Abstract
Fluorescent and colorimetric reporter genes are valuable tools for drug screening models, since microscopy is labor intensive and subject to observer variation. In this work, we propose a fluorimetric method for drug screening using red fluorescent parasites. Fluorescent Leishmania amazonensis were developed after transfection with integration plasmids containing either red (RFP) or green fluorescent protein (GFP) genes. After transfection, wild-type (LaWT) and transfected (LaGFP and LaRFP) parasites were subjected to flow cytometry, macrophage infection, and tests of susceptibility to current antileishmanial agents and propranolol derivatives previously shown to be active against Trypanosoma cruzi. Flow cytometry analysis discriminated LaWT from LaRFP and LaGFP parasites, without affecting cell size or granulosity. With microscopy, transfection with antibiotic resistant genes was not shown to affect macrophage infectivity and susceptibility to amphotericin B and propranolol derivatives. Retention of fluorescence remained in the intracellular amastigotes in both LaGFP and LaRFP transfectants. However, detection of intracellular RFP parasites was only achieved in the fluorimeter. Murine BALB/c macrophages were infected with LaRFP parasites, exposed to standard (meglumine antimoniate, amphotericin B, Miltefosine, and allopurinol) and tested molecules. Although it was possible to determine IC50 values for 4 propranolol derivatives (1, 2b, 3, and 4b), all compounds were considered inactive. This study is the first to develop a fluorimetric drug screening test for L. amazonensis RFP. The fluorimetric test was comparable to microscopy with the advantage of being faster and not requiring manual counting.
Keywords: Leishmania amazonensis, Green fluorescent protein, Red fluorescent protein, Drug screening, Chemotherapy
1. Introduction
Leishmaniasis is an infectious disease caused by the protozoa Leishmania and presenting a spectrum of clinical manifestations. It is widely distributed in 88 countries in Africa, Southern Europe, Central and South America, the Middle East, and Asia. Leishmania (Leishmania) amazonensis, a dermotropic species, is the etiologic agent of cutaneous leishmaniasis (CL) and diffuse cutaneous leishmaniasis (DCL) (Bittencourt et al., 1989). The latter is a chronic, progressive, polyparasitic variant exhibiting disseminated nonulcerative skin lesions (Desjeux, 2004).
Despite control methods, leishmaniasis cases have been increasingly reported in many urban areas (Gontijo and Melo, 2004). Factors involved in this expansion may include the lack of a vaccine, the adaptation of vectors and reservoirs to human environments, ineffective drug treatments, and the therapeutic failures (Croft et al., 2006a). Leishmania amazonensis is often associated with drug resistance, and conventional treatments may involve immunotherapy (Convit et al., 1989). In the Americas, for over 6 decades, parenteral administration of pentavalent antimonials (Sb-V), sodium stibogluconate (Pentostam®, GlaxoSmithKline, UK), and meglumine antimoniate (Glucantime®, Sanofi, Brazil) has been used for treating leishmaniasis. In India, where resistance to antimonials is common, other available chemotherapeutic agents include amphotericin B and pentamidine (Croft et al., 2006b; Mishra et al., 2007). However, the high toxicity and the lack of safe oral drugs underline the need for new antileishmanial treatments.
For many years, the classic microscopic method (Berman and Lee, 1984) has been used for screening compounds for efficacy against intracellular amastigote forms of Leishmania. Although it is labor intensive and cannot be automated, direct counting assay enables determination of the percent of infected cells and the number of amastigotes per cell. Half inhibitory concentration (IC50) values can be ascertained either by monitoring reduction in the mean percent of infected macrophages or by the mean reduction in the number of amastigotes per macrophage. This method requires well-trained personnel and is subject to individual observer variation (Sereno et al., 2007). More importantly, intra- and interspecies variations in susceptibility may occur (Croft et al., 2006b). For example, Leishmania donovani (Old World) is more susceptible to Miltefosine® (Sigma, St. Louis, MO, USA) than are New World species (Leishmania infantum, L. amazonensis, L. (Viannia) braziliensis, and L. (V.) guyanensis) (De Morais-Teixeira et al., 2011). The development of a semi-automated method using fluorescent parasites could more rapidly detect inter- and perhaps intraspecies variations.
Many efforts have been dedicated to the development of drug screening procedures to increase performance, efficacy, and reliability compared to the labor-intensive microscopy. These may include use of green fluorescent protein (GFP), bioluminescent (firefly luciferase), and colorimetric (chloramphenicol acetyl transferase, β-galactosidase and alkaline phosphatase) reporters (Dube et al., 2009; Gupta, 2011; Lang et al., 2005; Sereno et al., 2007). Emerging technologies using bioluminescent imaging have been adapted for the study of host-Leishmania chemotherapy. Consistent with this idea, 3 recent reports proposed the use of high-throughput screening (HTS) methods using Leishmania (De Muylder et al., 2011; Sharlow et al., 2010; Siqueira-Neto et al., 2010). Fluorescent reporter genes are promising tools for chemotherapeutic screening methods. They encode proteins in which expression is quantifiable and distinguishable from endogenous cell background. Other advantages include low cost, sensitivity, rapidity, no radioactivity, potential for bioimaging, higher efficiency, low toxicity, no substrate required, no need for permeabilization and fixation of cells, no additional steps required, and easy detection in a fluorimeter or by flow cytometry (Dube et al., 2009; Mehta et al., 2010; Varela et al., 2009).
In Leishmania, GFP parasites have been developed for Leishmania major (Bolhassani et al., 2011; Kram et al., 2008; Mißlitz et al., 2000; Plock et al., 2001), Leishmania donovani, (Kaur et al., 2010; Singh and Dube, 2004; Singh et al., 2009) Leishmania infantum, (Bolhassani et al., 2011; Kamau et al., 2001) Leishmania mexicana (Mißlitz et al., 2000), and L. amazonensis (Boeck et al., 2006; Chan et al., 2003; Costa et al., 2011; Demicheli et al., 2004; Mehta et al., 2008; Mehta et al., 2010; Okuno et al., 2003). Although red fluorescent protein (RFP) parasites of L. major have been developed (Ng et al., 2008), no chemotherapeutic method is available using those transfectants. However, chemotherapeutics employing RFP parasites have been produced for Trypanosoma brucei (Gibson et al., 2008; Peacock et al., 2007), Trypanosoma cruzi (DaRocha et al., 2004; Guevara et al., 2005; Pires et al., 2008), and Plasmodium berghei (Frevert et al., 2005). Although these parasites could be readily used for many purposes in cell biology, their biological fitness has not been properly assessed.
In this work, we generated transgenic L. amazonensis stably expressing GFP and/or RFP and compared their biological fitness to wild-type (WT) parasites. Subsequently, a host cell–based screening test using murine macrophages infected with LaRFP was conducted. Although both transfectants produced significant fluorescent signals in vitro, only LaRFP intracellular parasites were reliably detected by the fluorimeter. The intracellular detection of fluorescently viable Leishmania amastigote forms provides a more accurate approach for drug screening tests. This test was reproducible, in comparison to microscopy.
2. Material and methods
2.1. Mammalian cells and parasite strain
Animals were kept in the Animal Facility of the Centro de Pesquisas René Rachou/FIOCRUZ in strict accordance with the Guide for the Care and Use of Experimental Animals (Olfert et al., 1993). The procedures were approved by the Internal Ethics Committee in Animal Experimentation (CEUA) of Fundação Oswaldo Cruz (FIO-CRUZ), Brazil (Protocol L-042/08). Mice were euthanized with CO2 in an induction chamber prior to macrophage removal. The cell lineage Hep G2 A16 was derived from a human hepatocellular carcinoma cell line HepG2 (ATCC HB-8065) and obtained from the America Type Culture Collection line (ATCC) (Darlington et al., 1987). The World Health Organization (WHO) reference strain Leishmania (Leishmania) amazonensis (IFLA/BR/1967/PH8) was used in this work. The strain was typed as previously described (Rocha et al., 2010). To ensure infectivity, parasites were continuously passaged in BALB/c mice (Mus musculus) prior to isolation of amastigotes from foot-pad lesions. Those forms were differentiated from promastigotes and grown at 25 °C in M199 medium (Sigma, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal calf serum (Cultilab, Campinas, Brazil), 40 mmol/L Hepes (Amresco, Solon, OH, USA), 0.1 mmol/L adenine (Sigma, St. Louis, MO, USA), 0.0005% hemin (Sigma), 0.0002% biotin (Sigma), 50 U/mL penicillin (Invitrogen, Carlsbad, CA, USA), and 50 mg/mL streptomycin (Invitrogen) (Soares et al., 2002). Parasites were seeded in triplicate (1 × 105 cells/mL), and growth curves of LaWT, LaGFP, and LaRFP parasites determined daily using a Beckman Coulter Counter (Beckman Coulter, Brea, CA, USA) until cells reached a stationary phase (>4.0 × 107 cells/mL).
