Abstract
Adult stem cells are inextricably linked to whole-body physiology and nutrient availability through complex systemic signaling networks. A full understanding of how stem cells sense and respond to dietary fluctuations will require identifying key systemic mediators, as well as elucidating how they are regulated and integrated with local and intrinsic factors across multiple tissues. Studies focused on the Drosophila germline have generated valuable insights into how stem cells are controlled by diet-dependent pathways, and increasing evidence suggests that diverse adult stem cell populations respond to nutrients through similar mechanisms. Systemic signals, including nutrients themselves and diet-regulated hormones such as Insulin/Insulin-like growth factor or steroid hormones, can directly or indirectly affect stem cell behavior by modifying local cell-cell communication or intrinsic factors. The physiological regulation of stem cells in response to nutritional status not only is a fascinating biological problem, but also has clinical implications, as research in this field holds the key to non-invasive approaches for manipulating stem cells in vivo. In addition, given the known associations between diet, stem cells, and cancer risk, this research may inspire novel anti-cancer therapies.
Keywords: diet, adult stem cells, Insulin, AMPK, TOR, steroid hormone, nuclear hormone receptor, ecdysone, FOXO, PTEN, Drosophila, C. elegans, mouse, human
Introduction
The ability of a species to adapt to nutrient availability is crucial for the success of future generations; therefore, the physiological response to diet involves ancient, conserved cellular functions. In multicellular organisms, changes in nutrient intake at the organismal level are translated into specific cellular responses via an intricate web of metabolic pathways, circulating factors, and signaling relays to ensure physiological homeostasis. Stem cells in particular must be tightly linked to nutrient sensing systems to adjust the production of new differentiated cells to varying physiological constraints and demands.
Evidence ranging from human epidemiological to model organism experimental data suggests that nutrients impact a variety of adult stem cells. Diet influences wound healing, hematopoietic transplants, and cancer risk in humans1-3. In Drosophila melanogaster, germline stem cells (GSCs) have well-characterized dietary responses4-6. This review focuses on in vivo experimental systems (Figure 1) to illustrate our current state of knowledge on how adult stem cells sense and respond to multiple interconnected diet-dependent systemic signals that are integrated with local and intrinsic factors to determine stem cell behavior. While many questions remain regarding how diet controls adult stem cells, it is clear that this complex web of regulation is an essential part of their basic biology. Further, the remarkable evolutionary conservation across diverse organisms and the primal nature of dietary responses point to research using genetically tractable model organisms as the logical avenue towards future fundamental and broadly relevant discoveries concerning stem cell regulation by diet.
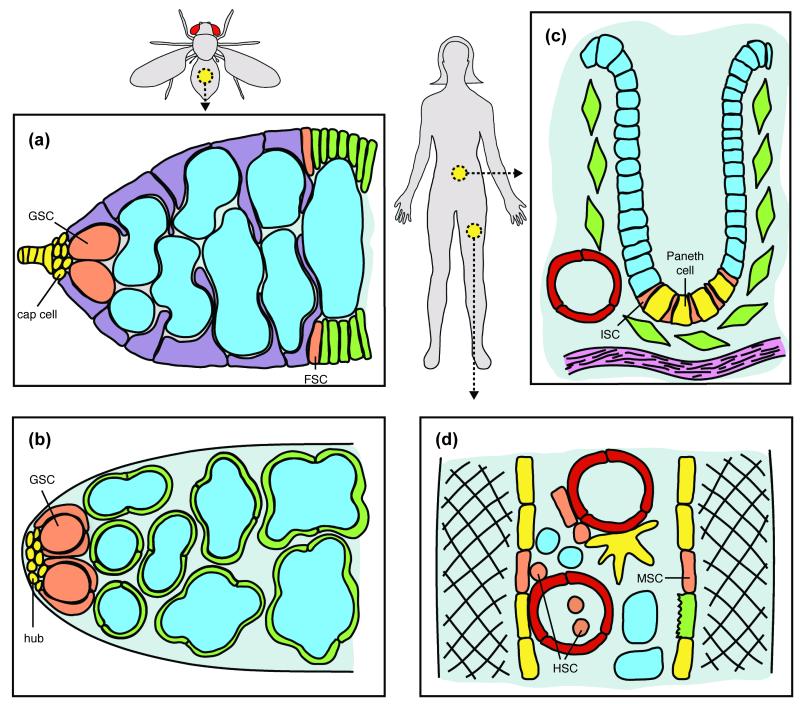
FIGURE 1.
Examples of adult stem cells influenced by whole-body physiology. (a) Drosophila female GSCs reside in a specialized niche (yellow), and their differentiating progeny (blue) are intimately associated with somatic escort cells (purple). FSCs give rise to follicle cells (green) that surround germ cells to form follicles. (b) GSCs (enveloped by cyst progenitor cells) in the Drosophila testis reside in the hub niche; the differentiating GSC progeny (blue) are enveloped by cyst cells. (c) Mammalian ISCs in the intestinal crypts generate transit amplifying cells (blue) that give rise to differentiated cells including Paneth cells, thought to serve as the ISC niche. Crypts are in close proximity to blood vessels (red) and other differentiated cells. (d) Mammalian HSCs reside in a niche composed of MSCs, vasculature (red), and other differentiated cells in the bone marrow, and HSCs can also be mobilized into the bloodstream. See references7 for detailed descriptions.
ADULT STEM CELLS RESPOND TO DIET VIA MULTIPLE MECHANISMS
The in vivo response of adult stem cells to dietary changes was first described in Drosophila female GSCs4, and growing evidence demonstrates that stem cells in many tissues and organisms respond to diet. Drosophila female and male GSCs reside in niches composed of cap and hub cells, respectively, that create a local signaling milieu7 (Figure 1a,b), but GSCs also respond to nutritional inputs4-6, 8. Specifically, female GSCs proliferate robustly under a yeast-rich diet, but without yeast, GSCs divide slowly and are frequently lost from the niche4, 5, 8. Male GSCs also show reduced numbers and proliferation rates under a yeast-free diet6, although halving the yeast concentration relative to a control diet increases GSC number9. Drosophila intestinal stem cells (ISCs) respond to diet by altering proliferation rates and modulating the balance between asymmetric and symmetric divisions6, 10, 11. In the nematode Caenorhabditis elegans, a small population of mitotic germ cells is protected from death during starvation and can later reconstitute the germline9. In mammals (Figure 1c,d), diet impacts multiple tissues1, 9, 12, but specific in vivo stem cell effects are largely unknown. In most cases, the effects of diet on stem cells are at least partially reversible, demonstrating that this is a dynamic process.
