Abstract
Because centrosomes were enriched in the bile canaliculi fraction from the chicken liver through their association with apical membranes, we developed a procedure for isolation of centrosomes from this fraction. With the use of the centrosomes, we generated centrosome-specific monoclonal antibodies. Three of the monoclonal antibodies recognized an antigen of ∼90 kDa. Cloning of its cDNA identified this antigen as a chicken homologue of outer dense fiber 2 protein (Odf2), which was initially identified as a sperm outer dense fiber-specific component. Exogenously expressed and endogenous Odf2 were shown to be concentrated at the centrosomes in a microtubule-independent manner in various types of cells at both light and electron microscopic levels. Odf2 exhibited a cell cycle-dependent pattern of localization and was preferentially associated with the mother centrioles in G0/G1-phase. Toward G1/S-phase before centrosome duplication, it became detectable in both mother and daughter centrioles. In the isolated bile canaliculi and centrosomes, Odf2, in contrast to other centrosomal components, was highly resistant to KI extraction. These findings indicate that Odf2 is a widespread KI-insoluble scaffold component of the centrosome matrix, which may be involved in the maturation event of daughter centrioles.
INTRODUCTION
Centrosomes are duplicated once per cell cycle in a semiconservative manner and function as the major microtubule (MT)-organizing centers in most animal cells. In mitotic cells, they play a central role in organizing the mitotic spindles to separate chromosomes. At the interphase they determine the polarized organization of MTs (for reviews see Kellogg et al., 1994; Stearns and Winey, 1997; Zimmerman et al., 1999). Studies with ultrathin-section electron microscopy have shown that in most animal cells centrosomes are composed of a pair of centrioles and a surrounding electron-dense cloud of pericentriolar material (PCM) (Rieder and Borisy, 1982; Bornens et al., 1987; Vorobjev and Nadezhdina, 1987; Paintrand et al., 1992; Kenney et al., 1997). Centrioles are cylindrical structures composed of nine groups of three MTs that are fused into triplets. The PCM appears to be composed of a mixture of fibrous, amorphous, and ring-like structures.
Our knowledge regarding the molecular architecture of centrosomes is still fragmentary, whereas the list of known centrosomal proteins is rapidly growing through antibody production and yeast genetic analyses as well. Three isotypes of tubulins, such as α-, β- and δ-tubulins, were identified as components of centrioles. However, the MT nucleation ability of centrosomes depends on PCM but not dependent on centrioles. Various kinds of structural or enzymatic proteins have been reported to be localized at PCM, i.e., γ-tubulin, ε-tubulin, pericentrin, centrin, cyclins, ninein, cenexin, katanin, centrosomin, kinases/phosphatases, and proteasomal components (Rieder and Borisy, 1982; Bornens et al., 1987; Vorobjev and Nadezhdina, 1987; Paintrand et al., 1992; Kellogg et al., 1994; Lange and Gull, 1995; Kenney et al., 1997; Stearns and Winey 1997; Whitehead and Salisbury, 1999; Zimmerman et al., 1999; Chang and Stearns, 2000). Among these, γ-tubulin has been exceptionally well characterized. The isotype of this tubulin is known to form the γ-tubulin ring complex (γ-TuRC), together with additional components, and confers the MT nucleation activity on centrosomes (Oakley and Oakley, 1989; Joshi et al., 1992; Felix et al., 1994; Stearns and Kirschner, 1994; Zheng et al., 1995; Moritz et al., 1995; Marschall and Stearns, 1997; Dictenberg et al., 1998; Moritz et al., 1998; Schnackenberg et al., 1998; Schiebel 2000).
When centrosomes were treated with 2 M KI, all of the centrosomal components identified to date, including γ-tubulin as well as centriolar constituents, were extracted, leaving fibrous anastomosing networks (Klotz et al., 1990; Moritz et al., 1998; Schnackenberg et al., 1998, 2000). These KI-insoluble structures have been examined morphologically in detail in Drosophila embryos and Spisula oocytes, and they are now referred to as the “centromatrix” or “centrosome scaffold,” but their molecular bases are totally unknown. The KI-resistant centrosome scaffold itself showed no MT nucleation activity, but when incubated with the γ-TuRC in the presence of the crude extract of Drosophila embryos or Spisula oocytes, the MT nucleation activity was restored to normal. The KI-resistant centrosome scaffold may recruit centrosomal components, including γ-TuRCs and γ-TuRC-interacting factors such as the pericentrin complex (Dictenberg et al., 1998) and Spc97p/Spc98p (Knop and Schiebel, 1998; Murphy et al., 1998; Tassin et al., 1998).
During the past decade, we have developed a protocol for isolation of the bile canaliculi and intercellular junctions from the liver and have identified various proteins concentrated in the junctional fraction (Tsukita and Tsukita, 1989; Furuse et al., 1993). During the course of this study, we noticed that the centrosomes were probably enriched in the bile canaliculi fraction through their direct association with apical/junctional membranes of chicken hepatocytes, and we developed a procedure for mass isolation of centrosomes. With the use of the isolated centrosomes as antigens, we obtained monoclonal antibodies (mAbs) that specifically recognized centrosomes. Here, we report that three of these mAbs recognized outer dense fiber 2 protein (Odf2), which was initially identified as a major component of sperm-specific outer dense fibers (Brohmann et al., 1997; Shao et al., 1997). Centrosomal Odf2 shows a cell cycle-dependent localization pattern at the centrosomes, marking the functional maturation of the centrioles. In sperm cells, the electron-dense cloud of PCM has been reported to be structurally continuous toward the outer dense fibers of sperm tails, as revealed by conventional electron microcopy (Fawcett, 1975). Taken together with the results we obtained here that Odf2 is a general component of the KI-insoluble centrosome scaffold, but not a sperm-specific protein, it is likely that in sperm tails Odf2 is specifically utilized to form the outer dense fibers.
