Summary
The aim of this review is to analyse the evidence related to the relationship between facial expression and pain assessment tools in the critically ill non-communicative patients. Pain assessment is a significant challenge in critically ill adults, especially those who are unable to communicate their pain level. During critical illness, many factors alter verbal communication with patients including tracheal intubation, reduced level of consciousness and administration of sedation and analgesia. The first step in providing adequate pain relief is using a systematic, consistent assessment and documentation of pain. However, no single tool is universally accepted for use in these patients. A common component of behavioural pain tools is evaluation of facial behaviours. Although use of facial expression is an important behavioural measure of pain intensity, there are inconsistencies in defining descriptors of facial behaviour. Therefore, it is important to understand facial expression in non-communicative critically ill patients experiencing pain to assist in the development of concise descriptors to enhance pain evaluation and management. This paper will provide a comprehensive review of the current state of science in the study of facial expression and its application in pain assessment tools.
Keywords: Pain assessment, Facial expression, Pain, Critically ill, Non-communicative, Facial Action Coding System
Introduction
Pain assessment is a significant challenge in critically ill adults, especially those who are unable to communicate their pain level. In 1968, Margo McCaffery defined pain as, “whatever the experiencing person says it is, existing whenever the experiencing person say it does” (McCaffery and Pasero, 1999). Unfortunately in critical care, many factors alter verbal communication with patients including endotracheal intubation, reduced level of consciousness, sedation, and administration of paralysing drugs. There is no question that critically ill patients experience acute pain manifested by the patient’s underlying disease, invasive procedures, catheters and drains, endotracheal tubes, suctioning, wound care and turning or other preexisting disease processes (Arroyo-Novoa et al., 2008; Erstad et al., 2009; Gelinas et al., 2004, 2006; Granja et al., 2005; Mularski, 2004; Puntillo et al., 2009, 1997, 2001, 2004; Stanik-Hutt, 2003). The International Pain Guidelines require that pain be assessed in “all patients” and that tools to evaluate pain should be specific to the age and disease state of the patient and to the site of pain (ANZCA, 2007; British Pain Society, 2007; Charlton, 2005; Chou, 2009; JCAHO, 2003, 2006). The first step in providing adequate pain relief for patients is systematic and consistent assessment and documentation of pain. Identification of the optimal pain scales for non-communicative patients have been the focus of several studies. To date, however, no one tool is universally accepted for use in these patients.
When patients cannot express themselves, observable indicators, both physiological and behavioural, have been labelled as ‘pain behaviours’ (Christoph, 1991; Hadjistavropoulos et al., 2002; Harrison and Cotanch, 1987; Herr et al., 2006a; Prkachin et al., 2002; Puntillo et al., 1997). Since the term ‘pain behaviour’ was first described by Fordyce (Fordyce, 1976) as a one-dimensional construct of chronic pain, there have been several attempts to develop systems for assessing pain behaviour (Gelinas et al., 2004; Keefe et al., 1984, 1990; Prkachin et al., 1977, 2002; Puntillo et al., 1997, 2004). One of the most frequently used pain behaviour incorporated in a variety of pain scales for the non-communicative patients is facial expression (Ambuel et al., 1992; De Jonghe et al., 2003; Gelinas et al., 2006; Merkel et al., 1997; Odhner et al., 2003; Payen et al., 2001; Puntillo et al., 2002; Salmore, 2002; Warden et al., 2003). Although use of facial expression is an important behavioural measure of pain intensity, precise and accurate methods for interpreting the facial expression of pain in critically ill adults has not been identified. Therefore, this review will provide an analysis of the use of facial expressions in non-communicative critically ill patients and the variation of facial expression descriptors used in pain assessment tools.
Pain in critically ill patients
Pain is a complex multidimensional concept that is difficult to define. Individual pain experiences influence cognitive, emotional, and behavioural responses. Pain is a subjective experience that is described as “the unpleasant sensory and emotional experience associated with actual or potential tissue damage” (IASP, 2010). The most reliable and valid indicator of pain is the patient’s self-report (Carr and Jacox, 1992; Jacobi et al., 2002; JCAHO, 2000; McCaffery and Pasero, 1999). In numerous studies, it has been reported that seriously ill patients experience pain and some patients can recall their dissatisfaction with pain control (Ahlers et al., 2010; Desbiens et al., 1996; Gelinas et al., 2008; Gelinas and Johnston, 2007; Hamill-Ruth and Marohn, 1999; Puntillo, 1997; Puntillo, 1990; Topolovec-Vranic et al., 2010). The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) (Desbiens et al., 1996) evaluated the pain experience of seriously ill hospitalized patients and their satisfaction with control of pain. Of the 9105 patients admitted to five teaching hospitals in the United States (US), 5176 patients provided interviews of their pain experience. The SUPPORT results indicated that seriously ill, hospitalised patients demonstrated a high prevalence of pain. Specifically, approximately 50% of patients reported pain and 14.9% reported extremely severe pain or moderately severe pain occurring at least half of the time, and nearly 15% of those patients with pain were dissatisfied with its control.