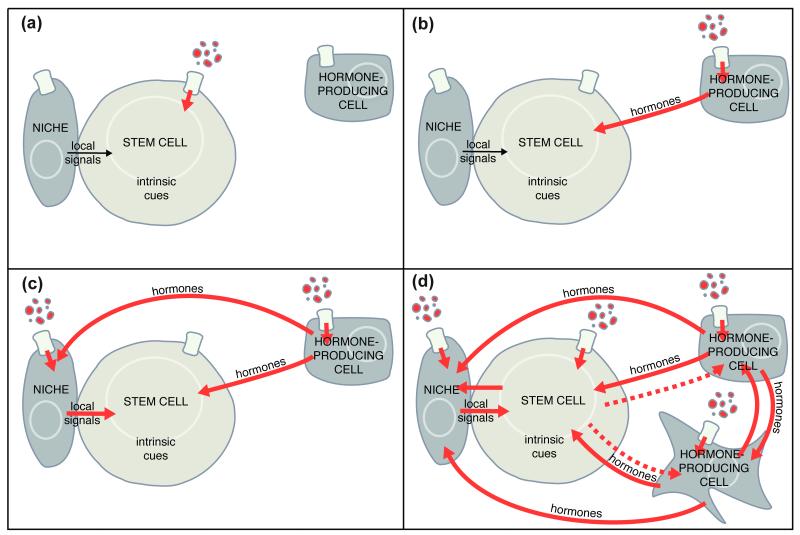
Stem cells could hypothetically sense and respond to diet in different ways (Figure 2). Nutrients might signal directly to stem cells (Figure 2a). Alternatively, nutrients might have indirect effects on stem cells through any of three general strategies. First, hormones produced downstream of nutrients by endocrine cells may directly stimulate stem cells (Figure 2b). Second, either diet-dependent hormones or nutrients may act on adjacent support cells (e.g. the stem cell niche), inducing a secondary signal to stem cells (Figure 2c). Third, more complex systemic hormonal relays may impose increasing degrees of separation between nutrients and their effects on stem cells, incorporating the impact of diet on multiple tissues into the final stem cell response (Figure 2d). Most likely, dietary factors shape stem cell behavior using all of these mechanisms, thereby generating a complex physiological network that coordinates a fine-tuned response of multiple types of stem cells with specific changes in the availability of nutrients.
FIGURE 2.
Possible mechanisms for dietary regulation of adult stem cells. (a) Nutrients may directly stimulate stem cells. (b-d) Alternatively, nutrients may affect stem cells through the direct action of systemic hormones (b), or through indirect effects of nutrients and/or systemic hormones acting on adjacent cells to modulate local signaling (c). Most likely, stem cell activity is regulated by a complex web of signaling (d), including all of the above mechanisms and additional hormonal crosstalk and signaling relays. The stem cell itself may also signal back to the niche or influence hormonal production indirectly (hatched lines) as a result of the function of its differentiated progeny.
Direct nutrient-sensing pathways
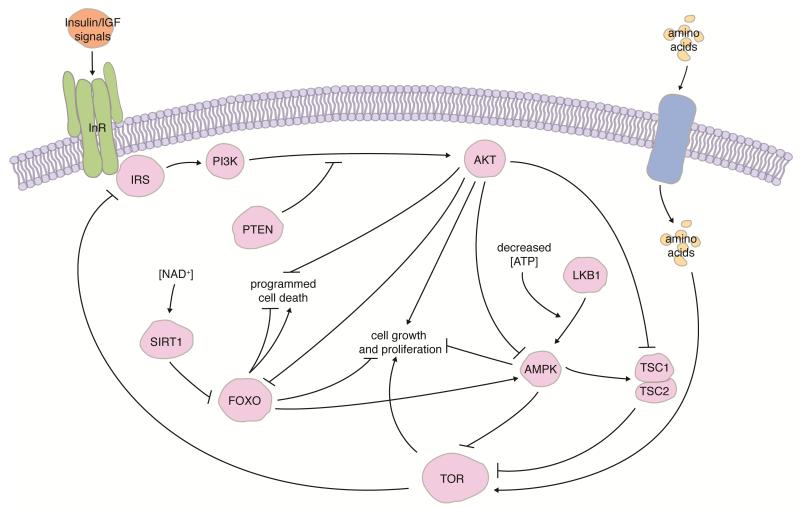
In general, nutrients signal through conserved intracellular pathways to regulate various cellular processes (Figure 3). Target of rapamycin (TOR) signaling is activated by amino acids, promoting protein synthesis and cell growth13. AMP-activated protein kinase (AMPK) is stimulated by upstream kinases such as Serine/threonine kinase 11 (LKB1/STK11) in response to low intracellular ATP levels12. AMPK phosphorylation of upstream regulators inhibits TOR and downstream energy-intensive processes, and AMPK also downregulates anabolism and upregulates catabolism to restore ATP levels within the cell12, 13. The Sirtuin (SIRT) family of protein deacetylases/mono-ADP-ribosyltransferases, which require nicotinamide adenine dinucleotide (NAD+) for their enzymatic activity, also links metabolism to nutrient availability12. The integration of TOR, AMPK, and SIRT signaling into the Insulin/Insulin-like growth factor (IGF) pathway (described below) via crosstalk and common downstream effectors couples multiple nutritional inputs to metabolism and other diet-dependent cellular processes (Figure 3). Recent studies indicate that direct nutrient sensing via these pathways plays an important role in modulating stem cell number and activity.
FIGURE 3.
Integration of conserved nutrient-sensing pathways. Cells respond to Insulin/IGF and amino acids via the Insulin receptor (InR) and TOR pathways, which share common downstream effectors to control cell growth, proliferation, and survival. Insulin receptor substrate (IRS) is a major direct target of InR. AMPK, which is activated by LKB1 under low intracellular ATP levels, and SIRTs, which require NAD+, also interact with the InR and TOR pathways. See references12, 13, 103 for in-depth description of pathways.
Target of rapamycin (TOR)
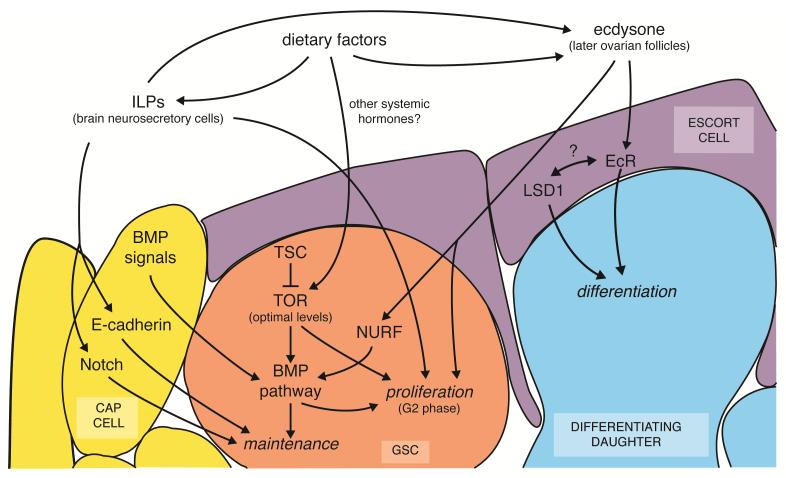
TOR activity has profound effects on Drosophila GSCs (Figure 1a,b). Tor function within female GSCs is required for proper rates of GSC proliferation through an effect on the G2 cell cycle phase14 (Figure 4). Tor mutant GSCs in genetic mosaic females are also frequently lost from the niche14. Interestingly, hyperactivation of TOR through the removal of its upstream inhibitors Tuberous sclerosis (TSC)1 or TSC2 (Figure 3) results in even more severe GSC loss14, 15, which is rescued by treatment with the TOR inhibitor rapamycin15. These studies suggest that tightly regulated TOR activity is crucial for GSC maintenance (Figure 4). Indeed, GSCs lacking Tsc1 function have markedly reduced levels of phosphorylated Mothers against decapentaplegic (MAD)15, a downstream target activated in response to niche-derived bone morphogenetic protein (BMP) signals and required for stem cell self-renewal7. How intrinsic TOR activity regulates BMP signaling within GSCs, however, is unknown. It also remains unclear what effect TOR has on other GSC populations, including Drosophila male GSCs, C. elegans mitotically dividing germ cell precursors, and male spermatogonial stem cells in mammals.
FIGURE 4.
Working model for Drosophila female GSC regulation by diet-dependent signaling. Insulin-like peptides (ILPs) act directly on GSCs to control their proliferation, but indirectly promote GSC maintenance through the niche. TOR signaling controls GSC proliferation, and optimal TOR activity is also required for GSC maintenance by modulating BMP signaling. Ecdysone directly stimulates GSC division and maintenance; ecdysone signaling functions with the NURF chromatin remodeler to stimulate the GSC response to niche BMP signals. In addition, ecdysone also acts on escort cells to promote differentiation of GSC daughters; this function is similar to that described for LSD1. For details, see text and references5, 7, 8, 14, 15, 37, 39, 51, 52, 92.
