Abstract
Apoptosis, a form of cellular suicide is a key mechanism involved in the clearance of cells that are dysfunctional, superfluous or infected. For this reason, the cell needs mechanisms to sense death cues and relay death signals to the apoptotic machinery involved in cellular execution. In the intrinsic apoptotic pathway, a subclass of BCL-2 family proteins called the BH3-only proteins are responsible for triggering apoptosis in response to varied cellular stress cues. The mechanisms by which they are regulated are tied to the type of cellular stress they sense. Once triggered, they interact with other BCL-2 family proteins to cause mitochondrial outer membrane permeabilization which in turn results in the activation of serine proteases necessary for cell killing. Failure to properly sense death cues and relay the death signal can have a major impact on cancer. This chapter will discuss our current models of how BH3-only proteins function as well as their impact on carcinogenesis and cancer treatment.
Introduction
In multicellular organisms, various cells of differing specialized functions work together to ensure the survival of the whole organism. In this complex cooperative network, it is essential to ensure the clearance of dysfunctional cells that may pose a risk to the collective. Thus the machinery required to carry out the cellular suicide program known as apoptosis is programmed genetically into each cell. Apoptosis is a form of programmed cell death that is essential in the clearance of cells that are infected, dislocated from their normal positions, damaged, superfluous or have reached the end of their useful life span. Once the apoptotic pathway is engaged, cells are efficiently dismantled and cleared. This efficient process is mediated by the activation of caspases, which are a family of specialized serine proteases that effectively cleave various protein substrates within the cell. One result of their proteolytic activity is the activation of the endonuclease CAD (caspase-activated DNase) which goes on to dismantle the cellular genome, preventing replication of the undesirable clone.1 The dying cells also exhibit cell surface markers that flag them for engulfment and clearance by macrophages. Once caspases have cleaved their downstream substrates, the destruction is irreparable and cell death is inescapable. For this reason, the pathways that lead to caspase activation are critical in determining cell fate.
Apoptosis can be executed by two major pathways called the extrinsic and intrinsic pathways. In the extrinsic pathway, extracellular death signals in the form of ligands bind and activate cell membrane-anchored death receptors like FAS (also known as CD95) receptor, TNF (tumor necrosis factor) receptor and TRAIL (TNF-related apoptosis-inducing ligand) receptor.2 After ligand binding, death receptors aggregate and recruit the adaptor molecule FADD (Fas Associated Death Domain). FADD interacts with pro-caspase-8 to form a complex known as the Death Inducing Signaling Complex (DISC). This complex places several pro-caspase-8 proteins in proximity to each other, causing them to activate by cleavage.2 Fully activated caspase-8 is an initiator caspase that goes on to cleave and activate effector caspases needed to kill the cell.
The intrinsic pathway relies on the mitochondria and thus is also referred to as the mitochondrial pathway. In this pathway, the cell internally senses death cues and usually relays the death signal through a subclass of BCL-2 family proteins called the BH3-only members. These BH3-only proteins interact with other pro-apoptotic and anti-apoptotic proteins members of the BCL-2 family to decide cell fate. If the cell commits to death, pro-apoptotic BCL-2 family members cause the mitochondrial outer membrane to become permeabilized (MOMP) and apoptogenic factors like cytochrome c are released into the cytosol.3–5 Once in the cytosol, cytochrome c interacts with APAF-1 (apoptotic protease activating factor 1) and pro-caspase-9 to form a complex termed the apoptosome.6 The apoptosome complex facilitates the proximity induced auto-cleave of pro-caspase-9 to the active caspase-9.7,8 Similar to caspase-8, caspase-9 is an initiator caspase that goes on to cleave and activate other effector caspases to kill the cell.
In many cells, activation of the extrinsic pathway alone is insufficient to induce apoptosis.2 Instead, recruitment of the intrinsic pathway is also required. Caspase-8 can amplify the extrinsic death signal by cleaving and activating the BH3-only protein Bid to trigger activation of the intrinsic pathway. Thus, BH3-only proteins are important players responsible for communicating death signals originating in both the extrinsic and intrinsic pathways. Understanding how these BH3-only proteins function will help us not only understand how cells survive to become cancerous but also how to trigger these death cues for better chemotherapeutics.
Categories of BCL-2 Family Proteins and Their Apoptotic Functions
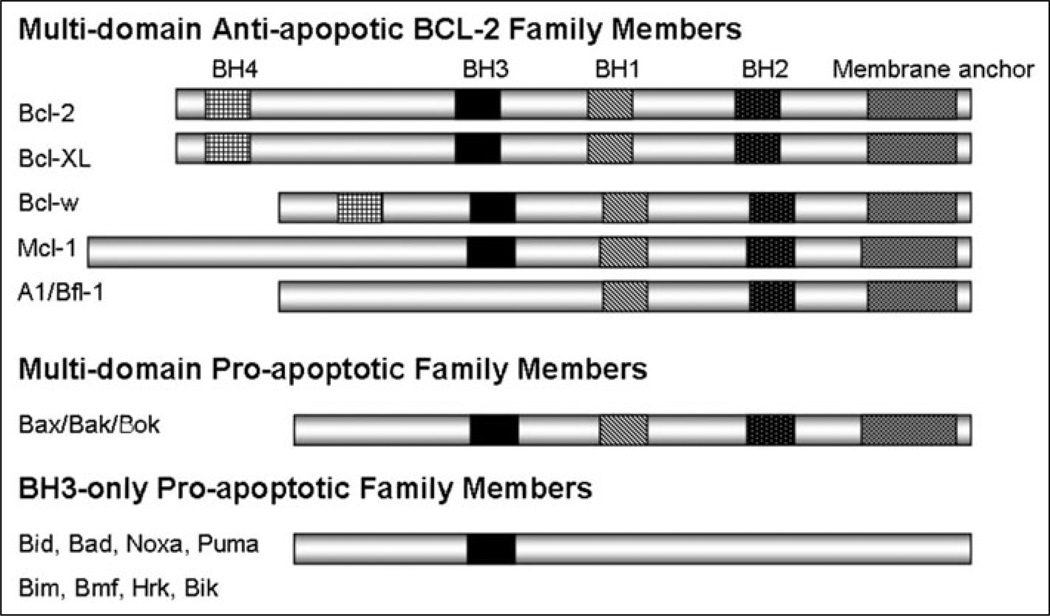
The BCL-2 family of proteins consists of three main categories based on their function and sequence homolog y as shown on Figure 1. Each member of this family of proteins shares at least one of four regions of homolog y with their founding member BCL-2. These BCL-2 homology regions are often denoted as BH1 through BH4. The multidomain anti-apoptotic members share all four regions (except Mcl-1 and Bfl-1). The multidomain pro-apoptotic members share domains BH1, BH2 and BH3. The final group consists of pro-apoptotic proteins that only share the BH3 domain and are thus referred to as the BH3-only proteins.
Figure 1.
BCL-2 homology domains of BCL-2 family members.
