Abstract
Two ideas have dominated the neuropsychology of the orbitofrontal cortex (OFC). One holds that OFC regulates emotion and enhances behavioral flexibility through inhibitory control. The other ascribes to OFC a role in updating valuations based on current motivational states. Neuroimaging, neurophysiological and clinical observations are consistent with either or both hypotheses. Although these hypotheses are compatible in principle, the present results support the latter view of OFC function and argue against the former. We show that excitotoxic, fibersparing lesions confined to OFC in monkeys do not alter either behavioral flexibility, as measured by object reversal learning, or emotion regulation, as assessed by snake fear. A follow-up experiment indicates that previous reports of a loss of inhibitory control resulted from damage to nearby fiber tracts and not from OFC dysfunction. Thus, OFC plays a more specialized role in reward-guided behavior and emotion than currently thought, a function that includes value updating.
INTRODUCTION
The orbital prefrontal cortex (OFC) is seen as a central node in the emotional circuits of the brain. Profoundly altered emotion regulation is a hallmark of damage or dysfunction within OFC 1, 2. At the extremes, patients with damage to the OFC exhibit ‘acquired sociopathic’ behavior 3. Monkeys with lesions of OFC similarly show altered emotional responsiveness to fear-inducing stimuli 4. OFC is also critical for behavioral flexibility 5. Neuropsychological studies have repeatedly found that damage to OFC leads to an inability to rapidly alter object-reward associations, as assessed in the object reversal learning task. This influential result has achieved ‘classic’ status and has been replicated many times in humans 6, 7, monkeys 8–11, rodents 12, 13 and other mammals.
In part because patients with OFC damage show a correlation between the severity of impairments on the object reversal learning task and the degree of emotional disruption 14, some theories posit that emotion regulation and behavioral flexibility reflect a single, OFC-dependent process15 corresponding to inhibitory control 16, 17. This ‘inhibitory control’ hypothesis has had a wide-ranging impact, influencing thinking not only about how the prefrontal cortex integrates emotion and cognition, but also about the pathophysiology of several psychiatric and neurological disorders 18, 19.
An alternative view of OFC function has also found support in the neuropsychological and neurophysiology literature. It holds that OFC is critical for representing and updating specific outcome expectancies to guide decisions 20–22. This idea agrees with neurophysiological 23–26 and neuropsychological27, 28 studies of OFC, which have emphasized the role of this area in encoding specific stimulus–outcome expectancies as well as in revaluing such expectancies based on current motivational states 9, 29.
These two hypotheses about OFC function—inhibitory control and updating valuations—are not incompatible, of course, and results from neuroimaging and neurophysiology agree with both. However, recent results from neuropsychology have cast doubt upon the former 20. In particular, two recent studies have found that partial lesions of OFC have no effect on object reversal learning, long assumed to assess behavioral flexibility and inhibitory control 29, 30. Because these experiments involved excitotoxic lesions, which spared fibers passing through or near OFC, we investigated the possibility that the ‘classic’ effects of OFC damage have depended on inadvertent damage to fiber tracts running near or through OFC and not on the function of OFC per se. In so doing we reassessed the two hypotheses of OFC function outlined above. Specifically, we tested behavioral flexibility, emotion regulation and revaluation in monkeys that had received complete, excitotoxic, fiber-sparing lesions of OFC.
Here we report that damage limited to neurons within OFC fails to produce the deficits in behavioral flexibility and emotion regulation that are observed after aspiration lesions of this region. Instead, our data show that OFC in monkeys is necessary for the ability to revalue objects in line with current biological needs. An additional experiment confirmed that the ‘classic’ effects of OFC lesions depend on damage to proximate fiber tracts.
RESULTS
OFC, behavioral flexibility and emotion regulation
We tested monkeys with complete, bilateral excitotoxic lesions of OFC (OFCEXC, n=7, Fig. 1) and a group of unoperated controls (CONEXC, n=12) on the key tests of behavioral flexibility and emotion regulation used in earlier studies. Based on MRI assessment we estimated that the injections of excitotoxins destroyed a mean of 77.2% (range: 64.3 – 96.3) of OFC (Supplementary Table S1). Inadvertent damage to adjacent prefrontal cortical and subcortical structures was minimal. Importantly, the extent and location of the lesions matched that used in earlier studies that had employed aspiration lesions of OFC (OFCASP, one-way ANOVA, total lesion volume: OFCEXC vs OFCASP, F(1,9)=0.4, p=0.85), and that had produced severe impairments in behavioral flexibility and emotion regulation 4, 9. The methods used to assess reward-guided behavior in the current and previous studies were virtually identical (see Online Methods); accordingly, we were able to directly compare the scores of monkeys in the present study with those of monkeys with aspiration lesions of OFC (OFCASP, n=3) and their corresponding controls (CONASP, n=10)4, 9.
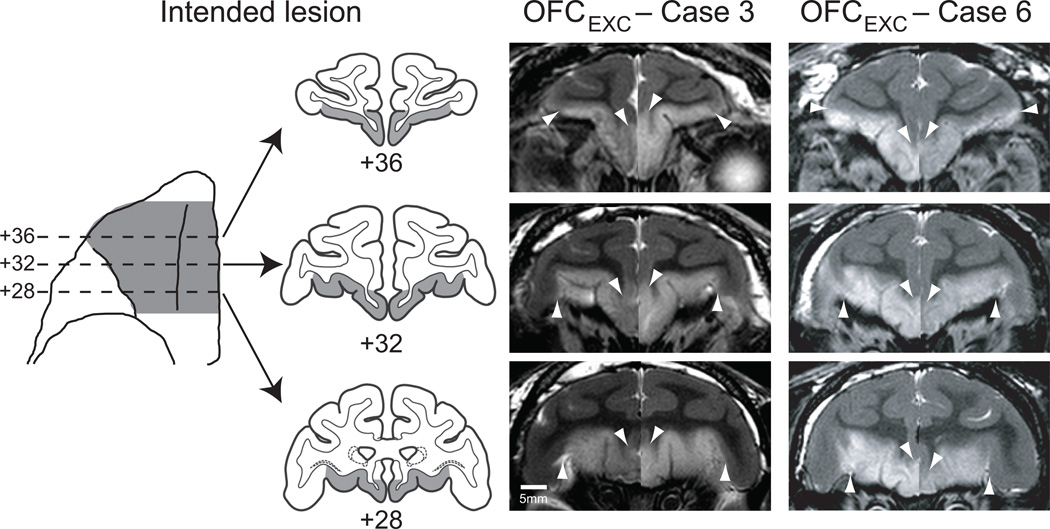
Figure 1.