Mammalian hematopoietic stem cells (HSCs), which neighbor mesenchymal stem cells (MSCs; see Sidebar 1) and their progeny in a vascularized niche in the bone marrow7 (Figure 1d), are also sensitive to TOR activity16-19. Conditional Tsc1 or phosphatase and tensin homolog (Pten) deletion or expression of a constitutively active, myristoylated AKT1 (Figure 3) increases mouse HSC proliferation and depletes the HSC pool16-19. These effects are reversible by rapamycin, indicating that they occur via TOR hyperactivation17-19. Reactive oxygen species may contribute to these phenotypes16, but additional mechanisms are likely involved because mutation of a cell cycle inhibitor reverses Pten-deficient HSC depletion20. Intriguingly, myristoylated AKT1 expression in endothelial cells triggers the release of factors that support HSC expansion21, suggesting that TOR may control HSCs via additional indirect mechanisms.
TOR signaling may regulate downstream targets in a stem cell-dependent context, potentially reflecting the varied nutritional demands of different stem cell populations, even within the same tissue. For example, despite the requirement for optimal TOR activity levels for GSC maintenance in Drosophila ovaries, neither Tor nor Tsc1 are required for the maintenance of nearby follicle stem cells (FSCs)14 (Figure 1a). In contrast, Tor is required for proliferation of both GSCs and FSCs, although the cell cycle of FSC descendents is surprisingly insensitive to TOR signaling14. In the Drosophila midgut, Tsc1/2 knockdown causes TOR- and MYC-dependent ISC overgrowth and S phase defects, with no apparent ISC number reduction. These ISC phenotypes appear to require intrinsic TOR activity based on genetic mosaic analysis22. These results suggest that widely conserved nutrient-sensing pathways regulate stem cell activity in specific ways.
AMP-activated protein kinase (AMPK)
Adult stem cell roles for AMPK (Figure 3) remain unclear. Recent studies suggest that Lkb1 is required for mouse HSC activity23-25 (Figure 1d). Conditional Lkb1 inactivation leads to abnormal proliferation of HSCs, but not of their more differentiated progeny, and to HSC depletion23-25. Despite similarities to the Tsc1 phenotype, Lkb1 mutant defects are independent of TOR23-25. Further, genetic or chemical inhibition of AMPK does not rescue the Lkb1 phenotypes, suggesting that LKB1 controls HSC proliferation and survival largely independently of AMPK23-25. AMPK, however, might be required in other stem cell populations. For example, AMPK has roles in neural precursor proliferation during development26. Multiple genes encode each of the three AMPK subunits in mammals12, while in Drosophila, a single gene encodes each subunit and the nutrient-sensing function of AMPK is conserved27; therefore, future studies on potential AMPK roles in Drosophila adult stem cells should be informative.
Sirtuins (SIRTs)
Although SIRTs (Figure 3) control organismal metabolism12, it is unclear how they function in adult stem cells. Neural SIRT1 expression increases under caloric restriction28, and AMPK enhances SIRT1 activity by raising NAD+ levels29, 30 (see Figure 3), suggesting potential roles in NSCs. Mouse Sirt1 global mutants have decreased hematopoietic progenitor cell numbers based on in vitro culture of bone marrow cells31. It is unknown, however, if SIRT function is specifically required in adult HSCs or other stem cells.
Diet-dependent hormonal pathways
Dietary factors also induce global physiological responses through changes in hormone levels. The peptide hormone Insulin is secreted by vertebrate pancreatic β cells in response to glucose or specific amino acids and signals through the Insulin receptor (InR)32 (Figure 3). Insulin-like growth factors (IGFs) produced mainly by the liver and also locally within tissues stimulate closely related IGF receptors, acting through a very similar pathway32. In Drosophila and C. elegans, multiple Insulin-like peptides produced mainly by neuroendocrine cells act through single Insulin/IGF receptor homologs32. Insulin/IGF signaling has highly conserved roles in metabolism, growth, proliferation and survival, and shares downstream effectors with the TOR and AMPK pathways13 (Figure 3).
Steroid hormones are diverse molecules derived from sterol precursors33. Mammals acquire cholesterol from their diet and synthesize it de novo, while Drosophila and C. elegans are solely dependent on dietary cholesterol-related molecules33. Other dietary factors can also serve as steroid hormone precursors (e.g., vitamin A for retinoic acid), and hormone biosynthetic enzyme expression can be nutritionally regulated34. Steroid hormones achieve cellular responses by binding to nuclear hormone receptors (NHRs), a family of proteins with highly conserved DNA-binding and variable ligand-binding domains35, 36. Some nutrients act as NHR ligands, including cholesterol, fatty acids, and vitamin D33, 34, 36. Finally, extensive crosstalk adds to the complexity of NHR-diet connections33, 35, 36. As discussed below, Insulin/IGF hormones and NHR signaling are important regulators of adult stem cells.
Insulin, Insulin-like growth factor (IGF), and other Insulin-like signals
Insulin-like signals have extensive roles in GSC regulation (Figure 1a,b). Insulin/IGF signaling controls proliferation and maintenance of Drosophila female GSCs through clearly distinct mechanisms4, 5, 37 (Figure 4). Insulin-like peptides act directly on GSCs to control their proliferation rates, based on genetic mosaic lineage tracing8, 37, and this occurs through Phosphoinositide 3-kinase (PI3K)-mediated inhibition of the downstream transcription factor Forkhead box, subgroup O (FOXO) (see Figure 3) to modulate G28. Independent evidence that Insulin promotes GSC progression through G2 comes from cultured Drosophila ovary studies38. In contrast, Insulin-like signals indirectly control GSC numbers through niche effects5, 39, suggesting that diet-dependent hormones can affect a single stem cell system in diverse ways. Genetic inhibition of Insulin/IGF signaling leads to reduced numbers and slowed G2 progression of Drosophila male GSCs9. InR mutant GSCs are lost at high rates in genetic mosaics, suggesting an intrinsic requirement for GSC maintenance in males6 (in contrast to females5, 39). In C. elegans, InR/daf-2 mutants have a smaller population of mitotically-dividing germ cells than wildtype, yet have normal proliferation rates in adult gonads; instead, daf-2 acts through Pten/daf-18 and Foxo/daf-16 to control larval germ cell division rates40. Cultured mouse spermatogonial stem cell studies suggest that Leydig cell-secreted IGF-1 may promote their maintenance41; future in vivo studies should carefully examine how Insulin/IGFs affect mammalian GSCs.
Insulin-like signals also control diverse populations of somatic stem cells. Both systemic and locally produced Insulin-like peptides modulate Drosophila ISC proliferation6, 9-11 via direct effects on the ISC itself and indirect effects through its immediate daughter10. In mammals, conditional Foxo1, Foxo3 and Foxo4 deletion causes increased proliferation and apoptosis of HSCs42 Peripherally administered IGF-1 enhances neurogenesis in the adult rat hippocampus, and intraventricular infusion of an inhibitory IGF-1 antibody inhibits ischemia-induced proliferation of neural progenitors in the subgranular zone of the hippocampal dentate gyrus43, 44. Consistent with these results, Pten deletion in neural progenitors stimulates self-renewal and proliferation, although simultaneous deletion of Foxo1, Foxo3, and Foxo4, or Foxo3 alone increases proliferation and, paradoxically, inhibits self-renewal12, 45-47. Specific effects on NSCs versus their immediate progeny, however, remain unclear.
