.29500. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro A, Bronk SF, Roberts PJ, et al. The transforming growth factor beta (1)-inducible transcription factor TIEG1 mediates apoptosis through oxidative stress. Hepatology. 1999;30:1490–1497. doi: 10.1002/hep.510300620. [DOI] [PubMed] [Google Scholar]
- 31••.Sanvito F, Herrera PL, Huarte J, et al. TGFβ influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120:3451–3462. doi: 10.1242/dev.120.12.3451. This paper describes the role that TGFβ plays during pancreatic development in vitro. It shows that TGFβ regulates the balance between endocrine and exocrine portions of the pancreas. TGFβ promotes the development of the endocrine cells but inhibits the development of the exocrine pancreas. [DOI] [PubMed] [Google Scholar]
- 32.Lee MS, Gu D, Feng L, et al. Accumulation of extracellular matrix and developmental dysregulation in the pancreas by transgenic production of transforming growth factor-beta 1. Am J Pathol. 1995;147:42–52. [PMC free article] [PubMed] [Google Scholar]
- 33.Miralles F, Battelino T, Czernichow P, et al. TGFβ plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol. 1998;143:827–836. doi: 10.1083/jcb.143.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111–127. doi: 10.1101/gad.859401. This review describes the interactions between intercellular signals that mediate pancreatic development. The authors emphasize the relationship between cellextrinsic pancreatic signaling and transcriptional regulators of pancreatic gene expression and their implications in the mechanism underlying human pancreatic exocrine and endocrine diseases. [DOI] [PubMed] [Google Scholar]
- 35.Akhurst RJ, Balmain A. Genetic events and the role of TGFβ in epithelial tumour progression. J Pathol. 1999;187:82–90. doi: 10.1002/(SICI)1096-9896(199901)187:1<82::AID-PATH248>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncol. 1999;10:303–360. [PubMed] [Google Scholar]
- 37••.de Caestecker MP, Piek E, Roberts AB. Role of transforming growth factor-beta signaling in cancer. J Natl Cancer Inst. 2000;92:1388–1402. doi: 10.1093/jnci/92.17.1388. The authors discuss the significant role of recently identified proteins that modulate the Smad-signaling pathway and illustrate the relevance of signaling interactions between the Smads and other signaling cascades in carcinogenesis. [DOI] [PubMed] [Google Scholar]
- 38•.Markowitz SD, Roberts AB. Tumor suppressor activity of the TGFβ pathway in human cancers. Cytokine Growth Factor Rev. 1996;7:93–102. doi: 10.1016/1359-6101(96)00001-9. This excellent review describes the frequency and functional implication of TGFβ receptor mutations in human cancer diseases. In addition, it discusses the therapeutic potential of receptor restoration in gene therapy approaches. [DOI] [PubMed] [Google Scholar]
- 39.Chen T, Carter D, Garrigue-Antar L, et al. Transforming growth factor beta type I receptor kinase mutant associated with metastatic breast cancer. Cancer Res. 1998;58:4805–4810. [PubMed] [Google Scholar]
- 40.Goggins M, Shekher M, Turnacioglu K, et al. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res. 1998;58:5329–5332. [PubMed] [Google Scholar]
- 41.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 42.Miyaki M, Iijima T, Konishi M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999;18:3098–3103. doi: 10.1038/sj.onc.1202642. [DOI] [PubMed] [Google Scholar]
- 43.Kleeff J, Ishiwata T, Maruyama H, et al. The TGFβ signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene. 1999;18:5363–5372. doi: 10.1038/sj.onc.1202909. [DOI] [PubMed] [Google Scholar]
- 44•.Oft M, Peli J, Rudaz C, et al. TGFβ1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. This paper describes the significance of Ha-Ras in TGFβ mediated epithelial mesenchymal transdifferentiation and tumor progression in mouse mammary carcinoma cells. [DOI] [PubMed] [Google Scholar]
- 45.Geng MM, Ellenrieder V, Wallrapp C, et al. Use of representational difference analysis to study the effect of TGFB on the expression profile of a pancreatic cancer cell line. Genes Chromosomes Cancer. 1999;26:70–79. doi: 10.1002/(sici)1098-2264(199909)26:1<70::aid-gcc10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 46.Wagner M, Kleeff J, Friess H, et al. Enhanced expression of the type II transforming growth factor-beta receptor is associated with decreased survival in human pancreatic cancer. Pancreas. 1999;19:370–376. doi: 10.1097/00006676-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Friess H, Yamanaka Y, Buchler M, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 48.Ellenrieder V, Adler G, Gress TM. Invasion and metastasis in pancreatic cancer. Ann Oncol. 1999;10:46–50. [PubMed] [Google Scholar]
- 49.Welch DR, Fabra A, Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci USA. 1990;87:7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wikstrom P, Stattin P, Franck LI, et al. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37:19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 51.Ellenrieder V, Hendler, Boeck W, et al. TGFβ 1 treatment leads to an epithelial-mesenchymal-transdifferentiation of pancreatic cancer cells requiring ERK2 activation. Cancer Res. 2001;61:4222–4228. [PubMed] [Google Scholar]
- 52••.Oft M, Heider KH, Beug H. TGFβ signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–1252. doi: 10.1016/s0960-9822(07)00533-7. Theauthors provideevidencesthat cell-autonomous TGFβ signaling is required for both the induction and the maintenance of in vitro invasiveness during late-stage tumorigenesis. [DOI] [PubMed] [Google Scholar]
- 53.Ellenrieder V, Hendler SF, Ruhland C, et al. TGFβ induced invasiveness of pancreatic cancer cells is mediated by MMP-2 and the urokinase plasminogen activator system. Int J Cancer. 2001;93:204–211. doi: 10.1002/ijc.1330. [DOI] [PubMed] [Google Scholar]