MATERIALS AND METHODS
Antibodies and Cells
Mouse anti-β-tubulin mAb (E7; Chu and Klymkowsky, 1989), mouse anti-γ-tubulin mAb (GTU-88; Sigma Chemical, St. Louis, MO), rabbit anti-γ-tubulin polyclonal antibody (pAb; Sigma Chemical, St. Louis, MO), mouse anti-hemagglutinin (HA) mAb (12CA5; Roche Molecular Biochemicals, Gipf-Oberfrick, Switzerland), rabbit anti-HA pAb (Medical and Biological Laboratories, Nagoya, Japan), and rabbit anti-ZO-1 pAb (Zymed Laboratories, San Francisco, CA) were purchased from the sources shown in the parentheses. Rabbit anti-ninein pAb was provided as a generous gift from Dr. M. Bornens. Chicken LMH cells were cultured in Waymouth's MB 752/1 medium supplemented with 10% fetal calf serum. Human HeLa, mouse Eph4, and L cells were cultured in DMEM supplemented with 10% fetal calf serum.
Isolation of Centrosomes from Bile Canaliculi Fraction
The procedure that we used to isolate bile canaliculi and junction fractions from 1- or 2-d-old male chick liver was the same as the one Furuse et al. (1993) described previously. An isolated junction fraction was washed three times with A solution (10 mM HEPES, pH 7. 5/10 μg/ml leupeptin) by centrifugation (15,000 × g, 10 min). A pellet from 200 chicks was suspended in 500 μl of A solution and sonicated four times on ice for 10 s with the use of a Sonifier 250 (Branson Ultrasonics, Danbury, CT) at the lowest output. The sample was centrifuged at 5000 × g for 2 min, and the supernatant was centrifuged at 20, 000 × g for 10 min to recover centrosomes into the pellet. Then, this pellet was repeatedly resuspended, sonicated twice for 10 s, and centrifuged as described above. The pellet that we finally obtained was sonicated for 8 s and mixed with 65% (wt/wt) sucrose solution to make a 50% (wt/wt) sucrose solution. This solution was then centrifuged through a discontinuous gradient consisting of 70, 65, 60, 58, 55, 52, and 51% sucrose solutions (wt/wt) in an SW-28 rotor (Beckman Instruments, Fullerton, CA) at 70,000 × g for 12 h at 4°C. The 52–60% sucrose solution was carefully collected with the use of a glass capillary, combined, and diluted with A solution. Centrosomes were recovered as a pellet after centrifugation at 70,000 × g for 5 h at 4°C. At this point, ∼20 μg of centrosome-enriched fraction was obtained from 500 chicks. In some cases, a part of the 52–60% sucrose solution was mixed with distilled water to make a 50% (wt/wt) sucrose solution and loaded onto a second gradient of 70, 62.5, 60, 58, 57, 56, 55, 54, 53, and 52%. The fractions of 54–58% sucrose solution contained centrosomes of high purity.
Generation of mAbs against Isolated Centrosomes
The isolated centrosomes were used as antigens to produce mAbs in rats. Hybridomas were prepared by fusion between rat lymphocytes and mouse P3 myeloma cells in accordance with the method described previously (Tsukita and Tsukita, 1989). The cultured supernatant of each hybridoma was assayed for antibody production by immunofluorescence microscopy with the use of isolated bile canaliculi.
Immunoscreening
With the use of a chicken liver λgt11 cDNA expression library (Clontech Laboratories, Palo Alto, CA), clones were immunoscreened with the use of mAb101 as described previously (Tsukita et al. , 1994). One cDNA clone was isolated. Its insert was subcloned into pBluescript SK(−) and sequenced with the use of a Taq terminator cycle sequencing kit (DyeDeoxy, Applied Biosystems, Foster City, CA).
Production of Glutathione S-Transferase (GST)-Fusion Proteins
The full-length (amino acids [aa] 1–659), N-terminal half (aa 1–296), and C-terminal half (aa 297–659) of chicken Odf2 were cloned in frame into pGEX (Amersham Pharmacia Biotech, Piscataway, NJ) to produce GST-fusion proteins in Escherichia coli. To determine the epitopes of anti-Odf2 mAbs in the C-terminal half, the cDNAs that encode aa 297–530 and aa 297–588 of chicken Odf2 were also cloned in frame into pGEX. The full-length cDNA encoding mouse Odf2/1 (610 aa) was obtained from a mouse F9 cell cDNA library by polymerase chain reaction (PCR) and was cloned in frame into pGEX. The GST-fusion proteins were expressed and purified in accordance with the method described previously (Maeda et al., 1999).
Construction and Transfection of Odf2 cDNA
The cDNA fragments that encode the full-length chicken Odf2 or mouse Odf2/1 were engineered to have an influenza HA epitope tag at their 5′-ends. The constructs were then cloned into CAG promoter-driven mammalian expression vector pCAG. These expression vectors were transfected into HeLa cells with the use of LipofectAMINE (GIBCO BRL, Grand Island, NY).
KI Extraction of Bile Canaliculi and Isolated Centrosomes
The isolated bile canaliculi were attached to polylysine-coated coverslips, treated with a 300-μl drop of 2 M KI in PEM solution (80 mM PIPES, pH 6. 8/1 mM EGTA/1 mM MgCl2/1 mM p-amidinophenylmethylsulfonyl fluoride/10 μg/ml leupeptin) and processed for immunofluorescence microscopy. For the KI treatment of bile canaliculi and isolated centrosomes, they were first pelleted down to remove the sucrose from −80°C stock solution. Their pellets (50–500 μg) were incubated in ∼3 ml of 2 M KI in PEM solution. KI-treated samples of the bile canaliculi and centrosomes were recovered as a pellet 20 min after centrifugation at 400,000 × g at 4°C.