Ecdysone
The Drosophila steroid hormone ecdysone is structurally similar to human sex steroids and regulates many developmental processes33, 35. In adult females, ecdysone is produced by vitellogenic ovarian follicles in a diet- and Insulin-dependent manner48, 49. Ecdysone signals through the Ecdysone receptor (EcR), which functions as a heterodimer with the Retinoid X receptor (RXR) homolog Ultraspiracle (USP)35. USP can also heterodimerize with Hormone receptor-like in 38 (Hr38)35. USP/Hr38 can be activated by several ecdysteroids, and Hr38 expression is diet-dependent during larval development35, 50.
Ecdysone controls ovarian GSCs51, 52 (Figure 4). Reduction in ecdysone signaling using temperature-sensitive mutants causes decreased GSC numbers and proliferation51. GSCs deficient for usp or Ecdysone-induced protein 74EF (Eip74EF), a direct transcriptional target of EcR, are rapidly lost in genetic mosaics, reflecting an intrinsic requirement51. Interestingly, GSCs lacking usp or Eip74EF show reduced BMP signaling, suggesting that ecdysone modulates the GSC response to niche signals51. Adult-specific dominant-negative EcR expression in surrounding somatic cells disrupts early germ cell differentiation52, indicating an additional indirect role for ecdysone in promoting differentiation of GSC daughters outside of the stem cell niche.
EcR/USP signaling in GSCs involves distinct mechanisms relative to those in other cells. Mutation of the known EcR co-activator taiman has no effect on GSC maintenance51, 52; however, taiman downregulation in surrounding cells phenocopies dominant-negative EcR expression52. As another example, Eip74EF, but not Ecdysone-induced protein 75B (Eip75B; another direct transcriptional target of EcR), promotes GSC proliferation and maintenance51, while both Eip74EF and Eip75B are required at later stages of oogenesis35. Identifying additional direct and indirect targets of ecdysone in GSCs and in other potential ecdysone-sensitive stem cell populations, and studying how they control stem cell behavior will shed light on how steroid hormones may induce specific responses in different types of stem cells.
Retinoic Acid
Mammalian Retinoic acid receptors (RARs) heterodimerize with RXRs to regulate retinoic acid transcriptional responses34, 53. Although multiple RAR and RXR isoforms exist53, Rara and Rarg phenotypes reveal isoform-specific roles in the mouse HSC lineage54 (Figure 1d). Rara controls granulocyte terminal differentiation but has no apparent role in HSCs, while Rarg-deficient mice have decreased HSC numbers54. Mechanisms underlying this specificity, however, are unknown.
Conflicting data suggest that retinoic acid might regulate adult neural progenitors. Rats fed retinol palmitate display increased numbers of proliferating subventricular neural progenitors55 and enhanced ischemia-induced neurogenesis56. In apparent contrast, retinoic acid intraperitoneal injection suppresses hippocampal and subventricular zone proliferation in mice57. Specific effects of retinoic acid on NSCs versus their diverse progeny, however, are unclear. For example, retinoid acid-treated neurospheres show increased BrdU incorporation in neuronal cells, but not in astrocytes58, and retinoic acid is required in vivo for an early step in neuronal differentiation59. Future studies analyzing the effects of retinoic acid specifically on NSCs will require cell type-specific markers and lineage tracing analysis.
Peroxisome proliferator-activated receptors (PPARs)
Mammalian Peroxisome proliferator-activated receptors (PPARs) may control multiple adult stem cell populations. In general, PPAR/RXR heterodimers function as lipid sensors that regulate transcription in response to various fatty acids to regulate triglyceride metabolism and fatty acid oxidation36. Systemic administration of a PPARG agonist in adult rats increases proliferating neural progenitor numbers60, suggesting a potential role for PPARG in adult NSCs. PPARB/D is expressed throughout intestinal crypts, and pparb/d-deficient mice have decreased numbers of Paneth cells61, which are thought to serve as the ISC niche and are themselves ISC-derived62 (Figure 1c). It is therefore conceivable that PPARB/D might regulate ISCs, either by intrinsic activation of transcriptional programs, or indirectly via the regulation of Paneth cell numbers or function.
Sex steroids
Sex steroids play key roles in regulating adult stem cells and are, in some cases, influenced by diet. Estrogen levels are increased in postmenopausal females on a high fat diet, resulting in a higher risk for breast cancer63. Estrogen and progesterone control mouse mammary stem cell (MaSC) proliferation64, 65. Mice deficient for estrogen and testosterone production have fewer MaSCs, which also perform poorly in transplantation assays64. MaSCs do not express detectable levels of estrogen or progesterone receptors, suggesting that MaSCs respond indirectly to these hormones, perhaps by alterations in local signaling64, 65. Nevertheless, these studies do not rule out potential direct effects of estrogen or progesterone on MaSCs.
The role of sex steroids in other stem cell populations is less clear. Estrogen and androgen receptors are expressed in sites of neurogenesis within the adult rat brain, and estrogen injection rescues the decreased hippocampal proliferation displayed by ovariectomized rats66-69. Androgen administration, however, results in decreased neural proliferation in both sexes66. Estrogen requirements apparently differ between brain regions: estrogen is not required for postnatal neurogenesis in the subventricular zone, but is necessary for the survival of new neurons in the main and accessory olfactory bulbs70. It will be important to examine the role of estrogen and testosterone specifically in NSCs and also in other stem cell populations including MSCs, which appear to be regulated by sex steroids in culture71 (see Sidebar 1), and in the muscle, where these hormones promote satellite cell proliferation72.
THE COMPLEX DIETARY RESPONSE OF STEM CELLS REQUIRES INTEGRATION OF SIGNALS
Diet-dependent systemic factors control stem cells via many different mechanisms. As discussed (Table 1), nutrients themselves and multiple hormones each have the potential to impact various stem cell lineages in similar or distinct ways, underscoring the complexity of systemic influences. Local signals and intrinsic factors also control stem cell self-renewal and proliferation7. The ultimate behavior of any given stem cell population under specific dietary and physiological conditions thus results from the integration of these various interconnected systemic factors with local and intrinsic regulators (see Figure 2d). As illustrated below, stem cells have elaborate ways of integrating these diverse signals into a distinct cellular response.
Table 1.
Summary of the main roles of diet-dependent regulators of adult stem cells
| MODEL SYSTEM | FACTOR | REPORTED ROLES | |
|---|---|---|---|
| Drosophila | GSC (ovary) | TOR | Intrinsically promotes proliferation, maintenance14, 15 |
| Insulin | Directly promotes proliferation, indirectly promotes maintenance (via the niche)5, 8, 37, 39 |
||
| Ecdysone | Directly promotes proliferation, maintenance; additional indirect roles in differentiation of progeny (via adjacent escort cells)51, 52 |
||
|
|
|||
| FSC | TOR | Intrinsically promotes proliferation14 | |
|
|
|||
| GSC (testes) | Insulin | Directly promotes proliferation, maintenance6, 9 | |
|
|
|||
| ISC | TOR | Intrinsically promotes growth, proliferation22 | |
| Insulin | Directly and indirectly (through effects on enterocyte growth) promotes proliferation6, 9-11 |
||
|
| |||
| C. elegans | Germline | Insulin | Promotes proliferation of germline progenitors during development, but not required in adult germ cells40* |
|
| |||
| Mouse/Rat | Spermatogonial stem cell (testes) |
IGF-1 | Promotes maintenance41 |
|
|
|||
| ISC | PPAR | Unknown (expression in crypts and reduction of Paneth cell numbers upon global pparb/d deficiency61) |
|
|
|
|||
| HSC | TOR | Intrinsically suppresses proliferation and promotes survival; indirectly promotes proliferation (via regulation of adjacent endothelial cells)16-21 |
|
| AMPK | Unknown (LKB1 intrinsically suppresses proliferation and promotes survival, but effects apparently independent of AMPK 24, 25, 104) |
||
| SIRTs | Unknown (decreased hematopoietic progenitors from global Sirt1 mutants in bone marrow culture31) |
||
| IGF-1 | Unknown (FOXO intrinsically inhibits proliferation and promotes survival of HSCs12, 42, 79, but upstream signals not determined) |
||
| Retinoic Acid | Promotes proliferation*; additional roles in differentiation of progeny54 |
||
|
|
|||
| NSC | AMPK | Unknown (promotes proliferation and survival of neural progenitor cells during development26) |
|
| SIRTs | Unknown (neural SIRT expression increases during caloric restriction28) |
||
| Insulin/IGF-1 | Promotes proliferation12, 43-47* | ||
| Retinoic Acid | Promotes neurogenesis*; additional roles in differentiation of progeny55-59 |
||
| PPAR | Promotes proliferation of progenitors during development and adults60* |
||
| Estrogen | Promotes hippocampal proliferation66-70* | ||
| Androgen | Suppresses neuronal proliferation66* | ||
|
|
|||
| MaSC | Estrogen plus Progesterone |
Promotes proliferation and maintenance (an indirect mechanism has been proposed)64, 65, 76, 78 |
|
|
|
|||
| MSC | Estrogen | Promotes proliferation, differentiation to osteoblastic lineage71* |
|
| Testosterone | Inhibits differentiation to adipogenic lineage71, 75* | ||
In these experiments, it is unclear whether the effect is on the stem cells or their immediate daughters.