In a more recent study, Topolovec-Vranic et al. (2010) described patients’ perspective of pain management in the ICU. The study included 52 patients who had recollection of their ICU stay and agreed to complete the Patient Pain Management Questionnaire. They compared patient satisfaction with pain management before and after implementation of the Non-verbal Pain Scale (NVPS). Although the “worst” level of pain was reduced after use of the NVPS (8.5 vs 7.2 on 10 point scale, P = 0.04), the reported level of pain was still very high. Gelinas et al. (2008) found that more than 50% of 99 intubated conscious patients reported pain whilst at rest and 80% during nociceptive exposure such as turning. In critically ill adults, Ahlers et al. (2010) found that nurses tended to report patient’s pain higher 16% of the time and lower 12% of the time when compared to patient self-report.
Unconscious or sedated patients cannot communicate their level of pain using numeric pain rating scales (NRS) (0-to-10) and are therefore at risk for being inadequately medicated for pain (Ferguson et al., 1997; Hall-Lord et al., 1998). Furthermore, optimal sedation/analgesia is difficult to achieve in the critically ill and data shows that nurses adjust sedation/analgesia based on a wide range of information, including subjective assessments related to patient amnesia and comfort needs, need for prevention of self-injurious behaviour and efficiency of care (Ahlers et al., 2008; Dasta et al., 1994; Egerod, 2002; Payen et al., 2007; Weinert et al., 2001). Inaccurate pain assessments and resulting inadequate treatment of pain in critically ill adults can lead to significant physiologic consequences such as increased myocardial workload which can lead to myocardial ischaemia or impaired gas exchange which can result in respiratory failure (McArdle, 1999). Therefore, it is imperative that health care providers assess pain accurately in the non-communicative critically ill patients.
Pain assessment in the non-communicative/unconscious patient
The first step in providing adequate pain relief for patients is systematic and consistent assessment and documentation of pain (Chanques et al., 2006; Herr et al., 2006b). Identification of the optimal pain scales for non-communicative (cognitively impaired, sedated, paralysed or mechanically ventilated) patients have been the focus of several studies. To date, however, no one tool is universally accepted for use in the non-communicative patient (Herr et al., 2006b; Jacobi et al., 2002). Pain intensity may be quantified using behavioural-physiological scales in the non-communicative patients but healthcare workers’ bias may influence perceptions of the patient’s suffering (Christoph, 1991; Harrison and Cotanch, 1987; Kappesser et al., 2006; Puntillo et al., 2003). Puntillo et al. (1997) found that the pain behaviours most frequently reported by nurses in the critically ill abdominal or thoracic surgery patients (n = 105) were grimacing, frowning, or wincing (34%); vocalisation (24%); and restlessness (19%); no movement (38%).
The 2004 Thunder Project II, developed by the American Association of Critical-Care Nurses Task Force, identified behaviours displayed during procedures in 5957 critically ill adult patients at 169 sites (Puntillo et al., 2004). In this comprehensive examination of procedural pain-related behaviours, patients (n = 4278) who reported pain during a procedure (turning, suctioning, wound care, device removal) displayed five behaviours: grimacing (43%), rigidity (27%), wincing (24%), shutting of eyes (34%) and verbalisation of complaints (24%). In addition, they showed that patient’s age and ethnicity or amount of sedation did not contribute to behavioural activity during a procedure. The presumption that sedation would decrease behavioural activity was not supported.
To identify pain behaviours in critically ill intubated patients, Gelinas et al. (2004) conducted a retrospective review of 183 pain episodes that occurred in the first 72 hours after the patients were intubated. Pain behaviours such as facial expressions, agitation, movement, compliance with ventilator, etc, were identified in nurses’ notes 73% of the time, whilst physiologic indicators (BP, HR, arrhythmia) were found only 24% of the time. Specifically, facial expressions were identified 6% of the time, whereas, body movement occurred 59% of the time. These studies (Gelinas et al., 2004, 2006; Puntillo et al., 2002, 2004) led to the development of pain assessment tools in the non-communicative critically ill patients.
Adult behavioural pain assessment tools
In a recent critical review, Li et al. (2008) identified psychometric properties of six objective pain measures that were developed to assess pain in non-communicative critically ill patients. A common component of these behavioural pain tools is facial expressions. However the descriptors used to identify facial expression in these tools varies across tools. The most common tools in use today that include facial expression are summarised in Table 1.
Table 1.
Pain assessment tools used in the non-communicative patients.
| Scale | Facial Behaviour Descriptors and Scoring | Validity and Reliability Studies for Facial Behaviour Component |
|---|---|---|
| The Pain Assessment and Intervention Notation (PAIN) Algorithm | Checklist
|
Puntillo et al. (1997)
|
| Pain Behaviour Assessment Tool (PBAT) | Checklist
|
Puntillo et al. (2004)
|
| PACU Behavioural Pain Rating Scale (BPRS) |
|
|
| Non-verbal Pain Scale (NVPS) |
|
Odhner et al. (2003)
|
| Face, Legs, Activity, Cry, Consolability Observational Tool (FLACC) |
|
Merkel et al. (1997)
|
| Behavioural Pain Scale (BPS) |
|
Payen et al. (2001)
|
Young et al. (2006)a
| ||
| Critical Care Pain Observation Tool (CPOT) |
|
Gelinas et al. (2006) (French version)a
|
Gelinas and Johnston (2007) (English Version)a
|
Facial expression component not separately evaluated or reported.