Immunofluorescence Microscopy
For indirect immunofluorescence microscopy, the isolated bile canaliculi were dried on coverslips or placed on polylysine-coated coverslips. The bile canaliculi attached to coverslips were then fixed with methanol at −20°C for 10 min, washed with PBS, and processed for immunofluorescence microscopy (Tsukita et al. , 1991). Fluorescein isothiocyanate-conjugated goat anti-rat immunoglobulin (Ig) G, rhodamine-conjugated goat anti-mouse IgG, and rhodamine-conjugated goat anti-rabbit IgG (Chemicon, Temecula, CA) were used as secondary antibodies. Cultured cells were fixed with methanol at −20°C for 10 min and processed for indirect immunofluorescence microscopy (Tsukita et al. , 1991).
Immunoelectron Microscopy and Ultrathin-Section Electron Microscopy
The isolated bile canaliculi from the chicken liver were incubated with rat mAb101, mAb1019, or mAb184 at 4°C overnight, washed three times with PBS, and fixed with 3% formaldehyde in PBS at room temperature for 10 min. After three washes with PBS, the samples were labeled with anti-rat IgG pAb-conjugated 15- or 10-nm gold particles (Britisch BioCell International, Cardiff, United Kingdom) at 4°C overnight and washed three times with PBS. Immunolabeled and unlabeled bile canaliculi as well as the pellet of isolated centrosomes were fixed with a fixative consisting of 2. 5% glutaraldehyde, 0. 1 M cacodylate buffer, pH 7.3, and 0.1% tannic acid and processed for electron microscopy (Tsukita and Tsukita, 1989).
SDS-PAGE and Immunoblotting
The isolated bile canaliculi and a total lysate of E. coli expressing GST-fusion proteins were resolved by SDS-PAGE according to the method of Laemmli (1970) and transferred onto nitrocellulose membranes. The membranes were incubated with first antibodies. Bound antibodies were visualized with alkaline phosphatase-conjugated goat anti-rat IgG and the appropriate substrates as described by the manufacturer (Amersham Pharmacia Biotech, Piscataway, NJ).
RESULTS
Association of Centrosomes with Isolated Apical Membranes from the Chicken Liver
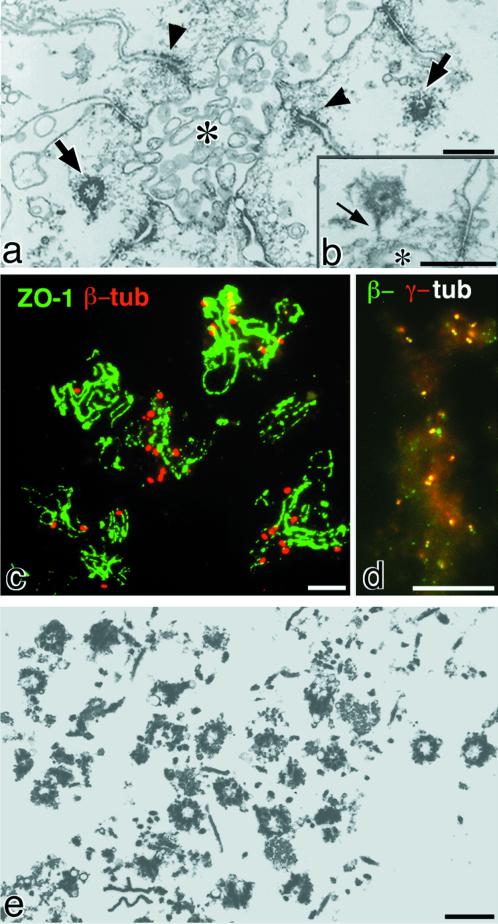
On ultrathin-section electron microscopy, the bile canaliculi fraction isolated from the chicken liver was shown to be enriched in centrosomes (Figure 1a). Close inspection identified some linking fibrous structures between the centrosomes and apical membranes/intercellular junctions (Figure 1b). When the isolated bile canaliculi were attached to coverslips, fixed, and double stained with anti-β-tubulin mAb/anti-ZO-1 pAb, anti-ZO-1 stained two parallel lines (tight junctions) for each bile canaliculus, because it has been clarified that ZO-1 is associated with the tight junctional membrane proteins, claudins and occludin (Tsukita and Tsukita, 1989; Furuse et al., 1993; Itoh et al., 1999). Interestingly, many β-tubulin-positive dots were associated with individual isolated bile canaliculi (Figure 1c). Most of these dots, which were occasionally resolved into two paired dots, were also recognized by anti-γ-tubulin mAb, indicating that numerous centrosomes are associated with the isolated bile canaliculi (Figure 1d).
Figure 1.
Centrosomes in the isolated bile canaliculi from chicken liver. (a) Ultrathin sections of the isolated bile canaliculi. The lumen of the bile canaliculus (asterisk) is delineated with apical membranes of several surrounding hepatocytes. Note several junctional complexes (arrowheads) and two centrosomes (arrows). (b) Fibrous structures (arrow) were occasionally detected between centrosomes and apical membranes (asterisk). (c and d) Double immunofluorescence staining of the isolated bile canaliculi on coverslips with anti-ZO-1 pAb (green)/anti-β-tubulin (tub) mAb (red) (c) or with anti-β-tubulin mAb (green)/anti-γ-tubulin pAb (red) (d). Many β-/γ-tubulin double-positive dots, i.e., centrosomes, were associated with individual isolated bile canaliculi, the tight junctions of which were visualized as parallel lines with anti-ZO-1 pAb. (e) Ultrathin sections of isolated centrosomes from the bile canaliculi fraction. In addition to centrosomes, contamination with fragmented membranous structures, fibrous structures such as collagen fibers, and amorphous structures can be seen. Bars: (a, b, and e) 0. 5 μm; (c and d) 10 μm.