Systemic signals and local signaling pathways
Examples of diet-dependent systemic factors influencing stem cells by altering local signaling are evident for Drosophila female GSCs (Figure 4). Insulin-like peptides stimulate niche cap cells to indirectly control GSC maintenance via two separate mechanisms5, 39. Insulin signaling via PI3K/FOXO promotes locally-induced Notch activation to maintain cap cell number (and thereby niche size)5, 39 and, in parallel, it enhances GSC-cap cell attachment and E-cadherin levels at their junction5, 39. Both TOR and ecdysone signaling control how GSCs respond to niche-secreted BMP signals, as indicated by reduced BMP signaling in the absence of Tsc115, usp, or Eip74EF51, and by strong genetic interactions between EcR and BMP receptor genes51. It will be important to understand how systemic factors are integrated with local signals in other adult Drosophila stem cell systems.
In mammals, ovarian sex hormones activate local signals in the mammary luminal epithelium64, 65. Estrogen plus progesterone induces Tumor necrosis factor receptor superfamily member 11a (RANK/TNFRSF11a) and the WNT receptor Low density lipoprotein receptor-related protein 5 (LRP5) in MaSCs, and the ligands RANKL/TNFSF11 and WNT4 in adjacent luminal cells64, 65. RANK-deficient mammary glands have impaired proliferation during mouse pregnancy76, while ductal cells from Lrp5-deficient mice are defective in transplantation assays77. Progesterone may therefore stimulate MaSC proliferation at least in part through RANKL and WNT ligands secreted from luminal cells64, 65; however, this model has not been directly tested. Finally, WNT signaling is activated in Pten-deficient MaSCs78, suggesting that both steroid hormones and Insulin/IGF signaling might regulate MaSCs.
Systemic signals appear to cooperate with WNTs in several mammalian stem cell systems. WNT and PI3K signaling synergize to promote mouse HSC division, self-renewal, and survival79, and the NHR NR2E1/TLX activates WNT signaling to stimulate adult mouse NSC proliferation and maintenance80. Intriguingly, the putative ISC marker LGR581 associates with WNT receptors and binds systemic R-spondins, which could perhaps potentiate the ISC response to local WNTs secreted by Paneth cells62, 82 (Figure 1c). Thus, systemic signals may employ diverse mechanisms to alter stem cell behavior.
Systemic factors and the intrinsic chromatin modifying machinery
Increasing evidence indicates that stem cell function is epigenetically regulated, and broad control over gene expression in response to dietary cues might be achieved via the integration of systemic factors with the intrinsic chromatin modifying machinery. Progressive epigenetic changes accompany the differentiation of mammalian HSC progeny83 and early germ cells in the adult Drosophila testes and ovary84, 85. Mouse Lysine(K)-specific demethylase 1 (LSD1/KDM1A) functions with NR2E1/TLX in adult NSCs to control their proliferation80. Histone methyltransferases are required for HSC and NSC self-renewal86, for maintenance of the adult C. elegans proliferative germ cell population87, 88, and for Drosophila female GSC progeny differentiation85. The Drosophila histone ubiquitin protease scrawny is required for GSC, FSC, and ISC maintenance89, and the SWI/SNF complex Nucleosome Remodeling Factor (NURF) promotes reception of niche factors necessary for GSC self-renewal in males and females90, 91. In Drosophila females, ecdysone appears to control the levels of the NURF complex as a potential mechanism to modulate BMP signaling in GSCs51, suggesting that systemic factors might regulate local signaling by modifying the intrinsic epigenetic state of stem cells (Figure 4).
The chromatin landscape of surrounding cells also contributes to stem cell regulation92. Down-regulation of the Drosophila histone demethylase Su(var)3-3 (LSD1) in escort cells abutting niche cap cells results in ectopic BMP signals and excessive GSC numbers, suggesting that LSD1 plays a key role in restricting BMP signal expression to the niche to allow differentiation of GSC daughters92. Although the similarity with the dominant-negative EcR phenotype52 is intriguing, it is not known if systemic hormones or dietary factors modulate LSD1 function (Figure 4).
Future studies should address how NHRs and other diet-regulated signals interact with the epigenetic machinery of stem cells (or neighboring cells) to broadly control transcriptional programs required for their proliferation, survival, or self-renewal. It is possible that stimulation by certain systemic factors actively modifies the overall epigenetic state of the cell. For example, NHRs are known to physically interact with epigenetic regulators including histone modifiers and chromatin remodellers80. In response to NHR ligand stimulation, these types of interactions may prime cells for a specific transcriptional response by other signaling molecules (Figure 5). Other systemic factors may not directly modify the stem cell chromatin, but the specificity of their responses in each cell type may instead be dictated by the particular epigenetic signature set by NHRs or other intrinsic factors.
FIGURE 5.
Model for interaction between NHRs and the epigenetic machinery. (a) In the absence of ligand, heterodimeric NHRs are bound by co-repressors, inhibiting transcription at target promoters (pink line). (b) Upon ligand-induced activation, chromatin remodelers replace co-repressors, promoting relaxation of the histone (light green)-DNA (blue) interaction or an open chromatin configuration via nucleosome sliding. (c,d) Transcription is facilitated by (c) open DNA at target promoters to allow the binding of additional target-specific transcription factors (e.g., FOXO, MAD, STAT, or Notch), thus promoting indirect transactivation of target genes, or by (d) direct transactivation of target genes by the NHR complex. Chromatin remodelers could also be exchanged for other NHR co-activators (c) that potentiate the transcriptional response.
Crosstalk between systemic signals
Stem cells simultaneously receive signals from multiple sources in response to diet, and these signals likely crosstalk to induce an adequate stem cell response for the physiological state of the organism (see Figure 2d). As described earlier, Drosophila female GSCs intrinsically require InR, TOR, and EcR activity for timely cell cycle progression, and signals activating these pathways originate from various sources14, 37, 51 (Figure 4). TOR and EcR appear to act largely in parallel to InR signaling to control GSC proliferation, despite the common targeting of G214, 51. Based on genetic interactions, TOR activation in GSCs appears to involve input(s) other than Insulin signaling14, such as perhaps circulating amino acids or other systemic factors. Although it is unclear whether or how TOR and EcR signaling interact, they are both intrinsically required for GSC maintenance14, 51 (as opposed to InR, which is required in the niche5, 39) and both pathways appear to regulate BMP signaling levels15, 51, suggesting that different systemic inputs can be integrated by impinging on a common local signaling pathway.
