Abstract
Transglutaminase (TGase)-induced activation of small G proteins via 5-HT2A receptor signaling leads to platelet aggregation (Walther et al., 2003). We hypothesize that stimulation of 5-HT2A receptors in neurons activates TGase, resulting in transamidation of serotonin to a small G protein, Rac1, thereby constitutively activating Rac1. Using immunoprecipitation and immunoblotting, we show that in a rat cortical cell line, A1A1v cells, serotonin increases TGase-catalyzed transamidation of Rac1. This transamidation occurs in both undifferentiated and differentiated cells. Treatment with a 5-HT2A/2C receptor agonist, 2,5-dimethoxy-4-iodoamphetamine (DOI), but not the 5-HT1A receptor agonist, 5-hydroxy-2-dipropylamino tetralin (DPAT), increases transamidation of Rac1 by TGase. In A1A1v cells, 5-HT2A receptors mediate the transamidation reaction since expression of 5-HT2C receptors was not detectable and the selective 5-HT2A receptor antagonist blocked transamidation. Time course studies demonstrate that transamidation of Rac1 is significantly elevated after 5 and 15 minutes of serotonin treatment, but returns to control levels after 30 minutes. The activity of Rac1 is also transiently increased following serotonin stimulation. Inhibition of TGase by cystamine or siRNA reduces TGase-modification of Rac1 and cystamine also prevents Rac1 activation. Serotonin itself is bound to Rac1 by TGase following 5-HT2A receptor stimulation as demonstrated by co-immunoprecipitation experiments and a dose-dependent decrease of serotonin-associated Rac1 by cystamine. These data support the hypothesis that Rac1 activity is transiently increased due to TGase-catalyzed transamidation of serotonin to Rac1 via stimulation of 5-HT2A receptors. Activation of Rac1 via TGase is a novel effector and second messenger of the 5-HT2A receptor signaling cascade in neurons.
Introduction
5-HT2A receptors are G-protein coupled receptors which are widely expressed in the brain, peripheral vasculature, platelets and skeletal muscle. 5-HT2A receptors are involved in diverse physiological functions, from platelet aggregation to neuroendocrine release (Van de Kar et al., 2001; Walther et al., 2003). Pathophysiologically, 5-HT2A receptor signaling is implicated in a number of disorders including hypertension, atherosclerosis, anxiety and depression (Roth, 1994; Weisstaub et al., 2006). The classical signal transduction pathway of 5-HT2A receptors is Gq/11-coupled activation of phospholipase C (PLC) (Sanders-Bush et al., 2003), which hydrolyzes phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate (IP3) and diacyglycerol. IP3 mobilizes calcium from the endoplasmic reticulum and thereby increases intracellular Ca2+ (Julius, 1991).
TGases (EC 2.3.2.13) are a family of Ca2+-dependent enzymes. Once activated, TGases can catalyze the cross-linking of proteins via the γ-carboxamide group of peptide-bound glutamine and the ε-amino group of peptide-bound lysine, forming an inter- or intramolecular isodipeptide bond (Griffin et al., 2002). TGases can also covalently link biogenic amines and polyamines, such as serotonin or spermine, to a peptide-bound glutamine residue in a transamidation reaction (Folk et al., 1980; Dale et al., 2002). In platelets, activation of 5-HT2A receptors stimulates TGase-catalyzed transamidation of serotonin to small G proteins, such as RhoA and Rab4, rendering them constitutively active (Walther et al., 2003). Furthermore, neuronal differentiation of SH-SY5Y cells induced by retinoic acid increases the expression/activation of TGases, resulting in transamidation of putrescine to RhoA and activation of RhoA (Singh et al., 2003).
Rac1 belongs to the Rho family of small G proteins, a subgroup of the Ras superfamily. Members of the Rho family (e.g. RhoA, Rac1, Cdc42) are associated with a wide array of cellular processes such as cytoskeletal organization, vesicular transport, cell cycle progression, cell adhesion and migration, neuronal differentiation and a variety of enzymatic activities (Etienne-Manneville and Hall, 2002; Burridge and Wennerberg, 2004). Like other small G proteins, Rac1 functions as a molecular switch which cycles between two conformational states: a GTP-bound, active state and a GDP bound, inactive state. The GTP/GDP cycling is controlled by many regulator molecules such as GTPase-activating proteins (GAPs), guanine nucleotide exchange factors (GEFs) and GDP dissociation inhibitors (GDIs) (Hakoshima et al., 2003). Post-translation modification of Rac1 in the regulator-interaction sites or in the GTP-hydrolyzing domain may have an enormous effect on its activation cycle. Bearing five glutamine residues in the amino acid sequence (Matos et al., 2000), Rac1 might serve as a suitable substrate of TGases resulting in the transamidation of primary amines to Rac1 and rendering this small G protein constitutively active.
A1A1v cells derived from embryonic rat cortex (Scalzitti et al., 1998) provide an excellent experimental model to study 5-HT2A receptor signaling. In the present study, we use A1A1v cells to test whether 5-HT2A receptor stimulation increases TGase-catalyzed transamidation and activation of Rac1 since they endogenously express 5-HT2A/1A receptors, Gαq/11, PLC β, TGase 2, the serotonin transporter, and small G proteins including Rac1, RhoA and Cdc42.
Methods
Cell Culture
A1A1v cells, a rat cortical cell line, were grown on 100 mm2 plates coated with poly-L-ornithine (Sigma, St Louis, MO) and maintained in 5% CO2 at 33°C, in Dulbecco’s modified Eagle medium (DMEM) (Fisher Scientific, Pittsburgh, PA) containing 10% fetal bovine serum (FBS) (Fisher Scientific, Pittsburgh, PA). Cells were induced to differentiate by incubation at 37°C for 4 days. Before each experiment, cells were maintained in DMEM with 10% charcoal-treated FBS for 48 hours. Charcoal adsorption removes most but not all serotonin in the medium. The maximal final concentration of serotonin in the medium is approximately 3 nM (Unsworth and Molinoff, 1992). Cells from passages 8–15 were used for all experiments.
Drugs
The following drugs were used in this study: (−)-1-(2,5-dimethoxy-4-lodophenyl)-2-aminopropane HCl (DOI) and serotonin (Sigma, St Louis, MO). (R)-(+)-8-hydroxy-2-dipropylamino tetralin hydrobromide (DPAT) (Tocris, Ellisville, MO). 2-aminoethyl disulfide dihydrochloride (cystamine) (MP biomedicals, Costa Mesa, CA). ≥-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidine methanol (MDL 100907) (a gift from Hoechst Marion Roussel Research Institute, Cincinnati, OH). Serotonin was dissolved in 10 μM HCl and MDL 100907 was dissolved in a minimal volume of dimethyl sulfoxide (DMSO) then diluted with saline. The remaining drugs were dissolved in purified water. All compounds were further diluted (at least 1:100) in cell culture media before they were applied to the cells and washed away prior to lysing the cells.
