Abstract
Herein is described a method of accessing indole/triazole and benzothiophene/triazole analogues that selectively promote or inhibit biofilm formation by Gram-positive and Gram-negative bacteria. Structure/function studies revealed that the addition of a bromine atom at the 2-position of the indole/triazole scaffold altered activity against both Gram-negative and Gram-positive bacteria and could transform a biofilm inhibitor into a biofilm inducer. Isosteric replacement of the indole core by a benzothiophene significantly impaired anti-biofilm activity. A competition assay exposing Escherichia coli to the most potent biofilm inducer and an inhibitor of E. coli biofilm formation was performed. The inducer exhibited the ability to mute the effect of the anti-biofilm compound for this targeted bacterial population.
Bacterial biofilms are structured communities of bacteria attached to each other and/or to a surface and encased in a self-produced matrix of extracellular polymeric substance.1 It is known that biofilms offer protective advantages to the embedded population as they provide shelter, and nutrient and metabolic diversity.2 Bacteria within a biofilm have been shown to be upwards of 1000-fold more resistant to antibiotics as compared to their planktonic counterparts, rendering their eradication difficult.3 It has been estimated that bacterial biofilms cause 80% of microbial infections in the human body, including lung infections of cystic fibrosis patients, gingivitis and infections of indwelling medical devices.4, 5 Although strategies to develop small molecules to modulate biofilm development typically focus on pathogenic bacteria, biofilm formation by a number of commensal bacteria is beneficial for their host.6 Extensive studies of the human microbiome have established the symbiotic relationship between human health and changes in the microbiota. For example, bacterial species of the commensal flora present in the gastrointestinal tract such as Escherichia coli provide crucial health benefits, strengthening the mucosal barrier, promoting polysaccharide digestion and protecting the host against colonization by pathogenic bacteria.7 Antimicrobial agents often eradicate commensal bacteria as well as pathogenic bacterial populations, which can lead to opportunistic infections and a temporarily weakened immune system.8 In this regard, it is potentially important to generate selective therapies that disrupt pathogenic biofilms while leaving biofilm communities of commensal bacteria unaffected or actually promoting their development.
In this regard, we have been exploring indole derivatives as potential agents for the selective modulation of biofilm development. Indole is a ubiquitous small molecule signal that controls a variety of phenotypes in both Gram-positive and Gram-negative bacteria. Over 85 species of bacteria have been documented to produce indole, while both indole-producing and non-producing species will modify their behavior in response to exogenous indole.9, 10, 11 Indole has been shown to play a role in acid tolerance, antibiotic resistance and biofilm formation. Indole decreases the biofilm formation of E. coli in a non-toxic manner by repressing motility, chemotaxis and cell adherence.12 In contrast with E. coli, indole increases biofilm formation in Vibrio cholerae and the non-indole producing Pseudomonas aeruginosa.13 Many bacteria produce oxygenases that oxidize indole to generate indole derivatives that may also affect biofilm formation.14
Our approach to harnessing the potential of synthetic indole derivatives to control bacterial behavior is underpinned by studying the activity of marine natural product derivatives containing a deep-seated indole core. To this end, the indole derived flustramine family of natural compounds has been previously investigated in our lab and we have demonstrated that simple flustramine derivatives have the ability to influence the formation of bacterial biofilms.15,16 We established a pyrroloindoline-triazole-amide scaffold inspired by flustramine C (Figure 1) and identified several compounds in this class that inhibit E. coli, Acinetobacter baumanniii, Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) biofilm formation.15
Figure 1.
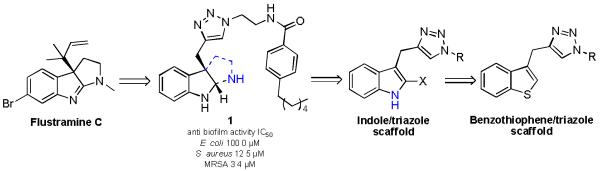
Generated scaffold incorporating flustramine C inspiration.
The lead compound of the pyrroloindoline-triazole library, compound 1, exhibits non-toxic anti-biofilm activity against E. coli and MRSA. Based upon this initial study, a number of structure/function questions arose. Specifically, we were interested in probing: 1) the necessity of the tricyclic pyrroloindoline scaffold; 2) the impact of halogenation on activity; 3) the effect of an isostere replacement of the indole core, and 4) whether small molecules based upon this scaffold could be employed as selective biofilm agents (Figure 1).