The facial expression component of these tools varies in their behavioural descriptors and scoring ranges. Each tool describes wincing, frowning and grimacing differently with a different intensity of pain score. The development of facial expression component in most of these tools were derived from previously described instruments (Mateo and Krenzischek, 1992; McGrath et al., 1985; Payen et al., 2001; Puntillo et al., 1997), chart review (Gelinas et al., 2004), focus groups interviews (Gelinas et al., 2005), or nurses’ intuitive knowledge of pain (Mateo and Krenzischek, 1992).
The Pain Assessment and Intervention Notation Algorithm (PAIN) (Puntillo et al., 1997) checklist of behavioural and physiological indicators of pain was derived from research literature and content validity was established by a panel of experts in critical care practice and pain. The Pain Behaviour Assessment Tool (PBAT) (Puntillo et al., 2004) was then adapted from the PAIN tool and Children’s Hospital Eastern Ontario Pain Scale (CHEOPS) (McGrath et al., 1985). Even though, the PBAT’s was extensively researched for reliability and validity of the facial expressions component of the tool, many of the research used was based on paediatric studies. Both the PAIN and PBAT algorithm were developed not as a scoring instrument but an observation tool to identify specific pain-related behaviours in patients who could respond to questions and were able to use a numeric rating scale of pain intensity.
The Post-Anaesthesia Care Unit Behavioural Pain Rating Scale (PACU BPRS) (Mateo and Krenzischek, 1992) and Non-verbal Pain Scale (NVPS) (Odhner et al., 2003) were adopted from previously established tools (Chambers and Price, 1967; Merkel et al., 1997). These tools were pilot tested in a specialised population of the Post-Anaesthesia Care Unit and Burn Trauma Unit, respectively. The PACU BPRS has four of the original eight categories for assessing three types of pain (acute, chronic and progressive pain) that were developed by Chambers and Price (1967). The four dimensions (restlessness, tense muscles, frowning or grimacing and patient sounds) range from none to severe (0–3) with total pain score ranging 0–12.
The NVPS consists of five dimensions (face, activity, guarding, physiological I and II). Each dimension ranges from 0 to 2 with total pain score ranging 0–10. The face and activity dimension of NVPS was patterned after the face, legs, activity, cry, consolability (FLACC) (Merkel et al., 1997) pain assessment tool. The FLACC was developed by clinicians to provide a simple, consistent method to identify, document, and evaluate pain in the paediatric population. One of the major limitations of the FLACC tool is the applicability of cry and consolability which are not appropriate for the critically ill, intubated adults. Thus, the NVPS used only the face and activity dimensions of FLACC tool.
The Behavioural Pain Scale (BPS) (Payen et al., 2001) was developed to assess pain in the mechanically ventilated patients. The BPS consists of three dimensions (facial expressions, upper limbs movement and compliance with ventilation) ranging from 1 to 4 points with total pain score ranging 3–12. The scoring of each facial expression from 1 (no response) to 4 (full response) was based on assumptions that these behaviours reflect increases in pain intensity in the critically ill as well. The facial expressions were derived from Prkachin’s (1992a,b) study of specific facial muscle actions related to pain states. Prkachin used the Facial Action Coding System (Ekman and Friesen, 1978) to measure facial actions during painful and pain-free periods on healthy adult volunteers. He divided facial expressions of pain into four groups by graded pain intensity: brow lowering, tightening and closing of the eye lids and nose wrinkling/upper lip raising. Payen et al. (2001) modified these facial expressions (Table 1) in the BPS to make it easy for the paired evaluators to rate.
A more recently developed tool, the Critical Care Pain Observation Tool (CPOT) (Gelinas et al., 2006) includes components of facial expressions that were derived from previous established tools, such as the PACU BPRS (Mateo and Krenzischek, 1992), PAIN Tool (Puntillo et al., 1997), and Behavioural Pain Scale (BPS) (Payen et al., 2001). The CPOT consist of 4 components with 0–2 rating for each behaviour: facial expression, body movements, muscle tension and compliance with the ventilator for intubated patients or vocalization for extubated patients.
In summary, facial expressions have not been rigorously tested in any of the above tools. If facial expressions are an essential component of pain evaluation tools, then scoring should be based on objective data related to facial expression during pain in the critically ill. Tools in use today include a wide range of facial expression descriptors such as no facial response, relaxed, smile to most extreme wince, frown, and grimacing. Experts (Li et al., 2008) suggest that more research is needed to identify facial indicators that reflect pain-related affective distress, to identify changes in facial pain behaviour that may occur with ageing to determine the effects of sedatives and the presence of an endotracheal tube and/or its securing device have on facial expressions of pain. Systematic identification of facial expression during pain is therefore crucial.
Study of facial expressions
The face reveals a wealth of information about human behaviour and emotions. The most frequently used pain behaviour in pain evaluation scales for patients who cannot orally communicate is facial expression (Ambuel et al., 1992; De Jonghe et al., 2003; Gelinas et al., 2006; Merkel et al., 1997; Odhner et al., 2003; Payen et al., 2001; Puntillo et al., 2002; Salmore, 2002; Warden et al., 2003). Facial expression has been studied for centuries, dating back to Charles Darwin’s “The Expression of Emotions in Man and Animals” (Darwin, 1998) reporting observations and detailed explanations of why particular facial expressions occur with particular emotions (Black, 2002; Darwin, 1998). Ekman et al., experts in facial expressions studies, conducted extensive cross-cultural studies in determining if facial expressions are universal or specific to each culture (Ekman et al., 1969, 1987; Ekman, 1972, 1993, 1999; Ekman and Friesen, 1971). They demonstrated that observers’ judgments of anger, disgust, fear, sadness, happiness and surprise made by preliterate people as isolated as New Guineans (Ekman and Friesen, 1971) were no different than judgments made by college students in eight literate cultures around the world (Ekman et al., 1987). They concluded that regardless of age, gender, and race/ethnicity, facial expressions are evidence of universal expressions across cultures with variation due to the expression itself, and in what the expression signifies to the person showing the expression and to others (Ekman, 1999). Their studies led to the development of the Facial Action Coding System (FACS) (Ekman and Friesen, 1978), which identifies distinct facial muscle movements during an emotional response. These facial muscle movements are typically identified through the use of slow action video and stop-frame feedback.