Immunoprecipitation of TGase-modified Protein
A1A1v cells were harvested and lysed using lysis buffer A (25 mM Tris-HCl, pH 7.5, 250 mM NaCl, 5 mM EDTA, 1% Triton X-100 and 1:1000 protease inhibitor cocktail (Sigma, St Louis, MO) containing 104 μM AEBSF, 0.08 μM aprotinin, 2 μM leupeptin, 4 μM bestatin, 1.5 μM pepstatin A and 1.4 μM E-64). Protein concentration was determined using the BCA Protein Assay kit (Pierce, Rockford, IL). Immunopurification of proteins containing TGase-catalyzed bonds was performed using 81D4 mAb (mouse IgM) prebound to Sepharose beads (Covalab, Lyon, France) using a protocol developed by Covalab and as described previously (Norlund et al., 1999; Zainelli et al., 2005). The 81D4 antibody is well characterized and has been previously shown to be specific for the N epsilon-(gamma-L-glutamy)-L-lysine isopeptide and N epsilon-(gamma-L-glutamy)-L-lysine isopeptide cross-link generated by TGase (Sarvari et al., 2002; Thomas et al., 2004). The 81D4 antibody has been extensively used to demonstrate increases in TGase-catalyzed bonds (Citron et al., 2002; Junn et al., 2003; Andringa et al., 2004). The use of the 81D4 antibody in a competitive ELISA assay was shown to result in isodipeptide cross-link measurements that correlate well to those measured by HPLC analysis but provide more sensitivity than the HPLC approach (Sarvari et al., 2002). Transfection of cells to over-express TGases, including TGase 1, 2 and 3, and a substrate protein such as mutant huntingtin protein results in increased presence of the isodipeptide cross-link in mutant huntingtin protein as demonstrated with immunoprecipitation with the 81D4 antibody (Zainelli et al., 2005).
Briefly, 20μl of sepharose-81D4 beads were washed three times in TBS/0.1% Tween 20 with gentle shaking for 15 minutes, followed by adding 200μg cell lysate (1 μg/μl) to the washed beads and incubating for 2 hours at 37°C. After incubation, the pellets were washed four times in TBS/0.1% Tween 20 for 15 minutes. Then 20μl of loading buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 0.1% bromophenol blue, 10% glycerol and 5% β-mercaptoethanol) was added to the washed pellets followed by 5 minutes incubation at 90°C. The samples were then centrifuged at 9,000 × g for 2 minutes and the supernatant was transferred and stored at −80°C until immunoblot analysis.
Immunoprecipitation of Rac1
200 μg of protein from each sample was brought up to a total volume of 100 μl with IP buffer (50 mM Tris-HCl, pH 7.4, 10 mM EGTA, 100 mM NaCl, 0.5% Triton-X 100 and 0.1% protease inhibitor cocktail), then the samples were precleared using 10μl of recombinant protein G (rProtein G) agarose (Invitrogen, Carlsbad, CA) for 1 hour. The samples were centrifuged at 9,000 × g for 10 minutes and the supernatant was incubated for 1.5 hour at 4ºC with either 2 μg of Rac1 antibody (Upstate, Lake Placid, NY) or 2 μg of normal mouse IgG (Santa Cruz, Santa Cruz, CA) as a control for non-specific binding. The immuno-complexes were precipitated using 20 μl of rProtein G agarose at 4ºC for 1 hour. The agarose-immuno complexes were washed three times in IP buffer and centrifuged after each wash at 100 × g for 3 minutes. After the last wash, bound proteins were eluted by adding 2× PAGE sample buffer and heating for 5 minutes at 90ºC. The samples were centrifuged at 13,000 × g for 5 minutes and the supernatant was transferred and stored at −80°C until immunoblot analysis.
Immunoblot
Immunoaffinity purified proteins and cell lysates were separated on 12% SDS-polyacrylamide gels and then electrophoretically transferred to nitrocellulose membranes. Membranes were then incubated in blocking buffer (5% non-fat dry milk, 0.1% Tween 20, 1× TBS) for 1 hour at room temperature. Membranes were incubated overnight at 4°C with primary antibodies on a shaker. Primary antibodies (Upstate Biotechnology, NY: anti-Rac1, mouse IgG, 1:700; anti-Na+/K+ ATPase, mouse IgG, 1: 10000; Abcam, Cambridge, MA: anti-serotonin, rabbit IgG, 1:1000; BD Pharmingen, San Jose, CA: anti-5-HT2C receptor, mouse IgG, 1:300; Cell Signaling, Danvers, MA: anti-phosphorylated ERK1/2 at residues Thr202/Tyr204, mouse IgG, 1:1,000; anti-ERK1/2, rabbit IgG, 1:1,000; MP Biomedicals, Aurora, OH: anti-actin, mouse IgG, 1:20,000; CovalAb, Lyon, France: 81D4, mouse IgM, 1: 500; NeoMarkers, Fremont, CA: TG100, mouse IgG, 1:100) were diluted in antibody buffer (1% non-fat dry milk, 0.1% Tween 20, 1× TBS). The next day, membranes were washed with TBS/0.1% Tween 20 and then incubated with goat-anti-mouse or goat-anti-rabbit secondary antibody conjugated to HRP (Jackson ImmunoResearch, West Grove, PA) diluted in antibody buffer. Membranes were washed and signal was detected using enhanced chemiluminescence (ECL) western blotting detection reagents (Amersham Biosciences, Piscataway, NJ). Using Scion Image for Windows (Scion, Frederick, MD), immunoblots were quantified by calculating the integrated optical density (IOD) of each protein band on the film.
Expression and Purification of Glutathione S-Transferase (GST)-PAK1
The pGEX-2T plasmid was used to express Rac1 interactive domain of human PAK1 (residues 51–135) fused to GST. The BL21 (DE3) E. coli (Invitrogen life technologies, Carlsbad, CA) were transformed with plasmid expressing GST-PAK1 and grown overnight at 37°C on LB-ampicillin plates. Then a single colony was used to inoculate 25ml LB-ampicillin (100 μg/ml) which was shaken overnight at 37°C. 10 ml of the overnight culture was used to inoculate 500ml LB-ampicillin which was grown for 3 h at 37°C while shaking. After induction with 0.4 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 2 h at 30–32ºC, bacteria were lysed in lysis buffer B (50 mM Hepes, pH 7.6, 1% Triton X-100, 100 mM NaCl, 5 mM MgCl2, 10% glycerol, 1 mM PMSF and 0.1% protease inhibitor cocktail). The lysates were sonicated and centrifuged for 15 minutes at 9,000 × g at 4ºC. Then the supernatant was added to glutathione-Sepharose 4B (Amersham Biosciences, Piscataway, NJ) and the mixture was incubated at 4ºC for 2 hours. The beads were then centrifuged at 4ºC at 2,000 × g and were washed four times with lysis buffer B. The beads were resuspended in lysis buffer B and were stored at −80ºC for at most two weeks.
Rac1 Activity Assay
A1A1v cells were treated for 5 or 15 minutes with 14 μM serotonin or vehicle (10 μM HCl). The cells were harvested in lysis buffer C (50 mM Tris–HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.1% SDS, 1 mM PMSF and 0.1% protease inhibitor cocktail). Cell lysates were centrifuged at 14,500 × g for 20 minutes at 4°C and 200 μl of the supernatant was incubated with 20 μl of GST-PAK1-Sepharose beads for 40 minutes at 4°C under constant agitation. The beads were washed three times with lysis buffer C, and 2× Laemmli sample buffer was added to the washed beads followed by 5 minutes incubation at 90°C. The samples were then centrifuged at 9,000 × g for 2 minutes and equivalent amounts of proteins in the supernatants were loaded on 12% SDS-PAGE as well as 20 μg cell lysates (in order to detect total Rac1 protein levels), followed by immunoblot analysis as described above.