2.2. Transfection
Leishmania amazonensis parasites were transfected with the constructs pIR1Phleo-GFP+(a)(sense) (B-5793) and pIR1SAT-LUC(a) DsRed2(b) (B5947) (Ng et al., 2008) containing GFP and RFP genes, respectively. The integrated constructs were linearized using SwaI prior to electroporation with 5–10 µg of DNA. Promastigotes in logarithmic phase (2 × 108 cells) were centrifuged at 2100 × g for 10 min. The pellet was resuspended in Cytomix buffer (120 mmol/L KCl; 0.15 mmol/L CaCl2; 10 mmol/L K2HPO4; 25 mmol/L Hepes; 2 mmol/L EDTA; and 5 mmol/L MgCl2; pH 7.6) and washed twice in the same buffer. The parasites were electroporated in 500 µL of cytomix in 4-mm gap cuvettes (BTX, Holliwton, MA, USA) at 1500 V, 25 µF (2 pulses between 10 s) (Robinson and Beverley, 2003) (with modifications, we used 10 µg/mL instead of 100 µg/mL of nourseothricin). Parasites were kept in M199 without antibiotics for 24 h. For selection, parasites were centrifuged and plated in semi-solid Noble aga (1%) in M199 medium containing phleomycin (10 µg/mL) (Sigma) and nourseothricin (SAT) (10 µg/mL) (Sigma) for GFP and RFP constructs, respectively. Colonies were selected and seeded in liquid M199 medium supplemented with fetal bovine serum (FBS) (Cultilab) (Ha et al., 1996). Live fluorescent promastigotes and intracellular amastigotes were placed on a slide for examination using a fluorescent microscope (Zeiss, Thornwood, NY, USA). LaGFP parasites were detected with an excitation wavelength of 490–494 nm and emission of LP 515 nm. LaRFP parasites were exposed to an excitation wavelength of BP546/12 nm and emission of LP590 nm. The acquired images were stored for AxioCam MRC electronically.
2.3. Flow cytometry analyses
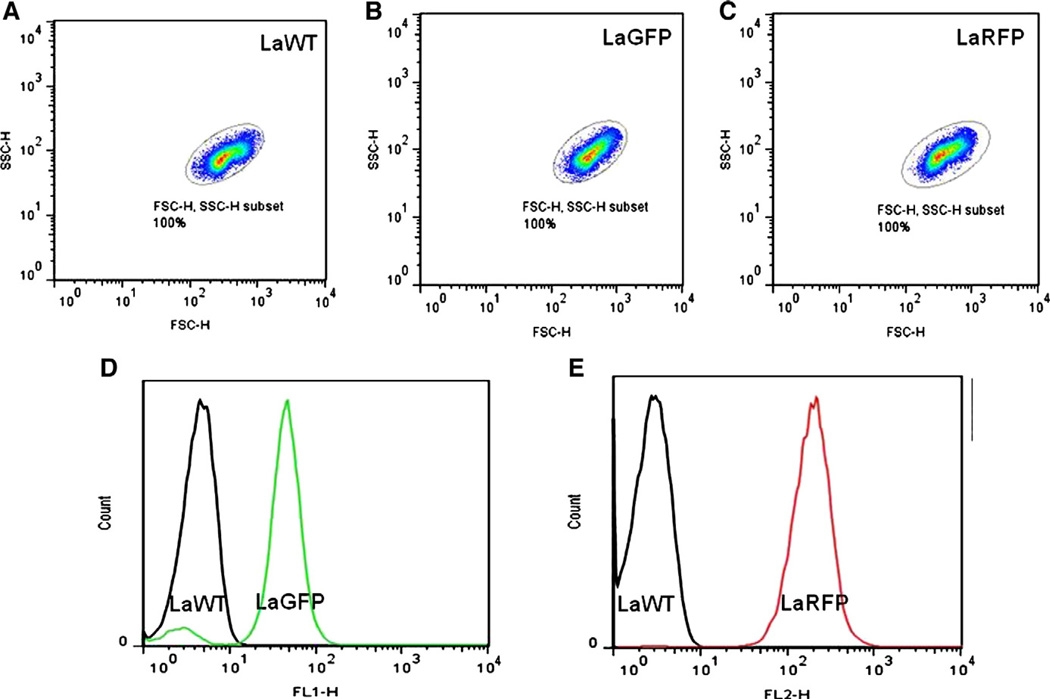
To evaluate whether transfection affected the morphology of the cells, LaWT, LaGFP, and LaRFP L. amazonensis (1 × 106/mL) were compared by flow cytometry. Measures were performed on a BD FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA, USA). Fluorescence, size (forward scatter—FSC-H), and granulosity (side scatter—SSC-H) of LaWT, LaGFP, and LaRFP parasites were visualized using FL1 and FL2 modes. Twenty thousand events were obtained for each preparation (Ha et al., 1996). FlowJo software 7.6.4 (Tree Star, Ashland, OR, USA) was used for data analysis.
2.4. Optical microscopy analyses
To evaluate whether transfection affected macrophage infectivity and susceptibility to tested molecules, WT, GFP, and RFP clones were compared microscopically (Berman and Lee, 1984). BALB/c mice were injected intraperitoneally with 2 mL of 3% sodium thioglycollate medium (Merck, Darmstad, Germany). Thioglycollate-elicited peritoneal macrophages were removed by peritoneal washing with RPMI 1640 (Sigma, St. Louis, MO, USA) and enriched by plastic adherence for 18 h. Cells (2 × 105 cells/well) were cultured in RPMI 1640 (Sigma),2 mmol/L glutamine (Sigma),50 U/mL of penicillin (Invitrogen), and 50 µg/mL streptomycin (Invitrogen) on 13-mm sterile glass coverslips (500 µL/well) (37 °C, 5% CO2). Promastigote forms (LaWT, LaGFP, and LaRFP) in stationary phase (2 × 106/well) were used for macrophage infection (ratio 1:10 macrophage/parasite). Plates were incubated for 4–5 h at 37 °C in 5% CO2. Noninternalized free-floating parasites were removed prior to drug exposure (Pinheiro et al., 2011). Macrophage infection (%) among LaWT, LaGFP, and LaRFP parasites was compared after 24 and 72 h.
In the microscopy test, propranolol derivatives and allopurinol were 2-fold serially diluted with RPMI 1640 medium supplemented with 10% FBS at final concentrations of 50 → 3.12 µg/mL. Amphotericin B (a reference antileishmanial drug) was used at 2-fold decreasing concentrations of 1 → 0.062 µg/mL. Meglumine antimoniate (Glucantime®) was used at 2-fold decreasing concentrations of 2000 → 3.2 µg/mL. Hexadecylphosphocholine (Miltefosine) was used at 2-fold decreasing concentrations of 10 → 0.016 µg/mL. Infected macrophages were exposed to the compounds daily for 3 consecutive days. Fresh compounds were added each day. After this period, coverslips were collected, stained with Panoptic (Laborclin, Pinhais, Brazil), and subsequently mounted with Entellan® (Merck, Darmstadt, Germany) on glass slides. Negative controls included only infected macrophages and medium. Incubations were tested in duplicate in 2 independent experiments. After the determination of IC50 values, data were transformed in micromoles per liter (μmol/L).
2.5. Spectrofluorometric analyses
Macrophages were obtained as described above and plated in dark 96-well plates with clear well bottoms (Corning, New York, NY, USA) in RPMI 1640 medium supplemented with 5% FBS (37 °C,5%CO2). In order to evaluate fluorescence, LaGFP promastigotes were incubated with macrophages (2 × 105/well) for 5 h (MOI 10:1) (Fig. 6A). On the other hand, LaRFP parasites were incubated with different macrophage concentrations (10 → 1 × 105 cells/well) using also MOI (10:1) in order to determine the best viable cell concentration prior to drug tests.