Preparation of Centrosome-enriched Fraction from Isolated Bile Canaliculi and Production of Centrosome-specific mAbs
The bile canaliculi fraction isolated from the chicken liver thus appeared to provide a useful source for mass isolation of centrosomes from animal cells. When the isolated bile canaliculi were sonicated and applied to discontinuous sucrose density gradient centrifugation, ultrathin-section electron microscopy revealed that the centrosomes were mostly recovered into the 52–60% sucrose solution in the first gradient, together with the major contaminants of extracellular matrix components and membranous structures. The centrosomes were further purified by the second sucrose density gradient centrifugation in 54–58% sucrose solution. As roughly estimated by thin-section electron microscopy (Figure 1e), almost 200 million centrosomes were recovered per preparation from 200 chicks. However, the quantity and purity of this fraction were not adequate for identification of centrosomal components by directly determining the aa sequences of individual bands on SDS-PAGE. Therefore, we used the 52–60% sucrose solution of the first density gradient as antigens, which contained a sufficient amount of centrosomes to produce centrosome-specific mAbs in rats. The centrosome-specific mAbs were screened by immunofluorescence microscopy with the isolated bile canaliculi on coverslips and a chicken liver cell line, LMH cells. Among a large number of centrosome-specific mAbs obtained through this type of screening procedure, we characterized three independent mAbs that recognized the same centrosomal component.
Identification of Odf2 as a Centrosomal Component
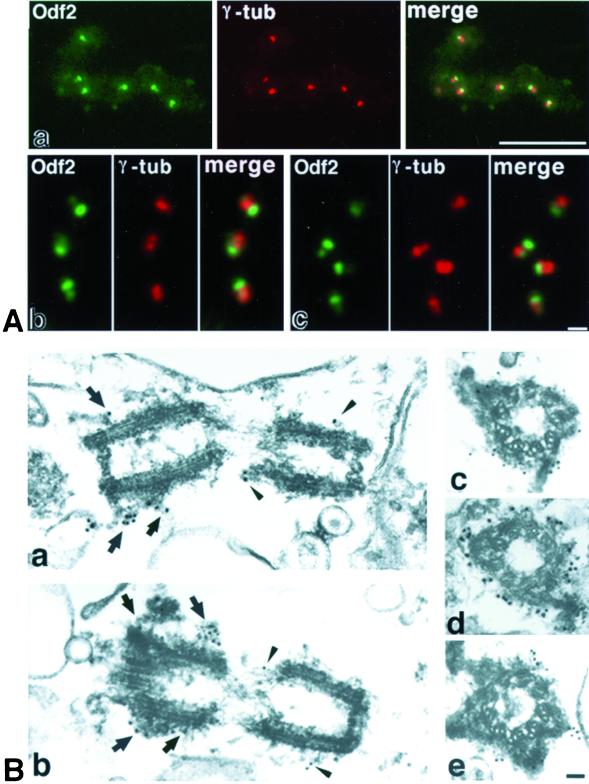
When the isolated chicken bile canaliculi were attached to the polylysine-coated coverslips and processed for immunofluorescence microscopy, the three independent mAbs, such as mAb101, mAb184, and mAb1019, stained the centrosomal region in which a pair of γ-tubulin-positive dots were seen (Figure 3Aa). The mAbs detected a band of ∼90 kDa after immunoblotting of isolated bile canaliculi, although it was not clear whether these mAbs recognized the same antigen (Figure 2A). With the use of mAb101, we then screened ∼3 × 105 plaques from a λgt11 cDNA library established from the chicken liver, and we cloned one positive phage recombinant, 101, which included a full-length cDNA. Its deduced aa sequence (659 aa) indicated that 101 encoded the chicken homologue of Odf2 (Figure 2B), which was initially identified as a major constituent of the outer dense fiber of mouse/rat sperm (Brohmann et al., 1997; Shao et al., 1997; Hoyer-Fender et al., 1998). The aa sequence of the C-terminal region of the polypeptide encoded by 101 was different from that of mouse Odf2. Because various forms of alternatively spliced Odf2 have been reported, it may be reasonable to conclude that 101-encoded ∼90-kDa protein is chicken Odf2.
Figure 2.
Identification of Odf2 as a centrosomal component. (A) Immunoblotting analyses of the isolated bile canaliculi from the chicken liver with three independent anti-centrosome mAbs, mAb101, mAb184, and mAb1019. All of the mAbs detected a band of approximately 90 kDa (arrow). CBB, Coomassie brilliant blue staining. (B) The deduced aa sequence of the antigen for mAb101. This antigen showed striking similarity to mouse Odf2/1(M. Odf2/1) (an isotype of Odf2), although its C-terminal fragment sequence is different from that of mouse Odf2/1. Conserved aa are boxed. This antigen was designated as chicken Odf2 (C. Odf2). (C) Reactivities of mAb101, mAb184, and mAb1019 with chicken Odf2 and mouse Odf2/1. GST-fusion proteins with full-length (c-F), parts of C-terminal half (aa 297–659 [c-C1], aa 297–630 [c-C2], and aa 297–588 [c-C3]) of chicken Odf2 and full-length of mouse Odf2/1 (m-F) were produced in E. coli, and then the crude lysate of E. coli was immunoblotted with three mAbs. D, Localization of exogenously expressed Odf2. HA-tagged chicken Odf2 (a) or mouse Odf2/1 (b) was expressed in human HeLa cells, followed by double immunofluorescence staining with anti-HA mAb (HA-tag; green)/anti-γ-tubulin pAb (γ-tub; red). In cells transiently expressing a large amount of chicken Odf2 (a), Odf2 formed huge fibrous aggregates throughout the cytoplasm in which centrosomes were detected by γ-tubulin staining. In stable transfectants expressing lower levels of Odf2 (b), mouse Odf2/1 showed centrosomal localization. Bars, 10 μm.