Small Interfering RNA
To induce TGase2 gene silencing, two siRNA duplexes to target the coding sequence of rat TGase2 mRNA were designed and synthesized by QIAGEN (Germantown, MD). The target sequence is 5′-AAGAGCGAGATGATCTGGAAT-3′ for siRNA1 and is 5′-AGAGCCAACCACCTGAACAAA-3′ for siRNA2. The sense strand of each siRNA was labeled at 3′ end with Alexa Fluor 488 to monitor transfection efficiency. At 30~50% confluence, A1A1v cells were transfected with siRNA at a final concentration of 90 nM using Lipofectamine™ 2000 (Qiagen, Germantown, MD) according to the manufacturer’s instructions. 24 hours after transfection, siRNA-lipid complexes were removed by changing medium. Transfection efficiency was assessed based on the percentage of fluorescent cells observed under Nikon Eclipse TE 2000U microscopy. 48 hours after transfection, cells were stimulated with DOI or vehicle for 5 minutes, and then cell lysates were collected to measure TGase2 expression and TGase-modified Rac1 by immunoblot and immunoprecipitation. Cells incubated with Lipofectamine™ 2000 alone were used as the nontransfected control.
Statistical Analyses
All data are presented as group mean ± the standard error of the mean (SEM) and analyzed by one-way or two-way ANOVA. Post hoc tests were conducted using Newman-Keuls Multiple Comparison Test. SYSTAT 11 (Systat Software, Inc., San Jose, CA) and GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA) were used for all statistical analyses. A probability level of p<0.05 was considered to be statistically significant for all statistical tests.
Results
Serotonin treatment induces Rac1 transamidation in A1A1v cells
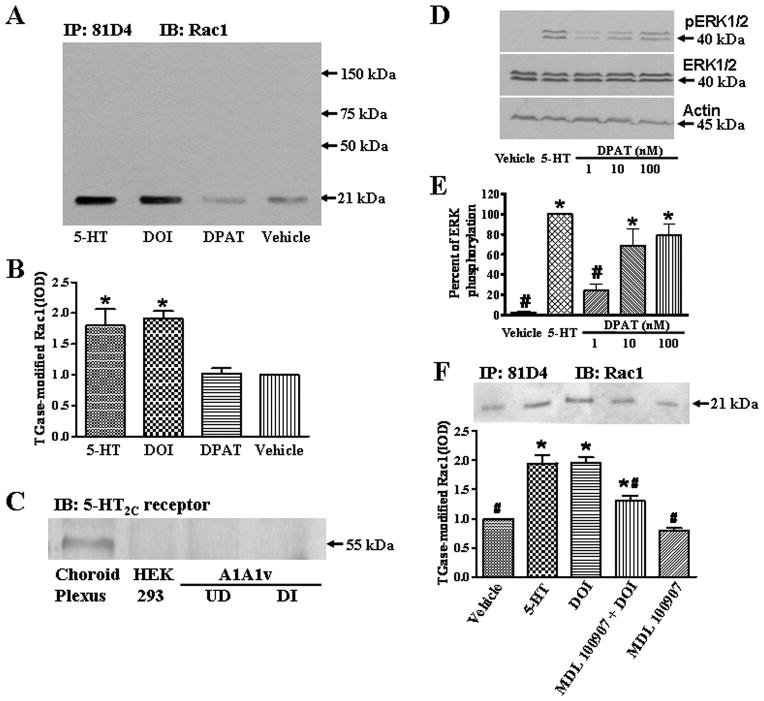
In order to determine whether TGase-catalyzed transamidation of Rac1 is increased after serotonin treatment, we treated A1A1v cells with 14 μM serotonin for 5, 15 or 30 minutes. The transamidation of Rac1 is significantly elevated after 5 or 15 minutes of serotonin treatment by approximately 2~2.5 fold as compared to vehicle (HCl)-treated cells (Fig. 1). However, there is no significant difference in the amount of TGase-modified Rac1 in cells treated with serotonin for 30 minutes as compared to vehicle-treated cells (Fig. 1A, B).
Fig. 1.
Serotonin-induced increase of Rac1 transamidation in A1A1v cells. (A) After 5, 15 or 30 minutes of 14 μM serotonin treatment, TGase-modified proteins were immunoprecipitated with 81D4 antibody coupled to sepharose. TGase-modified Rac1 was then detected on immunoblots with anti-Rac1 antibody. (B) Quantitation of immunoblots for 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to vehicle-treated control levels. One-way ANOVA (F(3,8)=7.31, p<0.05) indicates a significant difference among groups. Newman-Keuls Multiple Comparison Test indicates p<0.05* as compared to vehicle treatment.
5-HT2A receptor stimulation increases the transamidation of Rac1
In order to explore the serotonin receptor specificity for induction of TGase-catalyzed transamidation, A1A1v cells were stimulated with 14 μM serotonin, 3 μM DOI (5-HT2A/2C receptor agonist), 10 nM DPAT (5-HT1A receptor agonist) or 10 μM HCl (vehicle) for 15 minutes. Treatment with serotonin or DOI significantly (p<0.05) increases the amount of TGase-modified Rac1 2-fold as compared to vehicle-treated cells, whereas treatment with DPAT has no effect on the amount of TGase-modified Rac1 compared to vehicle-treated cells (Fig. 2A, B). Expression of 5-HT2A receptors in A1A1v cells has been previously confirmed using anti-sense oligodeoxynucleotide strategies and radioligand binding assays (Scalzitti et al., 1998). Here, 5-HT2C receptor expression in undifferentiated and differentiated A1A1v cells was examined by immunoblot analysis. Choroid plexus tissue from the fourth ventricle of a rat was used as a positive control and HEK 293 cell lysates were used as a negative control. We found that 5-HT2C receptors are expressed in rat choroid plexus, but 5-HT2C receptor expression was not detected in either HEK293 cells or undifferentiated or differentiated A1A1v cells (Fig. 2C). To exclude the possibility that the lack of involvement of the 5-HT1A receptors in Rac1 transamidation is not due to insufficient concentration of DPAT, we treated cells with 14 μM serotonin and increasing concentrations of DPAT (1 nM, 10 nM, and 100 nM) for 5 minutes, and then detected ERK phosphorylation by immunoblot. Treatment with 14 μM serotonin and 10 nM or 100 nM DPAT significantly increased phosphorylation of ERK (Fig. 2D, E), indicating 10 nM DPAT is enough to activate 5-HT1A receptors in A1A1v cells. To further confirm that the effect of DOI on Rac1 transamidation in A1A1v cells is due to stimulation of the 5-HT2A receptors, cells were pretreated with 100 nM MDL 100907 (a selective 5-HT2A receptor antagonist) for 15 minutes, and then stimulated with DOI for 5 minutes. We found the pretreatment of MDL 100907 significantly reduced DOI-induced Rac1 transamidation without itself having any effect on Rac1 transamidation (Fig. 2F).
Fig. 2.