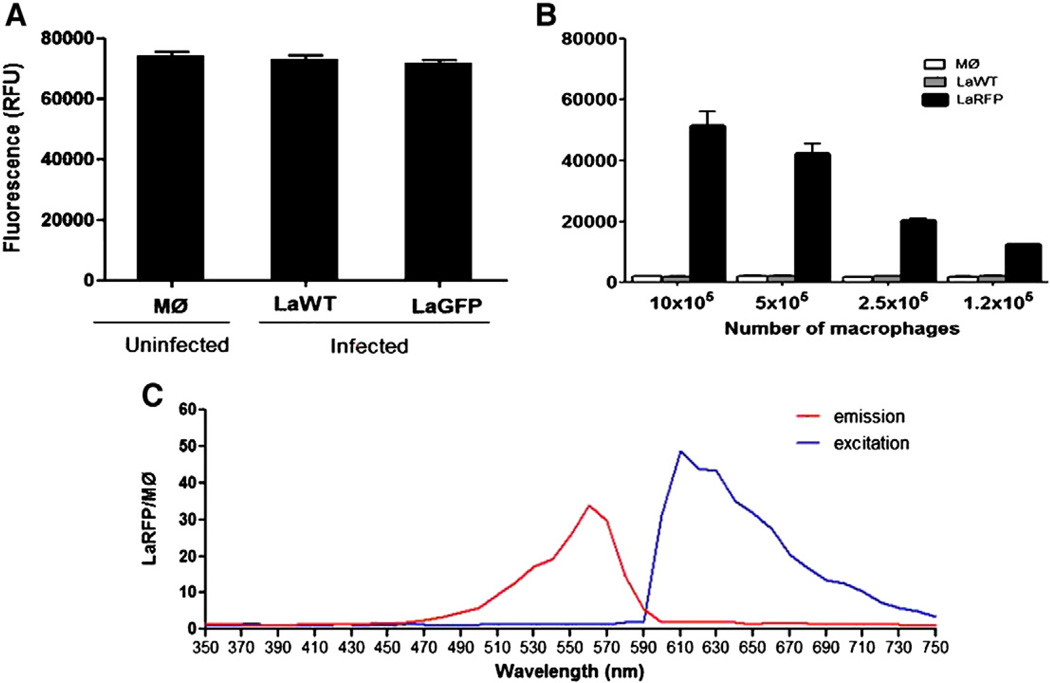
Fig. 6.
Evaluation of infected macrophage (MØ) with LaWT and fluorescent parasites (LaGFP and LaRFP) in the fluorimeter. (A) Macrophages (concentration2 ×105/well) containing LaWT and LaGFP parasites (excitation/emission of 472/512 nm). (B) Macrophages containing LaWT and LaRFP parasites (excitation/emission of 560/620 nm). (C) Wavelength spectra to determine the best excitation/emission (red/blue lines) values for LaRFP intracellular parasites in relation to uninfected macrophages (RFP/MØ).
The concentration of 2 × 105 macrophages, which was the same as for the microscopy method, enabled parasite detection (~20,000 relative fluorescence units [RFUs]) and was chosen for the experiments (Fig. 6B). The optimal emission/excitation wavelengths were determined after a screening spectrum ranging from 350 to 750 nm (Fig. 6C). Initially, our standardization experiments using the fluorimeter used the same drug concentrations as the microscopy test (50 → 3.12 µg/mL). However, it was necessary to increase the concentrations (to 500 µg/mL) in order to obtain IC50 curves. Propranolol derivatives and amphotericin B were 5-fold diluted (500 → 0.8 and 5 → 0.008 µg/mL, respectively). Meglumine antimoniate and allopurinol were also 5-fold diluted (2000 → 16 µg/mL), and Miltefosine was 5-fold diluted (50 → 0.016 µg/mL). Infected macrophages were exposed daily to the compounds for 3 consecutive days (freshly added each day), and analysis was performed after 72 h. Each dose of propranolol derivatives and control drugs was tested in triplicate in at least 2 experiments. The negative control included uninfected macrophages, and positive controls consisted of LaRFP- and LaGFP-infected macrophages. After the determination of IC50 values, the data were transformed in micromoles per liter. The optimal wavelengths for LaRFP were 560 nm (excitation) and 620 nm (emission) (Fig. 6C), and for LaGFP were 472 and 512 nm, respectively (not shown). RFUs were determined in the fluorimeter (SpectraMaxM5, Molecular Devices, Sunnyvale, CA, USA).
Inhibition of parasite growth was calculated from the percent of infected macrophages (100 cells) and RFU values using microscopy and the fluorescence method, respectively. The values were used for plotting curves and calculation of IC50 values using MicroCal Origin software (MicroCal, Northampton, MA, USA) (Pinheiro et al., 2011). IC50 values above 10 μmol/L were considered inactive using L. donovani as reference (Nwaka and Hudson, 2006).
2.6. Cytotoxicity assay and selective index
Cytotoxicity was determined using the MTT method (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (Sigma) in the hepatoma cell lineage Hep G2 A16. Cells were kept in RPMI medium supplemented with 10% FBS, and confluent monolayers were trypsinized, washed in RPMI, and transferred to tissue culture plate 96 well (4 ×104 cells/well). Active compounds and amphotericin B in the same conditions described for microscopy were incubated with the cells (37 °C, 5% CO2, 24 h). Colorimetric reaction was developed following incubation with MTT (37 °C, 4 h) and addition of acidified isopropanol (Denizot and Lang, 1986). The reaction was read spectrophotometrically (Spectramax M5) with a 570-nm filter and a background of 670 nm. Incubations were tested in triplicate in 2 independent experiments. The minimum dose that killed 50% of the cells (MLD50) was determined (Madureira et al., 2002), and the values were plotted to generate dose–response curves as described above. The selective indexes (SI) were calculated using the MLD50/IC50 ratios (Ioset et al., 2009; Nwaka and Hudson, 2006).
2.7. Statistical analyses
Statistical analyses were conducted using GraphPad Prism 5.0 software (Graph Prism, San Diego, CA, USA). The Shapiro–Wilk test was used to test the null hypothesis that the data were sampled from a Gaussian distribution (Shapiro and Wilk, 1965). The P value (P > 0.05) showed that the data did not deviate from Gaussian distribution. For this reason, analysis of variance and Tukey's multiple comparison test were used.
2.8. Chemistry
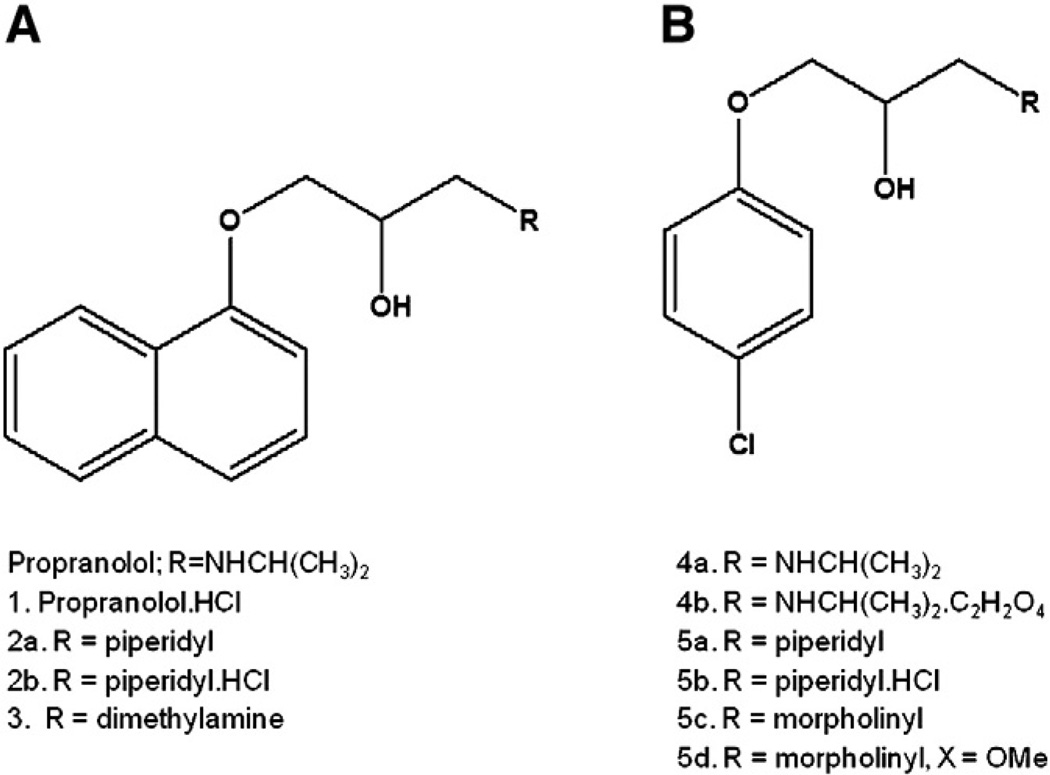
The propranolol derivatives were prepared using a standard synthetic methodology (Ing and Ormerod, 1952; Kaiser et al., 1977). The propranolol derivatives 2a, 3, 4a, 5a, 5c, and 5d were isolated as the free base. The compounds 2a, 4a, and 5a were converted to the corresponding hydrochloride 1, 2b, and 5b and oxalate (4b) salts prior to biological testing (Fig. 1).