The basic elements of FACS are 44 action units (AUs). Each AU represents the movement of a single facial muscle or a group of muscles, which move as a unit. The 44 AUs can be reliably identified by trained FACS coders and can also be reliably graded on a 5-point scale for intensity (degree of muscle excursion). Once the pain expressions are identified, data on number of expressions per minute over the course of each condition can be derived (Ekman and Friesen, 1978). The FACS has been shown to be highly reliable in many studies and shows a distinct pattern of facial actions that are characteristic of pain (Ambuel et al., 1992; Craig et al., 1991, 1994, 2001; Craig and Patrick, 1985; De Jonghe et al., 2003; Prkachin, 1992a,b; Terai et al., 1998). Facial Action Coding is a complex manual process but advances in automated face analysis using computer vision are being developed (Cohn et al., 1999). Cohn et al. (1999) reported high concurrent validity with automated face analysis by feature point tracking and manual FACS in the brow, eye and mouth regions.
Facial expression in pain
Facial expression specific to pain has been studied (Craig et al., 1991, 2001; Craig and Patrick, 1985; Patrick et al., 1986; Poole and Craig, 1992; Prkachin, 1992a,b; Prkachin and Craig, 1985; Prkachin and Mercer, 1989) using the Facial Action Coding System (FACS) developed by Ekman and Friesen (1978). Several facial actions that correlated with pain that have been identified include lowered brows, raised cheeks, tightened eyelids, a raised upper lip or opened mouth, and closed eyes (Fig. 1) (Craig et al., 1991, 2001; Prkachin, 1992a,b). In the general population, Craig and Patrick identified facial expressions of acute pain-related facial activity (brow lowering, narrowing of the eye aperture from below, raising the upper lip, and blinking) (Craig and Patrick, 1985; Patrick et al., 1986). They used the FACS to identify facial activity associated with exposure to one noxious stimulus in healthy adults and identified six action units (AU) categories that occurred more frequently during exposure to the noxious stimulus than during a baseline experience.
Figure 1.
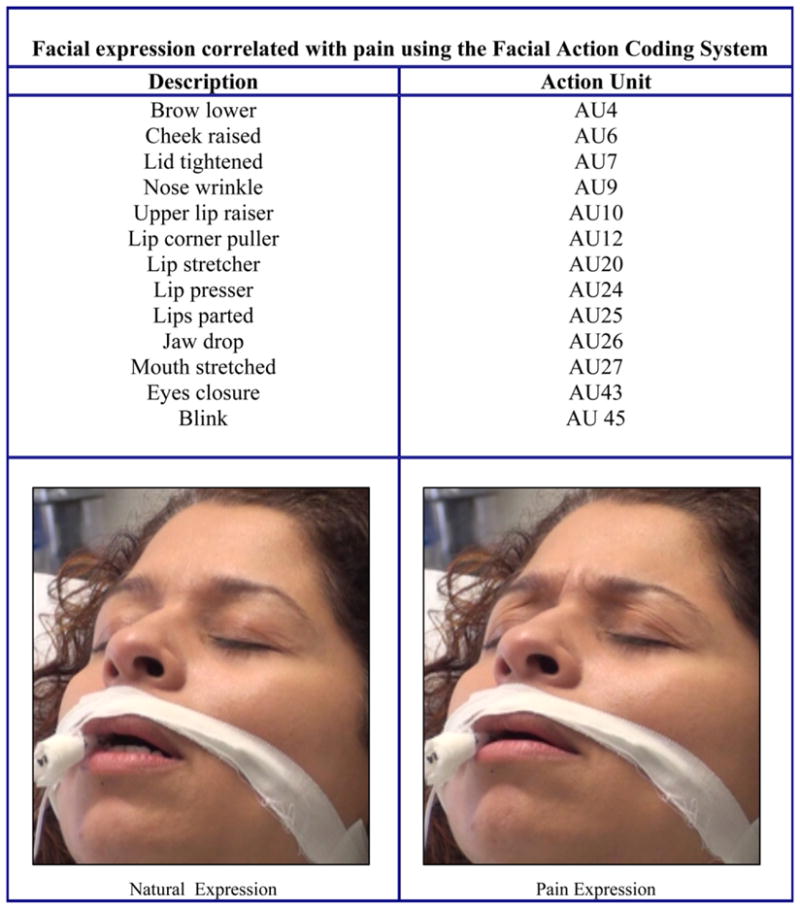
Facial expression correlated with pain using the Facial Action Coding System.