Serotonin receptor specificity on induction of Rac1 transamidation in A1A1v cells. (A) Cells were stimulated with 14 μM serotonin, 3 μM DOI (5-HT2A/2C receptor agonist), 10 nM DPAT (5-HT1A receptor agonist) or vehicle for 15 minutes. TGase-modified Rac1 was detected by immunoprecipitation and immunoblot analysis. (B) Quantitation of immunoblots for 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to vehicle-treated control levels. One-way ANOVA (F(3,8)=10.05, p<0.01) indicates a significant difference among various treatments. Newman-Keuls Multiple Comparison Test indicates p<0.05* as compared to vehicle treatment. (C) Immunoblot analysis of 5-HT2C receptor expression in undifferentiated (UD) and differentiated (DI) A1A1v cells. Rat choroid plexus was used as a positive control and HEK 293 cell was used as a negative control. (D) A1A1v cells were treated with vehicle, 14 μM serotonin or DPAT (1 nM to 100 nM) for 5 minutes. Cell lysates were then resolved by SDS-PAGE, followed by immunoblotting with an anti-phosphorylated ERK antibody. Equal loading was verified by reprobing the same membrane with antibodies to total ERK and actin. (E) Quantitation of immunoblots for 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to serotonin-treated control levels. Each phospho-ERK value was normalized using the corresponding total ERK value to control for the possibility of differential loading. One-way ANOVA (F(4,10)=18.12, p<0.001) indicates a significant difference among various treatments. Newman-Keuls Multiple Comparison Test *indicates p<0.05 as compared to vehicle treatment and #indicates p<0.05 as compared to serotonin treatment. (F) Upper, cells were stimulated with vehicle, 14 μM serotonin, 3 μM DOI. Some cells were pretreated with 100 nM MDL 100907 prior to treatment with DOI or treated with MDL100907 alone. TGase-modified Rac1 was detected by immunoprecipitation and immunoblot analysis. Lower, quantitation of immunoblots for 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to vehicle-treated control levels. One-way ANOVA (F(4,10)=38.96, p<0.001) indicates a significant difference among various treatments. Newman-Keuls Multiple Comparison Test indicates p<0.05* as compared to vehicle treatment and p<0.05# as compared to DOI treatment.
DOI increases Rac1 transamidation in both undifferentiated and differentiated A1A1v cells
After differentiation of A1A1v cells, the cytoskeleton undergoes a dramatic reorganization and the cells acquire a neuronal-like cell shape with long processes similar to axons and dendrites compared to undifferentiated cells (Fig. 3A). Rho family GTPases are critical regulators of the actin cytoskeleton organization (Etienne-Manneville and Hall, 2002; Burridge and Wennerberg, 2004). This prompted us to explore the effects of cell differentiation on Rac1 transamidation stimulated by 5-HT2A receptor activation. Stimulation of 5-HT2A receptors with DOI increased TGase-catalyzed transamidation of Rac1 in both undifferentiated and differentiated cells (Fig. 3B). Treatment with DOI in A1A1v cells significantly increased the amount of TGase-modified Rac1 by approximately 2~2.5 fold as compared to vehicle-treated cells (p<0.01). In vehicle-treated groups, the amount of TGase-modified Rac1 in differentiated cells was similar to the amount in undifferentiated cells, indicating that cell differentiation has no effect on basal level of Rac1 transamidation. Although the amount of TGase-modified Rac1 in DOI-treated differentiated cells was increased compared to DOI-treated undifferentiated cells, this increase was not significant, suggesting that cell differentiation also does not have a significant effect on DOI-induced transamidation of Rac1 (Fig. 3C).
Fig. 3.
The effects of cell differentiation on Rac1 transamidation. (A) A1A1v cells were incubated at 37°C for 4 days to induce differentiation. Compared to undifferentiated cells (left), differentiated cells (right) develop a neuron-like cell shape with long neurites. (B) Cells were treated with 3 μM DOI for 15 minutes, followed by immunoprecipitation and immunoblot analysis. Stimulation of 5-HT2A receptors by DOI increased the TGase-catalyzed transamidation of Rac1 in both undifferentiated and differentiated cells. (C) Quantitation of immunoblots for 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to vehicle-treated control levels in undifferentiated cells. Two-way ANOVA indicates a significant main effect of DOI treatment (F(1,8)=49.48, p<0.01). However, there was no significant main effect of cell differentiation on TGase-modified Rac1 (F(1,8)=4.07, p=0.08). The interaction between DOI treatment and cell differentiation was also not significant for TGase-modified Rac1 (F(1,8)=0.34, p=0.58). Newman-Keuls Multiple Comparison Test indicates p<0.01* as compared to vehicle treatment in undifferentiated cells, p<0.01# as compared to vehicle treatment in differentiated cells. Scale bar, 105 μm.
The activity of Rac1 is transiently increased following serotonin stimulation
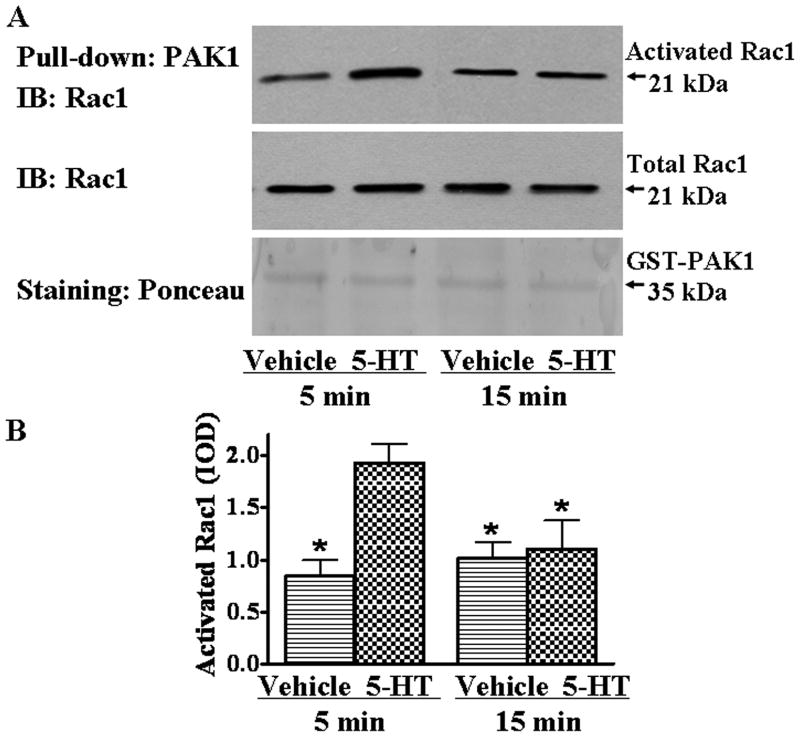
Racl is GDP-bound in the inactive state and GTP-bound when activated. Activated Rac1 binds to its downstream effectors such as PAK1, therefore a GST-PAK1 fusion protein purified from E. coli was used to determine if serotonin stimulation increases the activated from of Rac1. A1A1v cells were treated with serotonin for 5 or 15 minutes, the cells were harvested and cell lysates were incubated with GST-PAK1 prebound to glutathione-Sepharose beads. Then equivalent amounts of the purified proteins were resolved by SDS-PAGE. Immunoblot analysis was performed using an antibody against Rac1. As shown in Fig. 4, the activity of Rac1 is increased by 80 percent after 5 minutes of serotonin treatment, but returns back to baseline after 15 minutes in the presence of serotonin, indicating that Rac1 becomes transiently activated after serotonin treatment in A1A1v cells. In addition, there is no significant change in the total amount of Rac1 in the cell lysate after serotonin treatment (Fig. 4A), suggesting that the activity increase is not due to synthesis of new Rac1.
Fig. 4.