We then produced the recombinant full-length, N-terminal half (1–256 aa), and C-terminal half (aa 257–659) of chicken Odf2 as well as full-length mouse Odf2/1 (one isotype of mouse Odf2) in E. coli and examined whether the recombinant proteins were detected by mAb101, mAb184, and mAb1019 in immunoblotting (Figure 2C). The mAb101, mAb184, and mAb1019 recognized the GST-fusion protein of full-length and the C-terminal half (aa 257–659) of chicken Odf2 and were cross-reacted with recombinant mouse Odf2/1. Next, to determine the epitopes of the mAbs in the C-terminal half, GST-fusion proteins containing residues 297–588 and 297–530 of chicken Odf2 were produced (Figure 2C). MAb1019 recognized the residues 297–588 and 297–530 of chicken Odf2. In contrast, mAb184 recognized the residues 297–588, but not 297–530, whereas mAb101 did not recognize any residues. These findings indicated that the three centrosome-specific mAbs recognized distinct epitopes of the same antigen, i.e., chicken and mouse Odf2.
To confirm the centrosomal localization of Odf2, the cDNA that encodes HA-tagged chicken Odf2 or mouse Odf2/1 was introduced into HeLa cells, and the transfectants were double stained with anti-HA pAb/anti-γ-tubulin mAb. In transfectants transiently expressing a large amount of Odf2, Odf2 formed large aggregates and fibrous networks throughout the cytoplasm (Figure 2Da). When the expression level of Odf2 was relatively low, the exogenously expressed HA-tagged mouse/chicken Odf2 was concentrated at centrosomes (Figure 2Db). Curiously, Odf2 with a molecular mass of ∼90 kDa was originally believed to be exclusively expressed in spermatogenic cells to form very specialized fibrous structures, such as outer dense fibers, in the sperm tail (Brohmann et al., 1997; Hoyer-Fender et al., 1998; Petersen et al., 1999). To clarify this matter, we performed reverse transcription-PCR analyses to detect Odf2 mRNA in various mouse cell lines and tissues and found that Odf2 was widely expressed, although the levels of its expression in other tissues were lower than that in sperm cells (Nakagawa, Yamane, Okanoue, Tsukita, and Tsukita, unpublished results).
Centrosomal Localization of Odf2 in Isolated Bile Canaliculi
To examine the detailed localization of Odf2 in centrosomes, we first double stained the isolated bile canaliculi on coverslips with anti-γ-tubulin pAb and one of anti-Odf2 mAbs, such as mAb101, mAb184, and mAb1019. Anti-Odf2 mAbs labeled the centrosomes in essentially the same pattern. As shown in Figure 3A, each centrosome was resolved into two dots, both of which showed staining for γ-tubulin. In contrast, one dot in the pair was stained more intensely for Odf2 than the other dot. This peculiar distribution of Odf2 within the centrosomes was confirmed by immunoelectron microscopy. The isolated bile canaliculi were labeled with mAb101, followed by incubation with anti-rat IgG-conjugated 15- or 10-nm gold particles (Figure 3B). As reported in various types of cells (Rieder and Borisy, 1982) and also in the centrosomes associated with isolated bile canaliculi, the appearance of the electron-dense cloud or fibrous structures of the centrosome matrix varied between paired centrioles as well as along the proximal-distal axis of individual centrioles. In one of the paired centrioles, the surrounding fibrous structures were thicker, forming appendages in the distal region, which was thus judged to be the mother centriole. As shown in Figure 3B, anti-Odf2 mAb specifically recognized these fibrous structures surrounding centrioles including distal end appendages, indicating the preferential labeling of the mother centrioles.
Figure 3.
Localization of Odf2 in the isolated bile canaliculi. (A) Double immunofluorescence staining of the isolated bile canaliculi on coverslips with an anti-Odf2 mAb 101 (Odf2; green)/anti-γ-tubulin mAb (γ-tub; red). Each centrosome was resolved into two dots, both of which were stained equally for γ-tubulin at low (a) and high (b and c) magnifications. In sharp contrast, one dot of the pair was stained more intensely for Odf2 than the other dot. Bars: top, 10 μm; bottom, 1 μm. (B) Immunoelectron microscopic staining of the isolated bile canaliculi with anti-Odf2 mAb (mAb101) and then with anti-rat IgG pAb conjugated with 15-nm (a, b, and d) or 10-nm (c and e) gold particles. In the longitudinal (a and b) and transverse (c–e) sectional images of centrioles, the high levels of gold labeling of Odf2 were associated with the mother centrioles, especially with the distal appendages (arrows), whereas the lower levels were associated with daughter centrioles (arrowheads). Bar, 200 nm.