Prkachin (1992b) focused on pain behaviour of healthy adults (n = 41), specifically facial expression during three different types of pain stimulus (electric shock, cold, pressure, and muscle ischemia). He identified four actions during pain, increasing in intensity or duration across all modalities using the FACS: brow lowering (AU4), tightening and closing of the eye lids (AU6/AU7), and nose wrinkling/upper lip raising (AU9/AU10). Hadjistavropoulos et al. (2000) examined the validity of non-verbal measures in detecting pain amongst seniors who were experiencing movement-related exacerbations of musculoskeletal pain and documented the utility of behavioural coding of pain-related body/limb movements (e.g., bracing and guarding). The results demonstrate that FACS not only discriminates between pain and absence of pain but can also provide information about the variability of the pain experience (Hadjistavropoulos et al., 2000, 2002).
In another study to evaluate gender differences in facial expressiveness to pain, Kunz et al. (2006) used FACS, focusing on 4 AUs: brow lowering (AU 4), tightening of the orbital muscles surrounding the eye (AUs 6/7), nose wrinkling/upper lip raising (AUs 9/10) and eye closure (AU 43). They found that in young and pain-free individuals (male n = 20, female n = 20) that men and women were equally facially expressive during tonic heat stimulation at non-painful and at painful intensities. These observations are similar to previous findings of lack of gender differences in the facial expressiveness of pain (Craig et al., 1991; Prkachin, 1992a,b).
FACS provides an objective assessment of facial reactions that are most reflexive and automatic non-verbal indices of pain. Even though, facial expressions have been identified in infants, children, adults and the elderly using FACS, there is little empiric evidence of its utility in the critically ill patients. More research is needed to identify facial expressions during pain in the critically ill patients.
Conclusion
Pain assessment is a significant challenge in critically ill adults, especially those who are unable to communicate their pain level. Unfortunately in critical care, many factors alter verbal communication with patients including tracheal intubation, reduced level of consciousness, sedation and administration of paralysing drugs. Therefore, accurate assessment of non-verbal pain behaviours such as facial expression, especially in the critically ill, is important. Facial expressions provide a critical behavioural measure for the study of emotion, cognitive processes and social interaction (Ekman, 1999). Understanding facial expressions may assist in the development of strategies to enhance pain assessment tools. Tools currently available to assess pain in the non-communicative critically ill patient are not universally accepted and provide a wide range of descriptors of facial expressions. Interestingly, most of the facial descriptors identified in the pain assessment tools are of the upper face (eyes and brow) and using the Facial Action Coding System to study facial expressions in this region may be feasible since other facial areas (mouth, nose) are often distorted by the presence of an endotracheal or nasogastric tubes. Specifically, Facial Action Coding data may provide empirical evidence to use facial expressions accurately in assessment tools that are appropriate, practical, reliable and valid across patient populations.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahlers SJ, van GL, van d V, et al. Comparison of different pain scoring systems in critically ill patients in a general ICU. Crit Care. 2008;12:R15. doi: 10.1186/cc6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers SJ, van d V, van DM, Tibboel D, Knibbe CA. The use of the Behavioral Pain Scale to assess pain in conscious sedated patients. Anesth Analg. 2010;110:127–33. doi: 10.1213/ANE.0b013e3181c3119e. [DOI] [PubMed] [Google Scholar]
- Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Pediatr Psychol. 1992;17:95–109. doi: 10.1093/jpepsy/17.1.95. [DOI] [PubMed] [Google Scholar]
- ANZCA. Professional Standards 41: Guidelines on Acute Pain Management. Australian and New Zealand College of Anaesthetists. 2007 Ref Type: Electronic Citation (7-12-2010) [Google Scholar]
- Arroyo-Novoa CM, Figueroa-Ramos MI, Puntillo KA, et al. Pain related to tracheal suctioning in awake acutely and critically ill adults: a descriptive study. Intensive Crit Care Nurs. 2008;24:20–7. doi: 10.1016/j.iccn.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Black J. Darwin in the world of emotions. J R Soc Med. 2002;95:311–3. doi: 10.1258/jrsm.95.6.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Pain Society. Royal College of Physicians. British Geriatrics Society and British Pain Society. The assessment of pain in older people: national guidelines. In: Turner-Stokes Lynne, Higgins Bernard., editors. Concise guidance to good practice series. Royal College of Physicians; 2007. 7-12-2010. [Google Scholar]
- Carr DB, Jacox A. Clinical practice guideline. Rockville, MD: Agency for Health Care Policy and Research; 1992. Acute Pain Management Guideline Panel. Acute pain management: operative or medical procedures and trauma. AHCPR publication no. 92-0032. [Google Scholar]