Fig. 1.
Propranolol and synthetic derivatives tested for antileishmanial activity. (A) Basic structure of phenoxypropanolamine analogues. (B) Structure after simplification of naphthalene ring. Inserted radicals are represented below each structure.
3. Results
3.1. Fitness of transfected Leishmania
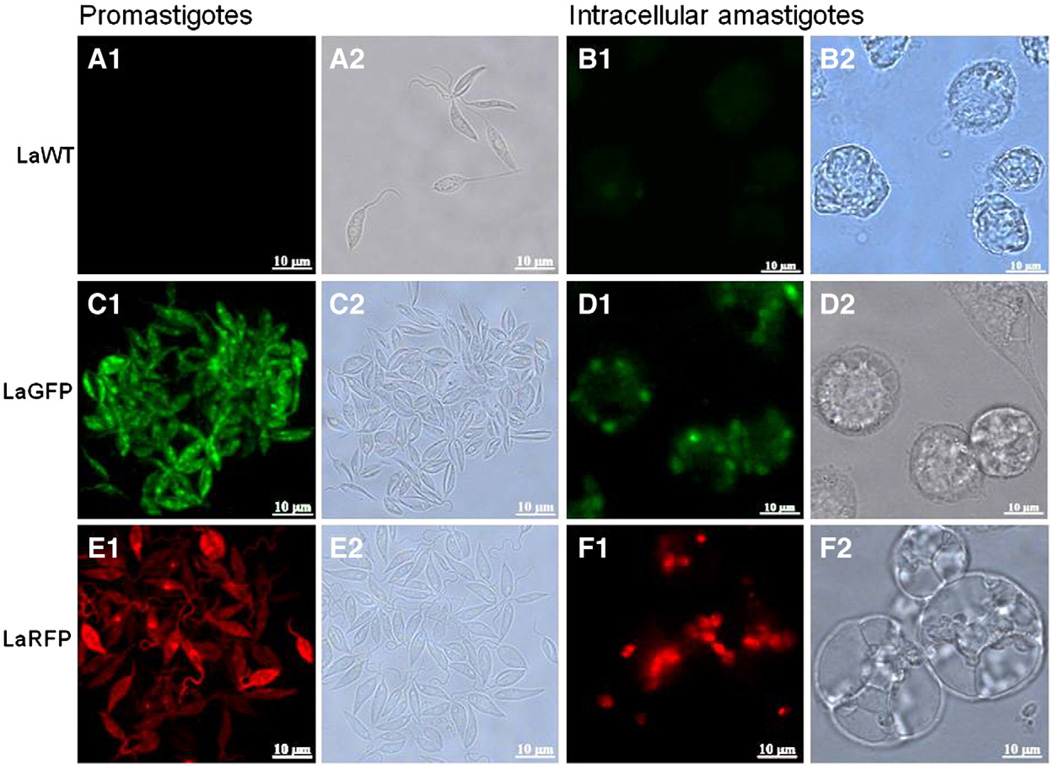
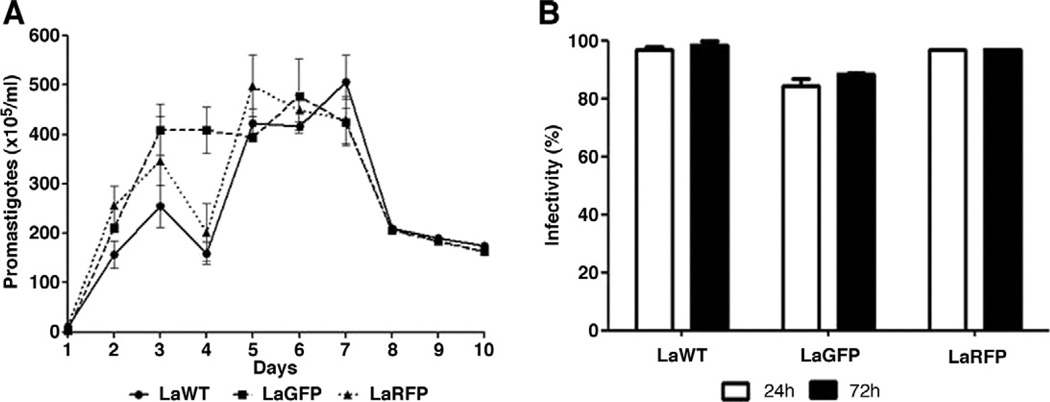
Leishmania amazonensis transfections with GFP and RFP were confirmed by direct microscopy in both promastigotes and amastigotes (Fig. 2). To verify parasite fitness, growth curves and infectivity were evaluated. No differences were observed in parasite densities among LaWT, LaGFP, and LaRFP in M199 medium (P > 0.05, Tukey) (Fig. 3A). Infectivity for all strains was above 85% after 24 h and 89% after 72 h (Fig. 3B). Parasites were analyzed by flow cytometry for size (FSC) and granulosity (SSC), and the morphometric profiles were similar amongst the isolates (Fig. 4A–C). Fluorescence clearly discriminated LaGFP/LaRFP parasites from LaWT (Fig. 4D and E). These results demonstrated that transfected parasites could be differentiated from LaWT by fluorescence alone.
Fig. 2.
Fluorescent and bright field images of L. (L.) amazonensis transfected with green and red fluorescent proteins (LaGFP and LaRFP). A1 and B1) Differential interference contrast (DIC); A2 and B2)wild-type (WT) promastigotes and amastigotes (filter LP515). C1 and D1) DIC; C2 and D2) LaGFP promastigotes and amastigotes (filter LP515). E1 and F1) DIC; E2 and F2; LaRFP promastigotes and amastigotes (filter LP590). Scale bar = 10 µm.
Fig. 3.
Growth curves and macrophage infectivity of LaWT and transfected (LaGFP and LaRFP) L. amazonensis. (A) Leishmania amazonensis parasites were grown in M199 medium and counts determined daily (initial concentration of 1 × 105/mL). (B) Macrophage infectivity (%) was determined at 24 and 72 h post-infection.
Fig. 4.
Flow cytometry analyses of WT and transfected (LaGFP and LaRFP) L. amazonensis. (A–C) Analyses of size (forward scatter—FSC-H) and granulosity (side scatter—SSC-H) of L. amazonensis promastigotes WT, LaGFP, and LaRFP, respectively. (D and E) Fluorescence comparison between WT (black line), LaGFP (green line) in FL1 and LaRFP (red line) in FL2.