Multidomain Proapoptotic Members
The multidomain proapoptotic BCL-2 family proteins consist of Bax, Bak and Bok. Bax and Bak are prevalent and expressed in a wide variety of tissues, whereas the less studied Bok is only known to be prevalent in reproductive tissue. Bax and Bak proteins consist of five amphipathic a-helices surrounding two central hydrophobic a-helices long enough to traverse a lipid bilayer.9 These proteins form pores in the outer membrane of the mitochondria in response to death signals. The resulting mitochondrial outer membrane permeability (MOMP) causes the release of death factors like cytochrome c into the cytosol to execute cell killing. Indeed, Bax was found to permeabilize giant liposomes releasing their fluorescence dye content.10 Furthermore, cytochrome c release is not observed in Bax/Bak null cells during drug treatments that normally evoke apoptosis.11 BH3-only proteins lack pro-death function in the absence of Bax and Bak.11 Thus, the roles of Bax and Bak in executing MOMP makes them essential for apoptosis to occur through the intrinsic apoptotic pathway.11
In the absence of a death signal, Bax and Bak mainly exist as monomers in their inactive forms. Inactive Bax can be found in the cytosol or loosely associated with the outer mitochondrial membrane. Inactive Bak is inserted into the mitochondrial outer membrane.12 In the presence of a death signal, Bax and Bak become activated and undergo a conformational change that exposes the N-terminal of the proteins. Upon activation, Bax translocates and inserts into the mitochondria. Both activated Bax and Bak homo-oligomerize and form pores to cause MOMP. As detailed below, the steps that lead to Bax and Bak induction of MOMP can be regulated by the other BCL-2 family proteins. Both the anti-apoptotic and BH3-only category of proteins have important roles in regulating Bax and Bak activity.
Anti-Apoptotic BCL-2 Family Members
The multidomain anti-apoptotic proteins include BCL-2, BCL-XL, BCL-2, Mcl-1 and Bfl-1/A1. BCL-2 and its two closest homologs BCL-XL and BCL-2 share the same core structure as the pore forming Bax and Bak, which consist of five amphipathic a-helices surrounding two central hydrophobic a-helices. These proteins possess three or four BH domains and a C-terminal transmembrane anchor. A hydrophobic groove formed by the BH1, BH2 and BH3 domains provide a binding site for the hydrophobic face of the amphipathic BH3 domain of pro-apoptotic proteins.
These anti-apoptotic family members have been observed to bind Bax and Bak resulting in the prevention of Bax/Bak oligomerization and MOMP. Modification of the hydrophobic pocket of BCL-XL inhibits this Bax interaction.13 This interaction is greatly increased in the presence of detergents, like Triton X100 and NP-40, that induce the active conformation of Bax.14 This suggests that anti-apoptotic BCL-2 family proteins preferentially inhibit the activated form of Bax and Bak. The BH3-domain of BH3-only proteins can also bind to the hydrophobic pockets of anti-apoptotic family members.15 Indeed, this may be the most important anti-apoptotic function of anti-apoptotic proteins. Two main line of evidence support this view. First, a mutant BCL-XL protein that lacked the ability to bind Bax or Bak, but could still bind BH3 only proteins, maintained nearly all of its anti-apoptotic function.16 Second, in living cells, when BCL-2 protects against cell death, it does so usually by preventing activation of Bax and Bak, suggesting that interception of Bax and Bak activating signals is the primary function of BCL-2 and related anti-apoptotic proteins.17 Nonetheless, cells may be found in which a portion of the total Bax and Bak is indeed bound to anti-apoptotic proteins. The significance of anti-apoptotic proteins binding to BH3-only proteins in regulating Bax and Bak activity is discussed further in the next section.
Proapoptotic BH3-Only Function
Proteins which can most confidently be referred to as BH3-only proteins include Bim,18 Bid,19 Bad,20 Bik,21 Noxa,22 Puma,23 Hrk,24 Bmf,25 Mule,26 Bcl-g.27 Others have been identified as possessing BH3-domains, but their function as pro-death molecules in the BCL-2 family remains less clear. These include Bnip3,28 Beclin-1,29 ApoL6,30 BRCC2,31 Spike32 and MAP-1.33
It is worth noting that the roughly 20 amino acid BH3 domain has only a few amino acids that are highly conserved across all members, so that searching for BH3 domains by sequence alone is challenging, if not impossible. This chapter will focus on the better studied BH3-only proteins Bim, Bid, Bad, Bik, Noxa, Puma, Hrk and Bmf. These proteins are induced by cell death cues and relay their death signal to the mitochondria. As their name denotes, BH3-only proteins share homolog y with BCL-2 only in their BH3 domain. The BH3 domain consists of an amphipathic a-helix. Mutating the BH3-domain of BH3-only proteins abrogates their pro-apoptotic function.18,19 Furthermore, short polypeptide oligomers made from the BH3 domain sequence are sufficient to induce Bax- and Bak-mediated MOMP.17,34 Thus the BH3 domain of the BH3-only proteins are both necessary and sufficient for their apoptotic function.
BH3-only proteins can be divided into two different groups based on their function, the activators and the sensitizers.34 BH3-only proteins require the presence of Bax or Bak proteins to perform their pro-apoptotic function.35 Data suggests that certain BH3-only proteins, called activators, may directly interact with Bax and Bak to promote their activation. These activator BH3-only proteins include Bim and the truncated form of Bid (tBid). Bid was initially cloned via interaction with both Bax and BCL-2, so it is perhaps not surprising that an interaction with Bax has biological relevance.19 Both tBid as well as the BH3 domain from Bid have been shown to induce Bax activation and membrane permeabilization in mitochondrial and liposomal systems.12,34,36 These observations have been greatly bolstered by more recent work directly observing interaction in a lipid membrane using full-length proteins and fluorescence resonance energy transfer (FRET).37 Also, structural studies show that the BH3 domain of Bim binds the a1 and a6 helices of Bax and not the hydrophobic pocket formed by the BH1, BH1 and BH3 domains.38 However, since loss of both Bim and Bid has only minor effects on select triggers of apoptosis,39 it seems likely that these factors are not the only activators. Indeed, activator function has also been attributed to p53 and the BH3-only protein Puma.40,41 Other uncharacterized proteins may play this role and even heat has been shown to foster Bax activation.42 Anti-apoptotic BCL-2 family proteins can bind and sequester activator BH3-only proteins, preventing them from activating Bax and Bak.34,43
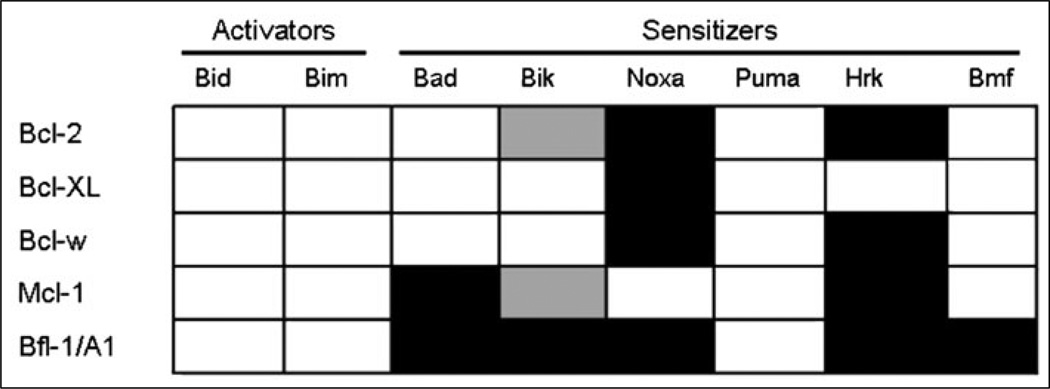
Sensitizer BH3-only proteins lack the ability to directly activate Bax and Bak but are still pro-apoptotic. They instead bind anti-apoptotic family proteins like BCL-2 and Mcl-1 to inhibit their function.18–22 The hydrophobic face of the BH3 a-helix inserts into the hydrophobic cleft formed by the BH1, BH2 and BH3 domains of the anti-apoptotic family members. By binding to anti-apoptotic members, BH3-only proteins prevent critical anti-apoptotic binding of pro-apoptotic molecules including activators like Bim and Bid, as well as activated, monomeric Bax and Bak. If the anti-apoptotic proteins are already binding select pro-death proteins such as activator BH3-only proteins (e.g., Bim or Bid) or activated Bax or Bak, the inhibition of anti-apoptotic function can induce death by freeing those molecules to trigger MOMP. It must be noted that not all BH3-only proteins can bind all anti-apoptotic members. Binding assays show BH3 peptides from the various BH3-only proteins have specificity for only certain anti-apoptotic BCL-2 family members.17,44 Some BH3-only proteins like Bim, Bid and Puma can bind all known anti-apoptotic family members. However, proteins like Bad, Bik, Noxa, Hrk and Bmf are more limited in the spectrum of anti-apoptotic proteins they can bind. Figure 2 displays the binding specificities of the BH3 peptide from each BH3-only protein to the anti-apoptotic family members.