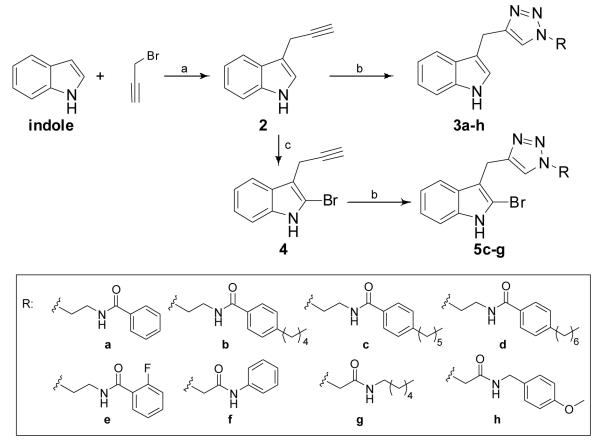
The synthetic approaches to probe these structure/function questions are outlined in Schemes 1 and 2. Indole/triazole analogues could be accessed rapidly in only two steps. The first step involved the installation of a terminal alkyne at the 3-position of indole via a zinc-mediated Barbier reaction,19 which entailed treatment of indole with propargyl bromide and zinc dust in THF at ambient temperature to deliver 3-propargylindole 2 in 30% yield. Other reported methods to synthesize 2 were explored; 20, 21 however, each method failed to deliver 2 or delivered it in inferior yields to the Barbier reaction. Formation of the triazole was achieved through Huisgen Cu (I) - catalyzed alkyne/azide cycloadditions (click chemistry) 22 under standard conditions to generate a small pilot library of indole/triazole analogues 3a-h in 23-82% yield. Since most biofilm modulators are amphipathic,15 various hydrophobic amides were introduced at the triazole tail. All amide azides were prepared using a two-step procedure from 2-bromoethylamine hydrobromide or chloroacetyl chloride via nucleophilic displacement and acylation.23
Scheme 1.
Synthesis of indole-triazole-amide analogues 3a-h and 5c-g. a) Zn dust, THF, 0°C to rt; b) azide, CuSO4, sodium ascorbate, H2O:tBuOH:DCM (2:2:1); c) NBS, HOAc:HCO2H (3:1), rt.
Scheme 2.
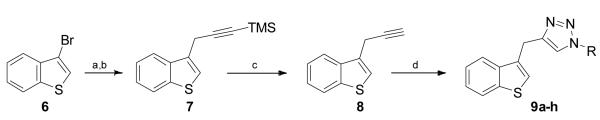
Synthesis of benzothiophene/triazole analogues 9a-h. a) Mg, I2 pellets, THF, reflux; b) 3-Bromo-1-(trimethylsilyl)-1-propyne; c)AgNO3 aq, CF3CO2H, acetone, dark, rt; d) azide, CuSO4, sodium ascorbate, H2O:tBuOH:DCM (2:2:1).
Since many antibiofilm agents also present a bromine atom in their structure,16, 24 we elected to study the impact of bromination upon activity in the context of our simplified flustramine derivatives by introducing a bromine atom at the 2-position of the indole ring. To this end, the treatment of 3-propargylindole 2 with NBS in a mixture of AcOH:HCO2H (3:1) afforded selective bromination and delivered intermediate 4 in 35% yield. Minor amounts of isomer brominated in the 4-position contributed to the observed moderate yield. Finally, the alkyne/azide cycloaddition was performed to afford the final products 5c-g in 18-61% yield (Scheme 1).
The synthetic route to the benzothiophene-triazole-amide conjugates allowed the generation of target compounds in bromobenzothiophene 6 was converted into the corresponding Grignard reagent by reaction with Mg, and I2, then subsequently reacted with 3-(trimethylsilyl)propargyl bromide to afford the TMS-protected alkyne 7 in 80% yield. Upon treatment with aqueous silver nitrate and trifluoroacetic acid, intermediate 8 was obtained in quantitative yield. Finally, the alkyne was elaborated through cycloaddition chemistry to generate the benzothiophene/triazole conjugates 9a-h in 11-73% yield (Scheme 2). To assess the effect of the benzothiophene core on the biological activity in comparison to an indole core, the same hydrophobic amides were employed as previously introduced in the indole/triazole scaffold.