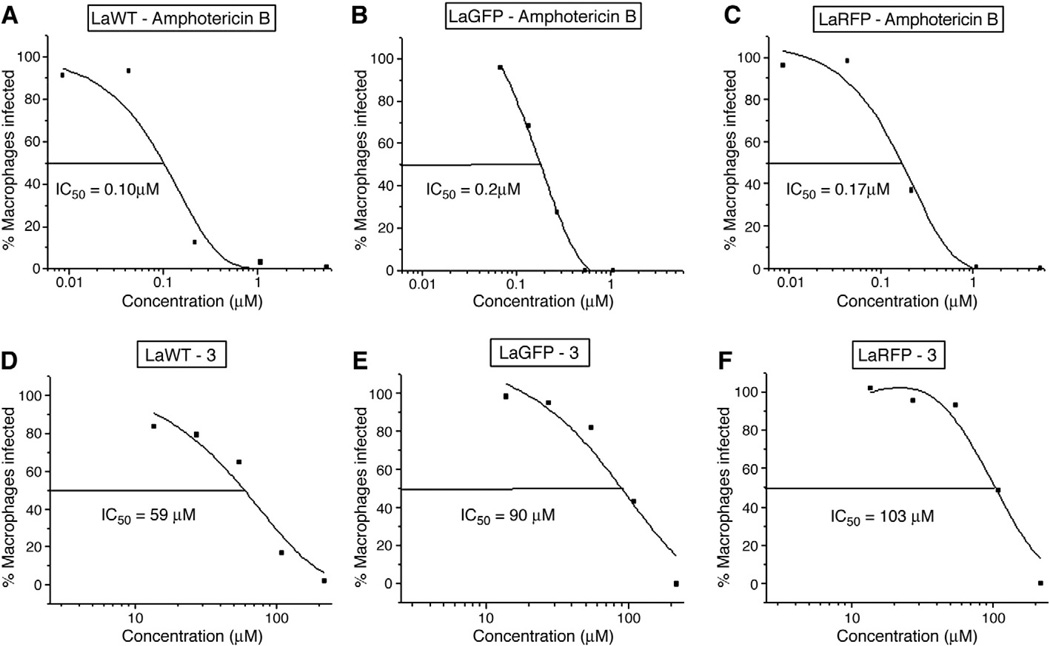
3.2. Susceptibility evaluation using the classic microscopic test
Transfection with GFP and RFP proteins also included the insertion of resistance genes phleomycin and nourseothrycin, respectively. To assess their influence on the resistance to amphotericin B and propranolol derivatives, LaGFP and LaRFP parasites were compared to LaWT using microscopy (Table 1). All propranolol derivatives were inactive against the parasite (IC50 > 10 μmol/L) (Fig. 5) (Table 1). The IC50 values obtained for control drugs (amphotericin B, meglumine antimoniate, and Miltefosine), and 1, 2b, 3, and 4 were compared among groups (LaWT × LaGFP, WT × LaRFP, and LaGFP × LaRFP). With the exception of meglumine antimoniate and 4b, statistical analysis did not reveal significant differences among groups (P > 0.05) (Table 1). Overall, transfection with GFP or RFP plasmids containing antibiotic resistance genes did not result in cross-resistance with the tested molecules.
Table 1.
In vitro antileishmanial activity (μmol/L) of tested compounds against WT and transfected (LaGFP and LaRFP) L. amazonensis in the microscopy test.
| IC50 (μmol/L)a |
|||
|---|---|---|---|
| WTb | LaGFPc | LaRFPd | |
| Amphotericin B | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.16 ± 0.02 |
| Meglumine antimoniate | 1133 ± 246 | NDe | 552 ± 18.38 |
| Miltefosine | 7.6 ± 0.8 | NDe | 9.3 ± 0.1 |
| Allopurinol | >250 | NDe | >250 |
| 1 | 32.3 ± 10.0 | 29.2 ± 7.0 | 46.6 ± 3.2 |
| 2a | >250 | >250 | >250 |
| 2b | 59.0 ± 19.5 | 41.9 ± 4.4 | 83.9 ± 23.5f |
| 3 | 80.5 ± 19.8 | 91.3 ± 7.0 | 86.0 ± 9.2 |
| 4a | 210.5 ± 97.7 | >250 | >250 |
| 4b | 56.3 ± 37.1 | 29.2 ± 5.0 | 139.2 ± 32.0f |
| 5a | >250 | >250 | >250 |
| 5b | >250 | >250 | >250 |
| 5c | >250 | >250 | >250 |
| 5d | >250 | >250 | >250 |
IC50 = Half-maximal inhibitory response.
WT = Wild type.
LaGFP = Parasites transfected with green fluorescent protein.
LaRFP = Parasites transfected with red fluorescent protein.
ND = Not determined.
Values are statistically higher P < 0.05, Tukey test).
Fig. 5.
Dose–response curves of tested molecules against intracellular L. amazonensis in the microscopy test. (A–C) Half inhibitory concentration (IC50) of amphotericin B; (D–F) IC50 of propranolol derivative 3; WT, wild type; LaGFP, transfected parasites with green fluorescent protein; LaRFP, transfected parasites with red fluorescent protein. Curves were obtained using MicroCal Origin Software. Results are a representation of 1 experiment.
3.3. In vitro fluorimetric test
Since transfected fluorescent parasites were comparable to LaWT with respect to biological fitness (growth curve, macrophage infection, morphology, and drug susceptibility), the next step was to standardize an in vitro drug screening test. Although LaGFP intracellular parasites could be easily seen under fluorescence microscopy (Fig. 2D), the RFUs of those parasites were indistinguishable from LaWT and from uninfected macrophages (Fig. 6A). LaGFP promastigotes could be detected in the fluorimeter at concentrations above 1 × 107 cells (50,000 RFUs) (data not shown). LaRFP could be distinguished from LaWT and uninfected macrophages. The concentration of 2 × 105 macrophages was chosen since it was the concentration used for the microscopy method and enabled the detection of LaRFP parasites (~20,000 RFUs) (Fig. 6B). After screening, the optimal excitation/emission wavelengths of 560/620 were determined (Fig. 6C). For this reason only, LaRFP parasites were tested for in vitro assays in the fluorimeter. Seven LaRFP clones were obtained and evaluated for infection and fluorescence. No substantial differences were observed among the tested clones, and a single clone was selected for subsequent experiments. To analyze fluorescence persistence during infection, infected macrophages were observed at 5, 24, 48, and 72 h post-infection (Table 2). Maximum fluorescence detection was observed 5 h post-infection (33,000 RFUs). After 24 h, a decrease (~40%) was seen, consistent with parasite killing by the macrophage. After 24 h, fluorescence stabilized at approximately 4–5-fold the control. Negative control (background) represented by uninfected macrophages remained constant during the course of the experiment. These data confirmed the use of LaRFP parasites as a model for a fluorescent drug screening test.
Table 2.
Evaluation of fluorescence persistence (RFUs) in macrophages (MØ) infected with L. amazonensis RFP (LaRFP) parasites according to time (h).
| 5 | 24 | 48 | 72 | |
|---|---|---|---|---|
| MØ | 3195 ± 1037 | 2297 ± 203 | 3029 ± 1036 | 2236 ± 537 |
| MØ + LaRFP | 33093 ± 5226 | 19368 ± 2806 | 16162 ± 2480 | 11654 ± 1988 |
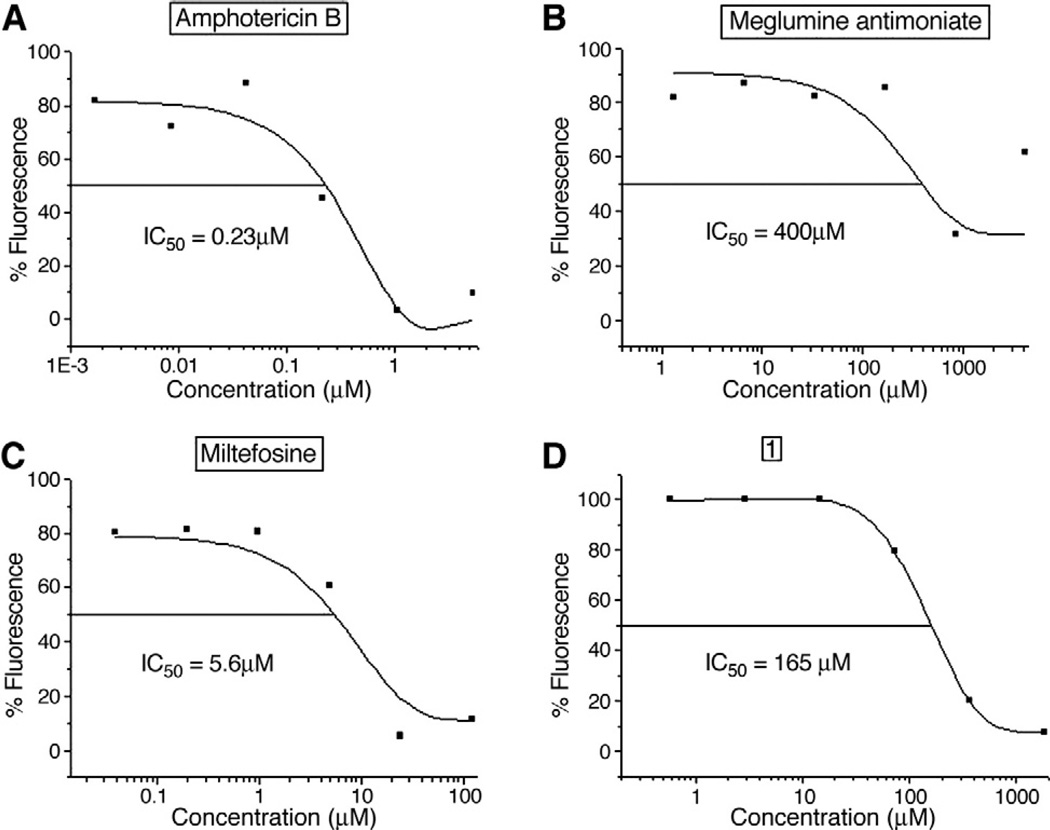
As expected, all antileishmanial control drugs (except allopurinol) were active against L. amazonensis. Comparing the microscopy test and the fluorimetric test, all antileishmanial reference drugs showed similar IC50 values (P > 0.05, t test) (Fig. 7A – D). Again, none of the propranolol derivatives tested was found active with the fluorimeter (IC50 >10 μmol/L) (Table 3). Selective indices for meglumine antimoniate and Miltefosine observed with microscopy were similar to those found using fluorimetry. For amphotericin B, the value calculated with the microscopic method was 2-fold that in the fluorimeter (Table 4) (Fig. 7A). These data indicate that the fluorimetric test is comparable to the microscopy assay.