Figure 2.
Binding specificity of various BH3-only protein oligomers for anti-apoptotic members. White blocks denotes strong affinity, grey blocks denote weak-affinity and black blocks denote no detectable binding. Adapted from: Certo M et al. Cancer Cell 2006; 9(5):351–365;17 © 2006 with permission from Elsevier.
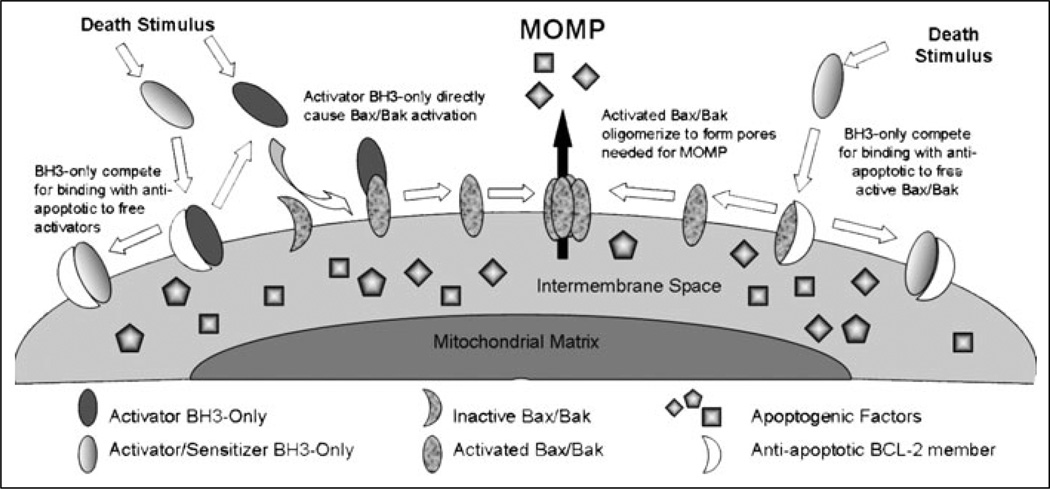
The presence of two distinct models of control over apoptosis, the so-called direct and indirect models, have received considerable attention in the field. However, with the now widespread appreciation that Bax and Bak require some kind of activating step to effect MOMP, the differences are quite slender. Proponents of the direct model have focused on the ability of activator BH3-only proteins to cause death by activating Bax and Bak. Proponents of the indirect pathway have focused more on the sensitizer function of BH3-only proteins, particularly in their ability to displace Bax and Bak that is already activated. Both mechanisms likely occur in cells, the relative degree no doubt varying according to context. A summary can be found in Figure 3.
Figure 3.
Model of BH3-only induction of Bax/Bak mediated outer mitochondrial membrane permeabilization.
Regulation of Various BH3-Only Proteins and Cell Stress Sensing
Genetic and biochemical data has shown that, depending on context, different BH3-only proteins are essential for cell death initiation through the intrinsic pathway. Each BH3-only protein member can be stimulated by many mechanisms that correspond to the type of cell stress the protein is able to sense. However, it must be also be noted that many stimuli activate multiple BH3-only proteins to ensure the death signal is carried forth.
Bim (BCL-2 interacting mediator of cell death)18 is constitutively expressed in several tissues and exists as a number of splice variants denoted as BimEL (the largest), BimL and Bims (the smallest). Bims has the most potent apoptosis-inducing activity.18,45 There are other splice isoforms that are not observed as often like a1–1, 1–4 and Bim AD46 and others.47 However, BimEL and BimL seem to be the most relevant in vivo. Bim is regulated by multiple known mechanisms. Bim can be localized to the microtubules by binding to dynein light chain (DLC) 1, a component of the microtubule dynein motor complex.48 This localization suggests that Bim may be activated during microtubule perturbation. Bim can also be regulated transcriptionally. After cytokine withdrawal, activation of Foxo3a (forkhead transcription factor) induces Bim expression in lymphocytes.49 Loss of cellular adhesion can also cause transcriptional upregulation of Bim via the Mek/Erk pathway.50 Phosphorylation by Jun N-terminal kinase (JNK) in the DLC-binding domain causes Bim to dissociate from the microtubule and induce cell death after UV irradiation.51 Bim can also be downregulated by proteasomal degradation after ERK-mediated phosphorylation and subsequent ubiquitination.52 With all of these mechanisms of regulation, Bim can serve as a sentinel for genotoxic stress, microtubule stress, loss of cellular adhesion and loss of growth factors.
Bid (BH3-interacting domain) is relatively inactive until proteolytically cleaved to the truncated form tBid. Bid can be cleaved by caspase-8 and thus serve as a means by which the extrinsic apoptotic pathway can activate the intrinsic pathway. Granzyme B, a protease released in secretory granules by cytotoxic T-lymphocytes and natural killer cells, can also activate Bid.53,54
These granules are often directed at virally transfected or transformed cells. The lysosomal protein cathepsin can also cleave Bid after lysosomal disruption.55 Once cleaved, Bid becomes myristoylated which promotes its targeting to the mitochondria.56 Aside from proteolytic activation, Bid was also shown to be transcriptionally induced by p53 during y-irradiation though much of it remains inactive and uncleaved.57 Since upregulation of total Bid was not accompanied with increased activated tBid, it is not clear how this mode of Bid activation contributes to p53-induced apoptosis. Data also suggests that Bid may be regulated by ATM kinase mediated phosphorylation during the DNA damage response.58 Nonphosphorylatable Bid mutant was more resistant to etoposide-induced apoptosis than wild-type.58 Thus Bid is a sentinel that detects internal death cues from DNA damage as well as relay external death signals.