Compounds were screened initially at 150 μM to investigate their effects on biofilm development using E. coli, A. baumannii, S. aureus and MRSA as our model bacteria. Biofilm development was monitored under static conditions using a crystal violet reporter assay.25 For compounds showing the ability to either inhibit biofilm formation, dose response studies were subsequently performed to quantify activity. Here we define the compound concentration to inhibit 50% of biofilm growth relative to an untreated control as the IC50 value. Analogues inducing biofilm formation less than 50% at 50 μM were qualified as “inducers” and analogues inducing biofilm formation more than 50% at 50 μM were qualified as “strong inducers”. For the strong inducers of E. coli biofilms, we generated dose-response curves and defined the concentration required to promote 50% of biofilm formation as the PC50 value. PC25 and PC75 values were also determined and define the concentration of a compound that promotes biofilm growth by 25% and 75% respectively. Growth curve and colony counts analyses were performed for each active compound to determine their toxicity towards planktonic bacteria at the IC50, PC25, PC50 and PC75 values. This analysis reveals if a compound inhibits or promotes biofilm formation via a biocidal or non-microbicidal mechanism. Molecules that modulate bioflm formation through non-microbicidal mechanisms would potentially be highly desirable, as they would have a reduced likelihood of exerting evolutionary pressure on the bacteria to adapt and become resistant.
The results of these studies are summarized in Table 1. Each compound affects biofilm formation in a unique way against the four strains of bacteria. Although most analogues exhibited moderate inhibitory activity, some of them displayed remarkable selectivity towards individual bacterial populations.
Table 1.
Biological activity of the indole-triazole analogues and the benzothiophene-triazole analogues.
| compound | E. coli | A. baumannii | S. aureus | MRSA |
|---|---|---|---|---|
| 3a | <5% | <5% | 23% at 150 μM | <5% |
| 3b | strong inducer | <5% | 44.4 ± 3.57μM | <5% |
| 3c | strong inducer | 25% at 150 μM | 173.6 ± 3.16 μM | 312.6 ± 1.89 μM |
| 3d | strong inducer | 30% at 150 μM | 174.8 ± 2.12 μM | <5% |
| 3e | <5% | 15% at 150 μM | inducer | 35% at 150 μM |
| 3f | inducer | <5% | <5% | <5% |
| 3g | inducer | 25% at 150 μM | <5% | <5% |
| 3h | <5% | 20% at 150 μM | <5% | <5% |
| 5c | inducer | 22% at 150 μM | 40% at 150 μM | 25% at 150 μM |
| 5d | inducer | inducer | inducer | strong inducer |
| 5e | inducer | inducer | inducer | 20% at 150 μM |
| 5f | 12% at 150 | 325.5 ± 4.63 μM | strong inducer | 151.4 ± 0.91 μM |
| 5g | <5 % | 17% at 150 μM | < 5% | inducer |
| 9a | 14% at 150 | <5% | <5% | <5% |
| 9b | strong inducer | <5% | <5% | <5% |
| 9c | <5% | <5% | <5% | <5% |
| 9d | strong inducer | inducer | 83.0 ± 4.45 μM | <5% |
| 9e | <5% | <5% | strong inducer | <5% |
| 9f | inducer | inducer | 214.9 ± 1.87 μM | 20% at 150 μM |
| 9g | <5% | 20% at 150 μM | <5% | <5% |
| 9h | <5% | 20% at 150 μM | <5% | <5% |
In general, compounds 3b-d strongly promote biofilm formation in E. coli while selectively inhibiting the formation of S. aureus biofilms with IC50 values of 44.4 μM, 173.6 μM and 174.8 μM respectively. The four analogues 3a-d structurally differ from each other in the para-alkylphenyl chain of the triazole amide substituent. Compounds 3b-d that present a long para-subsituted alkyl chain strongly promote biofilm formation of E. coli while compound 3a, which does not present an alkyl appendage at the 4-position, moderately inhibits biofilm formation of this strain. Analogue 5f promotes biofilm formation of S. aureus and inhibits biofilm formation of E. coli, A. baumannii and MRSA while analogue 5d induces biofilm growth for all four bacterial strains. Interestingly, each analogue except 5f demonstrated an opposite effect to that of indole by inducing biofilm formation of E. coli. For each compound presenting an IC50 value, growth curve analysis and colony counts were performed to evaluate their toxicity. None of these analogues affected planktonic bacterial growth. In order to determinate if promoters were inducing biofilm formation via a toxic mechanism, growth curve and colony counts analyses was completed at the PC25, PC50 and PC75 values. Inducers were shown to promote biofilm formation in a non-toxic manner.