Fig. 7.
Dose–response curves of tested molecules against intracellular L. (L.) amazonensis in the fluorimetric test. IC50 of amphotericin B (A), meglumine antimoniate (B), Miltefosine (C), and molecule 1 (D) against intracellular L. (L.) amazonensis transfected with red fluorescent protein (LaRFP). Curves were obtained using MicroCal Origin Software. Results are a representation of 1 experiment.
Table 3.
Evaluation of in vitro antileishmanial activity (μmol/L) of tested compounds against L. amazonensis transfected with red fluorescent protein (LaRFP).
| Microscopy methoda | Fluorimetric methoda | |
|---|---|---|
| Amphotericin B | 0.16 ± 0.02 | 0.31 ± 0.16 |
| Meglumine antimoniate | 552 ± 18.38 | 460 ± 351 |
| Miltefosine | 9.3 ± 0.1 | 9.0 ± 5.0 |
| Allopurinol | >250 | >250 |
| 1 | 46.6 ± 3.2 | 197.5 ± 39.9 |
| 2a | >250 | >250 |
| 2b | 83.9 ± 23.5 | 180.6 ± 5.0 |
| 3 | 86.0 ± 9.2 | 301.5 ± 17.0 |
| 4a | >250 | >250 |
| 4b | 139.2 ± 32.0 | 283.8 ± 83.9 |
| 5a | >250 | >250 |
| 5b | >250 | >250 |
| 5c | >250 | >250 |
| 5d | >250 | >250 |
Values obtained for LaRFP parasites in Table 1 for comparison.
Table 4.
Comparison of cytotoxicity and selective indexes of active substances in the microscopy and fluorimetric tests.
| Cytotoxicity (μmol/L) |
Selective index (SI)a |
||
|---|---|---|---|
| MLD50b | Microscopy | Fluorimeter | |
| Amphotericin B | 92.2 ± 29.8 | 595 | 294 |
| Meglumine antimoniate | 48451 ± 17355 | 88 | 105 |
| Miltefosine | 166.9 ± 45.3 | 18 | 19 |
Based on the MLD50/IC50 ratios.
MLD50 = The minimum lethal dose that killed 50% of the cells in μmol/L.
4. Discussion
The absence of research and development for new medicines targeting tropical diseases has become a global concern. The search for new drugs, drug combinations, and protocols against tropical and neglected diseases has recently been stimulated (Chirac and Torreele, 2006; Moran et al., 2009). There is no available vaccine for leishmaniases, and vector/reservoir control has limitations. Current control measures are based on patient treatment. Leishmaniasis chemotherapy is hindered by occurrence of side effects, treatment failure due to parasite resistance, HIV co-infection, and the need for intravenous administration (Croft et al., 2006a,b). A search for new chemotherapeutic compounds and methods of screening potential drugs against Leishmania is warranted (Alvar et al., 2006; Sereno et al., 2007).
Three recent reports have described high-throughput methods providing a more efficient way to identify candidate anti-parasitic compounds against Leishmania (De Muylder et al., 2011; Sharlow et al., 2010; Siqueira-Neto et al., 2010). An important advantage of those methods is their capability of screening a high number of compounds in promastigotes and in intracellular and axenic amastigotes. Our method requires no nuclear staining such as DAPI (De Muylder et al., 2011), Draq5 (Siqueira-Neto et al., 2010), or celltiter blue reagent (Sharlow et al., 2010). Since the plasmid is stably integrated, it is not necessary to keep the parasites in the presence of the selection antibiotic. More importantly, our method allows analysis concomitant with that of the host cell. Although our method could be considered medium throughput, it has the advantage of being less costly than the automated image system. A plate reader is more accessible and does not require sophisticated image capturing, analysis software, and expensive maintenance. Ours and the 3 above-mentioned HTS methods are summarized in Table 5. Here, it was described for the first time that transfection did not affect LaGFP or LaRFP parasite fitness in any parameter studied. It was shown that LaGFP parasites were not suitable for fluorimetry, whereas RFP L. amazonensis (LaRFP) were successfully detected. Many recent studies have determined the activity of chemical- or plant-derived compounds against L. amazonensis (Aguiar et al., 2010; Garcia et al., 2010; Junior et al., 2010; Khouri et al., 2010; Pinheiro et al., 2011; Souza-Fagundes et al., 2010). Although IC50 values could be determined for compounds 1, 2b, 3, and 4b using both assessed methods, none of our 10 propranolol derivatives was considered active (IC50 >10 μmol/L) (Ioset et al., 2009). It is important to point out that the cut-off value was established for L. donovani, an Old World viscerotropic species. However, for the dermotropic and viscerotropic New World species, the value may vary. For example, with the reference drug Miltefosine, IC50 values for L. amazonensis, L. infantum, L. braziliensis, and L. guyanensis were 19-fold that of L. donovani (De Morais-Teixeira et al., 2011).
Table 5.
Summary of drug screening protocols against Leishmania
| Type of screening |
Analyses type | Need for substrates/dyes |
Parasite stage | Applicability to any parasite strain/host cell |
Host cell | Species | Reference |
|---|---|---|---|---|---|---|---|
| HTS | Resazurin sodium salt (Sigma, St. Louis, MO, USA) | Yes | Promastigote | Yes/no | L. major | Siqueira-Neto et al., 2010 | |
| Draq5 (DNA marker) | Intracellular amastigote | Yes/yes | THP1 | L. major/L. donovani | |||
| HTS | CellTiter-Glo (Promega, Madison, WI, USA) (ATP-bioluminescence) | Yes | Promastigote | Yes/no | L. donovani | De Muylder et al., 2011 | |
| DAPI (DNA marker) | Intracellular amastigote | Yes/yes | THP1 | ||||
| CellTiter-Glo (Promega, Madison, WI, USA) | Axenic amastigote | Yes/no | |||||
| HTS | CellTiter Blue Reagent | Yes | Promastigote | Yes/no | Leishmania sp. | Sharlow et al., 2010 | |
| MTS | RFP fluorescence | No | Intracellular amastigote | Yes/yes | Murine macrophages | L. amazonensis |
With flow cytometry, LaGFP parasites could be detected in both amastigotes and promastigotes (Bolhassani et al., 2011; Siqueira-Neto et al., 2010); hence not only LaGFP, but also LaRFP parasites, could be distinguished from LaWT using this technique. This could only be achieved with LaGFP after macrophage lysis as previously demonstrated using a spectrofluorimeter (Costa et al., 2011). One of the main advantages of using LaRFP is the elimination of the lysis step. In our model, the macrophage green background fluorescence was a determinant in hindering fluorimeter detection of LaGFP parasites. Similar to our data, LaGFP promastigote detection was also observed by Boeck et al. (2006). However, those authors achieved intracellular detection of episomal LaGFP parasites and this may be due to differences in the wavelengths used (435 nm excitation/538 nm emission).
However, in the fluorimeter, LaGFP parasites could not be distinguished from infected or uninfected LaWT macrophages (Fig. 6A). This can be attributed to the slight green background fluorescence in mouse macrophages (Fig. 2B). In our study, only LaGFP promastigotes could be detected in the fluorimeter in numbers above 1 × 107 cells (data not shown).