- Chambers W, Price G. Influence of nurse upon effects of analgesics administered. Nurs Res. 1967;16:228–33. [Google Scholar]
- Chanques G, Jaber S, Barbotte E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34:1691–9. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- Charlton JE. Core Curriculum for Professional Education in Pain. 3. International Association for the Study of Pain/IASP Press; 2005. [Google Scholar]
- Chou R. Clinical Guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119:469–77. [PubMed] [Google Scholar]
- Christoph SB. Pain assessment. The problem of pain in the critically ill patient. Crit Care Nurs Clin North Am. 1991;3:11–6. [PubMed] [Google Scholar]
- Cohn JF, Zlochower AJ, Lien J, Kanade T. Automated face analysis by feature point tracking has high concurrent validity with manual FACS coding. Psychophysiology. 1999;36:35–43. doi: 10.1017/s0048577299971184. [DOI] [PubMed] [Google Scholar]
- Craig KD, Patrick CJ. Facial expression during induced pain. J Pers Soc Psychol. 1985;48:1080–91. doi: 10.1037/0022-3514.48.4.1089. [DOI] [PubMed] [Google Scholar]
- Craig KD, Hyde SA, Patrick CJ. Genuine, suppressed and faked facial behavior during exacerbation of chronic low back pain. Pain. 1991;46:161–71. doi: 10.1016/0304-3959(91)90071-5. [DOI] [PubMed] [Google Scholar]
- Craig KD, Hadjistavropoulos HD, Grunau RV, Whitfield MF. A comparison of two measures of facial activity during pain in the newborn child. J Pediatr Psychol. 1994;19:305–18. doi: 10.1093/jpepsy/19.3.305. [DOI] [PubMed] [Google Scholar]
- Craig KD, Prkachin KM, Grunau RVE. Handbook of pain assessment. New York: Guilford; 2001. The facial expression of pain. [Google Scholar]
- Darwin C. The expression of the emotions in man and animals/Charles Darwin; with an introduction, afterword, and commentaries by Paul Ekman. 3. New York: Oxford University Press; 1998. [Google Scholar]
- Dasta JF, Fuhrman TM, McCandles C. Patterns of prescribing and administering drugs for agitation and pain in patients in a surgical intensive care unit. Crit Care Med. 1994;22:974–80. doi: 10.1097/00003246-199406000-00016. [DOI] [PubMed] [Google Scholar]
- De Jonghe B, Cook D, Griffith L, et al. Adaptation to the Intensive Care Environment (ATICE): development and validation of a new sedation assessment instrument. Crit Care Med. 2003;31:2344–54. doi: 10.1097/01.CCM.0000084850.16444.94. [DOI] [PubMed] [Google Scholar]
- Desbiens NA, Wu AW, Broste SK, et al. Pain and satisfaction with pain control in seriously ill hospitalized adults: findings from the SUPPORT research investigations. For the SUPPORT investigators. Study to understand prognoses and preferences for outcomes and risks of treatment. Crit Care Med. 1996;24:1953–61. doi: 10.1097/00003246-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Egerod I. Uncertain terms of sedation in ICU. How nurses and physicians manage and describe sedation for mechanically ventilated patients. J Clin Nurs. 2002;11:831–40. doi: 10.1046/j.1365-2702.2002.00725.x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Sorger B, Friesen WV. Pancultural elements in facial displays of emotions. Science. 1969;164:86–8. doi: 10.1126/science.164.3875.86. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Constants across cultures in the face and emotion. J Pers Soc Psychol. 1971;17:124–9. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Ekman P, Cole J, editors. Universals and cultural differences in facial expressions of emotion. Lincoln: University of Nebraska Press; 1972. [Google Scholar]
- Ekman P, Friesen WV. Investigator’s guide to the Facial Action Coding System. Palo Alto, CA: Consulting Psychologists Press; 1978. [Google Scholar]
- Ekman P, Friesen WV, O’Sullivan M, et al. Universals and cultural differences in the judgments of facial expressions of emotion. J Pers Soc Psychol. 1987;53:712–7. doi: 10.1037//0022-3514.53.4.712. [DOI] [PubMed] [Google Scholar]
- Ekman P. Facial expression and emotion. Am Psychol. 1993;48:384–92. doi: 10.1037//0003-066x.48.4.384. [DOI] [PubMed] [Google Scholar]
- Ekman P. Handbook of cognition and emotion. Chichester, England, New York: Wiley; 1999. [Google Scholar]
- Erstad BL, Puntillo K, Gilbert HC, et al. Pain management principles in the critically ill. Chest. 2009;135:1075–86. doi: 10.1378/chest.08-2264. [DOI] [PubMed] [Google Scholar]
- Ferguson J, Gilroy D, Puntillo K. Dimensions of pain and analgesic administration associated with coronary artery bypass grafting in an Australian intensive care unit. J Adv Nurs. 1997;26:1065–72. doi: 10.1111/j.1365-2648.1997.tb00796.x. [DOI] [PubMed] [Google Scholar]
- Fordyce WE. Behavioral methods for chronic pain and illness. St. Louis, MO: Mosby; 1976. [Google Scholar]
- Gelinas C, Fortier M, Viens C, Fillion L, Puntillo K. Pain assessment and management in critically ill intubated patients: a retrospective study. Am J Crit Care. 2004;13:126–35. [PubMed] [Google Scholar]
- Gelinas C, Viens C, Fortier M, Fillion L. Pain indicators in critical care. Perspect Infirm. 2005;2:12–20. 22. [PubMed] [Google Scholar]
- Gelinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15:420–7. [PubMed] [Google Scholar]