Excitotoxic lesions of OFC. The first column shows the extent of the intended lesion (shaded region) on a ventral view and on standard coronal sections through the frontal lobe of a macaque brain. The lesions correspond approximately to Walker’s areas 11, 13 and 14. The second and third columns show coronal images at corresponding levels taken from T2-weighted MRI scans obtained within one week of surgery from OFCEXC cases 3 and 6. White hypersignal – set off by arrowheads – is associated with edema that follows injections of excitotoxins and indicates the extent of the lesion. Left and right sides of the MR images are from different scans and have been placed together for ease in viewing. Numerals indicate the distance in mm from the interaural plane.
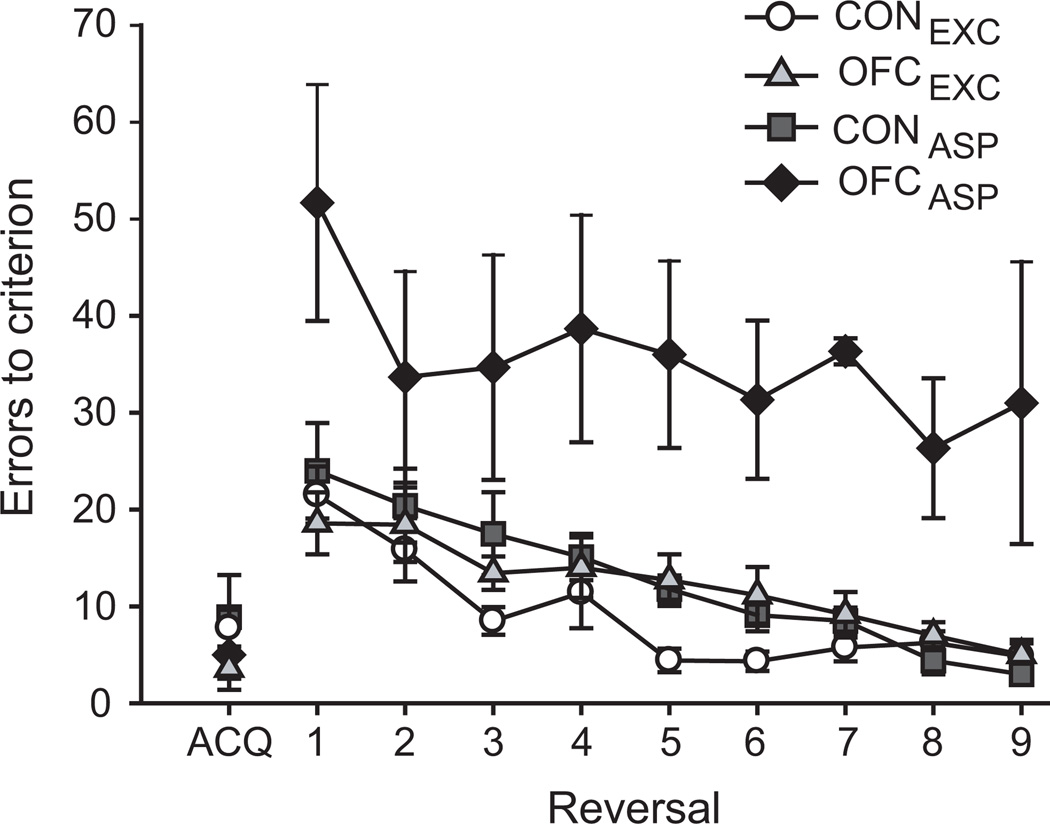
We first sought to determine the effect of excitotoxic lesions of OFC on object reversal learning, a widely used test of behavioral flexibility that has been linked to inhibitory control 10, 16. Initially monkeys learned to discriminate a single pair of objects, novel at the start of testing, for a food reward. After monkeys had attained criterion on this initial object discrimination problem, the contingencies were reversed (i.e., the rewarded object now became the unrewarded item of the pair, and vice versa) and animals were trained to the same criterion as before. This procedure was repeated until a total of nine serial reversals had been completed. The four groups of monkeys learned the initial discrimination at a similar rate (Fig. 2, ACQ; one-way ANOVA, F(3,28)=0.56, p=0.65). In addition, all monkeys, both operated and controls alike, made fewer errors as they completed more reversals (Fig. 2, reversal 1–9; repeated measures ANOVA, effect of reversal, F(8,224)=15.72, p<0.001). Over the course of nine serial reversals monkeys with excitotoxic lesions of OFC switched their responses to the rewarded object as quickly as controls, but were dramatically different from monkeys with aspiration lesions of OFC (effect of group, F(3,28)=15.25, p<0.001; post-hoc tests: OFCEXC vs OFCASP, p<0.001, OFCEXC vs either CONEXC/CONASP, p=1).
Figure 2.
Excitotoxic lesions of OFC fail to disrupt object reversal learning. The plot shows the number of errors to criterion scored by monkeys during acquisition (ACQ) and the nine subsequent serial reversals (1–9) in the object reversal learning task. In contrast to monkeys with aspiration lesions of OFC, monkeys with excitotoxic lesions of OFC scored in the same range as unoperated controls. Error bars show SEM. CONASP and CONEXC, unoperated control monkeys; OFCASP, monkeys with bilateral aspiration lesions of the orbital prefrontal cortex; OFCEXC, monkeys with bilateral excitotoxic lesions of the orbital prefrontal cortex. Data for groups OFCASP and CONASP are from an earlier study 9.
Given this unexpected result, we further probed whether monkeys with excitotoxic lesions of OFC used both positive (correct) and negative (error) feedback in a manner similar to the controls. This trial-by-trial analysis, which is arguably more sensitive than our main measure, also showed no effect of the excitotoxic OFC lesions on monkeys choices (repeated measures ANOVA, effect of group F(3,28)=5.91, p=0.003, posthoc tests OFCEXC vs CONEXC or CONASP, p=1). By contrast monkeys with aspiration lesions of OFC differed markedly from controls (CONEXC, p=0.002; CONASP, p=0.005 Supplementary Fig. S1a) and from monkeys with excitotoxic lesions of the OFC (p=0.011). These data indicate that excitotoxic lesions of OFC do not simply produce a milder deficit than aspiration lesions. Thus, at least in macaques, OFC is not necessary for the ability to flexibly assign rewards to particular objects, as required by the object reversal task.