Centrosomal Localization of Odf2 in Cultured Cells
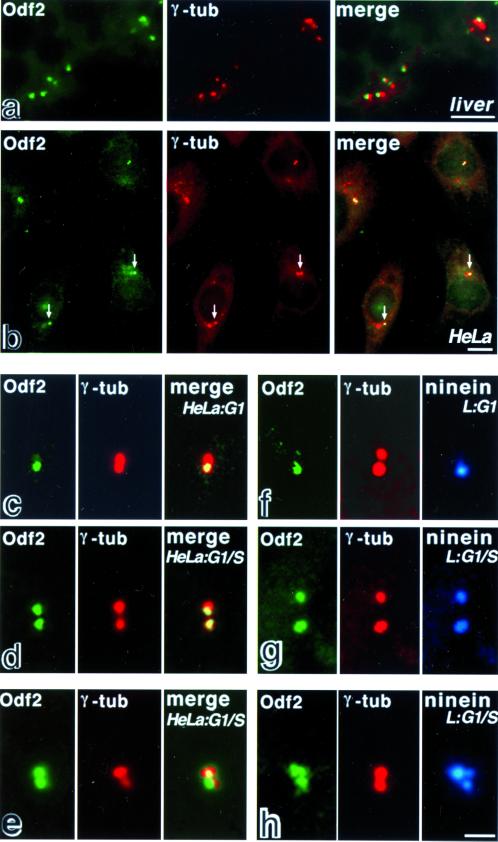
When frozen sections of the chicken liver were double stained with anti-Odf2 mAb (mAb101) and anti-γ-tubulin pAb, Odf2 of all centrosomes in hepatocytes appeared to be preferentially associated with one of the paired γ-tubulin-positive centrioles (Figure 4a). In contrast, in cultured cells, such as human HeLa cells and mouse L cells, the ratio of staining intensity with anti-Odf2 mAb between paired centrioles appeared to vary significantly among cells (Figure 4b). This finding led us to examine cell cycle-dependent changes in the localization of Odf2 within centrosomes, because another centrosomal protein, such as ninein, was reported to be associated primarily with the mother centriole during G1-phase but with the paired centrioles during S/G2-phase (Mogensen et al., 2000; Piel et al., 2000). We then partially synchronized HeLa and L cells at M phase by mitotic shake-off (Mariani et al., 1981). When double stained with anti-Odf2 mAb and anti-γ-tubulin pAb 4 h after replating, in most of the cells only one of the paired γ-tubulin-positive centrioles showed strong staining for Odf2 (Figure 4c). Triple staining with anti-Odf2 mAb, anti-γ-tubulin pAb and anti-ninein pAb revealed that within individual centrosomes ninein was preferentially associated with the Odf2-positive centriole (Figure 4f). These observations indicated that under these culture conditions the majority of cells were in G1-phase and that Odf2 was primarily associated with the mother centriole. At 8 h after replating, the cells were proceeded into G1/S-phase. In the cells before duplication of centrioles, Odf2 and ninein were associated equally with both paired γ-tubulin-positive centrioles (Figure 4, d and g), although the distributions of these three proteins were not precisely identical (Figure 4, e and h). Furthermore, when cultured human and mouse cells were treated with nocodazole to depolymerize intracellular MTs, Odf2 was still associated with centrosomes in the same pattern as that before nocodazole treatment. It suggested that Odf2 is localized at centrosomes in an MT-independent manner (Nakagawa, Yamane, Okanoue, Tsukita, and Tsukita, unpublished results).
Figure 4.
Subcellular localization of endogenous Odf2 in the liver and cultured cells. (a and b) Localization of Odf2 in frozen sections of the chicken liver and cultured human HeLa cells at low magnification. The samples were double stained with anti-Odf2 mAb101 (Odf2; green) and anti-γ-tubulin mAb (γ-tub; red). In the liver (a), Odf2 appeared to be preferentially associated with one of the paired γ-tubulin-positive centrioles. In HeLa cells (b), the ratio of staining intensity with anti-Odf2 mAb between paired centrioles appeared to vary among cells. In the majority of cells, both of the paired centrioles were stained equally. However, in some cells, one of the paired centrioles was stained more intensely than the other (arrows). (c–e) Cell cycle-dependent localization of Odf2 in the centrosomes of human HeLa cells at higher magnification. The cells were double stained with anti-Odf2 mAb101 (Odf2; green) and anti-γ-tubulin mAb (γ-tub; red). In G1-phase (c), Odf2 (Odf2; green) was preferentially associated with the mother centriole, whereas γ-tubulin (γ-tub; red) was equally associated with both centrioles. Toward G1/S-phase before duplication of centrioles, Odf2 (Odf2; green) was associated almost equally with both centrioles (γ-tub; red; d and e). The distributions of Odf2 and γ-tubulin were not precisely identical (e). (f–h) Cell cycle-dependent localization of Odf2 and ninein in the centrosomes of mouse L cells at higher magnification. The cells were triple stained with anti-Odf2 mAb101 (Odf2; green), anti-γ-tubulin mAb (γ-tub; red), and anti-ninein pAb(ninein; blue). In G1-phase (f), Odf2 (Odf2; green) was preferentially associated with the mother centriole, which was identified as the ninein staining. The Cy5 staining of ninein was pseudocolored in blue. Toward G1/S-phase before duplication (g and h), Odf2 and ninein were associated equally with both of the paired centrioles. The distributions of Odf2, γ-tubulin and ninein were not precisely identical (e and h). Bars: (a and b) 5 μm; (c–h) 2 μm.
Odf2 as a Constituent of the KI-resistant Centrosome Matrix That Recruits γ-Tubulin
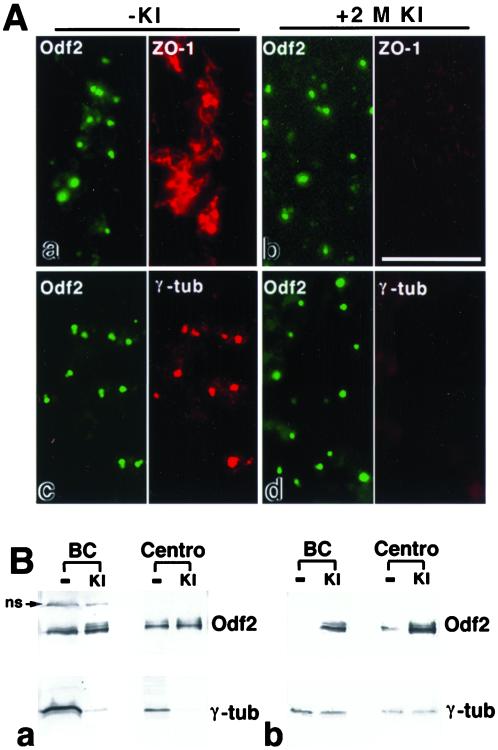
As described in the Introduction, PCM can be subdivided into two fractions, such as KI soluble and KI insoluble (Klotz et al., 1990; Moritz et al., 1998; Schnackenberg et al., 1998, 2000). To examine the KI solubility of Odf2, chicken bile canaliculi on coverslips were treated with 2 M KI at room temperature for 20 min and double stained with anti-Odf2 mAb (mAb101)/anti-ZO-1 pAb or with anti-Odf2 mAb/anti-γ-tubulin mAb. As shown in Figure 5A, 2 M KI completely removed γ-tubulin and ZO-1 from the isolated bile canaliculi. In contrast, Odf2 was highly resistant to KI extraction, maintaining the granular structural integrity. These results suggest that Odf2 is a KI-resistant component of the centrosome matrix.