- Gelinas C, Johnston C. Pain assessment in the critically ill ventilated adult: validation of the critical-care pain observation tool and physiologic indicators. Clin J Pain. 2007;23:497–505. doi: 10.1097/AJP.0b013e31806a23fb. [DOI] [PubMed] [Google Scholar]
- Gelinas C, Harel F, Fillion L, Puntillo KA, Johnston CC. Sensitivity and specificity of the critical-care pain observation tool for the detection of pain in intubated adults after cardiac surgery. J Pain Symptom Manage. 2008 doi: 10.1016/j.jpainsymman.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Granja C, Lopes A, Moreira S, Dias C, Costa-Pereira A, Carneiro A. Patients’ recollections of experiences in the intensive care unit may affect their quality of life. Crit Care. 2005;9:R96–109. doi: 10.1186/cc3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjistavropoulos T, LaChapelle DL, Hadjistavropoulos HD, Green S, Asmundson GJ. Using facial expressions to assess musculoskeletal pain in older persons. Eur J Pain. 2002;6:179–87. doi: 10.1053/eujp.2001.0327. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos T, LaChapelle DL, MacLeod FK, Snider B, Craig KD. Measuring movement-exacerbated pain in cognitively impaired frail elders. Clin J Pain. 2000;16:54–63. doi: 10.1097/00002508-200003000-00009. [DOI] [PubMed] [Google Scholar]
- Hall-Lord ML, Larsson G, Steen B. Pain and distress among elderly intensive care unit patients: comparison of patients’ experiences and nurses’ assessments. Heart Lung. 1998;27:123–32. doi: 10.1016/s0147-9563(98)90020-6. [DOI] [PubMed] [Google Scholar]
- Hamill-Ruth RJ, Marohn ML. Evaluation of pain in the critically ill patient. Crit Care Clin. 1999;15:35–vi. doi: 10.1016/s0749-0704(05)70038-5. [DOI] [PubMed] [Google Scholar]
- Harrison M, Cotanch PH. Pain: advances and issues in critical care. Nurs Clin North Am. 1987;22:691–7. [PubMed] [Google Scholar]
- Herr K, Bjoro K, Decker S. Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review. J Pain Symptom Manage. 2006a;31:170–92. doi: 10.1016/j.jpainsymman.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Herr K, Coyne PJ, Key T, et al. Pain assessment in the nonverbal patient: position statement with clinical practice recommendations. Pain Manage Nurs. 2006b;7:44–52. doi: 10.1016/j.pmn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- IASP. International association for the study of pain. Seattle: IASP; 2010. International Association for the Study of Pain (IASP): pain terminology. [Google Scholar]
- Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- JCAHO. Joint Commission on Accreditation of Healthcare Organizations: comprehensive accreditation manual for hospital: the official handbook. Oak Brook, IL: Joint Commission on Accreditation of Healthcare Organization; 2000. [Google Scholar]
- JCAHO. Pain assessment and management: a team solution. Joint Comm: Source. 2003;1:8–9. [Google Scholar]
- JCAHO. Accreditation Essentials: Top Compliance Issues for 2006 – Standards PI.3.20 and HR.4. 10. Joint Comm: Source. 2007;5(12):4–5. [Google Scholar]
- Kappesser J, Williams AC, Prkachin KM. Testing two accounts of pain underestimation. Pain. 2006;124:109–16. doi: 10.1016/j.pain.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Bradley LA, Crisson JE. Behavioral assessment of low back pain: identification of pain behavior subgroups. Pain. 1990;40:153–60. doi: 10.1016/0304-3959(90)90066-M. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Wilkins RH, Cook WA. Direct observation of pain behavior in low back pain patients during physical examination. Pain. 1984;20:59–68. doi: 10.1016/0304-3959(84)90811-X. [DOI] [PubMed] [Google Scholar]
- Kunz M, Gruber A, Lautenbacher S. Sex differences in facial encoding of pain. J Pain. 2006;7:915–28. doi: 10.1016/j.jpain.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Li D, Puntillo K, Miaskowski C. A review of objective pain measures for use with critical care adult patients unable to self-report. J Pain. 2008;9:2–10. doi: 10.1016/j.jpain.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Mateo OM, Krenzischek DA. A pilot study to assess the relationship between behavioral manifestations and self-report of pain in postanesthesia care unit patients. J Post Anesth Nurs. 1992;7:15–21. [PubMed] [Google Scholar]
- McArdle P. Intravenous analgesia. Crit Care Clin. 1999;14:89–104. doi: 10.1016/s0749-0704(05)70041-5. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- McCaffery M, Pasero C. Pain clinical manual. 2. St. Louis: Mosby; 1999. [Google Scholar]
- McGrath PJ, Johnson G, et al. CHEOPS: a behavioral scale for rating postoperative pain in children. Adv Pain Res Ther. 1985;9:395–402. [Google Scholar]
- Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- Mularski RA. Pain management in the intensive care unit. Crit Care Clin. 2004;20:381–401. viii. doi: 10.1016/j.ccc.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Odhner M, Wegman D, Freeland N, Steinmetz A, Ingersoll GL. Assessing pain control in nonverbal critically ill adults. Dimens Crit Care Nurs. 2003;22:260–7. doi: 10.1097/00003465-200311000-00010. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Craig KD, Prkachin KM. Observer judgments of acute pain: facial action determinants. J Pers Soc Psychol. 1986;50:1291–8. doi: 10.1037//0022-3514.50.6.1291. [DOI] [PubMed] [Google Scholar]