Next we assessed emotion regulation in these same monkeys. Monkeys are fearful of artificial or real snakes, even when they have not encountered them previously 31, and aspiration lesions of OFC blunt such emotional responses, rendering monkeys less fearful of snakes 4, 32. To assess emotional responsiveness, fear-inducing stimuli (toy spider or snake) and neutral objects were presented, one at a time, inside a Plexiglas box with a food reward placed on the back edge of the box. The dependent measure was the time taken by monkeys to reach over the object to retrieve the food reward. An important feature of the task is that on some trials there is a conflict between approach responses elicited by a motivational incentive, the food, and withdrawal responses engendered by a fear-inducing stimulus. Based on earlier results, we predicted that intact animals would show a greater latency to retrieve the food on trials with fear-inducing objects relative to those with neutral objects. Because differences in testing protocols preclude direct comparison of excitotoxic and aspiration lesions of OFC, comparisons are restricted to concurrently run controls (CONEXC).
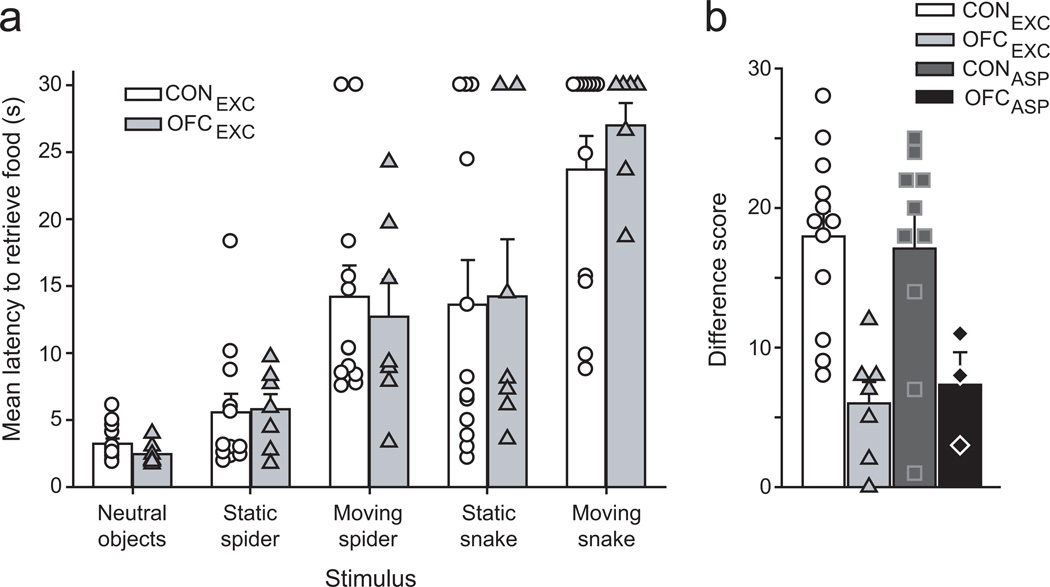
Monkeys with excitotoxic OFC lesions and unoperated controls readily retrieved the food reward in the presence of neutral objects but took increasingly longer in the presence of progressively more anxiogenic objects (repeated measures ANOVA, effect of object type, F(5,85)=45.3, p<0.001, Fig. 3a). Monkeys with excitotoxic lesions of OFC were just as fearful of the snake and spider stimuli as the controls, as measured by their retrieval latencies (group by object type interaction F(5,85)=0.45, p=0.74; effect of group, F(1,17)=0.008, p=0.93). This is in stark contrast to the behavior of monkeys with aspiration lesions of OFC, which fail to inhibit food retrieval responses in the presence of the fear-inducing objects 4 Thus, excitotoxic lesions of OFC in macaques fail to alter emotion regulation, at least in the domain tested.
Figure 3.
Excitotoxic lesions of OFC fail to alter emotional responses but disrupt monkeys’ ability to link objects with food value. a) Like unoperated controls, monkeys with excitotoxic lesions of OFC showed increasingly greater fear when in the presence of increasingly anxiogenic objects, arranged from left to right (neutral objects – moving snake), as indexed by their greater food-retrieval latencies across conditions. Symbols represent the scores of individual monkeys. Error bars show SEM. A trial limit of 30 sec was imposed. b) When required to link objects with food value, unoperated controls chose objects overlying the higher-value food on a high proportion of trials; higher Difference scores indicate greater sensitivity to changes in reward value. Monkeys with either excitotoxic or aspiration lesions of OFC, unlike controls, were unable to link objects with current food value. Symbols represent the scores of individual monkeys. Error bars show SEM.
OFC and the ability to revalue objects
To determine the role of the OFC in the updating of object-outcome value, we evaluated the monkey’s behavior on an object reinforcer devaluation task. In contrast to object reversal learning, this task measures the ability of monkeys to flexibly choose between rewarded objects based on the current biological value of associated foods (see Online Methods). Monkeys learned 60 object discrimination problems in which the positive objects of each pair were rewarded consistently with either food 1 (30 objects) or food 2 (30 objects). We then used a selective satiation procedure intended to devalue one of the two foods to test whether monkeys could update the value of specific object-outcome associations. In critical test sessions comprised of probe trials, monkeys were presented with pairs of objects associated with either food 1 or food 2 and were able to choose between the objects based on their subjective preferences. To determine the degree to which monkeys adaptively shifted their choice of objects following reinforcer devaluation, we computed a ‘difference score’ (object choices after satiation relative to object choices during baseline)29. Higher difference scores reflect a greater shift from baseline following selective satiation and therefore a greater sensitivity to the current value of the foods. In keeping with previous studies we conducted two devaluation tests (see Online Methods). To allow comparison with previously studied groups (CONASP and OFCASP), we compare group scores only on reinforcer devaluation test 2. Irrespective of which test was analyzed the statistical outcome was the same (see Supplementary Fig. S2a).
In sessions following selective satiation, unoperated controls (CONEXC and CONASP) adaptively shifted their choices relative to baseline sessions; specifically, they selected objects associated with the non-sated food on a high proportion of trials, a phenomenon known as the devaluation effect. By contrast, both groups of monkeys with lesions of OFC failed to shift their object choices to the same degree as controls following selective satiation (one-way ANOVA, F(3,28)=7.31, p=0.001; post-hoc tests: OFCEXC vs CONEXC, p=0.003 or CONASP, p=0.008; OFCEXC vs OFCASP, p=1; Fig. 3b). Thus, like monkeys with aspiration lesions of OFC, monkeys with excitotoxic lesions of OFC were unable to link objects with the current biological value of associated food rewards. Control procedures showed that the impairment was unlikely to be due to other factors such as gross changes in visual perception, motivation, food preferences or an inability to discriminate between the two foods (Supplementary Fig. S2b). In addition to showing a role for OFC in linking objects with the value of specific outcomes, and/or using that information to guide choices, these data also indicate that the lesions are effective.