Bad is best known for its role in apoptosis induced by cytokine or growth factor deprivation. The Bad gene results in two different size proteins (Bad long and short) due to alternate translation initiation and translation start sites.59 It is tightly regulated by phosphorylation through various pathways. In mice, serine 112, serine 136 and serine 155 are phosphorylated. In humans the residues phosphorylated are Ser-75, Ser-99 and Ser-118. Protein kinases that phosphorylate Bad include Akt,60,61 Raf-1,62 protein kinase A (PKA).63 All of these kinases are activated by growth survival signals. Phosphorylation at these sites promotes sequestration of Bad in the cytoplasm by 14-3-3 scaffold proteins away from the mitochondria.64 Phosphatases that dephosphorylate Bad include calcineurin,65 protein phosphatase 1 (PP1)66 and protein phosphatase 2A (PP2A).67 Dephosphorylation allows Bad to translocate to the mitochondria and inhibit select anti-apoptotic BCL-2 members.
Bik (also known as Nbk) is induced due to various apoptotic triggers. The mouse orthologue is called Blk. Bik expression can be upregulated by p53.68 Once induced, Bik translocates to the mitochondria and endoplasmic reticulum. Bik can be enhanced when phosphorylated possibly by a casein kinase II related enzyme.69 Nonphosphorylatable Bik displays less apoptotic activity though its ability to bind BCL-2 is maintained.69 Bik can also be transcriptionally upregulated by the oncogene E2F in a manor that is independent of p53.70
Puma (p53 upregulated modulator of apoptosis) was first discovered as a gene induced by p53.23 It was concurrently discovered by another lab which named it bbc3.71 The promoter contains two p53 binding sites allowing it to be directly affected by p53. The gene produces two proteins products (Puma-a and Puma-) that exhibit similar activity in binding BCL-2 and causing cytochrome c release.23 Puma substantially contributes to p53-dependent and independent apoptosis during glucocorticoid treatment, ionizing irradiation, deregulated c-Myc induced death and cell death induction during cytokine withdrawal.71,72 Though other BCL-2 family members are also transcriptionally upregulated by p53, data suggest that Puma plays a predominant role in mediating p53-induced apoptosis.72,73
Noxa (Greek for “damage”) was the first BH3-only protein reported to be a p53 transcriptional target.22 The human Noxa protein has one BH3 domain but the mouse Noxa is the only BCL-2 family member known to have two functional BH3 domains.22 Both human and mouse Noxa genes have a p53-binding site in their promoter causing them to be a direct effector of p53.22 Together with Puma, Noxa plays a role in p53-mediated death but seems to be less important than Puma in many cases. This may be due it is effects being more limited than Puma in regards to the anti-apopotic BCL-2 members it can neutralize(see Figure 2). Noxa is thought to induce apoptosis though antagonism MCL-1 and BFL-1.17,44
Bmf is reported to be regulated in a similar manner as Bim and also has a conserved dynein light chain-binding motif. However, instead of binding to the microtubules, BMF is bound to dynein light chain 2 (DLC2) which is a component of the myosin C actin motor complex found on the actin microfilament.25 Loss of cellular adhesion can mobilize Bmf to exert its pro-apoptotic function.74
Human Hrk has a murine homologue DP5, which was first discovered to induce death when rat sympathetic neurons were deprived of nerve growth factor (NGF).75 Murine DP5 is mainly only expressed in the nervous system75 while human Hrk is also found in hematopoietic tissues.24
Hrk can be regulated at the transcription level. It has been shown that E2F1 can transcriptionally upregulate Hrk. Hrk is also induced during growth factor withdrawal in haemopoietic progenitor cells lines.76 Thus, Hrk can serve as a sentinel for growth factor withdrawal and oncogene activation induced death.
Though much information is known regarding the regulation of BH3-only proteins, a full picture of what triggers cause each protein to be activated is far from complete. Also, each tissue type may utilize a different set of BH3-only to respond to the same death stimulus. Lastly, it must be noted that cancer cells exhibit at baseline many characteristics that would normally mobilize these BH3-only sentinels. Oncogenes like Myc upregulates Bim and E2F1 upregulates Hrk. Metastatic cancer cells also loose normal adhesion to their environment which should trigger Bim and Bmf. Tumors that grow outside of their normal tissue also lack normal survival factors that could result in induction of BH3-only proteins like Bim, Bad, Puma and Hrk. Thus, BH3-only proteins may play important roles in cancer prevention at very early stages of tumorigensis. In the next section, the possible impact of BH3-only proteins to cancer development will be assessed.
BH3-Only Proteins and Cancer Development
It has been postulated that inhibiting apoptosis is a prerequisite for cancer development.77
In this section, we will summarize data accumulated regarding how direct changes in BH3-only members contribution to carcinogenesis. The best studies to address their contribution are found in experimental mouse knockout models. These models will be compared with data obtained from human studies to also assess their effect on human cancer.
Bim-deficient lymphoid and myeloid cells facilitate tumorigenesis in mice with a tumor-prone background.78 In one study, half of the bim-null mice became terminally ill within a year.78 Loss of a single allele of Bim in the presence of a Myc transgene causes robust tumor formation especially in the form of acute B-cell leukemia.79 This is thought to be due to the observation that apoptosis triggered by Myc is mediated by its induction of Bim.79 Thus without Bim, Myc tumors have a great survival advantage. Lymphoma from Bim-deficient mice also show resistance to cytokine deprivation,78 which may help these cells grow unchecked. In human cancer, microarray data showed Bim is down regulated by exhibiting homozygous deletion in mantle cell lymphoma and promoter hypermethylation in Burkitt’s lymphoma.80 Thus Bim plays a significant role in tumor-suppression in controlled mouse models and may indeed be relevant in human cancer. Aging Bid-deficient mice develop a myeloproliferative disorder and progress to a fatal malignancy resembling chronic myelomonocytic leukemia.81 In 2 years, 53% of Bid-deficient mice have developed leukemia.81 Bid-deficient myeloid precursors reportedly show enhanced colony-formation potential in vitro.81 Thus Bid acts as a tumor suppressor in myeloid cells. The tumorigenetic contribution of Bid in humans is not as evident. An immunohistochemistry survey found increased Bid expression in prostate cancer, colon cancer, ovarian cancer, brain cancer and B-cell non-Hodgkin’s lymphoma.82 Therefore, even though mouse data supports the role of Bid as a tumor-suppressor, its role in human cancer is less evident.
Bad-deficient mice were viable and with age develop diffuse large B-cell lymphoma of germinal center origin.59 Tumors begin appearing about 15 months of age.59 Tumor development due to loss of BH3-only proteins generally requires substantial time which suggests that accumulation of other mutations are necessary to induce tumorigenesis. Indeed, increasing mutation rate by sublethally y-irradiating mice lacking Bad produced greater incidence of leukemia.59 Loss of Bad also offers advantage for cancer cells by allowing them to become independent of survival signals that control the growth of normal cells. Withdrawal of epidermal growth factor (EGF) increased death of wild-type mammary epithelial cells but Bad null cells were more resistant.59
Studies in human have found the Bad gene is methylated in multiple myeloma cells.83 Thus Bad loss may be tumorigenic in both mice and humans.