Figure 5.
KI insolubility of Odf2 in centrosomes. (A) immunofluorescence analysis of the untreated bile canaliculi (a and c; −KI) or 2 M KI-treated bile canaliculi (b and d; +2 M KI) on coverslips were doubly stained with anti-Odf2 mAb101 (Odf2; green)/anti-ZO-1 pAb (ZO-1; red; a and b) or with mAb101(Odf2; green)/anti-γ-tubulin mAb (γ-tub; red; c and d). Incubation (20 min) with 2 M KI removed ZO-1 and γ-tubulin from bile canaliculi, whereas Odf2 was highly resistant to KI extraction. Bar, 10 μm. (B) Western blot analysis of the untreated (−) or 2 M KI-treated (KI) samples of bile canaliculi (BC) or isolated centrosomes (Centro). The untreated or KI-extracted samples, prepared from the bile canaliculi (30 μg) and isolated centrosome fractions (3 μg), were applied to the SDS-PAGE and blotted with anti-Odf2 mAb1019 and anti-γ-tubulin mAbs (a). Because >90% of the total proteins of the bile canaliculi and isolated centrosomes were extracted by the KI treatment, the total protein applied on the gel is reduced to <10% in KI-treated samples as compared with the untreated samples. However, the signals for Odf2 were almost the same in their intensity between the KI-insoluble and the untreated samples in the bile canaliculi as well as the isolated centrosomes, indicating high resistance of Odf2 to the extraction by KI (ns; nonspecific signal; see Figure 2A). In contrast, the signals for γ-tubulin were extremely attenuated by the KI treatment, indicating high extractability of γ-tubulin by KI. Next, to estimate the degree of enrichment of Odf2 into the KI-insoluble fractions, the gels loaded with equal protein (3 μg) from the untreated and KI-treated samples of the bile canaliculi or isolated centrosomes were blotted with anti-Odf2 mAb1019 and anti-γ-tubulin mAb, with the result that Odf2 is highly enriched in the KI-treated samples versus the untreated samples (b).
Next, to examine the insolubility of Odf2 against KI by the biochemical gel/Western blot analysis, the same amounts of the bile canaliculi and isolated centrosome fractions, before or after the KI treatment, were applied to the SDS-PAGE (Figure 5Ba). Although >90% of the total proteins of the bile canliculi and isolated centrosomes were extracted by the KI treatment, the signals for Odf2 were almost the same in their intensity in the KI-insoluble fractions as compared with the untreated fractions in the bile canaliculi and isolated centrosomes. In sharp contrast, the signals for γ-tubulin were extremely attenuated by the KI treatment. These biochemical results were highly consistent with those obtained from immunofluorescence microscopy in that Odf2, but not γ-tubulin, was highly insoluble to KI. Then, to estimate the degree of enrichment, the gels loaded with equal protein from the untreated and KI-treated samples of bile canaliculi or isolated centrosomes, revealed that Odf2, but not γ-tubulin, is highly enriched in the KI-treated samples versus the untreated samples (Figure 5Bb). We, therefore, came to the conclusion that Odf2 is the first example of a KI-resistant component of the centrosome matrix.
DISCUSSION
Although centrioles have long been studied by cell biologists, our knowledge of the structure and function of centrioles is still fragmentary. How centrioles assemble, duplicate, and nucleate MTs is still unknown. This is partly due to a lack of information concerning the molecular components of centrosomes in animal cells. The major barrier against the biochemical identification of centrosomal components is the difficulty to isolate a sufficient quantity of centrosomes (Mitchison and Kirschner, 1984, 1986; Bornens et al., 1987; Moritz et al., 1995; Vogel et al., 1997; Gräf et al., 1998). In our study, we found that the centrosomes are enriched in the isolated bile canaliculi fraction through their direct association with apical/junctional membranes, and we succeeded for the first time in establishing a procedure for mass isolation of centrosomes from an animal organ. Because this fraction was obtained from chicken, the isolated centrosomes could be used as powerful antigens to produce mAbs in rats. Actually, we obtained a large number of mAbs that specifically stained centrosomes. In our study, three independent mAbs were characterized that recognized an antigen of ∼90 kDa. Cloning of cDNA identified the antigen as Odf2. This was originally believed to be exclusively expressed in the spermatogenic cells to form very specialized fibrous structures, such as outer dense fibers, in sperm tails (Brohmann et al., 1997; Hoyer-Fender et al., 1998; Petersen et al., 1999). However, as confirmed by immunolocalization and transfection analyses as well as RT-PCR analyses of Odf2 in various mouse cell lines and tissues, Odf2 appears to be a general component of centrosome matrices. As a centrosomal component, Odf2 showed two characteristic features. First, the distribution of this protein in individual centrosomes changed in a cell cycle-dependent manner and it changed between the mother and daughter centrioles. Second, this protein was highly resistant to KI extraction, showing an MT-independent centrosomal localization. In individual centrosomes detected in the isolated bile canaliculi as well as in frozen sections of the liver, one centriole was stained more intensely with anti-Odf2 mAb than the other. Immunoelectron microscopy of the isolated bile canaliculi revealed that anti-Odf2 mAb more strongly labeled the fibrous structures around the mother centrioles, which were characterized by the distal appendages (Paintrand et al., 1992). Thus, Odf2 is likely to be preferentially associated with the mother centriole in G0-phase. Also in G1-phase, as shown in synchronized cells such as HeLa and L cells, Odf2 was preferentially, but not exclusively, enriched around the mother centrioles, which were identified by labeling with anti-ninein pAb (Mogensen et al., 2000). Interestingly, again like ninein, toward S/G2-phase before duplication Odf2 was associated equally with the mother and daughter centrioles. In contrast, cenexin is also reportedly associated with the mother centrioles but not with the daughter centrioles in any phases during cell cycle (Lange and Gull, 1995). At present, the physiological relevance of this peculiar behavior of Odf2 and ninein as well as cenexin remains unknown. However, recent observations clearly showed that in G1-phase the mother centrioles, but not the daughters, reside in the center of the MT arrays (Mogensen et al., 2000; Piel et al., 2000). Therefore, it is likely that Odf2, together with ninein, plays some important role in anchoring MTs to centrosomes, i.e., the mother centriole (Mogensen et al., 2000). In this respect, the possible direct interaction, if any, between Odf2 and ninein should be elucidated, because the distribution of these proteins within centrosomes is very similar at the immunofluorescence level and the electron microscopic level as well.