White matter near OFC, behavioral flexibility and emotion
Taken together, the pattern of spared and impaired abilities in macaques with the two types of OFC lesions (either excitotoxic or aspiration) reveals that deficits in emotion regulation and behavioral flexibility observed after aspiration lesions of OFC are related but that they are not dependent on OFC. Instead, the ‘classic’ deficits reported in previous studies may be due to inadvertent damage to neuronal projection fibers passing nearby or through OFC en route to other regions, either alone or in concert with damage to the neurons residing in OFC. Notably, cortical and subcortical structures in the medial temporal lobe contain neurons that project to the prefrontal cortex, and these projections course near the posterior and ventromedial portions of OFC via the uncinate fascicle as well as the extreme capsule 33, 34. Furthermore, neurons in OFC send efferent projections to a host of targets, including the ventral striatum and medial thalamus, and these connections also travel near to the posterior OFC. If impairments seen after aspiration lesions of OFC are due to damage to fibers of passage, then an aspiration lesion limited to the posterior OFC should reproduce alterations in emotion regulation and reversal learning performance, regardless of the exact site(s) of origin or termination of those fibers.
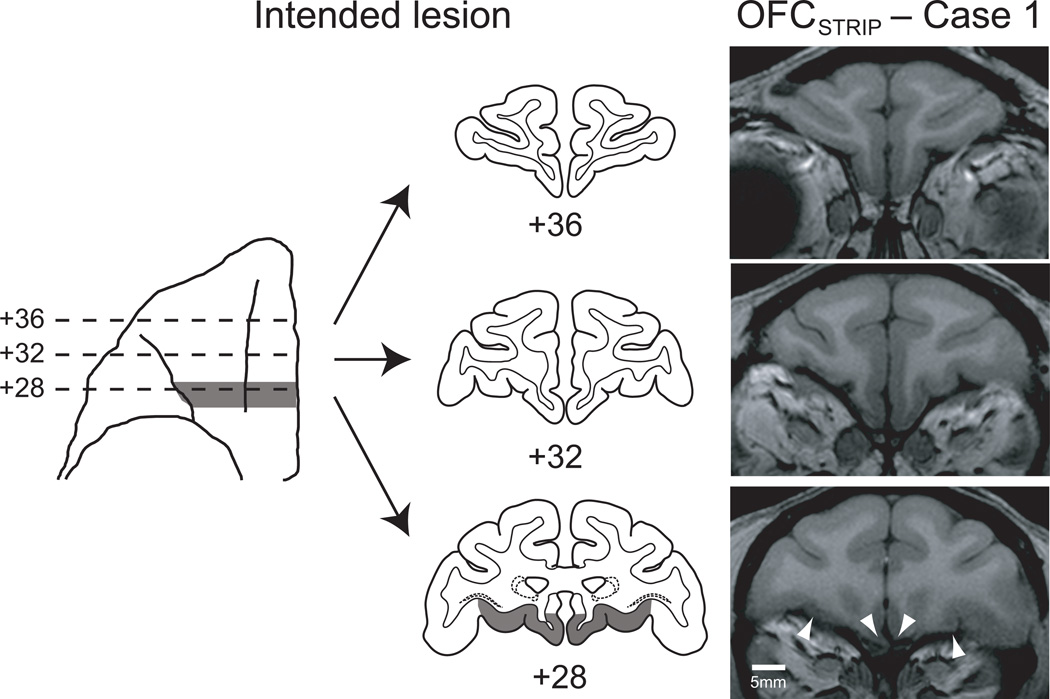
To test this hypothesis, a total of three monkeys from the CONEXC (n=2) and OFCEXC (n=1) groups received an aspiration removal of a narrow strip of cortex, oriented in the mediolateral plane and situated at the posterior boundary of our OFC lesion, at the most posterior extent of OFC (group OFCSTRIP, n=3). For convenience, we refer to these surgical removals as ‘strip lesions’. Lesions were assessed using T1-weighted MRI scans and found to reliably destroy a strip of cortex in posterior OFC; the lesion involved portions of areas 13l, 13m, 13a and 14c 35 (Fig. 4). These monkeys were retested on the object reversal learning task and then the emotional responsiveness task. Their scores were compared with those of the original unoperated control group (CONSTRIP, n=10) and the monkeys with aspiration lesions of OFC and their controls (OFCASP and CONASP).
Figure 4.
Strip lesions situated in posterior OFC. The left side of the figure shows the extent of the intended lesion (shaded region) on a ventral view and on standard coronal sections through the frontal lobe of a macaque brain. The right side shows coronal images at corresponding levels taken from a T1-weighted MRI scan obtained from OFCSTRIP case 1. Arrowheads mark the boundaries of the lesion at +28. Numerals indicate the distance in mm from the interaural plane.
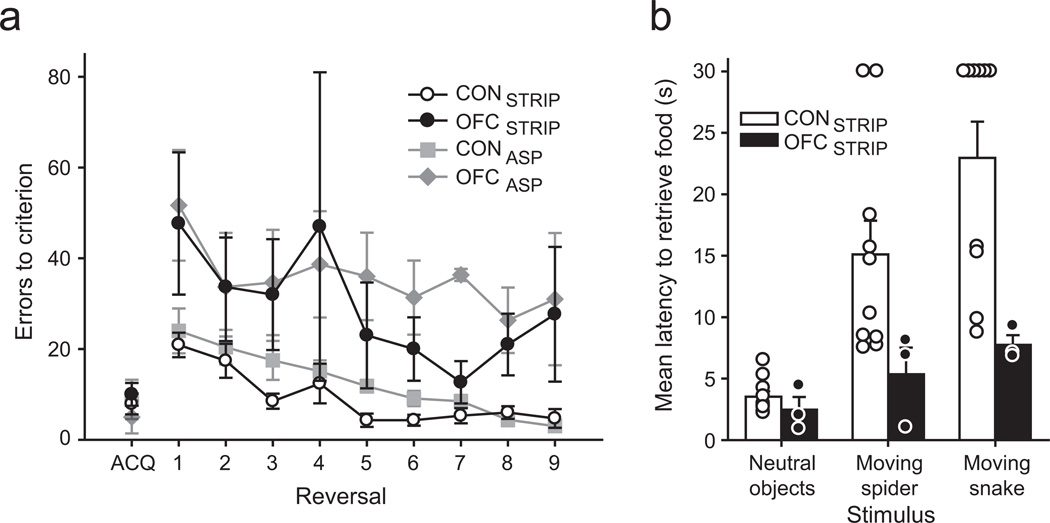
All groups performed similarly when acquiring the initial discrimination problem in the object reversal learning task (ACQ, Fig. 5a; F(3,22)=0.15, p=0.93). When the reward contingencies were reversed, however, monkeys with either strip lesions of the posterior OFC or complete aspiration lesions of OFC made many more errors than controls (repeated measures ANOVA, effect of group, F(3,22)=11.67, p<0.001; post-hoc tests, OFCSTRIP vs CONSTRIP , p=0.001; OFCSTRIP vs CONASP, p=0.021; OFCASP vs CONSTRIP or CONASP, p<0.0025). Monkeys with either strip lesions or aspiration lesions performed similarly (OFCSTRIP vs OFCASP, p=1). Notably, the difference between monkeys with strip lesions of the posterior OFC and unoperated controls was found despite the OFCSTRIP group being retested on object reversal learning, which might be expected to improve performance. Additional trial-by-trial analyses with data collapsed across reversals found that monkeys with strip lesions of the posterior OFC did not benefit from either correctly or incorrectly performed trials to the same degree as unoperated controls (effect of group, F(3,22)=5.74, p=0.005; Least Squared Difference post hoc tests , OFCSTRIP vs CONSTRIP, p=0.022; OFCSTRIP vs CONASP, p=0.034, Supplementary Fig. S1b). Notably, the scores of monkeys with strip lesions of posterior OFC did not differ from those of monkeys with complete aspiration lesions of OFC (p=0.426).