Transfection did not affect parasite morphology (Figs. 2 and 4), growth curves (Fig. 3), and infectivity in mouse (data not shown). The possibility of cross-resistance with the antibiotics used during selection is a concern when using transfected parasites. By using stable integrated plasmids, the requirement for selective drugs is avoided. With the exception of 2 molecules (Table 3), transfection did not affect drug susceptibility in either LaGFP or LaRFP parasites. However, these exceptions are likely to be due to variations in the microscopy method.
Preliminary results using propranolol have shown activity against trypomastigote forms of Trypanosoma cruzi (Hammond et al., 1984). Ten new propranolol derivatives were evaluated for their in vitro activity against intracellular amastigote forms of L. amazonensis using both microscopic and fluorimetric assays. All proved inactive by both methods.
This is the first description of a fluorimetric drug screening test for L. amazonensis using LaRFP parasites. This procedure was faster, suitable for medium throughput, accessible, and was comparable to microscopy with minimal modifications. Transfection of the parasites with GFP or RFP genes did not affect parasite biological fitness.
Acknowledgment
The authors thank Dr. Tânia Maria de Almeida Alves for support with the fluorimetric experiments through the Programme for Drug Development and Discovery (P3D).
Footnotes
Financial support: R. P. Soares, O. A. Martins-Filho, and M. N. Melo are research fellows supported by the National Council for the Development of Research of Brazil (CNPq; process 305042/2010-6). M. N. Rocha is supported by CNPq (process 142361/2009-7). This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (APQ-01123-09 PRONEX). S. M. Beverley is supported by the National Institutes of Health (AI21903 and 29646).
References
- Aguiar MG, Pereira AM, Fernandes AP, Ferreira LA. Reductions in skin and systemic parasite burdens as a combined effect of topical paromomycin and oral miltefosine treatment of mice experimentally infected with Leishmania (Leishmania) amazonensis. Antimicrob Agents Chemother. 2010;54:4699–4704. doi: 10.1128/AAC.00809-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, Croft S, Olliaro P. Chemotherapy in the treatment and control of leishmaniasis. Adv Parasitol. 2006;61:223–274. doi: 10.1016/S0065-308X(05)61006-8. [DOI] [PubMed] [Google Scholar]
- Berman JD, Lee LS. Activity of antileishmanial agents against amastigotes in human monocyte-derived macrophages and in mouse peritoneal macrophages. J Parasitol. 1984;70:220–225. [PubMed] [Google Scholar]
- Bittencourt A, Barral A, De Jesus AR, De Almeida RP, Grimaldi G., Jr In situ identification of Leishmania amazonensis associated with diffuse cutaneous leishmaniasis in Bahia, Brazil. Mem Inst Oswaldo Cruz. 1989;84:585–586. doi: 10.1590/s0074-02761989000400022. [DOI] [PubMed] [Google Scholar]
- Boeck P, Bandeira Falcao CA, Leal PC, Yunes RA, Filho VC, Torres-Santos EC, Rossi-Bergmann B. Synthesis of chalcone analogues with increased antileishmanial activity. Bioorg Med Chem. 2006;14:1538–1545. doi: 10.1016/j.bmc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bolhassani A, Taheri T, Taslimi Y, Zamanilui S, Zahedifard F, Seyed N, Torkashvand F, Vaziri B, Rafati S. Fluorescent Leishmania species: development of stable GFP expression and its application for in vitro and in vivo studies. Exp Parasitol. 2011;127:637–645. doi: 10.1016/j.exppara.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Chan MM, Bulinski JC, Chang KP, Fong D. A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitol Res. 2003;89:266–271. doi: 10.1007/s00436-002-0706-4. [DOI] [PubMed] [Google Scholar]
- Chirac P, Torreele E. Global framework on essential health R&D. Lancet. 2006;367:1560–1561. doi: 10.1016/S0140-6736(06)68672-8. [DOI] [PubMed] [Google Scholar]
- Convit J, Castellanos PL, Ulrich M, Castes M, Rondon A, Pinardi ME, Rodriquez N, Bloom BR, Formica S, Valecillos L, et al. Immunotherapy of localized, intermediate, and diffuse forms of American cutaneous leishmaniasis. J Infect Dis. 1989;160:104–115. doi: 10.1093/infdis/160.1.104. [DOI] [PubMed] [Google Scholar]
- Costa SS, de Assis Golim M, Rossi-Bergmann B, Costa FT, Giorgio S. Use of in vivo and in vitro systems to select Leishmania amazonensis expressing green fluorescent protein. Korean J Parasitol. 2011;49:357–364. doi: 10.3347/kjp.2011.49.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006a;123:399–410. [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006b;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington GJ, Kelly JH, Buffone GJ. Growth and hepatospecific gene expression of human hepatoma cells in a defined medium. In Vitro Cell Dev Biol. 1987;23:349–354. doi: 10.1007/BF02620991. [DOI] [PubMed] [Google Scholar]
- DaRocha WD, Silva RA, Bartholomeu DC, Pires SF, Freitas JM, Macedo AM, Vazquez MP, Levin MJ, Teixeira SM. Expression of exogenous genes in Trypanosoma cruzi: improving vectors and electroporation protocols. Parasitol Res. 2004;92:113–120. doi: 10.1007/s00436-003-1004-5. [DOI] [PubMed] [Google Scholar]
- De Morais-Teixeira E, Damasceno QS, Galuppo MK, Romanha AJ, Rabello A. The in vitro leishmanicidal activity of hexadecylphosphocholine (miltefosine) against four medically relevant Leishmania species of Brazil. Mem Inst Oswaldo Cruz. 2011;106:475–478. doi: 10.1590/s0074-02762011000400015. [DOI] [PubMed] [Google Scholar]
- De Muylder G, Ang KK, Chen S, Arkin MR, Engel JC, McKerrow JH. A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl Trop Dis. 2011;5:e1253. doi: 10.1371/journal.pntd.0001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli C, Ochoa R, da Silva JB, Falcao CA, Rossi-Bergmann B, de Melo AL, Sinisterra RD, Frezard F. Oral delivery of meglumine antimoniate-beta-cyclodextrin complex for treatment of leishmaniasis. Antimicrob Agents Chemother. 2004;48:100–103. doi: 10.1128/AAC.48.1.100-103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Dube A, Gupta R, Singh N. Reporter genes facilitating discovery of drugs targeting protozoan parasites. Trends Parasitol. 2009;25:432–439. doi: 10.1016/j.pt.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Frevert U, Engelmann S, Zougbede S, Stange J, Ng B, Matuschewski K, Liebes L, Yee H. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 2005;3:e192. doi: 10.1371/journal.pbio.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Monzote L, Montalvo AM, Scull R. Screening of medicinal plants against Leishmania amazonensis. Pharm Biol. 2010;48:1053–1058. doi: 10.3109/13880200903485729. [DOI] [PubMed] [Google Scholar]
- Gibson W, Peacock L, Ferris V, Williams K, Bailey M. The use of yellow fluorescent hybrids to indicate mating in Trypanosoma brucei. Parasit Vectors. 2008;1:4. doi: 10.1186/1756-3305-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontijo CM, Melo MN. Visceral leishmaniasis in Brazil: current status, challenges and prospects. Rev Bras Epidemiol. 2004;7:338–349. [Google Scholar]
- Guevara P, Dias M, Rojas A, Crisante G, Abreu-Blanco MT, Umezawa E, Vazquez M, Levin M, Anez N, Ramirez JL. Expression of fluorescent genes in Trypanosoma cruzi and Trypanosoma rangeli (Kinetoplastida: Trypanosomatidae): its application to parasite-vector biology. J Med Entomol. 2005;42:48–56. doi: 10.1093/jmedent/42.1.48. [DOI] [PubMed] [Google Scholar]
- Gupta S. Visceral leishmaniasis: experimental models for drug discovery. Indian J Med Res. 2011;133:27–39. [PMC free article] [PubMed] [Google Scholar]
- Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- Hammond DJ, Cover B, Gutteridge WE. A novel series of chemical structures active in vitro against the trypomastigote form of Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1984;78:91–95. doi: 10.1016/0035-9203(84)90184-6. [DOI] [PubMed] [Google Scholar]
- Ing HR, Ormerod WE. The synthesis and local anesthetic properties of aryloxypropa-nolamines. J Pharm Pharmacol. 1952;4:21–26. doi: 10.1111/j.2042-7158.1952.tb13106.x. [DOI] [PubMed] [Google Scholar]