Molecular crosstalk between NHR signaling and other diet-dependent pathways also occurs in mammals. Estrogen and IGF-1 crosstalk is important for neurogenesis and in breast cancer progression63, 97; however, it is largely unclear whether relevant mechanisms involve stem cells themselves. Interestingly, there is considerable crosstalk between nutrient-sensing pathways and NHRs in the control of circadian rhythms30. Many metabolic hormones exhibit circadian oscillation, and circadian rhythms can be modified by nutrients (e.g., glucose, amino acids, and retinoic acid) and changes in cellular energy levels (likely via AMPK and SIRTs)30. Conversely, the molecular oscillator driving circadian rhythms is regulated by the activity of two NHRs (NR1D1/REV-ERB and RORa) and targets enzymes required for cholesterol metabolism, amino acid regulation, and glycolysis30. HSC proliferation and mobilization are reportedly influenced by circadian rhythms98; however, these data must be carefully interpreted, as stem cell marker expression itself might exhibit circadian cycling.
Finally, there is considerable regulation of Drosophila and mammalian NHRs at the transcriptional level by autoregulatory and feedback mechanisms33, 35, 36. For example, NR2E1/TLX regulates RARB expression in mouse retinal cells99, and neuronal NR2E1/TLX-expressing cells are responsive to retinoic acid59. In fact, since some evidence suggests neural roles for NR2E1/TLX80, it might be interesting to examine potential crosstalk between RAR signaling and the NR2E1/TLX in NSC lineage control. Estrogen stimulates Progesterone receptor expression65, and EcR/USP likely regulates other NHRs35. It is therefore conceivable, for instance, that EcR/USP may be part of an as yet unidentified diet-responsive NHR network contributing to the dietary regulation of Drosophila GSCs. Developing a complete picture of how various systemic signals are integrated to control stem cells in response to nutrients will require the continued investigating of adult stem cell populations within their native physiological context.
NUTRIENT SENSING IN STEM CELLS: PARALLELS TO REGULATION OF TUMOR CELLS?
Notwithstanding the controversies regarding the model that cancer stem cells sustain tumor growth100, it is noteworthy that adult stem cells and cancers in general share an indefinite proliferative potential and the ability to produce differentiated cells, and appear to rely extensively on nutrient-sensing pathways. For example, PTEN mutations are common in human cancers, and the InR and TOR pathways are often constitutively active in cancer cells101, 102. There is also a high correlation between cancers and obesity, metabolic syndrome, and energy-rich diets3. Determining how stem cells receive and interpret systemic signals may therefore inspire innovative ideas and approaches for cancer prevention and treatment.
Conclusion
Coordinating adult stem cell behavior with the systemic environment is crucial to adjust the production of new cells for reproduction and tissue maintenance, remodelling, and repair to the organism’s physiological demand and the constraints imposed by diet. Initial lessons derived from studies in a variety of model systems suggest that despite the conserved and ubiquitous nature of many nutrient-sensing pathways, remarkably specific responses are achieved to finely regulate stem cell behavior (see Table 1). As exemplified by TOR function in Drosophila ovarian stem cell populations14, although stem cells and their progeny often reside in physical proximity in the same tissue and are thus exposed to similar environmental cues, stem cells may respond to those cues differently from their progeny or from other nearby stem cell types.
The response of stem cells to diet requires multiple layers of regulation integrating systemic, local, and intrinsic factors. Some important questions remain: How do changes in levels of individual types of macronutrients, such as proteins, carbohydrates, and lipids, affect the actions of specific systemic factors and nutrient-sensing pathways on stem cells? How do stem cells respond in vivo to micronutrients such as metals, vitamins, or other dietary components, such as flavonoids, sulforophane, curcuminoids, catechins, or resveratrol? How are different systemic signals integrated with each other? How do interactions with local and intrinsic factors control the specificity of stem cell responses? Do stem cells have a feedback effect on circulating factors and organismal physiology through the differentiated progeny they produce? Do different types of stem cells communicate with each other via systemic factors? Due to the very nature of the relationship between stem cells and whole-body physiology, further progress towards a more complete, detailed understanding of the complex regulatory mechanisms involved will require in vivo models in which gene function can be easily manipulated in specific cells and the behavior of stem cells monitored, and methods for detecting changes in stem cell metabolism in situ. This will facilitate identification of the sites of requirement for the production and reception of systemic signals in response to specific dietary factors, and elucidating how they are integrated at the cellular and molecular level with other systemic signals, local stimuli, and intrinsic regulators. These studies will illuminate some of the great mysteries of stem cell biology, and likely provide valuable insights into non-invasive strategies to manipulate stem cells in vivo.
Sidebar 1: A connection between adult MSCs and metabolic syndrome?
In addition to responding to systemic factors, stem cells may have profound effects on our physiology. It was recently proposed that MSC malfunction may contribute to the development of metabolic syndrome (a combination of hyperlipidemia, hypertension, hyperglycemia, Insulin resistance and other symptoms), which is often associated with obesity and hormonal alterations73. In adults, adipocytes are thought to arise from MSCs that can produce muscle, bone, and adipose lineages in culture74, and appear to be influenced by systemic sex steroids71, 75. Estrogen induces higher expression of osteogenic markers, including BMPs, in cultured MSCs, while ovariectomized rats display increased bone resorption and enlarged fat depots, which are reversed by estrogen or testosterone administration, respectively71. Overexpression of androgen receptor in MSCs results in significant reductions in fat mass in vivo75. Changes in the hormonal milieu might therefore alter MSC activity, potentially causing an imbalance in the production of different types of differentiated daughters and subsequent impaired function of various tissues that leads to metabolic syndrome73. Establishing causal relationships in in vivo models, however, will be essential to experimentally elucidate how systemic and local regulation of MSC lineages and metabolic syndrome impact each other.
Sidebar 2: Epigenetic effects of diet on the germ line.
The heritability of epigenetic modifications underscores the potential ramifications of diet-dependent GSC changes on subsequent generations. In fact, maternal nutrition has been linked to the development of adult-onset metabolic disease, and paternal nutrition is critical for normal β cell function in female offspring93. In addition, various nutrients impact the activity of histone modifying enzymes; for example, garlic and cinnamon polyphenols inhibit histone deacetylases, whereas green tea polyphenols inhibit histone acetyltransferases94. Dietary components also may affect the availability of methyl donors or DNA methylation enzyme (DNMT) activity94. For example, silencing Dnmt3 in honeybee larvae mimics feeding “royal jelly” to larval honeybees selected to become queens, biasing development towards the fertile queen rather than the sterile worker fate95. Further, adding the soy isoflavone genistein to the diet of pregnant obese agouti viable yellow (Avy) mice - in which a CpG methylation-sensitive retrotransposon promotes ectopic agouti expression leading to adult-onset diabetes and yellow fur in the absence of methylation - results in increased DNA methylation and decreased incidence of obesity and hyperinsulinemia in their adult offspring96 (see Figure). These studies suggest the interesting possibility that diet-dependent epigenetic changes might occur within parental GSCs that are stably inherited by their progeny, with long-term consequences for the physiology of the resulting offspring.
SIDEBAR 2 FIGURE: Epigenetic effects of diet on the germ line. Maternal genistein supplementation at levels comparable to a human high-soy diet leads to methylation of the IAP murine retrotransposon inserted in agouti viable yellow (Avy) mice. A gradient of CpG methylation in genetically identical Avy /a littermates inversely correlates with coat color and obesity, from yellow (left, unmethylated) to leaner pseudoagouti (right, hypermethylated). For details, see original reference96 from which figure was adapted.