It has been shown that the oncogene E1A induces apoptosis and upregulated Bik though a p53-dependent pathway.68 However, mice deficient in Bik showed no signs of developing cancer. In human studies, Bik has been found to be methylated in multiple myeloma cells.83
Immunohistochemistry studies show Bik loss of expression in malignant kidney epithelium compared to their adjacent normal tissue.84 Thus, Bik is associated with cell death during oncogene activation and cancer progression, but by itself loss of Bik may not be greatly tumorigenic.
Despite its role as an important effector of p53 mediated apoptosis, loss of Puma is not tumorigenic on its own since no spontaneous tumors arise in Puma-deficient mice over time. However, its loss does dramatically reduce the time required for lymphoma formation in Myc transgenic mice.85 These lymphomas usually maintain wild-type p53 that can still function in inducing cell cycle arrest due to y-irradiation.85 Puma loss also reduced time of tumor formation of MEFs transformed by E1A and ras-encoding retroviruses in nude mice.85 Similarly, loss of Puma greatly promotes apoptotic resistance in MEFs transformed by the Myc oncogene.72 In humans, no relationship was found between Puma expression level with HNSCC and lung cancer.86 A correlation was found where Puma decreased upon melanoma progression.87 Since p53 loss is relatively rare in melanoma,88 Puma loss in these cells may provide selective advantage from its p53-related activity. Thus, Puma loss may not be highly tumorigenic on its own but may provide many transformed cells with a survival advantage.
Mice deficient in Noxa showed no signs of developing cancer. Noxa was also down regulated by mutation and silenced in diffuse large B-cell lymphoma.80 Mutational analysis done on colon adenocarcinomas, advanced gastric adenocarcinomas, nonsmall-cell lung carcinomas, breast carcinomas, urinary bladder transitional cell carcinomas and hepatocellular carcinomas found no inactivating mutations associated with these cancers.89 Thus the evidence available does not conclusively show that Noxa loss significantly contributes to tumorigenesis.
The effect of Bmf on tumorigenesis remains to be determined. Microarray data showed levels of Bmf increase in anoikis conditions. Knock-down of Bmf prevented anoikis and promoted anchorage independent growth.74 Circumstantial evidence exists to suggest its involvement in cancer since its chromosomal location 15q14 harbors a tumor-suppressor found in patients with advanced breast, lung and brain cancer.90,91 Yet this evidence is not sufficient to make conclusions regarding the impact of Bmf as a tumor-suppressor.
Mice deficient in Hrk alone showed no signs of developing cancer. However, the Hrk gene is often inactivated by methylation in gastric and colorectal cancer compared to normal colon or stomach.92 Though the gene suppression of this BH3-only member is associated with cancer, these associations alone do not necessarily point to a causal relationship.
Throughout this survey of BH3-only proteins and their impact on cancer development, Bim and Bad show strongest evidence of being human tumor suppressors. In mouse models Bid also is a tumor suppressor but relevance to human cancer is not evident. This does not rule out the possibility that other BH3-only proteins may also be tumor suppressors as well. The weakness of certain BH3-only proteins as tumor-suppressors may be due to their redundancy of function during various apoptotic stimuli. For this reason, it is not surprising that upregulation of anti-apoptotic BCL-2 family proteins that can block a wide range of BH3-only members have a greater oncogenic ability93–95 than the loss of individual BH3-only members alone.
BH3-Only Proteins and Chemotherapy Response and Resistance
BH3-only proteins may not only have a profound effect on cancer development but also on cancer treatment. Many cancer therapeutics cause cellular stress that lead to apoptotic death.
However, in many cases the exact mechanism of how these therapeutics cause death is not very well studied. The following table summarizes some of the BH3-only proteins implicated in various cancer therapeutic treatments.
In most of the studies shown in Table 1, the BH3-only proteins listed was either knocked-down or knocked out. If treatment of the cells deficient in the BH3-only protein of interest resulted in survival then that protein is implicated as a BH3-only protein involved in cell killing. However, since BH3-only protein expression can be tissue specific, the set of BH3-only proteins actually involved in a certain drug-induced killing may be different for cancers of varying origin. Even cancer cells lines from the same tissue type can differ dramatically in regards to the BH3-only proteins that they may employ in cellular suicide. Yet despite these issues, some tentative generalizations can be drawn from our current understanding of BH3-only proteins and their involvement in cancer therapeutics.
Table 1.
BH3-only proteins implicated in apoptosis due to various treatments
| Cancer Treatment | Cellular Damage | BH3-Only Proteins |
|---|---|---|
| 5-Fluorouracil | DNA synthesis inhibitor | Bim,96 Puma,96 Bad97 and Bid57,98 |
| Adriamycin | DNA damaging agent | Bim,99 Bik,70 Bid100 and Puma23 |
| Bortezomib | Proteasome inhibitor | Bim,101 Noxa101 and Bik101 |
| Etoposide | DNA damaging agent | Bim,99 Bid,102,103 Puma73 and Noxa73 |
| Gefitnib | EGFR inhibitor | Bim104 and Bad105 |
| Glucocorticoid | Antagonize immune cells | Bim106–108 and Puma107 |
| HDAC inhibitor | Inhibit histone deacytylse | Bim109 Bmf110 |
| Imatinib | Kinase inhibitor | Bim,111 Bmf111 and Bad111 |
| Irradiation | DNA damaging agent | Bim,107 Bid112 and Puma73,107 |
| MEK inhibitors | MAPK inhibitor | Bim113 and Bmf113 |
| Paclitaxel | Microtubule inhibitor | Bim114–116 |
One noted trend is the prevalence of Bim involvement in a wide variety of cancer therapy responses. Since Bim is an activator and can also inhibit all anti-apoptotic BCL-2 family proteins, loss of its activity may more noticeably affect drug-induced death than the other BH3-only proteins. Likewise, Puma can inhibit a wide range of anti-apoptotic proteins and is also important in a wide range of cancer therapeutic killing.
One final item worth noting is the fact that each cancer treatment often mobilizes many BH3-only proteins to evoke apoptosis. For example, Imatinib was found to cause cell death through activation of several BH3-only proteins including Bim, Bmf and Bad.111 Imatinib induced Bim through transcriptional activation and inhibition of its proteasomal degradation.111 Imatinib also upregulate Bad by dephosphorylation and Bmf though transcriptional induction. Knock-down of Bim caused partial resistance to Imatinib.117 However, fetal liver cells lacking both Bim and Bad are nearly completely resistant to Imatinib.111 Thus, the effectiveness of chemotherapeutic treatment relies on many BH3-only proteins to ensure cell death.