Rac1 activity was transiently increased after serotonin treatment. (A) Top, activated Rac1 was purified using GST-PAK1 coupled to glutathione-Sepharose beads and detected on immunoblot with an antibody against Rac1. Middle, the total amount of Rac1 in the cell lysates used for pull-down of activated Rac1 was monitored by immunoblot. Bottom, Ponceau staining of GST-PAK1 documents equal amounts of protein loading in each lane. (B) Quantitation of immunoblot for 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to vehicle-treated control levels. The two-way ANOVA reveals a significant (F(1,8)=8.64, p<0.05) main effect for serotonin treatment, and a significant (F(1,8)=6.32, p<0.05) interaction between serotonin treatment and time course. Newman-Keuls Multiple Comparison Test indicates p<0.05* as compared to 5 minutes of serotonin treatment.
TGase inhibition reduces Rac1 transamidation and activity
The activity of Rac1 is under the direct control of a large set of regulatory proteins. To exam whether the serotonin-stimulated increase of Rac1 activity is due to TGase-catalyzed transamidation, A1A1v cells were treated with increasing concentrations (0 μM, 100 μM, 500 μM and 1000 μM) of the TGase inhibitor, cystamine, for 1 h, followed by treatment with 14 μM serotonin for 5 minutes. Precipitation of TGase-modified proteins and the activated Rac1 were performed as in previous experiments. Then immunopurified proteins and activated Rac1 were examined on immunoblots using anti-Rac1 and 81D4 antibodies. We found that pretreatment with cystamine significantly decreases Rac1 transamidation (Fig. 5) and activity (Fig. 6) in a dose-dependent manner. Treatment with 100 μM, 500 μM or 1000 μM of cystamine decreases the amount of TGase-modified Rac1 by approximately 50%, 70% or 80%, respectively, as compared to untreated cells (Fig. 5A, C). A similar dose-dependent decrease in TGase-modified Rac1 was also observed when cells were treated with serotonin for 15 minutes following cystamine inhibition (data not shown). Treatment with 500 μM or 1000 μM of cystamine decreases the amount of activated Rac1 by approximately 20% or 40%, respectively, as compared to untreated cells (Fig. 6A, C). These results indicate that TGase-catalyzed transamidation of Rac1 contributes to the increase in Rac1 activity upon serotonin stimulation. The ubiquitously expressed Na+/K+ ATPase is a well-established plasma membrane marker and its function underlies essentially all of mammalian cell physiology (Kaplan, 2002). In order to verify that the dose-dependent decrease of Rac1 transamidation and activation was not due to cystamine-induced total Rac1 reduction or cellular toxicity, cell lysates from the same experiment were examined on western blots with antibodies for Rac1 or Na+/K+ ATPase. There is no significant difference in total Rac1 or Na+/K+ ATPase levels between cystamine-treated and untreated cells, indicating that the concentrations of cystamine were not toxic to the cells (Fig. 5B, 6B).
Fig. 5.
Dose-dependent effects of cystamine on Rac1 transamidation. (A) Immunoprecipitation and immunoblot analyses reveal that cystamine causes a dose-dependent (100 to 1000 μM) inhibition of the transamidation of Rac1 as indicated by less intense bands. (B) Cells lysates from the same experiment were examined on immunoblots with antibodies for Rac1 and Na+/K+ ATPase, which indicate that cystamine has no effect of on total Rac1 and cell viability, respectively. (C) Quantitation of effects of cystamine on Rac1 transamidation in 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to untreated control levels. One-way ANOVA (F(3,8)=19.41, p<0.001) indicates a significant effect of cystamine on Rac1 transamidation. Newman-Keuls Multiple Comparison Test indicates p<0.05* as compared to untreated cells.
Fig. 6.
Rac1 activity is inhibited by cystamine. (A) Top, pull-down and immunoblot analyses reveal that cystamine causes a dose-dependent (100 to 1000 μM) inhibition of the activation of Rac1. Middle, the same membrane was reprobed with antibody 81D4, directed against TGase-catalyzed covalent bonds. Bottom, Ponceau staining of GST-PAK1 (~35 kDa) documents equal amounts of protein loading in each lane. (B) Cell lysates from the same experiment were examined on immunoblots with an antibody for Na+/K+ ATPase. (C) Quantitation of the effects of cystamine on Rac1 activation in 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to untreated control levels. One-way ANOVA (F(3,8)=20.94, p<0.001) indicates a significant effect of cystamine on Rac1 activity. Newman-Keuls Multiple Comparison Test indicates p<0.05* as compared to untreated cells, p<0.001** as compared to untreated cells.
Next, a second and more specific approach was used to determine the significance of TGase in Rac1 transamidation; we designed two siRNA duplexes to inhibit endogenous TGase expression. Several isoenzymes of TGase are found in the brain, of which TGase2 is the most abundant, therefore siRNAs were developed to silence rat TGase2 gene. At 24 hours post-transfection, transfection efficiency of both siRNA reached about 90%. 48 hours after transfection, siRNA1 and siRNA2 transfection of A1A1v cells resulted in 95% and 65% down-regulation of TGase2 protein expression, respectively (data not shown). Reprobing of membrane with anti-Rac1 antibody revealed that neither siRNA changed Rac1 protein level, indicating no off-target effect on Rac1 (Fig. 7A). DOI induced TGase-modification of Rac1 was significantly reduced by knocking down TGase2 expression with siRNA1 or siRNA2. As compared to DOI-stimulated nontransfected cells, siRNA1 and siRNA2 transfection caused 70% and 30% decreases in TGase-modified Rac1 responding to DOI, respectively (Fig. 7B, C). These results confirm that 5-HT2A receptor-mediated Rac1 transamidation is dependent on TGase2 expression in A1A1v cells.
Fig. 7.
Knockdown of TGase2 by siRNAs prevents Rac1 transamidation. (A) Cells were incubated with 90nM of TGase2-specific siRNAs for 24 hours, and then treated with 3 μM of DOI or vehicle for 5 minutes at 48 hours post-transfection. Immunoblot analyses confirmed that transfection of siRNA1 or siRNA2 significantly inhibited TGase2 protein expression compared to nontransfected control (NT). The membranes were stripped and reprobed with anti-Rac1 and anti-actin antibodies. (B) TGase-modified Rac1 was detected by immunoprecipitation and immunoblot analysis. DOI induced TGase-modification of Rac1 were significantly decreased in siRNAs-transfected cells compared to NT cells. (C) Quantitation of effects of siRNAs on Rac1 transamidation in 3 separate experiments. Data shown are the mean IOD ± SEM and normalized to DOI-stimulated nontransfected cells. Two-way ANOVA indicates a significant main effect of transfection (F(2,12)=24.68, p<0.0001), a significant main effect of DOI treatment (F(1,12)=63.23, p<0.0001) and a significant interaction between transfection and DOI treatment (F(2,12)=9.42, p<0.01) on TGase-modified Rac1. Newman-Keuls Multiple Comparison Test indicates p<0.01* or p<0.001** as compared to vehicle treatment in nontransfected cells, p<0.01# or p<0.001## as compared to DOI treatment in nontransfected cells.