- Ioset JR, Brun R, Wenzler T, Kaiser M, Yardley V. Drug screening for kinetoplastids diseases: a training manual for screening in neglected diseases. DNDi and Pan-Asian Screening Network. 2009:1–74. [Google Scholar]
- Junior CG, de Assis PA, Silva FP, Sousa SC, de Andrade NG, Barbosa TP, et al. Efficient synthesis of 16 aromatic Morita-Baylis-Hillman adducts: biological evaluation on Leishmania amazonensis and Leishmania chagasi. Bioorg Chem. 2010;38:279–284. doi: 10.1016/j.bioorg.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Kaiser C, Jen T, Garvey E, Bowen WD, Colella DF, Wardel JR., Jr Adrenergic agents. 4. Substituted phenoxypropanolamine derivatives as potential beta-adrenergic agonists. J Med Chem. 1977;20:687–692. doi: 10.1021/jm00215a014. [DOI] [PubMed] [Google Scholar]
- Kamau SW, Grimm F, Hehl AB. Expression of green fluorescent protein as a marker for effects of antileishmanial compounds in vitro. Antimicrob Agents Chemother. 2001;45:3654–3656. doi: 10.1128/AAC.45.12.3654-3656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Sundar S, Singh N. Molecular docking, structure–activity relationship and biological evaluation of the anticancer drug monastrol as a pteridine reductase inhibitor in a clinical isolate of Leishmania donovani. J Antimicrob Chemother. 2010;65:1742–1748. doi: 10.1093/jac/dkq189. [DOI] [PubMed] [Google Scholar]
- Khouri R, Novais F, Santana G, de Oliveira CI, Vannier dos Santos MA, Barral A, et al. DETC induces Leishmania parasite killing in human in vitro and murine in vivo models: a promising therapeutic alternative in leishmaniasis. PLoS One. 2010;5:e14394. doi: 10.1371/journal.pone.0014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram D, Thale C, Kolodziej H, Kiderlen AF. Intracellular parasite kill: flow cytometry and NO detection for rapid discrimination between anti-leishmanial activity and macrophage activation. J Immunol Methods. 2008;333:79–88. doi: 10.1016/j.jim.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lang T, Goyard S, Lebastard M, Milon G. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell Microbiol. 2005;7:383–392. doi: 10.1111/j.1462-5822.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- Madureira AM, Martins AP, Gomes M, Paiva J, Ferreira MJU, Cunha AP, Rosario VE. Antimalarial activity of medicinal plants used in traditional medicine in S. Tome and Principe Island. J Ethnopharmacol. 2002;81:23–29. doi: 10.1016/s0378-8741(02)00005-3. [DOI] [PubMed] [Google Scholar]
- Mehta SR, Huang R, Yang M, Zhang XQ, Kolli B, Chang KP, et al. Real-time in vivo green fluorescent protein imaging of a murine leishmaniasis model as a new tool for Leishmania vaccine and drug discovery. Clin Vaccine Immunol. 2008;15:1764–1770. doi: 10.1128/CVI.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SR, Zhang XQ, Badaro R, Spina C, Day J, Chang KP, Schooley RT. Flow cytometric screening for anti-leishmanials in a human macrophage cell line. Exp Parasitol. 2010;126:617–620. doi: 10.1016/j.exppara.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Saxena A, Singh S. Chemotherapy of leishmaniasis: past, present and future. Curr Med Chem. 2007;14:1153–1169. doi: 10.2174/092986707780362862. [DOI] [PubMed] [Google Scholar]
- Mißlitz A, Mottram JC, Overath P, Aebischer T. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol Biochem Parasitol. 2000;107:251–261. doi: 10.1016/s0166-6851(00)00195-x. [DOI] [PubMed] [Google Scholar]
- Moran M, Guzman J, Ropars AL, McDonald A, Jameson N, Omune B, et al. Neglected disease research and development: how much are we really spending? PLoS Med. 2009;6:e30. doi: 10.1371/journal.pmed.1000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LG, Hsu A, Mandell MA, Roediger B, Hoeller C, Mrass P, et al. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008;4:e1000222. doi: 10.1371/journal.ppat.1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- Okuno T, Goto Y, Matsumoto Y, Otsuka H, Matsumoto Y. Applications of recombinant Leishmania amazonensis expressing egfp or the beta-galactosidase gene for drug screening and histopathological analysis. Exp Anim. 2003;52:109–118. doi: 10.1538/expanim.52.109. [DOI] [PubMed] [Google Scholar]
- Olfert ED, Cross BM, McWilliam AA. Guide To The Care And Use of Experimental Animals. Saskatchewan, Canada: Canadian Council on Animal Care, Saskatoon; 1993. [Google Scholar]
- Peacock L, Ferris V, Bailey M, Gibson W. Dynamics of infection and competition between two strains of Trypanosoma brucei brucei in the tsetse fly observed using fluorescent markers. Kinetoplastid Biol Dis. 2007;6:4. doi: 10.1186/1475-9292-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro AC, Rocha MN, Nogueira PM, Nogueira TC, Jasmim LF, de Souza MV, Soares RP. Synthesis, cytotoxicity, and in vitro antileishmanial activity of mono-t-butyloxycarbonyl-protected diamines. Diagn Microbiol Infect Dis. 2011;71:273–278. doi: 10.1016/j.diagmicrobio.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Pires SF, DaRocha WD, Freitas JM, Oliveira LA, Kitten GT, Machado CR, et al. Cell culture and animal infection with distinct Trypanosoma cruzi strains expressing red and green fluorescent proteins. Int J Parasitol. 2008;38:289–297. doi: 10.1016/j.ijpara.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Plock A, Sokolowska-Kohler W, Presber W. Application of flow cytometry and microscopical methods to characterize the effect of herbal drugs on Leishmania Spp. Exp Parasitol. 2001;97:141–153. [Google Scholar]
- Robinson KA, Beverley SM. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2003;128:217–228. doi: 10.1016/s0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- Rocha MN, Margonari C, Presot IM, Soares RP. Evaluation of 4 polymerase chain reaction protocols for cultured Leishmania spp. typing. Diagn Microbiol Infect Dis. 2010;68:401–409. doi: 10.1016/j.diagmicrobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Sereno D, Cordeiro da Silva A, Mathieu-Daude F, Ouaissi A. Advances and perspectives in Leishmania cell based drug-screening procedures. Parasitol Int. 2007;56:3–7. doi: 10.1016/j.parint.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- Sharlow ER, Lyda TA, Dodson HC, Mustata G, Morris MT, Leimgruber SS, et al. A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity. PLoS Negl Trop Dis. 2010;4:e659. doi: 10.1371/journal.pntd.0000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Dube A. Short report: fluorescent Leishmania: application to anti-leishmanial drug testing. Am J Trop Med Hyg. 2004;71:400–402. [PubMed] [Google Scholar]
- Singh N, Gupta R, Jaiswal AK, Sundar S, Dube A. Transgenic Leishmania donovani clinical isolates expressing green fluorescent protein constitutively for rapid and reliable ex vivo drug screening. J Antimicrob Chemother. 2009;64:370–374. doi: 10.1093/jac/dkp206. [DOI] [PubMed] [Google Scholar]
- Siqueira-Neto JL, Song OR, Oh H, Sohn JH, Yang G, Nam J, Jang J, et al. Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl Trop Dis. 2010;4:e675. doi: 10.1371/journal.pntd.0000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares RP, Macedo ME, Ropert C, Gontijo NF, Almeida IC, Gazzinelli RT, et al. Leishmania chagasi: lipophosphoglycan characterization and binding to the midgut of the sand fly vector Lutzomyia longipalpis. Mol Biochem Parasitol. 2002;121:213–224. doi: 10.1016/s0166-6851(02)00033-6. [DOI] [PubMed] [Google Scholar]
- Souza-Fagundes EM, Cota BB, Rosa LH, Romanha AJ, Correa-Oliveira R, Rosa CA, et al. In vitro activity of hypnophilin from Lentinus strigosus: a potential prototype for Chagas disease and leishmaniasis chemotherapy. Braz J Med Biol Res. 2010;43:1054–1061. doi: 10.1590/s0100-879x2010007500108. [DOI] [PubMed] [Google Scholar]
- Varela MR, Munoz DL, Robledo SM, Kolli BK, Dutta S, Chang KP, Muskus C. Leishmania (Viannia) panamensis: an in vitro assay using the expression of GFP for screening of antileishmanial drug. Exp Parasitol. 2009;122:134–139. doi: 10.1016/j.exppara.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]