Figure 5.
Monkeys with aspiration lesions of a narrow strip of posterior OFC, like monkeys with complete aspiration lesions of OFC, were impaired on object reversal learning and showed reduced emotional responsiveness. a) When retested on object reversal learning, monkeys with a strip lesion of the posterior OFC (OFCSTRIP) performed significantly worse than unoperated controls (CONSTRIP). Plot shows number of errors to criterion scored by monkeys during acquisition (ACQ) and the nine subsequent serial reversals (1–9) in the object reversal learning task. Error bars show SEM. b) When retested on responses to neutral and fear-inducing objects, monkeys with a strip lesion of the posterior OFC showed reduced emotional responses relative to controls. This pattern matches that seen for monkeys with complete aspiration lesions of OFC. Symbols represent the scores of individual monkeys. Error bars show SEM.
Similarly, monkeys with strip lesions of the posterior OFC exhibited altered emotional responses to fear-inducing stimuli by comparison to unoperated controls (repeated measures ANOVA, group by object type interaction F(2,22)=4.56, p=0.022; effect of group, F(1,11)=6.01, p=0.031, Fig. 5b). This difference was primarily driven by scores on trials with the toy snake; monkeys with strip lesions of the posterior OFC readily retrieved the food reward in the presence of the anxiogenic moving snake object, in stark contrast to unoperated controls (moving snake trials, repeated measures ANOVA, effect of group F(1,11)=7.51, p=0.019). Given that only a small portion of OFC is removed in the strip lesions, these data support the idea that damage to fiber pathways is sufficient to produce deficits observed after complete aspiration lesions of OFC.
DISCUSSION
We assessed monkeys with excitotoxic lesions of OFC on two tests of inhibitory control (object reversal learning and snake fear) and one of updating object–outcome expectancies (reinforcer devaluation). Unlike aspiration lesions of OFC, damage limited to cells in this area, but sparing fibers, did not affect either behavioral flexibility, as measured by object reversal learning, or emotion regulation, as reflected in measures of snake fear. These excitotoxic lesions did, however, cause the same impairment in updating valuations that follows aspiration lesions of OFC. In a follow-up experiment, aspiration of a narrow strip of cortex in the posterior OFC reproduced the often-replicated impairments in object reversal learning and the blunting of snake fear caused by aspiration lesions of the entire OFC.
Taken together, these findings show that inadvertent damage to fibers passing near or through OFC causes the impairments in behavioral flexibility and emotion regulation typically attributed to OFC. The results also support the idea that the OFC performs a more specific function in reward-guided behavior and emotion than currently thought, and indicate that this function includes the updating of object valuations according to current motivational states. It could be argued that the pattern of results after excitotoxic OFC lesions – impairment on reinforcer devaluation but not on object reversal learning and emotional reactivity – simply reflects a difference in the sensitivity of the tasks to OFC damage. Against this idea, however, we note that lesions of the ventral prefrontal cortex impair reversal learning but not reinforcer devaluation36. Thus, the devaluation task is not intrinsically more sensitive to prefrontal cortex damage.
Although our results come from macaque monkeys, there is reason to believe that this conclusion applies to humans as well 37. Given that damage to structures in the temporal lobe leads to deficits in object reversal learning and emotional responses like those observed after OFC damage 4, 38, 39, it seems likely that temporal–frontal interactions underlie both flexible responding in reversal learning and regulation of emotional responses to artificial snakes and spiders. Many of the relevant temporal areas project to the medial frontal cortex via the uncinate fascicle 34. Thus, it is possible that disruption of white matter pathways linking the temporal and medial frontal cortex is responsible for the deficits that classically follow aspiration lesions of OFC. Consistent with this idea, lesions of one part of the medial frontal cortex in macaques, the anterior cingulate cortex (ACC), mildly disrupts object reversal learning 40. The OFC aspiration and ACC lesion groups have in common the inability to sustain the correct choice of object after a reversal.
Because the effects of OFC and ACC aspiration lesions on object reversal learning differ both quantitatively and qualitatively, however, damage to temporal–frontal connections directed to ACC cannot provide a complete account of the disconnection. An alternative possibility is that the deficits result from a more widespread temporal–frontal disconnection, one involving all of the medial frontal cortex and the ventral prefrontal cortex as well. This idea is consistent with identified contributions of the ventral prefrontal cortex to object reversal learning11. Finally, aspiration lesions of OFC may damage multiple circuits passing near or through posterior OFC. Indeed, this region appears to be a bottleneck not only for fibers passing between the temporal and frontal lobes, but also for fibers serving broadly directed neurotransmitter systems 41, 42.
One outstanding question is why our results seem to diverge from those reported in rodents and New World monkeys 10, 12, 13. It is important to note that deficits in rodents and New World monkeys reported after OFC lesions are qualitatively and quantitatively different from those seen in macaques. Specifically, aspiration lesions of the OFC in macaques have a long lasting effect on reversal learning performance, but this effect only becomes apparent around the third or fourth serial reversal 9, 11. By contrast, reversal learning deficits in rodents and New World monkeys are often transient and limited to the first few reversals (for example, ref. 43). It may be that different species learn or solve stimulus reversal tasks in slightly different ways or using different cognitive strategies. In addition, tasks are often tailored to individual species with respect to the cognitive demands of the task (e.g., number of reversals performed) or the sensory modality tested (e.g., odor reversal learning for rodents vs. visual reversal learning in macaques), factors that hamper cross-species comparisons.