Acknowledgements
We apologize to our colleagues whose original work could not be cited due to space limitations, and thank L. Sampson for valuable comments on this manuscript. Work from our laboratory reviewed here was supported by National Institutes of Health (NIH) R01 GM069875 and American Cancer Society RSG-DDC-112316 (D.D.B.), NIH National Research Service Award F32 GM086031 (E.T.A.), and NIH training grant T32 CA009110 (K.M.L.).
References
- 1.Martin-Salces M, de Paz R, Canales MA, Mesejo A, Hernandez-Navarro F. Nutritional recommendations in hematopoietic stem cell transplantation. Nutrition. 2008;24:769–775. doi: 10.1016/j.nut.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25:61–68. doi: 10.1177/0884533609358997. [DOI] [PubMed] [Google Scholar]
- 3.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28:4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 5.Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc Natl Acad Sci U S A. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, McLeod C, Jones DL. Regulation of adult stem cell behavior by nutrient signaling. Cell Cycle. 2011;10 doi: 10.4161/cc.10.16.17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 8.Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313:700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jasper H, Jones DL. Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 2010;12:561–565. doi: 10.1016/j.cmet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc Natl Acad Sci U S A. 2011;108:18702–18707. doi: 10.1073/pnas.1109348108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138:3343–3356. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaFever L, Feoktistov A, Hsu HJ, Drummond-Barbosa D. Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development. 2010;137:2117–2126. doi: 10.1242/dev.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun P, Quan Z, Zhang B, Wu T, Xi R. TSC1/2 tumour suppressor complex maintains Drosophila germline stem cells by preventing differentiation. Development. 2010;137:2461–2469. doi: 10.1242/dev.051466. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, Chin L, Williams DA, Kwiatkowski DJ, DePinho RA. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharas MG, Okabe R, Ganis JJ, Gozo M, Khandan T, Paktinat M, Gilliland DG, Gritsman K. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Nakada D, Yilmaz OH, Tothova Z, Joseph NM, Lim MS, Gilliland DG, Morrison SJ. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell. 2010;7:593–605. doi: 10.1016/j.stem.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amcheslavsky A, Ito N, Jiang J, Ip YT. Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J Cell Biol. 2011;193:695–710. doi: 10.1083/jcb.201103018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, Tzatsos A, Ozsolak F, Milos P, Ferrari F, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dasgupta B, Milbrandt J. AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev Cell. 2009;16:256–270. doi: 10.1016/j.devcel.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem J. 2002;367:179–186. doi: 10.1042/BJ20020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 29.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 31.Ou X, Chae HD, Wang RH, Shelley WC, Cooper S, Taylor T, Kim YJ, Deng CX, Yoder MC, Broxmeyer HE. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood. 2011;117:440–450. doi: 10.1182/blood-2010-03-273011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- 33.Wollam J, Antebi A. Sterol regulation of metabolism, homeostasis, and development. Annu Rev Biochem. 2011;80:885–916. doi: 10.1146/annurev-biochem-081308-165917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic Acid oxidation. J Nutr. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 35.King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- 36.Vacca M, Degirolamo C, Mariani-Costantini R, Palasciano G, Moschetta A. Lipid-sensing nuclear receptors in the pathophysiology and treatment of the metabolic syndrome. Wiley Interdiscip Rev Syst Biol Med. 2011 doi: 10.1002/wsbm.137. [DOI] [PubMed] [Google Scholar]
- 37.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 38.Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138:2207–2215. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu HJ, Drummond-Barbosa D. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev Biol. 2011;350:290–300. doi: 10.1016/j.ydbio.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137:671–680. doi: 10.1242/dev.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YH, Chin CC, Ho HN, Chou CK, Shen CN, Kuo HC, Wu TJ, Wu YC, Hung YC, Chang CC, et al. Pluripotency of mouse spermatogonial stem cells maintained by IGF-1-dependent pathway. FASEB J. 2009;23:2076–2087. doi: 10.1096/fj.08-121939. [DOI] [PubMed] [Google Scholar]
- 42.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- 45.Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz M, Kelly TJ, Imberski RB, Rubenstein EC. The efects of nurtrition and methoprene treatment on ovarian ecdysteroid synthesis in Drosophila melanogaster. Journal of Insect Physiology. 1985;31:947, 957. [Google Scholar]
- 49.Tu MP, Yin CM, Tatar M. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell. 2002;1:158–160. doi: 10.1046/j.1474-9728.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 50.Ruaud AF, Lam G, Thummel CS. The Drosophila NR4A nuclear receptor DHR38 regulates carbohydrate metabolism and glycogen storage. Mol Endocrinol. 2011;25:83–91. doi: 10.1210/me.2010-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ables ET, Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell. 2010;7:581–592. doi: 10.1016/j.stem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konig A, Yatsenko AS, Weiss M, Shcherbata HR. Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J. 2011;30:1549–1562. doi: 10.1038/emboj.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 54.Purton LE, Dworkin S, Olsen GH, Walkley CR, Fabb SA, Collins SJ, Chambon P. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203:1283–1293. doi: 10.1084/jem.20052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giardino L, Bettelli C, Calza L. In vivo regulation of precursor cells in the subventricular zone of adult rat brain by thyroid hormone and retinoids. Neurosci Lett. 2000;295:17–20. doi: 10.1016/s0304-3940(00)01580-9. [DOI] [PubMed] [Google Scholar]
- 56.Plane JM, Whitney JT, Schallert T, Parent JM. Retinoic acid and environmental enrichment alter subventricular zone and striatal neurogenesis after stroke. Exp Neurol. 2008;214:125–134. doi: 10.1016/j.expneurol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, McCaffery P. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci U S A. 2004;101:5111–5116. doi: 10.1073/pnas.0306336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang TW, Zhang H, Parent JM. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development. 2005;132:2721–2732. doi: 10.1242/dev.01867. [DOI] [PubMed] [Google Scholar]
- 59.Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morales-Garcia JA, Luna-Medina R, Alfaro-Cervello C, Cortes-Canteli M, Santos A, Garcia-Verdugo JM, Perez-Castillo A. Peroxisome proliferator-activated receptor gamma ligands regulate neural stem cell proliferation and differentiation in vitro and in vivo. Glia. 2011;59:293–307. doi: 10.1002/glia.21101. [DOI] [PubMed] [Google Scholar]
- 61.Varnat F, Heggeler BB, Grisel P, Boucard N, Corthesy-Theulaz I, Wahli W, Desvergne B. PPARbeta/delta regulates paneth cell differentiation via controlling the hedgehog signaling pathway. Gastroenterology. 2006;131:538–553. doi: 10.1053/j.gastro.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perks CM, Holly JM. Hormonal mechanisms underlying the relationship between obesity and breast cancer. Endocrinol Metab Clin North Am. 2011;40:485–507. doi: 10.1016/j.ecl.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 65.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 66.Brannvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci. 2005;21:871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- 67.Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- 68.Isgor C, Watson SJ. Estrogen receptor alpha and beta mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience. 2005;134:847–856. doi: 10.1016/j.neuroscience.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veyrac A, Bakker J. Postnatal and adult exposure to estradiol differentially influences adult neurogenesis in the main and accessory olfactory bulb of female mice. FASEB J. 2011;25:1048–1057. doi: 10.1096/fj.10-172635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex steroids and stem cell function. Mol Med. 2008;14:493–501. doi: 10.2119/2008-00004.Ray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y, Zajac JD, MacLean HE. Androgen regulation of satellite cell function. J Endocrinol. 2005;186:21–31. doi: 10.1677/joe.1.05976. [DOI] [PubMed] [Google Scholar]