TGase-mediated transamidation of serotonin to Rac1
TGase-modified Rac1 did not show a significant upward shift on immunoblots compared to native Rac1 in cell lysates (data not shown), indicating that the molecular weight of Rac1 does not significantly increase after modification by TGase. Therefore, a small amine such as serotonin is most likely incorporated into Rac1 upon stimulation of 5-HT2A receptors. To test this hypothesis, we stimulated A1A1v cells with serotonin or vehicle for 5 minutes then cells were harvested and cell lysates were used to immunoprecipitate Rac1 with an anti-Rac1 antibody, or immunoprecipitate TGase-modified proteins with the 81D4 antibody. The immunopurified proteins were examined on immunoblots using antibodies directed against serotonin. Immunoprecipitation of Rac1 and probing for serotonin reveals an association between Rac1 and serotonin in A1A1v cells (Fig. 8A). However we were unable to detect serotonin in TGase-modified proteins (Fig. 8A). This may be due to a compromise of the serotonin antibody epitope during the immunoprecipitation of TGase-modified proteins, since this procedure uses higher temperatures and longer incubation times compared to the Rac1 immunoprecipitation procedure. Therefore in order to confirm that serotonin is associated with Rac1 by a TGase-catalyzed covalent bond, we treated cells with increasing concentrations (0 μM, 100 μM, 500 μM, 1000 μM) of cystamine for 1 hour followed by stimulating the cells with serotonin for 5 minutes. Then we immunoprecipitated Rac1 and detected associated serotonin by immunoblot, as described above. We found that treatment with cystamine decreases the serotonin-associated Rac1 in a dose-dependent manner (Fig. 8B, D), thus supporting the hypothesis that serotonin is incorporated into Rac1 by a TGase-catalyzed covalent bond. To compare the effects of different agonists on the incorporated serotonin, cells were exposed to either serotonin or DOI for 5 min. Then Rac1 was immunoprecipitated and the incorporated serotonin was detected by immunoblot. There was no significant difference in serotonin incorporation between serotonin and DOI-treated cells. Pretreatment with cystamine also inhibited DOI-induced serotonin incorporation (Fig. 9).
Fig. 8.
Transamidation of serotonin to Rac1 is mediated by TGase. (A) Immunoprecipitation of Rac1, but not TGase-modified proteins, reveals an association between Rac1 and serotonin in A1A1v cells upon 5 minutes of 14 μM serotonin stimulation. (B) Immunoprecipitation and immunoblot analysis reveal that cystamine causes a dose-dependent (100 to 1000 μM) reduction of the serotonin-associated Rac1. Normal mouse IgG is used as a negative control (NC) for immunoprecipitation. (C) Cell lysates from the same experiment were examined on immunoblots with an antibody for Na+/K+ ATPase, which indicates cystamine has no effect of on cell viability. (D) Quantitation of the effects of cystamine on serotonin-induced transamidation of Rac1. Data shown are the mean IOD ± SEM and normalized to untreated control levels. One-way ANOVA (F(3,8) = 7.15, p<0.05) indicate a significant difference in serotonin-associated Rac1 among cells treated with different concentration of cystamine. Newman-Keuls Multiple Comparison Test indicates p<0.05* as compared to untreated cells.
Fig. 9.
DOI stimulates transamidation of Rac1 to serotonin to a similar extent as those treated with serotonin. (A) Cells were stimulated with vehicle, 14 μM 5-HT or 3 μM DOI for 5 minutes. Some cells were pretreated with 500 μM cystamine (Cys) for 1 hour prior to stimulation with DOI. Transamidation of serotonin to Rac1 was detected by immunoprecipitation and immunoblot analysis. (B) Quantitation of immunoblot for 3 separate experiements. Data shown are the mean IOD ± SEM and normalized to serotonin-treated levels. One-way ANOVA (F(3,8)=19.80, p<0.001) indicates a significant difference in Rac1-incorporated serotonin among groups. Newman-Keuls Multiple Comparison Test * indicates p<0.05 as compared to vehicle-treated cells and # indicates p<0.05 as compared to serotonin-treated cells.
Discussion
In platelets, 5-HT2A receptor activation causes TGase-catalyzed transamidation of RhoA and Rab4, leading to activation of these proteins and platelet aggregation (Walther et al., 2003). In aplysia ganglia, serotonin-treatment induces the activation of Cdc42 and its downstream effector PAK to regulate the actin cytoskeleton (Udo et al., 2005). In the present study, we extend these findings to a rat cortical cell model and find that 5-HT2A receptor stimulation increased TGase-catalyzed transamidation of Rac1 and Rac1 activity, both of which can be suppressed by TGase inhibition. In elucidating the mechanism further, we identify serotonin as an amine that becomes transamidated to Rac1 by TGase. Activation of Rac1 via TGase is a novel effector and second messenger of the 5-HT2A receptor signaling pathway in neurons.
To exam the serotonin receptor specificity on induction of Rac1 transamidation, we treated cells with different serotonin receptor agonists and found that the 5-HT2A/2C receptor agonist DOI, but not the 5-HT1A receptor agonist DPAT induces a significant increase in TGase-modified Rac1 with a similar magnitude to that observed in serotonin-treated cells. Since there is no 5-HT2C receptor expression in A1A1v cells and MDL 100907 reversed the effect of DOI on Rac1 transamidation (Fig. 2), serotonin or DOI-induced Rac1 transamidation is selectively mediated by activation of 5-HT2A receptors in A1A1v cells.
Previous studies demonstrated that TGase-mediated transamidation of the Rho family of small G proteins blocked the GTP-hydrolyzing activity of these proteins, rendering these small GTPases constitutively active for their respective signaling pathways (Masuda et al., 2000; Walther et al., 2003). Based on these results, we hypothesized that 5-HT2A receptor-stimulated transamidation of Rac1 by TGase will result in prolonged activation of Rac1. Unexpectedly, we found a transient increase in both Rac1 activity and TGase-catalyzed transamidation which returned to baseline levels following continuous exposure to serotonin (Figs. 1 and 4). The transient response may due to selective degradation of transamidated Rac1. Mammalian cells contain multiple proteolytic systems for degradation of various classes of intracellular proteins. Most abnormal proteins (mutant or misfolded) are degraded in the ubiquitin–proteasome pathway. Rac1 is normally a stable protein, but activation by cytotoxic necrotizing factor-1 (CNF-1) in rat bladder carcinoma cells (804G) and human umbilical vein endothelial cells resulted in increased sensitization to ubiquitination and proteasomal degradation (Doye et al., 2002; Munro et al., 2004). Further, dominant-positive forms of Rac1 appeared more susceptible to ubiquitin-mediated proteasomal degradation compared to both dominant-negative and wild-type Rac1(Doye et al., 2002). Activated and transamidated Rac1 may be targeted for proteasomal degradation, resulting in transient activation of Rac1. However, there is no significant change in total Rac1 protein 15 min after serotonin stimulation (Fig. 2A), suggesting that activated or transamidated Rac1 may only account for small fraction of total Rac1.
In this study, we found differences in the time course for Rac1 transamidation and for its increased activity. After serotonin stimulation for 15 minutes, Rac1 activity is reduced to baseline (Fig. 4) while TGase-catalyzed transamidation of Rac1 still remains at a relatively high level (Fig. 1). These results suggest that transamidation may not only occur at the GTPase activity-related residues, but also at residues which do not have an effect on Rac1 activity. Essentially, transamidation of Rac1 at some sites may induce activation of the small G proteins, while transamidation at other sites may not affect the GTPase activity of Rac1. If activated Rac1 is more sensitive to proteasomal degradation, the activated Rac1 may be degraded earlier than non-activated Rac1 that is transamidated at sites not involved in activation.