Irrespective of these differences, one possibility is that that the divergence between our findings and those from rodents may reflect differences in the expansion of the prefrontal cortex across species during their prolonged period of independent evolution44. Such differences could affect the dependence on the OFC for adaptive stimulus-reward related functions. On this view, in an intact macaque brain, the OFC would be involved in reversing stimulus-reward associations to a minor extent but is not necessary for this function. When the OFC is unavailable, stimulusreward contingency and reversal in macaques can be carried out by alternative brain structures such as amygdala, ventral striatum and other parts of the prefrontal cortex. By contrast, in rodents, the OFC might be more important for all stimulus-reward related functions, including reversing stimulus-reward associations.
It is more difficult, however, to account for the conflicting data from New World monkeys, specifically marmosets 10, 43. In primates, the expansion of the prefrontal cortex occurred in steps, and at least some of that expansion occurred in parallel in New World and Old World monkeys 44, 45. One possibility is that parts of the prefrontal cortex developed divergent functions between New and Old World monkeys. Another is that the apparent difference in the effects of OFC lesions in New and Old World monkeys reflects the distinct foraging niches of the two species studied (Callithrix jacchus and Macaca mulatta). Common marmosets feed mainly on tree gums and insects, which requires patient foraging 46, whereas rhesus monkeys feed on fruit, seeds, roots, buds, bark and cereals, foods obtained by more active foraging.
Our results have implications for psychiatry, computational modeling and theories of prefrontal cortex function. First, partly because of deficits in reversal learning and emotion regulation, several psychiatric disorders have been attributed to dysfunction of OFC, including major depression, obsessive-compulsive disorder and psychopathy 18, 19, 47. Given our finding that the reversal and emotion regulation impairments do not result from damage to OFC neurons, the association between inhibitory control, the OFC and psychiatric disorders needs to be reassessed.
Second, the ability to alter object–reward associations rapidly has often served as a starting point for hierarchical models of prefrontal cortex 48. Narrowing the range of functions attributed to OFC should contribute to more accurate computational models of prefrontal cortex and its role in the processing of rewards and risks.
Third, for decades, OFC has been held to exert inhibitory control over behavior. Although evidence in opposition to this view has been gradually accruing 20, our findings make it clear that inhibitory control is not the purview of OFC. Recent reconsiderations of prefrontal cortex function have led to the same conclusion 20, 44. In agreement with evidence from rats, monkeys and humans, OFC instead represents specific outcome expectancies 20, 21, 24, 49. These representations include the sensory properties and the motivational value of specific outcomes, such as foods 26, punishers 23 and social signals 50, all updated in terms of biological needs based on the animal’s current state 44. Thus, OFC enhances fitness by allowing an informed judgment about the relative merits of choices, in the contexts in which they occur.
METHODS
Subjects
Nineteen adult rhesus monkeys (Macaca mulatta), three female, served as subjects. All animals were naive at the start of the experiment and all experiments were conducted during the light cycle of the day. For the first experiment, seven monkeys sustained bilateral excitotoxic lesions of OFC (group OFCEXC) and the remaining twelve were retained as unoperated controls (group CONEXC). Monkeys were randomly assigned to each group. For the second experiment, a subset of the monkeys from experiment 1 were given ‘strip’ lesions of the posterior OFC (OFCSTRIP). The data from four of the unoperated controls has been previously published29. Data from thirteen monkeys, three with aspiration lesions of OFC and 10 unoperated controls, from two previous studies are also included for comparison9, 51. No statistical test was run to determine the sample size a priori. The sample sizes we chose are similar to those used in previous publications. Monkeys weighed between 5.1–10.0 kg and all were at least 4.5 years old at the start of testing. Each animal was individually or pair housed, was kept on a 12-h light dark cycle and had access to water 24 hours a day. All procedures were reviewed and approved by the NIMH Animal Care and Use Committee.
Apparatus and materials
All apparatus and materials were identical to those described in previous reports on the effects of lesions within the macaque OFC on reversal learning and reinforcer devaluation tasks4, 9, 29, 51. Briefly, all testing was conducted in a modified Wisconsin General Test Apparatus (WGTA) inside a darkened room. Monkeys occupied a wheeled transport cage in the animal compartment of the WGTA. The test compartment of the WGTA held the test tray, which contained two food wells spaced 235 mm apart. Test material for reinforcer devaluation consisted of 120 objects that varied in size, shape, color and texture. Two additional novel objects were used for object discrimination reversal learning. Food rewards for the devaluation task consisted of two of the following six foods: M & M’s (Mars candies, Hackettstown, NJ), half peanuts, raisins, craisins (Ocean Spray, Lakeville-Middleboro, MA), banana-flavored pellets (Noyes, Lancaster, NH) and fruit snacks (Giant Foods, Landover, MD). For object reversal learning a half peanut served as the food reward.
For the emotional response tests, a Plexiglas box measuring 70 × 11 × 11 cm with a hinged back was fixed to the WGTA 20 cm in front of the transport cage. Sixteen neutral, ‘junk’ objects were used across the two tests as well as four different fear inducing objects, either a static rubber spider (test 1), a static rubber snake (test 1), a moving toy spider (test 2), or a moving wooden snake (test 2). Two synchronized cameras recorded monkey’s behavioral and reaching responses.
Surgery
Standard aseptic surgical procedures were used throughout29. Under isoflurane anesthesia, a large bilateral bone flap was raised over the region of the prefrontal cortex and a dura flap was reflected toward the orbit to allow access to the orbital surface in one hemisphere. For the excitotoxic OFC lesion, a series of injections was made into the cortex corresponding to Walker’s areas 11, 13 and 14 in each hemisphere using a hand-held, 30-gauge Hamilton syringe. In a two-stage operation, injections were made bilaterally into the cortex on the orbital surface between the fundus of the lateral orbital sulcus and the rostral sulcus on the medial surface of the hemisphere. The rostral boundary of the injections was an imaginary line joining the tips of the medial and lateral orbital sulci. The caudal boundary of the injections was a line joining the most caudal points of the medial and lateral orbital sulci (Fig. 1). At each site 1.0 µl of ibotenic acid (OFC cases 1–3: 10–15 µg/µl; Sigma or Tocris) or a cocktail of ibotenic acid and N-Methyl-D-aspartic acid (NMDA) (OFC cases 4–7: ibotenic acid 10 µg/µl, NMDA 10 µg/µl; Sigma) was injected into the cortex as a bolus (Mean number of injections per hemisphere ±SEM: 92 ± 6; Range: 71 – 119). The needle was then held in place for 2–3 seconds to allow the toxin to diffuse away from the injection site. Injections were spaced approximately 2 mm apart.