- Payen JF, Bru O, Bosson JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29:2258–63. doi: 10.1097/00003246-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Payen JF, Chanques G, Mantz J, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007;106:687–95. doi: 10.1097/01.anes.0000264747.09017.da. [DOI] [PubMed] [Google Scholar]
- Persson K, Ostman M. The Swedish version of the PACU-Behavioural Pain Rating Scale: a reliable method of assessing postoperative pain? Scand J Caring Sci. 2004;18:304–9. doi: 10.1111/j.1471-6712.2004.00286.x. [DOI] [PubMed] [Google Scholar]
- Poole GD, Craig KD. Judgments of genuine, suppressed, and faked facial expressions of pain. J Pers Soc Psychol. 1992;63:797–805. doi: 10.1037//0022-3514.63.5.797. [DOI] [PubMed] [Google Scholar]
- Prkachin KM. Dissociating spontaneous and deliberate expressions of pain: signal detection analyses. Pain. 1992a;51:57–65. doi: 10.1016/0304-3959(92)90009-Z. [DOI] [PubMed] [Google Scholar]
- Prkachin KM. The consistency of facial expressions of pain: a comparison across modalities. Pain. 1992b;51:297–306. doi: 10.1016/0304-3959(92)90213-U. [DOI] [PubMed] [Google Scholar]
- Prkachin KM, Craig KD, Papageorgis D, Reith G. Nonverbal communication deficits and response to performance feedback in depression. JAbnorm Psychol. 1977;86:224–34. doi: 10.1037//0021-843x.86.3.224. [DOI] [PubMed] [Google Scholar]
- Prkachin KM, Craig KD. Influencing non-verbal expressions of pain: signal detection analyses. Pain. 1985;21:399–409. doi: 10.1016/0304-3959(85)90168-X. [DOI] [PubMed] [Google Scholar]
- Prkachin KM, Mercer SR. Pain expression in patients with shoulder pathology: validity, properties and relationship to sickness impact. Pain. 1989;39:257–65. doi: 10.1016/0304-3959(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Prkachin KM, Schultz I, Berkowitz J, Hughes E, Hunt D. Assessing pain behaviour of low-back pain patients in real time: concurrent validity and examiner sensitivity. Behav Res Ther. 2002;40:595–607. doi: 10.1016/s0005-7967(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Puntillo KA. Pain experiences of intensive care unit patients. Heart Lung. 1990;19:526–33. [PubMed] [Google Scholar]
- Puntillo KA. Stitch, stitch. creating an effective pain management program for critically ill patients. Am J Crit Care. 1997;6:259–60. [PubMed] [Google Scholar]
- Puntillo KA, Miaskowski C, Kehrle K, Stannard D, Gleeson S, Nye P. Relationship between behavioral and physiological indicators of pain, critical care patients’ self-reports of pain, and opioid administration. Crit Care Med. 1997;25:1159–66. doi: 10.1097/00003246-199707000-00017. [DOI] [PubMed] [Google Scholar]
- Puntillo KA, White C, Morris AB, et al. Patients’ perceptions and responses to procedural pain: results from Thunder Project II. Am J Crit Care. 2001;10:238–51. [PubMed] [Google Scholar]
- Puntillo KA, Stannard D, Miaskowski C, Kehrle K, Gleeson S. Use of a pain assessment and intervention notation (PAIN) tool in critical care nursing practice: nurses’ evaluations. Heart Lung. 2002;31:303–14. doi: 10.1067/mhl.2002.125652. [DOI] [PubMed] [Google Scholar]
- Puntillo KA, Neighbour M, O’Neil N, Nixon R. Accuracy of emergency nurses in assessment of patients’ pain. Pain Manage Nurs. 2003;4:171–5. doi: 10.1016/s1524-9042(03)00033-x. [DOI] [PubMed] [Google Scholar]
- Puntillo KA, Morris AB, Thompson CL, Stanik-Hutt J, White CA, Wild LR. Pain behaviors observed during six common procedures: results from Thunder Project II. Crit Care Med. 2004;32:421–7. doi: 10.1097/01.CCM.0000108875.35298.D2. [DOI] [PubMed] [Google Scholar]
- Puntillo K, Pasero C, Li D, et al. Evaluation of pain in ICU patients. Chest. 2009;135:1069–74. doi: 10.1378/chest.08-2369. [DOI] [PubMed] [Google Scholar]
- Salmore R. Development of a new pain scale: Colorado Behavioral Numerical Pain Scale for sedated adult patients undergoing gastrointestinal procedures. Gastroenterol Nurs. 2002;25:257–62. doi: 10.1097/00001610-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Stanik-Hutt JA. Pain management in the critically ill. Crit Care Nurse. 2003;23:99–103. [PubMed] [Google Scholar]
- Terai T, Yukioka H, Asada A. Pain evaluation in the intensive care unit: observer-reported faces scale compared with self-reported visual analog scale. Reg Anesth Pain Med. 1998;23:147–51. doi: 10.1097/00115550-199823020-00006. [DOI] [PubMed] [Google Scholar]
- Topolovec-Vranic J, Canzian S, Innis J, Pollmann-Mudryj MA, McFarlan AW, Baker AJ. Patient satisfaction and documentation of pain assessments and management after implementing the adult nonverbal pain scale. Am J Crit Care. 2010;19:345–54. doi: 10.4037/ajcc2010247. [DOI] [PubMed] [Google Scholar]
- Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4:9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- Weinert CR, Chlan L, Gross C. Sedating critically ill patients: factors affecting nurses’ delivery of sedative therapy. Am J Crit Care. 2001;10:156–65. [PubMed] [Google Scholar]
- Young J, Siffleet J, Nikoletti S, Shaw T. Use of a Behavioural Pain Scale to assess pain in ventilated, unconscious and/or sedated patients. Intensive Crit Care Nurs. 2006;22:32–9. doi: 10.1016/j.iccn.2005.04.004. [DOI] [PubMed] [Google Scholar]