When comparing brominated and non-brominated analogues, it was established that incorporating a bromine atom at the 2-position of the indole core attenuated anti-biofilm activity. Compound 3d, which exhibited an IC50 value of 174.8 μM against S. aureus, becomes a strong inducer of this bacterial strain when brominated at the 2-position (5d). This result is also observed for Gram-negative bacteria, as moderate A. baumannii anti-biofilm analogues 3d-e become inducers for this bacterial strain when brominated (5d-e).
Interestingly, benzothiophene-triazole-amide 9d was shown to be selectively active against the formation of S. aureus biofilms in a non-toxic manner while promoting biofilm formation of the three other bacterial strains. However, this activity appeared to be unique as most of the benzothiophene derivatives displayed marginal ability to modulate biofilm development. Therefore, it appears that there is no bioisosteric relationship between indole and benzothiophene since the analogues of these two libraries do not have similar biological activity. Growth curve analysis was performed for each inducer at a concentration of 50 μM. It was determined that promotion of biofilm was occurring in a non-toxic manner.
Since it is desirable to tune the activity of small molecules that can selectively inhibit biofilm formation in specific bacterial strains and promote biofilm formation in a targeted bacterial population we decided to further investigate the inducing effect upon biofilm formation by E. coli, a bacterial species present in the gut flora. Of all the synthesized analogues, compound 3c exhibits the most potent anti-biofilm activity towards A. baumannii, S. aureus and MRSA while strongly promoting the biofilm formation of E. coli. A competitive assay was performed by mixing inducer 3c with inhibitor 1, the lead anti-biofilm compound of the previously investigated pyrroloindoline-triazole-amide library. When compound 3c is mixed at its PC25 PC50 and PC75 concentrations in the presence of compound 1 at its IC50 value, we observe a dose dependant reversal of biofilm inhibition. The mixture of 3c at concentrations PC25, PC50 or PC75 with 1 at its IC50 lowers the activity of the inhibitor by 7.7%, 47% and 87% respectively (Figure 2). Growth curve analysis were performed for these combined concentrations and revealed that none of these mixtures affected the planktonic bacterial growth. By counteracting the anti-biofilm effect of a potent inhibitor, compound 3c demonstrates its efficacy as an E. coli biofilm promoter and could be a valuable adjuvant to counteract the unwanted effect antibiotics that do not differentiate between biofilms derived from commensal or pathogenic bacteria.
Figure 2.
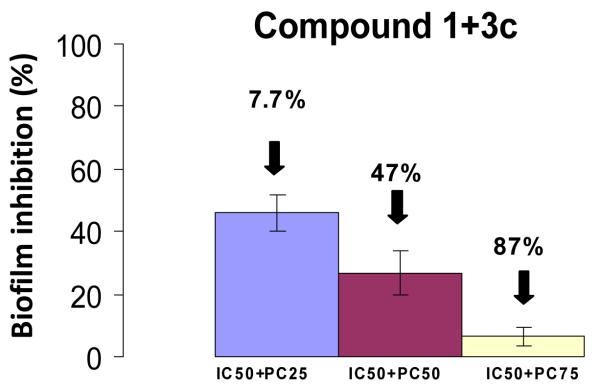
Effect of the addition of inducer 3c to inhibitor 1 at different concentrations.
Conclusions
In summary, we have developed a synthetic approach to rapidly access indole/triazole and benzothiophene/triazole conjugates. Using this approach, a set of 21 analogues was screened against E. coli, A. baumannii, S. aureus and MRSA. We noted that indole/triazole conjugates presenting a long alkyl chain are potent inducers towards E. coli biofilms via a non-toxic mechanism with the most selective inhibitor, 3c, promoting biofilm formation by 50% at 82.6 μM. This compound presented strong activity towards the promotion of E. coli biofilms while inhibiting the biofilm formation of the pathogenic bacteria A. baumannii, S. aureus and MRSA without affecting planktonic bacterial growth. Compound 3c was also able to counteract the biofilm inhibition activity of 1 and reduced the effect of the anti-biofilm effect of 1 by 87% for an IC50+PC75 mixture. We are currently exploring whether the activity of 3c can be augmented further through analogue design and the activity of these indole-derived agents in vivo.
Supplementary Material
Table 2.