For the OFCSTRIP lesion, a 3 mm strip of cortex was removed by a combination of electrocautery and suction from the posterior OFC (Fig. 4). The strip lesion was placed at the caudal extent of the medial orbital sulcus, which is at or near the caudal limit of the OFC lesions employed in previously studied groups from this laboratory. In the medial to lateral axis, the lesion extended from the fundus of the lateral orbital sulcus to the rostral sulcus on the medial surface.
Lesion assessment
Injections of excitotoxins into OFC resulted in hypersignal – visible in T2-weighted MR scans – in the cortex on the orbital surface extending from the fundus of the lateral orbital sulcus, laterally, to the rostral sulcus, medially (Fig. 1). The location and extent of excitotoxic lesions is reliably indicated by white hypersignal on T2-weighted scans_ENREF_53. Accordingly, for each operated monkey the extent of hypersignal on coronal MR images between approximately 40 to 26 mm anterior to the interaural plane was plotted onto a standard set of drawings of coronal sections from a macaque brain. The volume of the lesions was then estimated using a digitizing tablet (Wacom, Vancouver, WA). For the monkeys in the OFCSTRIP group, lesions were assessed using T1-weighted MRI scans (Fig. 4).
Behavioral testing
Prior to surgery all animals were habituated to the WGTA and were allowed to retrieve food from the test tray. Following preliminary training and initial food preference testing, monkeys either received excitotoxic lesions of OFC or were retained as unoperated controls. Following surgery, monkeys were tested on reinforcer devaluation test 1, emotional response test 1, reinforcer devaluation test 2, object reversal learning and then emotional test 2. The testing order was highly similar to previous experiments from the laboratory. In the case of the monkeys that received OFCSTRIP lesions, surgery was conducted at the conclusion of the five initial tests. Monkeys were then retested on object reversal learning and emotional test 2. Testers conducting the behavioral experiments were, where possible, blind to group assignments.
Food preference testing
After habituation to the WGTA, each monkey’s preference for six different foods was assessed over a 15-day period. Every day monkeys received 30 trials consisting of pairwise presentation of the six different foods, one each in the left and right wells of the test tray. The left-right position of the foods was counterbalanced. Preferences were determined by analyzing choices within each of the 15 possible pairs of foods over the final five days of testing.
Reinforcer devaluation
The behavioral methods used were highly similar to those reported before, so that direct statistical comparisons between could be made9. The procedure employed object discrimination learning, which set up particular object-outcome associations, followed by reinforcer devaluation tests, in which probe trials gauged the monkeys’ ability to link objects with current food value.
Object discrimination learning
Monkeys were trained to discriminate 60 pairs of novel objects. For each pair, one object was randomly designated as the positive object (S+, rewarded) and the other was designated as negative (S−, unrewarded). Half of the positive objects were baited with food 1. The other half were baited with food 2. For each monkey, the identity of foods 1 and 2 was based on the monkey’s previously determined food preferences. The foods selected were those that the monkey valued highly and which were roughly equally palatable as judged by choices in the food preference test.
On each trial, monkeys were presented with a pair of objects, one each overlying a food well, and were allowed to choose between them. If they displaced the S+ they were allowed to retrieve the food. The trial was then terminated. If they chose the S−, no food was available, and the trial was terminated. The left-right position of the S+ followed a pseudorandom order. Training continued until monkeys attained the criterion of a mean of 90% correct responses over 5 consecutive days. (i.e., 270 correct responses or greater in 300 trials).
Reinforcer devaluation test 1
Monkey’s object choices were assessed under two conditions: after one of the foods was devalued, and in normal (baseline) conditions. On separate days we conducted four test sessions, each consisting of 30 trials. Only the positive (S+) objects were used. On each trial, a food-1 object and a food-2 object were presented together for choice; each object covered a well baited with the appropriate food. With the constraint that a food-1 object was always paired with a food-2 object, the object pairs were generated randomly for each session.
Preceding two of the test sessions a selective satiation procedure, intended to diminish the value of one of the foods, was conducted. For the other two test sessions, which provided baseline scores, monkeys were not sated on either food before being tested. The order in which the test sessions occurred was the same for all monkeys and was as follows: 1) baseline test 1; 2) food 1 devalued by selective satiation prior to test session; 3) baseline test 2; 4) food 2 devalued by selective satiation prior to test session.
For the selective satiation procedure a food box filled with a pre-weighed quantity of either food 1 or food 2 was attached to the front of the monkey’s home cage. The monkey was given a total of 30 minutes to consume as much of the food as it wanted, at which point the experimenter started to observe the monkey’s behavior. Additional food was provided if necessary. The selective satiation procedure was deemed to be complete when the monkey refrained from retrieving food from the box for 5 minutes. The amount of time taken in the selective satiation procedure and the total amount of food consumed by each monkey was noted. The monkey was then taken to the WGTA within 10 minutes and the test session conducted.
Reinforcer devaluation test 2
A second devaluation test, identical to the first, was conducted between 26 to 77 days after reinforcer devaluation test 1. Monkeys were retrained on the same 60 pairs to the same criterion as before. After relearning, the reinforcer devaluation test was conducted in the same manner as before.
Food choices after selective satiation
Shortly after reinforcer devaluation test 2, we assessed the effect of selective satiation on monkey’s choices of foods alone. This test was conducted to evaluate whether satiety transferred from the home cage to the WGTA, and whether behavioral effects of the lesion (if any) were due to an inability to link objects with food value as opposed to an inability to discriminate the foods. This test was identical to both reinforcer devaluation tests 1 and 2, but with the important difference that no objects were presented over the two wells where foods were placed. On each trial of the 30-trial sessions, monkeys could see the two foods and were allowed to choose between them. As was the case for reinforcer devaluation tests 1 and 2, there were four critical test sessions; two were preceded by selective satiation and two were not.