BH3-Only Mimetic Drugs
Due to the significant effect of BH3-only proteins on drug response, various companies have developed small molecules that mimic the BH3 domain and induce apoptotic death. One such drug, ABT-737 is the most potent and specific inhibitor (Ki < 1 nM) of BCL-2, BCL-XL and BCL-2.118 Studies have shown significant cytotoxic effects of ABT-737 as a single agent on primary follicular lymphoma and B-CLL cells.118,119 As a single agent ABT-737 is not as effective in solid tumor cell lines but does show activity in small cell lung cancer (SCLC) cells.118 For a review see Labi et al.120 In vivo, ABT-737 is not only well tolerated but showed a sixfold increase in event-free survival of immune compromised mice harboring transplanted human pediatric ALL cells when combined with vincristine, dexamethasone and L-ASP.121
Based on the activator/sensitizer model, the therapeutic window that allows normal cells to resist ABT-737 treatment and cancer cells to succumb to apoptosis is a phenomenon often termed “BCL-2 addiction.” Cancer cells often exhibit characteristics that would evoke the activity of BH3-only proteins like genomic instability and oncogene activation. One way for these cancer cells to survive is to upregulate levels of anti-apoptotic proteins to protect them from death. Indeed it has been shown that many cancer cells over-expressing anti-apoptotic BCL-2 are preloaded with the activator Bim even prior to drug treatment.119 These cells are in a state that is referred to as “primed” for death and only require sensitizer mediated displacement of activators to trigger cell death.17 Based on the activator/sensitizer model, ABT-737 would be classified as a sensitizer mimetic since it is not able to activate Bax and Bak on its own. Indeed, ABT-737 has been observed to displace the activator Bim from BCL-2 to cause cytochrome c release.119 Moreover, the status of being “primed” has been positively correlated with ABT-737 sensitivity.17,119,122 For this reason, BH3-only mimetics are extremely useful chemotherapeutics against cancer cells.
Despite the effectiveness of ABT-737, its inability to inhibit Mcl-1 can limits its efficacy. Cells displaying resistance to ABT-737 are often shown to be protected by Mcl-1.123,124 For this reason development of BH3 mimetics which target Mcl-1 and other antiapoptotic proteins are currently explored.
Conclusion
As the sentinels of cellular dysfunction, BH3-only proteins play an important role in the detection and clearance of potentially cancerous cells. A better understanding of BH3-only protein function has already yielded an effective drug ABT-737 that can mimic their activity in the selective killing of cancer cells. However, many solid tumors are not responsive when treated with ABT-737 alone. Since ABT-737 has sensitizer function, it likely requires the presence of other BH3-only proteins to induce cell killing. Indeed, in combination with other chemotherapeutic drugs that can induce activation of other BH3-only proteins, ABT-737 does show greater effectiveness. For this reason, a deeper understanding of BH3-only protein regulation and response to drug action is needed to develop more effective treatment options. Furthermore, understanding how various oncogenes effect the BH3-only proteins mobilization can help physicians tailor drug treatment to the oncogenes involved in individual patient tumors. Standing in the cross roads between detection of cellular derangement and the cellular death machinery, BH3-only proteins serve as important allies in cancer cell killing and rational treatment design.
References
- 1.Enari M, Sakahira H, Yokoyama H, et al. A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature. 1998;391(6662):43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 2.Khosravi-Far R, Esposti MD. Death receptor signals to mitochondria. Cancer Biol Ther. 2004;3(11):1051–1057. doi: 10.4161/cbt.3.11.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du C, Fang M, Li Y, et al. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Kim CN, Yang J, et al. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 5.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922–2933. [PubMed] [Google Scholar]
- 6.Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000;275(40):31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez J, Lazebnik Y. Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev. 1999;13(24):3179–3184. doi: 10.1101/gad.13.24.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou H, Li Y, Liu X, et al. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274(17):11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Saez AJ, Mingarro I, Perez-Paya E, et al. Membrane-insertion fragments of Bcl-xL, Bax and Bid. Biochemistry. 2004;43(34):10930–10943. doi: 10.1021/bi036044c. [DOI] [PubMed] [Google Scholar]
- 10.Schlesinger PH, Saito M. The Bax pore in liposomes, Biophysics. Cell Death Differ. 2006;13(8):1403–1408. doi: 10.1038/sj.cdd.4401991. [DOI] [PubMed] [Google Scholar]
- 11.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei MC, Lindsten T, Mootha VK, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14(16):2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 13.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the BCL-2 family of proteins. Biochim Biophys Acta. 2004;1644(2–3):83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273(17):10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 15.Petros AM, Nettesheim DG, Wang Y, et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis and biophysical studies. Protein Sci. 2000;9(12):2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng EH, Levine B, Boise LH, et al. Bax-independent inhibition of apoptosis by BCL-XL. Nature. 1996;379(6565):554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 17.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor L, Strasser A, O’Reilly LA, et al. Bim: a novel member of the BCL-2 family that promotes apoptosis. EMBO J. 1998;17(2):384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Yin XM, Chao DT, et al. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10(22):2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 20.Yang E, Zha J, Jockel J, et al. Bad, a heterodimeric partner for BCL-XL and BCL-2, displaces Bax and promotes cell death. Cell. 1995;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 21.Boyd JM, Gallo GJ, Elangovan B, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with BCL-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11(9):1921–1928. [PubMed] [Google Scholar]
- 22.Oda E, Ohki R, Murasawa H, et al. Noxa, a BH3-only member of the BCL-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 23.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 24.Inohara N, Ding L, Chen S, et al. Harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins BCL-2 and Bcl-X(L) EMBO J. 1997;16(7):1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puthalakath H, Villunger A, O’Reilly LA, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293(5536):1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 26.Zhong Q, Gao W, Du F, et al. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121(7):1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Guo B, Godzik A, Reed JC. Bcl-G, a novel pro-apoptotic member of the BCL-2 family. J Biol Chem. 2001;276(4):2780–2785. doi: 10.1074/jbc.M005889200. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda M, Theodorakis P, Subramanian T, et al. Adenovirus E1B-19K/BCL-2 interacting protein BNIP3 contains a BH3 domain and a mitochondrial targeting sequence. J Biol Chem. 1998;273(20):12415–12421. doi: 10.1074/jbc.273.20.12415. [DOI] [PubMed] [Google Scholar]
- 29.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the BCL-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282(17):13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Lu H, Jiang Z, et al. Apolipoprotein l6, a novel proapoptotic BCL-2 homology 3-only protein, induces mitochondria-mediated apoptosis in cancer cells. Mol Cancer Res. 2005;3(1):21–31. [PubMed] [Google Scholar]
- 31.Broustas CG, Gokhale PC, Rahman A, et al. BRCC2, a novel BH3-like domain-containing protein, induces apoptosis in a caspase-dependent manner. J Biol Chem. 2004;279(25):26780–26788. doi: 10.1074/jbc.M400159200. [DOI] [PubMed] [Google Scholar]
- 32.Mund T, Gewies A, Schoenfeld N, et al. Spike, a novel BH3-only protein, regulates apoptosis at the endoplasmic reticulum. FASEB J. 2003;17(6):696–698. doi: 10.1096/fj.02-0657fje. [DOI] [PubMed] [Google Scholar]