Cystamine has been shown to decrease TGase activity and TGase-catalyzed ε-(γ-glutamyl) lysine bonds in cultured cells and animal models of neurodegenerative diseases (Karpuj et al., 2002; Ientile et al., 2003; Zainelli et al., 2005). As a primary amine, cystamine is a potential substrate for TGase, and acts as a competitive inhibitor for TGase by blocking access to the active site of the enzyme for the glutamine residues in proteins which would otherwise participate in forming ε-(γ-glutamyl) lysine bonds (Lorand et al., 1979). In our study, pretreatment with cystamine reduced both serotonin-stimulated Rac1 transamidation (Fig. 5A, C) and activation (Fig. 6A, C) in a dose-dependent manner. Those observations were further supported by the use of TGase2-specific siRNAs, which significantly suppressed DOI induced TGase-modification of Rac1 (Fig. 7B, C). These results suggest that cystamine prevented Rac1 transamidation through TGase inhibition, and that transamidation of Rac1 by TGase may contribute to the increase in Rac1 activity.
Post-translational modifications, such as transamidation and phosphorylation of Rac1 may affect its ability to interact with regulatory proteins (e.g. GAPs, GEFs and GDIs) or to convert GTP back to GDP, resulting in increased activation of Rac1 and stimulation of its signaling cascade in the cells. The Conserved Domain Database (CDD) (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=cdd) provides an interactive tool to identify conserved domains present in protein sequences. We searched the CDD for GTP/Mg2+ binding sites, and GAPs, GEFs and GDIs interaction sites in the Rac1 sequence (PSSM-Id: 57957) because they will be most likely to modify Rac1 activity after TGase-catalyzed transamidation at these sites. We identified two glutamine residues (Gln61, Gln74) located within these domains. It has been reported that site-specific deamidation of a Gln residue in the Rho family G proteins (Gln61 in Rac and Cdc42, Gln63 in RhoA) by CNF-1 inhibits both intrinsic and GAPs-stimulated GTP hydrolysis activity, resulting in constitutive activation of these proteins (Flatau et al., 1997; Schmidt et al., 1997). CNF-1 has also been shown to possess in vitro TGase activity. In the presence of primary amines, RhoA is transamidated in vitro at Gln63 by CNF-1 and at positions 52, 63 and 136 by guinea pig liver TGase (Schmidt et al., 1998). Similarly, in addition to Gln61 which is critical in regulating Rac1 activity, the other four glutamine residues in Rac1 may also be modified by TGase. It is not surprising, therefore, that the same dosage of cystamine prevents Rac1 transamidation and its activation to different extents (Fig. 5C, 6C).
Walther et al. found that in platelets TGase covalently cross-links serotonin to RhoA or Rab4 at a position in the phosphate-binding site that is conserved in the sequences of all Ras-related small GTPases (Walther et al., 2003). It has also been shown that polyamines such as putrescine, spermidine and spermine could be incorporated into Rac1 by bacterial TGase in vitro (Masuda et al., 2000). In our study, co-immunoprecipitation assays demonstrated that serotonin is associated with Rac1 in A1A1v cells after serotonin stimulation (Fig. 8A). To explore the association mechanism further, we inhibited TGase by cystamine and found a reduction of serotonin-associated Rac1 with increasing concentrations of cystamine (Fig. 8B, D), indicating that serotonin is incorporated into Rac1 by a TGase-catalyzed bond. Since both serotonin and DOI stimulation can lead to serotonin incorporation to similar levels (Fig. 9), the serotonin bound to 5-HT2A receptors is not likely the source of Rac1-incorporated serotonin; the serotonin bound to Rac1 may originate from endogenously synthesized serotonin or serotonin transported into the cell from serum in the cell culture media.
The first signal transduction mechanism identified for the 5-HT2A receptor was Gq/11 mediated activation of PLC, leading to increased accumulation of IP3 and an increase in intracellular Ca2+ (Boess and Martin, 1994). In addition to activation of PLC, extensive evidence suggests that 5-HT2A receptors couple to other effector pathways, such as phospholipase A2/arachidonic acid cascade (Berg et al., 1994), mitogen-activated protein kinase signaling (Watts, 1998) and phospholipase D/protein kinase C pathway (Mitchell et al., 1998). On the other hand, TGases are subjected to transcriptional regulation by retinoic acid and steroid hormones (Fujimoto et al., 1996; Ou et al., 2000), and require the binding of Ca2+ for their activity (Burgoyne and Weiss, 2001). Evidence indicates that TGase activity can be significantly enhanced in response to increased intracellular Ca2+ through IP3 generation (Zhang et al., 1998). In aplysia, serotonin mediated activation of Cdc42 was dependent on PLC and phosphatidylinositol 3-kinase (PI3K) (Udo et al., 2005). In RA-induced neuronal differentiation of SH-SY5Y cells, TGase-catalyzed transamidation is required for activation of RhoA (Singh et al., 2003), whereas activation of Rac1 is mediated by PI3K in a transamidation-independent manner (Pan et al., 2005). Further studies are needed to elucidate the underlying molecular mechanisms by which the 5-HT2A receptor signaling regulates TGase-catalyzed activation of Rac1.
The present study provides the first evidence in neurons that stimulation of 5-HT2A receptors induces an increase in TGase-catalyzed transamidation of serotonin into Rac1 and constitutive activation of Rac1. Further studies using alternative approaches to measure Rac1 transamidation, such as mass spectroscopy, are needed to confirm this hypothesis. Rac1 activation via 5-HT2A receptor stimulation may have an important functional impact given the plethora of pathways that utilize this small G protein. For example, Rac1 is best known as a regulator for the assembly of the actin cytoskeleton, thereby playing a role in neurite outgrowth and neuronal differentiation. We demonstrate that stimulation of 5-HT2A receptor can increase the transamidation of Rac1 in both undifferentiated and differentiated A1A1v cells, suggesting that transamidation and constitutive activation of this signal transducer are not altered by neuronal differentiation of cells. However, the effect of 5-HT2A receptor-mediated activation of Rac1 on cytoskeleton organization, cell cycle progression, transcriptional activation or other crucial cellular functions in neurons has yet to be explored.
Acknowledgments
We thank Dr. William Clarke and Kelly Berg (University of Texas Health Science Center, San Antonio, TX) for providing A1A1v cells.
This work was supported by United States Public Health Service Grant MH068612.