Emotional responsiveness test
Monkeys were pretrained until they readily retrieved a food reward from the top, back edge of the Plexiglas box. Two emotional response tests were conducted. In the first test, eight neutral objects and two fear inducing objects (static rubber spider and snake), all novel, were presented inside the Plexiglas box, one object per trial. Each test session comprised ten 30-second trials, each separated by 20 seconds. In the second test, 10 novel objects, including eight neutral objects and two fear-inducing objects (moving spider and snake), were presented. As for test 1, the objects were presented for a total of five consecutive sessions. For both tests, sessions were run once every other day. For analysis, the two tests were combined as monkeys showed consistent food-retrieval latencies on trials with neutral objects (neutral objects, effect of test, F(1,17)=3.84, p=0.067). The food-retrieval latencies (i.e., time that monkeys took to retrieve the food reward) were determined offline using frame-by-frame video analysis. Latencies for each trial were calculated by determining the difference between the trial start and retrieval times.
Object reversal learning
A single pair of objects, novel at the start of testing, was used throughout object reversal learning. To prevent object preferences from biasing learning scores, both objects were either baited (for half the monkeys in each group) or unbaited on the first trial of the first session of acquisition of the object discrimination. If the object chosen on the first trial was rewarded, it was designated the S+; if not, it was designated the S−. Through trial and error monkeys learned which object was associated with a food reward. Monkeys were tested for 30 trials per daily session for 5–6 days per week. Criterion was set at 93% (i.e., 28 correct responses in 30 trials) for one day followed by at least 80% (i.e., 24/30) the next day. Once monkeys had attained criterion on the initial object discrimination problem, the contingencies were reversed and animals were trained to the same criterion as before. This procedure was repeated until a total of nine serial reversals had been completed.
Data analysis
The data were analyzed in SPSS statistical software using one-way or repeated-measures ANOVA (two-tailed) with Hynh-Feldt correction and where appropriate with test (emotional response test [neutral object comparison], 2 levels), object type (emotional response test, 6 levels), session (emotional response test, 5 levels), reversal (object reversal learning, 9 levels), as within subject factors and group (2/4 levels) as a between subjects factor. Further post hoc analyses with Bonferroni correction were used, unless otherwise stated, to explore any significant main effects or interactions (p<0.05).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dawn Lundgren and Emily Howland for assistance with data collection, and Rachel Reoli for help performing surgery. We also thank Steve Wise and Mark Walton for helpful comments on an earlier version of the manuscript. This work was supported by the Intramural Research Program of the National Institute of Mental Health. PHR designed the experiment, assisted in surgery, analyzed the data and wrote the manuscript. RCS performed the surgeries and edited the manuscript. ATP and LSC collected and analyzed the data. EAM designed the experiment, performed the surgeries, supervised the project and wrote the manuscript. The authors have no competing interests.
Footnotes
COMPETITING INTERESTS: The authors declare no competing financial interests.
REFERENCES
- 1.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 2.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29:1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- 4.Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. Journal of Neuroscience. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornak J, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 7.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 8.Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta . Physiology and Behavior. 1969;4:163–171. [Google Scholar]
- 9.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 11.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Experimental Brain Research. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 12.Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning & Memory. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology Neurosurgery and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolls ET. The Brain and Emotion. Oxford: Oxford University Press; 1999. [Google Scholar]
- 16.Mishkin M. Perseveration of central sets after frontal lesions in monkeys. In: Warren JM, Akert K, editors. The Frontal Granular Cortex and behavior. New York: McGraw-Hill; 1964. pp. 219–241. [Google Scholar]
- 17.Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cerebral Cortex. 2000;10:252–262. doi: 10.1093/cercor/10.3.252. [DOI] [PubMed] [Google Scholar]
- 18.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain and cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 19.Blair RJ. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. British Journal of Psychology. 2010;101:383–399. doi: 10.1348/000712609X418480. [DOI] [PubMed] [Google Scholar]
- 20.Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 23.Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. Journal of Neuroscience. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 25.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. Journal of Neuroscience. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walton ME, Behrens TE, Buckley MJ, Rudebeck PH, Rushworth MF. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke KA, Franz TM, Miller DN, Schoenbaum G. The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature. 2008;454:340–344. doi: 10.1038/nature06993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. Journal of Neuroscience. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazama A, Bachevalier J. Selective aspiration or neurotoxic lesions of orbital frontal areas 11 and 13 spared monkeys' performance on the object discrimination reversal task. Journal of Neuroscience. 2009;29:2794–2804. doi: 10.1523/JNEUROSCI.4655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mineka SA. primate model of phobic fears. In: Eysenck HJ, Martin I, editors. Theoretical Foundations of Behavior Therapy. New York: Plenum; 1987. pp. 81–111. [Google Scholar]
- 32.Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 33.Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmahmann JD, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. Journal of Comparative Neurology. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 36.Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Ventrolateral prefrontal cortex is required for performance of a strategy implementation task but not reinforcer devaluation effects in rhesus monkeys. European Journal of Neuroscience. 2009;29:2049–2059. doi: 10.1111/j.1460-9568.2009.06740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croxson PL, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. Journal of Neuroscience. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray EA, Baxter MG, Gaffan D. Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behavioral Neuroscience. 1998;112:1291–1303. doi: 10.1037//0735-7044.112.6.1291. [DOI] [PubMed] [Google Scholar]
- 39.Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. European Journal of Neuroscience. 2009;30:2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chudasama Y, et al. The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitt CA, Mitchell SJ, DeLong MR, Wainer BH, Price DL. Fiber pathways of basal forebrain cholinergic neurons in monkeys. Brain Research. 1987;406:192–206. doi: 10.1016/0006-8993(87)90783-9. [DOI] [PubMed] [Google Scholar]
- 42.Morrison JH, Foote SL, O'Connor D, Bloom FE. Laminar, tangential and regional organization of the noradrenergic innervation of monkey cortex: dopamine-beta-hydroxylase immunohistochemistry. Brain Res Bull. 1982;9:309–319. doi: 10.1016/0361-9230(82)90144-7. [DOI] [PubMed] [Google Scholar]
- 43.Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. Journal of Neuroscience. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Passingham RE, Wise SP. The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight. USA: Oxford University Press; 2012. [Google Scholar]
- 45.Bush EC, Simons EL, Allman JM. High-resolution computed tomography study of the cranium of a fossil anthropoid primate, Parapithecus grangeri: new insights into the evolutionary history of primate sensory systems. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004;281:1083–1087. doi: 10.1002/ar.a.20113. [DOI] [PubMed] [Google Scholar]
- 46.Stevens JR, Hallinan EV, Hauser MD. The ecology and evolution of patience in two New World monkeys. Biology Letters. 2005;1:223–226. doi: 10.1098/rsbl.2004.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray EA, Wise SP, Drevets WC. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry. 2011;69:e43–54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 49.Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 50.Watson KK, Platt ML. Social signals in primate orbitofrontal cortex. Curr Biol. 2012;22:2268–2273. doi: 10.1016/j.cub.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. Journal of Neuroscience. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.