- 33.Tan KO, Tan KM, Chan SL, et al. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax throug h its BCL-2 homology domains. J Biol Chem. 2001;276(4):2802–2807. doi: 10.1074/jbc.M008955200. [DOI] [PubMed] [Google Scholar]
- 34.Letai A, Bassik MC, Walensky LD, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2(3):183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 35.Zong WX, Lindsten T, Ross AJ, et al. BH3-only proteins that bind pro-survival BCL-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15(12):1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuwana T, Mackey MR, Perkins G, et al. Bax and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111(3):331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 37.Lovell JF, Billen LP, Bindner S, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135(6):1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Gavathiotis E, Suzuki M, Davis ML, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455(7216):1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple BCL-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 40.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303(5660):1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Rafiuddin-Shah M, Tu HC, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8(12):1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 42.Pagliari LJ, Kuwana T, Bonzon C, et al. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA. 2005;102(50):17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng EH, Wei MC, Weiler S, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival BCL-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17(3):393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 45.O’Reilly LA, Cullen L, Visvader J, et al. The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal and germ cells. Am J Pathol. 2000;157(2):449–461. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marani M, Tenev T, Hancock D, et al. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22(11):3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adachi M, Zhao X, Imai K. Nomenclature of dynein light chain-linked BH3-only protein Bim isoforms. Cell Death Differ. 2005;12(2):192–193. doi: 10.1038/sj.cdd.4401529. [DOI] [PubMed] [Google Scholar]
- 48.Puthalakath H, Huang DC, O’Reilly LA, et al. The proapoptotic activity of the BCL-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3(3):287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 49.Dijkers PF, Medema RH, Lammers JW, et al. Expression of the pro -apoptotic BCL-2 family member Bim is regulated by the forkhead transcription factor FK HR-L1. Curr Biol. 2000;10(19):1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 50.Reginato MJ, Mills KR, Paulus JK, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5(8):733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 51.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100(5):2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akiyama T, Bouillet P, Miyazaki T, et al. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only BCL-2 family member Bim. EMBO J. 2003;22(24):6653–6664. doi: 10.1093/emboj/cdg635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barry M, Heibein JA, Pinkoski MJ, et al. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol. 2000;20(11):3781–3794. doi: 10.1128/mcb.20.11.3781-3794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutton VR, Davis JE, Cancilla M, et al. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J Exp Med. 2000;192(10):1403–1414. doi: 10.1084/jem.192.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Droga-Mazovec G, Bojic L, Petelin A, et al. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic BCL-2 homologues. J Biol Chem. 2008;283(27):19140–19150. doi: 10.1074/jbc.M802513200. [DOI] [PubMed] [Google Scholar]
- 56.Zha J, Weiler S, Oh KJ, et al. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290(5497):1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 57.Sax JK, Fei P, Murphy ME, et al. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4(11):842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 58.Kamer I, Sarig R, Zaltsman Y, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122(4):593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 59.Ranger AM, Zha J, Harada H, et al. Bad-deficient mice develop diffuse large B-cell lymphoma. Proc Natl Acad Sci USA. 2003;100(16):9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 61.del Peso L, Gonzalez-Garcia M, Page C, et al. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278(5338):687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 62.Wang HG, Rapp UR, Reed JC. BCL-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87(4):629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 63.Harada H, Becknell B, Wilm M, et al. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3(4):413–422. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 64.Zha J, Harada H, Yang E, et al. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87(4):619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 65.Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284(5412):339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 66.Ayllon V, Martinez AC, Garcia A, et al. Protein phosphatase 1alpha is a Ras-activated Bad phosphatase that regulates interleukin-2 deprivation-induced apoptosis. EMBO J. 2000;19(10):2237–2246. doi: 10.1093/emboj/19.10.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiang CW, Harris G, Ellig C, et al. Protein phosphatase 2A activates the proapoptotic function of BAD in interleukin-3-dependent lymphoid cells by a mechanism requiring 14-3-3 dissociation. Blood. 2001;97(5):1289–1297. doi: 10.1182/blood.v97.5.1289. [DOI] [PubMed] [Google Scholar]
- 68.Mathai JP, Germain M, Marcellus RC, et al. Induction and endoplasmic reticulum location of BIK/NBK in response to apoptotic signaling by E1A and p53. Oncogene. 2002;21(16):2534–2544. doi: 10.1038/sj.onc.1205340. [DOI] [PubMed] [Google Scholar]
- 69.Verma S, Zhao LJ, Chinnadurai G. Phosphorylation of the pro-apoptotic protein BIK: mapping of phosphorylation sites and effect on apoptosis. J Biol Chem. 2001;276(7):4671–4676. doi: 10.1074/jbc.M008983200. [DOI] [PubMed] [Google Scholar]
- 70.Real PJ, Sanz C, Gutierrez O, et al. Transcriptional activation of the proapoptotic bik gene by E2F proteins in cancer cells. FEBS Lett. 2006;580(25):5905–5909. doi: 10.1016/j.febslet.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 71.Han J, Flemington C, Houghton AB, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci USA. 2001;98(20):11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4(4):321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 73.Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302(5647):1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 74.Schmelzle T, Mailleux AA, Overholtzer M, et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proc Natl Acad Sci USA. 2007;104(10):3787–3792. doi: 10.1073/pnas.0700115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imaizumi K, Tsuda M, Imai Y, et al. Molecular cloning of a novel polypeptide, DP5, induced during programmed neuronal death. J Biol Chem. 1997;272(30):18842–18848. doi: 10.1074/jbc.272.30.18842. [DOI] [PubMed] [Google Scholar]
- 76.Sanz C, Benito A, Inohara N, et al. Specific and rapid induction of the proapoptotic protein Hrk after growth factor withdrawal in hematopoietic progenitor cells. Blood. 2000;95(9):2742–2747. [PubMed] [Google Scholar]
- 77.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 78.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic BCL-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis and to preclude autoimmunity. Science. 1999;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 79.Egle A, Harris AW, Bouillet P, et al. Bim is a suppressor of Myc-induced mouse B-cell leukemia. Proc Natl Acad Sci USA. 2004;101(16):6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mestre-Escorihuela C, Rubio-Moscardo F, Richter JA, et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood. 2007;109(1):271–280. doi: 10.1182/blood-2006-06-026500. [DOI] [PubMed] [Google Scholar]
- 81.Zinkel SS, Ong CC, Ferguson DO, et al. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17(2):229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krajewska M, Zapata JM, Meinhold-Heerlein I, et al. Expression of BCL-2 family member Bid in normal and malignant tissues. Neoplasia. 2002;4(2):129–140. doi: 10.1038/sj.neo.7900222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pompeia C, Hodge DR, Plass C, et al. Microarray analysis of epigenetic silencing of gene expression in the KAS-6/1 multiple myeloma cell line. Cancer Res. 2004;64(10):3465–3473. doi: 10.1158/0008-5472.CAN-03-3970. [DOI] [PubMed] [Google Scholar]