Efficiency of compounds 3b-d, 9b and 9d in promoting the formation of E. coli biofilms.
| compound | PC25 | PC50 | PC75 |
|---|---|---|---|
| 3b | 48.3 ± 1.17 μM | 74.8 ± 0.08 μM | 111.6 ± 0.17 μM |
| 3c | 47.8 ± 1.20 μM | 82.6 ± 2.80 μM | 160.7 ± 6.13 μM |
| 3d | 50.5 ± 1.82 μM | 80.7 ± 2.76 μM | 119.7 ± 2.19 μM |
| 9b | 26.7± 0.06 μM | 99.8 ± 5.03 μM | 151.9 ± 4.85 μM |
| 9d | 22.2 ± 0.62 μM | 56.3 ± 5.57 μM | 93.8 ± 1.36 μM |
Acknowledgements
The authors would like to thank the National Institutes of Health (GM055769) for their support.
Footnotes
Electronic Supplementary Information (ESI) available. See DOI: 10.1039/b000000x/
Notes and references
- 1.Rodney MD, Costerton JW. Clin. Microbiol. Rev. 2002;15:167. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards JJ, Melander C. ChemBioChem. 2009;10:2287. doi: 10.1002/cbic.200900317. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen TB, Givkov M. Int. J. Med. Microbiol. 2006;296:149. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Davies D. Nat. Rev. Drug Discov. 2003;2:114. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 5.Okimoto N, Hayashi T, Ishiga M, Nanba F, Kishimoto M, Yagi J, Kurihara J, Asaoka N, Tamada S. J. Infect. Chemoter. 2010;16:216. doi: 10.1007/s10156-010-0034-z. [DOI] [PubMed] [Google Scholar]
- 6.Sansonetti PJ. Curr. Opin. Gastroenterol. 2008;24:435. doi: 10.1097/MOG.0b013e32830007f7. [DOI] [PubMed] [Google Scholar]
- 7.Fang Y, Polk B. Curr. Opin. Gastroenterol. 2004;6:565. doi: 10.1097/00001574-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Blaser M. Nature. 2011;476:393. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Lee J. FEMS Microbiol. Rev. 2010;34:426. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Molla M, Cantor C, Collins J. Nature. 2010;467:82. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vega N, Allison K, Khalil A, Collins J. Nat. Chem. Biol. 2012;8:431. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal T, Englert D, Lee J, Hegde M, Wood T, Jayaraman A. Infect. Immun. 2007;75:4597. doi: 10.1128/IAI.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller R, Beyhan S, Saini S, Yildiz F, Bartlett D. J. Bacteriol. 2009;191:3504. doi: 10.1128/JB.01240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Bansal T, Jayaraman A, Bentley W, Wood T. Appl. Environ. Microbiol. 2007;73:4100. doi: 10.1128/AEM.00360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunders C, Cavanagh J, Melander C. Org. Biomol. Chem. 2011;9:5476. doi: 10.1039/c1ob05605k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunders C, Minvielle M, Worthington R, Ortiz M, Cavanagh J, Melander C. J. Am. Chem. Soc. 2011;133:20160. doi: 10.1021/ja209836z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govan J, Deretic V. Microbiol. Rev. 1996;60:539. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klevens M, Morrison M, Nadle J, Petit S, Gershman K, Ray S, Harrison L, Lynfield R, Dumyati G, Townes J, Craig A, Zell E, Fosheim G, McDougal L, Carey R, Fridkin S. J. Amer. Med. Assoc. 2007;15:1763. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 19.Yu RT, Friedman RK, Rovis T. JACS. 2009;131:13250. doi: 10.1021/ja906641d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prajapati D, Gohain U, Gogoi B. Tetrahedron Lett. 2006;47:3535. [Google Scholar]
- 21.Zhu X, Ganesan A. J. Org. Chem. 2002;67:2705. doi: 10.1021/jo010996b. [DOI] [PubMed] [Google Scholar]
- 22.Kolb HC, Finn MG, Sharpless KB. Angew. Chem. 2001;113:2056. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Reed C, Huigens RW, Rogers SA, Melander C. Bioorg. Med. Chem. Lett. 2010;20:6310. doi: 10.1016/j.bmcl.2010.08.075. [DOI] [PubMed] [Google Scholar]
- 24.Worthington RJ, Richards JJ, Melander C. Org. Biomol. Chem. 2012;10:7457. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Toole GA, Kolter R. Mol. Microbiol. 1998;28:449. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.