Non-standard abbreviations
- DOI
2,5-dimethoxy-4-iodoamphetamine
- DPAT
5-hydroxy-2-dipropylamino tetralin
- CNF-1
cytotoxic necrotizing factor-1
- GDIs
GDP dissociation inhibitors
- GAPs
GTPase-activating proteins
- GEFs
guanine nucleotide exchange factors
- IP3
inositol 1,4,5-trisphosphate
- PLC
phospholipase C
- 5-HT
serotonin
- TGase
transglutaminase
References
- Andringa G, Lam KY, Chegary M, Wang X, Chase TN, Bennett MC. Tissue transglutaminase catalyzes the formation of alpha-synuclein crosslinks in Parkinson’s disease. Faseb J. 2004;18:932–934. doi: 10.1096/fj.03-0829fje. [DOI] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol Pharmacol. 1994;46:477–484. [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Citron BA, Suo Z, SantaCruz K, Davies PJ, Qin F, Festoff BW. Protein crosslinking, tissue transglutaminase, alternative splicing and neurodegeneration. Neurochem Int. 2002;40:69–78. doi: 10.1016/s0197-0186(01)00062-6. [DOI] [PubMed] [Google Scholar]
- Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, Jackson KW, Clemetson KJ, Alberio L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415:175–179. doi: 10.1038/415175a. [DOI] [PubMed] [Google Scholar]
- Doye A, Mettouchi A, Bossis G, Clement R, Buisson-Touati C, Flatau G, Gagnoux L, Piechaczyk M, Boquet P, Lemichez E. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell. 2002;111:553–564. doi: 10.1016/s0092-8674(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- Folk JE, Park MH, Chung SI, Schrode J, Lester EP, Cooper HL. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980;255:3695–3700. [PubMed] [Google Scholar]
- Fujimoto M, Kanzaki H, Nakayama H, Higuchi T, Hatayama H, Iwai M, Kaneko Y, Mori T, Fujita J. Requirement for transglutaminase in progesterone-induced decidualization of human endometrial stromal cells. Endocrinology. 1996;137:1096–1101. doi: 10.1210/endo.137.3.8603579. [DOI] [PubMed] [Google Scholar]
- Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature’s biological glues. Biochem J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakoshima T, Shimizu T, Maesaki R. Structural basis of the Rho GTPase signaling. J Biochem (Tokyo) 2003;134:327–331. doi: 10.1093/jb/mvg149. [DOI] [PubMed] [Google Scholar]
- Ientile R, Campisi A, Raciti G, Caccamo D, Curro M, Cannavo G, Li Volti G, Macaione S, Vanella A. Cystamine inhibits transglutaminase and caspase-3 cleavage in glutamate-exposed astroglial cells. J Neurosci Res. 2003;74:52–59. doi: 10.1002/jnr.10702. [DOI] [PubMed] [Google Scholar]
- Julius D. Molecular biology of serotonin receptors. Annu Rev Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- Junn E, Ronchetti RD, Quezado MM, Kim SY, Mouradian MM. Tissue transglutaminase-induced aggregation of alpha-synuclein: Implications for Lewy body formation in Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 2003;100:2047–2052. doi: 10.1073/pnas.0438021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na, K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Karpuj MV, Becher MW, Springer JE, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med. 2002;8:143–149. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- Lorand L, Parameswaran KN, Stenberg P, Tong YS, Velasco PT, Jonsson NA, Mikiver L, Moses P. Specificity of guinea pig liver transglutaminase for amine substrates. Biochemistry. 1979;18:1756–1765. doi: 10.1021/bi00576a019. [DOI] [PubMed] [Google Scholar]
- Masuda M, Betancourt L, Matsuzawa T, Kashimoto T, Takao T, Shimonishi Y, Horiguchi Y. Activation of rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. Embo J. 2000;19:521–530. doi: 10.1093/emboj/19.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos P, Skaug J, Marques B, Beck S, Verissimo F, Gespach C, Boavida MG, Scherer SW, Jordan P. Small GTPase Rac1: structure, localization, and expression of the human gene. Biochem Biophys Res Commun. 2000;277:741–751. doi: 10.1006/bbrc.2000.3743. [DOI] [PubMed] [Google Scholar]
- Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, Fink G, Zhou W, Sealfon SC. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- Munro P, Flatau G, Doye A, Boyer L, Oregioni O, Mege JL, Landraud L, Lemichez E. Activation and proteasomal degradation of rho GTPases by cytotoxic necrotizing factor-1 elicit a controlled inflammatory response. J Biol Chem. 2004;279:35849–35857. doi: 10.1074/jbc.M401580200. [DOI] [PubMed] [Google Scholar]
- Norlund MA, Lee JM, Zainelli GM, Muma NA. Elevated transglutaminase-induced bonds in PHF tau in Alzheimer’s disease. Brain Res. 1999;851:154–163. doi: 10.1016/s0006-8993(99)02179-4. [DOI] [PubMed] [Google Scholar]
- Ou H, Haendeler J, Aebly MR, Kelly LA, Cholewa BC, Koike G, Kwitek-Black A, Jacob HJ, Berk BC, Miano JM. Retinoic acid-induced tissue transglutaminase and apoptosis in vascular smooth muscle cells. Circ Res. 2000;87:881–887. doi: 10.1161/01.res.87.10.881. [DOI] [PubMed] [Google Scholar]
- Pan J, Kao YL, Joshi S, Jeetendran S, Dipette D, Singh US. Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Neurochem. 2005;93:571–583. doi: 10.1111/j.1471-4159.2005.03106.x. [DOI] [PubMed] [Google Scholar]
- Roth BL. Multiple serotonin receptors: clinical and experimental aspects. Ann Clin Psychiatry. 1994;6:67–78. doi: 10.3109/10401239409148985. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Fentress H, Hazelwood L. Serotonin 5-ht2 receptors: molecular and genomic diversity. Mol Interv. 2003;3:319–330. doi: 10.1124/mi.3.6.319. [DOI] [PubMed] [Google Scholar]
- Sarvari M, Karpati L, Fesus L, Deli L, Muszbek L, Nemes Z. Competitive enzyme-linked immonosorbent assay for N epsilon gamma-glutamyl lysine. Anal Biochem. 2002;311:187–190. doi: 10.1016/s0003-2697(02)00422-0. [DOI] [PubMed] [Google Scholar]
- Scalzitti JM, Berg KA, Kratowicz SA, Hensler JG. Regulation of serotonin2A receptor expression by an antisense oligodeoxynucleotide. J Neurochem. 1998;71:1457–1463. doi: 10.1046/j.1471-4159.1998.71041457.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Selzer J, Lerm M, Aktories K. The Rho-deamidating cytotoxic necrotizing factor 1 from Escherichia coli possesses transglutaminase activity. Cysteine 866 and histidine 881 are essential for enzyme activity. J Biol Chem. 1998;273:13669–13674. doi: 10.1074/jbc.273.22.13669. [DOI] [PubMed] [Google Scholar]
- Singh US, Pan J, Kao YL, Joshi S, Young KL, Baker KM. Tissue transglutaminase mediates activation of RhoA and MAP kinase pathways during retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Biol Chem. 2003;278:391–399. doi: 10.1074/jbc.M206361200. [DOI] [PubMed] [Google Scholar]
- Thomas V, Fournet G, Simonet F, Roch AM, Ceylan I, El Alaouia S, Quash G. Definition of the fine specificity of the monoclonal antibody 81D4: its reactivity with lysine and polyamine isopeptide cross-links. J Immunol Methods. 2004;292:83–95. doi: 10.1016/j.jim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Udo H, Jin I, Kim JH, Li HL, Youn T, Hawkins RD, Kandel ER, Bailey CH. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron. 2005;45:887–901. doi: 10.1016/j.neuron.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Unsworth CD, Molinoff PB. Regulation of the 5-hydroxytryptamine1B receptor in opossum kidney cells after exposure to agonists. Mol Pharmacol. 1992;42:464–470. [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang Y, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- Watts SW. Activation of the mitogen-activated protein kinase pathway via the 5-HT2A receptor. Ann N Y Acad Sci. 1998;861:162–168. doi: 10.1111/j.1749-6632.1998.tb10187.x. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Zainelli GM, Dudek NL, Ross CA, Kim SY, Muma NA. Mutant huntingtin protein: a substrate for transglutaminase 1, 2, and 3. J Neuropathol Exp Neurol. 2005;64:58–65. doi: 10.1093/jnen/64.1.58. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lesort M, Guttmann RP, Johnson GV. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J Biol Chem. 1998;273:2288–2295. doi: 10.1074/jbc.273.4.2288. [DOI] [PubMed] [Google Scholar]