- 73.Mansilla E, Diaz Aquino V, Zambon D, Marin GH, Martire K, Roque G, Ichim T, Riordan NH, Patel A, Sturla F, et al. Could metabolic syndrome, lipodystrophy, and aging be mesenchymal stem cell exhaustion syndromes? Stem Cells Int. 2011;2011:943216. doi: 10.4061/2011/943216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222:268–277. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- 75.Semirale AA, Zhang XW, Wiren KM. Body composition changes and inhibition of fat development in vivo implicates androgen in regulation of stem cell lineage allocation. J Cell Biochem. 2011;112:1773–1786. doi: 10.1002/jcb.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 77.Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 78.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perry JM, He XC, Sugimura R, Grindley JC, Haug JS, Ding S, Li L. Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 2011;25:1928–1942. doi: 10.1101/gad.17421911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gui H, Li ML, Tsai CC. A tale of tailless. Dev Neurosci. 2011;33:1–13. doi: 10.1159/000321585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 82.de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011 doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 83.Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, Zhao K. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X, Lu C, Prado JR, Eun SH, Fuller MT. Sequential changes at differentiation gene promoters as they become active in a stem cell lineage. Development. 2011;138:2441–2450. doi: 10.1242/dev.056572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rangan P, Malone CD, Navarro C, Newbold SP, Hayes PS, Sachidanandam R, Hannon GJ, Lehmann R. piRNA Production Requires Heterochromatin Formation in Drosophila. Curr Biol. 2011;21:1373–1379. doi: 10.1016/j.cub.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–418. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 87.Li T, Kelly WG. A role for Set1/MLL-related components in epigenetic regulation of the Caenorhabditis elegans germ line. PLoS Genet. 2011;7:e1001349. doi: 10.1371/journal.pgen.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiao Y, Bedet C, Robert VJ, Simonet T, Dunkelbarger S, Rakotomalala C, Soete G, Korswagen HC, Strome S, Palladino F. Caenorhabditis elegans chromatin-associated proteins SET-2 and ASH-2 are differentially required for histone H3 Lys 4 methylation in embryos and adult germ cells. Proc Natl Acad Sci U S A. 2011;108:8305–8310. doi: 10.1073/pnas.1019290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buszczak M, Paterno S, Spradling AC. Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science. 2009;323:248–251. doi: 10.1126/science.1165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cherry CM, Matunis EL. Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell. 2010;6:557–567. doi: 10.1016/j.stem.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xi R, Xie T. Stem cell self-renewal controlled by chromatin remodeling factors. Science. 2005;310:1487–1489. doi: 10.1126/science.1120140. [DOI] [PubMed] [Google Scholar]
- 92.Eliazer S, Shalaby NA, Buszczak M. Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc Natl Acad Sci U S A. 2011;108:7064–7069. doi: 10.1073/pnas.1015874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferguson-Smith AC, Patti ME. You are what your dad ate. Cell Metab. 2011;13:115–117. doi: 10.1016/j.cmet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 94.McKay JA, Mathers JC. Diet induced epigenetic changes and their implications for health. Acta Physiol (Oxf) 2011;202:103–118. doi: 10.1111/j.1748-1716.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- 95.Kucharski R, Maleszka J, Foret S, Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319:1827–1830. doi: 10.1126/science.1153069. [DOI] [PubMed] [Google Scholar]
- 96.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- 98.Mendez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16:235–242. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobayashi M, Yu RT, Yasuda K, Umesono K. Cell-type-specific regulation of the retinoic acid receptor mediated by the orphan nuclear receptor TLX. Mol Cell Biol. 2000;20:8731–8739. doi: 10.1128/mcb.20.23.8731-8739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 101.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hafen E. Cancer, type 2 diabetes, and ageing: news from flies and worms. Swiss Med Wkly. 2004;134:711–719. doi: 10.4414/smw.2004.09885. [DOI] [PubMed] [Google Scholar]
- 104.Gan Q, Chepelev I, Wei G, Tarayrah L, Cui K, Zhao K, Chen X. Dynamic regulation of alternative splicing and chromatin structure in Drosophila gonads revealed by RNA-seq. Cell Res. 2010;20:763–783. doi: 10.1038/cr.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading/Resources
- [accessed August 29, 2011];StemBook: An open-access collection of peer-reviewed chapters covering topics related to stem cell biology. maintained by the Harvard Stem Cell Institute. http://www.stembook.org/
- [accessed August 29, 2011];Society for Developmental Biology, Interactive Fly: An on-line guide to Drosophila development, including a detailed atlas of Drosophila development and a tutorial on oogenesis and spermatogenesis. http://www.sdbonline.org/fly/aimain/1aahome.htm.
- National Human Genome Research Institute model organism ENCyclopedia Of DNA Elements [accessed August 29, 2011];Open-access database of genomic datasets from Drosophila and C. elegans, assembled by a consortium of 11 primary projects, identifying transcription factor binding sites, histone modifications, chromatin structure, and other sequence-based functional elements. modENCODE. http://www.modencode.org/
- [accessed August 29, 2011];Mouse Phenotype Database: Comprehensive database containing phenotypic characteristics of commonly used inbred mouse strains. maintained by The Jackson Laboratory. http://phenome.jax.org/
- Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Sensing of. energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S–896. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- Hietakangas V, Cohen SM. Regulation of tissue growth through nutrient sensing. Annu Rev Genet. 2009;43:389–410. doi: 10.1146/annurev-genet-102108-134815. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Mangelsdorf DJ. Nuclear receptor regulation of stemness and stem cell differentiation. Exp Mol Med. 2009;41:525–537. doi: 10.3858/emm.2009.41.8.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura M. Royalactin induces queen differentiation in honeybees. Nature. 2011;473:478–483. doi: 10.1038/nature10093. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Ward S. Why do worms need cholesterol? Nat Cell Biol. 2003;5:684–688. doi: 10.1038/ncb0803-684. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Benomar Y, Staels B, Retinoid X. receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab. 2010;21:676–683. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318, 326. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol. 2010;28:1079, 1088. doi: 10.1038/nbt.1684. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Levi BP, Morrison SJ. Integrating physiological regulation with stem cell and tissue homeostasis. Neuron. 2011;70:703–718. doi: 10.1016/j.neuron.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH, Hochedlinger K. Chromatin connections to pluripotency and cellular reprogramming. Cell. 2011;145:835–850. doi: 10.1016/j.cell.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Molofsky AV, He S, Morrison SJ. Stem cell self-renewal and cancer cell proliferation are regulated by common networks that balance the activation of proto-oncogenes and tumor suppressors. Cold Spring Harb Symp Quant Biol. 2005;70:177–185. doi: 10.1101/sqb.2005.70.057. [DOI] [PubMed] [Google Scholar]
- Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Stillman B, Stewart DJ, Grodzicker T, Cold Spring Harbor Laboratory . Control and regulation of stem cells. Cold Spring Harbor Laboratory Press; Woodbury, NY: 2008. [Google Scholar]
- Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam Horm. 2000;60:1–73. doi: 10.1016/s0083-6729(00)60016-x. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011 doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Speder P, Liu J, Brand AH. Nutrient control of neural stem cells. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr MR, Pietras EM, Passegue E. Mechanisms controlling hematopoietic stem cell functions during normal hematopoiesis and hematological malignancies. Wiley Interdiscip Rev Syst Biol Med. 2011 doi: 10.1002/wsbm.145. [DOI] [PubMed] [Google Scholar]
- Wolf IM, Heitzer MD, Grubisha M, DeFranco DB. Coactivators and nuclear receptor transactivation. J Cell Biochem. 2008;104:1580–1586. doi: 10.1002/jcb.21755. [DOI] [PubMed] [Google Scholar]
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–681. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]