- 84.Sturm I, Stephan C, Gillissen B, et al. Loss of the tissue-specific proapoptotic BH3-only protein Nbk/Bik is a unifying feature of renal cell carcinoma. Cell Death Differ. 2006;13(4):619–627. doi: 10.1038/sj.cdd.4401782. [DOI] [PubMed] [Google Scholar]
- 85.Hemann MT, Zilfou JT, Zhao Z, et al. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci USA. 2004;101(25):9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoque MO, Begum S, Sommer M, et al. PUMA in head and neck cancer. Cancer Lett. 2003;199(1):75–81. doi: 10.1016/s0304-3835(03)00344-6. [DOI] [PubMed] [Google Scholar]
- 87.Karst AM, Dai DL, Martinka M, et al. PUMA expression is significantly reduced in human cutaneous melanomas. Oncogene. 2005;24(6):1111–1116. doi: 10.1038/sj.onc.1208374. [DOI] [PubMed] [Google Scholar]
- 88.Chin L, Merlino G, DePinho RA. Malignant melanoma: modern black plague and genetic black box. Genes Dev. 1998;12(22):3467–3481. doi: 10.1101/gad.12.22.3467. [DOI] [PubMed] [Google Scholar]
- 89.Lee SH, Soung YH, Lee JW, et al. Mutational analysis of Noxa gene in human cancers. APMIS. 2003;111(6):599–604. doi: 10.1034/j.1600-0463.2003.1110602.x. [DOI] [PubMed] [Google Scholar]
- 90.Schmutte C, Tombline G, Rhiem K, et al. Characterization of the human Rad51 genomic locus and examination of tumors with 15q14-15 loss of heterozygosity (LOH) Cancer Res. 1999;59(18):4564–4569. [PubMed] [Google Scholar]
- 91.Wick W, Petersen I, Schmutzler RK, et al. Evidence for a novel tumor suppressor gene on chromosome 15 associated with progression to a metastatic stage in breast cancer. Oncogene. 1996;12(5):973–978. [PubMed] [Google Scholar]
- 92.Obata T, Toyota M, Satoh A, et al. Identification of HRK as a target of epigenetic inactivation in colorectal and gastric cancer. Clin Cancer Res. 2003;9(17):6410–6418. [PubMed] [Google Scholar]
- 93.Nieborowska-Skorska M, Hoser G, Kossev P, et al. Complementary functions of the antiapoptotic protein A1 and serine/threonine kinase pim-1 in the BCR/ABL-mediated leukemogenesis. Blood. 2002;99(12):4531–4539. doi: 10.1182/blood.v99.12.4531. [DOI] [PubMed] [Google Scholar]
- 94.Packham G, White EL, Eischen CM, et al. Selective regulation of BCL-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12(16):2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou P, Levy NB, Xie H, et al. MCL1 transgenic mice exhibit a high incidence of B-cell lymphoma manifested as a spectrum of histologic subtypes. Blood. 2001;97(12):3902–3909. doi: 10.1182/blood.v97.12.3902. [DOI] [PubMed] [Google Scholar]
- 96.Sinicrope FA, Rego RL, Okumura K, et al. Prognostic impact of bim, puma and noxa expression in human colon carcinomas. Clin Cancer Res. 2008;14(18):5810–5818. doi: 10.1158/1078-0432.CCR-07-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sinicrope FA, Rego RL, Foster NR, et al. Proapoptotic Bad and Bid protein expression predict survival in stages II and III colon cancers. Clin Cancer Res. 2008;14(13):4128–4133. doi: 10.1158/1078-0432.CCR-07-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JH, Soung YH, Lee JW, et al. Inactivating mutation of the pro-apoptotic gene BID in gastric cancer. J Pathol. 2004;202(4):439–445. doi: 10.1002/path.1532. [DOI] [PubMed] [Google Scholar]
- 99.Zantl N, Weirich G, Zall H, et al. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene. 2007;26(49):7038–7048. doi: 10.1038/sj.onc.1210510. [DOI] [PubMed] [Google Scholar]
- 100.Kohler B, Anguissola S, Concannon CG, et al. Bid participates in genotoxic drug-induced apoptosis of HeLa cells and is essential for death receptor ligands’ apoptotic and synergistic effects. PLoS ONE. 2008;3(7):e2844. doi: 10.1371/journal.pone.0002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008;27(9):1189–1197. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- 102.Shelton SN, Shawgo ME, Robertson JD. Cleavage of bid by executioner caspases mediates feed forward amplification of mitochondrial outer membrane permeabilization during genotoxic stress-induced apoptosis in jurkat cells. J Biol Chem. 2009 doi: 10.1074/jbc.M809392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song G, Chen GG, Chau DK, et al. Bid exhibits S phase checkpoint activation and plays a pro-apoptotic role in response to etoposide-induced DNA damage in hepatocellular carcinoma cells. Apoptosis. 2008;13(5):693–701. doi: 10.1007/s10495-008-0195-8. [DOI] [PubMed] [Google Scholar]
- 104.Cragg MS, Kuroda J, Puthalakath H, et al. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4(10):1681–1689. doi: 10.1371/journal.pmed.0040316. discussion 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gilmore AP, Valentijn AJ, Wang P, et al. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem. 2002;277(31):27643–27650. doi: 10.1074/jbc.M108863200. [DOI] [PubMed] [Google Scholar]
- 106.Abrams MT, Robertson NM, Yoon K, et al. Inhibition of glucocorticoid-induced apoptosis by targeting the major splice variants of BIM mRNA with small interfering RNA and short hairpin RNA. J Biol Chem. 2004;279(53):55809–55817. doi: 10.1074/jbc.M411767200. [DOI] [PubMed] [Google Scholar]
- 107.Erlacher M, Michalak EM, Kelly PN, et al. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005;106(13):4131–4138. doi: 10.1182/blood-2005-04-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L, Insel PA. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem. 2004;279(20):20858–20865. doi: 10.1074/jbc.M310643200. [DOI] [PubMed] [Google Scholar]
- 109.Gillespie S, Borrow J, Zhang XD, et al. Bim plays a crucial role in synergistic induction of apoptosis by the histone deacetylase inhibitor SBHA and TRAIL in melanoma cells. Apoptosis. 2006;11(12):2251–2265. doi: 10.1007/s10495-006-0283-6. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Adachi M, Kawamura R, et al. Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ. 2006;13(1):129–140. doi: 10.1038/sj.cdd.4401686. [DOI] [PubMed] [Google Scholar]
- 111.Kuroda J, Puthalakath H, Cragg MS, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA. 2006;103(40):14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pradhan S, Kim HK, Thrash CJ, et al. A critical role for the proapoptotic protein bid in ultraviolet-induced immune suppression and cutaneous apoptosis. J Immunol. 2008;181(5):3077–3088. doi: 10.4049/jimmunol.181.5.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.VanBrocklin MW, Verhaegen M, Soengas MS, et al. Mitogen-activated protein kinase inhibition induces translocation of Bmf to promote apoptosis in melanoma. Cancer Res. 2009;69(5):1985–1994. doi: 10.1158/0008-5472.CAN-08-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tan TT, Degenhardt K, Nelson DA, et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7(3):227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 115.Li R, Moudgil T, Ross HJ, et al. Apoptosis of nonsmall-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 2005;12(3):292–303. doi: 10.1038/sj.cdd.4401554. [DOI] [PubMed] [Google Scholar]
- 116.Janssen K, Pohlmann S, Janicke RU, et al. Apaf-1 and caspase-9 deficiency prevents apoptosis in a Bax-controlled pathway and promotes clonogenic survival during paclitaxel treatment. Blood. 2007;110(10):3662–3672. doi: 10.1182/blood-2007-02-073213. [DOI] [PubMed] [Google Scholar]
- 117.Kuribara R, Honda H, Matsui H, et al. Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitors. Mol Cell Biol. 2004;24(14):6172–6183. doi: 10.1128/MCB.24.14.6172-6183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of BCL-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 119.Del Gaizo Moore V, Brown JR, Certo M, et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117(1):112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Labi V, Grespi F, Baumgartner F, et al. Targeting the BCL-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ. 2008;15(6):977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kang MH, Kang YH, Szymanska B, et al. Activity of vincristine, L-ASP and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood. 2007;110(6):2057–2066. doi: 10.1182/blood-2007-03-080325. [DOI] [PubMed] [Google Scholar]
- 122.Deng J, Carlson N, Takeyama K, et al. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12(2):171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 123.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective BCL-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]