PCM can be subdivided into two fractions, such as KI soluble and KI insoluble (Klotz et al., 1990; Moritz et al., 1998; Schiebel, 2000; Schnackenberg et al., 2000). The KI-insoluble remnant of centrosomes, the centrosome scaffold, was reported to look like fine fibrous structures under the electron microscope, although its molecular components remained to be identified (Schnackenberg et al., 1998). On the other hand, Odf2 was initially identified as a major component of very stable fibrous structures in sperm tails, and our present immunoelectron microscopic study identified Odf2 as a component of the PCM fibrous structures (Figure 3). These findings led to the speculation that Odf2 is a general constituent of the centrosome matrix. Actually, Odf2 in the isolated bile canaliculi was highly resistant to extraction with 2 M KI, as shown by the gel/western blot and immunofluorescence analyses. Thus, we concluded that Odf2 is a general constituent of the KI-insoluble centrosome matrix. Because it has been reported that γ-TuRC in the crude extracts of Drosophila embryos and Spisula oocytes is recruited to the KI-insoluble centrosome matrix, a question has arisen as to whether Odf2 functions as a scaffold to recruit centrosomal proteins, including γ-tubulin. This point should be clarified in the future. However, in our preliminary experiments, when KI-treated bile canaliculi on coverslips were incubated with the low-salt extract of isolated bile canaliculi, which included a substantial amount of γ-tubulin, γ-tubulin was shown to be efficiently recruited to the Odf2-positive granular structures, as shown by immunofluorescence for Odf2 and γ-tubulin (Nakagawa, Yamane, Okanoue, Tsukita, and Tsukita, unpublished results). This recruitment of γ-tubulin was suppressed by the preincubation with one of the anti-Odf2 mAbs, mAb1019, but with neither of the other mAbs nor rat IgG (Nakagawa, Yamane, Okanoue, Tsukita, and Tsukita, unpublished results). It suggests that Odf2 is involved in the recruitment of γ-tubulin into centrosomes, directly or indirectly. Thus, taken together with the result that Odf2 is localized at centrosomes in a nocodazole-insensitive, MT-independent manner, it is likely that Odf2 plays a role in anchoring nucleating sites rather than MTs to the centrioles.
Finally, we should discuss the relationship between Odf2 in centrosomes and that in sperm tails. Because of the structural continuity of the outer dense fiber toward the outer dense fibers in sperm cells as revealed by conventional electron microcopy (Fawcett, 1975), it is likely that Odf2 is a general scaffold protein of centrosome matrices and that in sperm tails this protein is utilized to form outer dense fibers. To date, two unrelated proteins, Odf1 and Odf2, have been identified as the major components of outer dense fibers of sperm tails (Schalles et al., 1998; Shao et al., 1999; Mogensen et al., 2000). Recently, as an Odf1-binding protein, Spag4 was identified as a molecular linker between axonemes and outer dense fibers (Shao et al., 1999). If the outer dense fiber is a specialized variation of PCM in sperm cells, Odf1, Spag4, or related proteins may also constitute the centrosome matrix. Furthermore, recent analyses have suggested that outer dense fibers in sperm cells are involved in the regulation of the motility of axonemes through not only their elastic properties but also their affinity to Rho-dependent signaling molecules (Hinsch et al., 1993; Fujita et al., 2000). These findings suggest that Odf2 in the centrosome matrix would be more actively involved in regulation of centrosomes in nonsperm cells. These points should be examined in future studies.
ACKNOWLEDGMENTS
We thank Dr. M. Furuse for his valuable suggestions regarding the procedure for isolating centrosomes from the chicken bile canaliculi fraction and Ms. J. Yamane for her assistance in isolating bile canaliculi. We also thank Dr. M. Bornens for generously providing the anti-ninein pAb. The E7 hybridoma developed by Dr. M. Klymkowsky was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, under contract NO1-HD-7-3263 from the National Institute of Child Health and Human Development. This work was supported in part by a Grant-in-Aid for Scientific Research (B) (to Sa. Tsukita) and a Grant-in-Aid for Cancer Research (to Sh. Tsukita) from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- Bornens M, Paintrand M, Berges J, Marty MC, Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil Cytoskeleton. 1987;8:238–249. doi: 10.1002/cm.970080305. [DOI] [PubMed] [Google Scholar]
- Brohmann H, Pinnecke S, Hoyer-Fender S. Identification and characterization of new cDNAs encoding outer dense fiber proteins of rat sperm. J Biol Chem. 1997;272:10327–10332. doi: 10.1074/jbc.272.15.10327. [DOI] [PubMed] [Google Scholar]
- Chang P, Stearns T. Delta-tubulin and epsilon-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat Cell Biol. 2000;2:30–35. doi: 10.1038/71350. [DOI] [PubMed] [Google Scholar]
- Chu DTW, Klymkowsky MW. The appearance of acetylated alpha-tubulin during early development and cellular differentiation in Xenopus. Dev Biol. 1989;136:104–117. doi: 10.1016/0012-1606(89)90134-6. [DOI] [PubMed] [Google Scholar]
- Dictenberg JB, Zimmerman W, Sparks CA, Young A, Vidair C, Zheng Y, Carrington W, Fay FS, Doxsey SJ. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW. The mammalian spermatozoon. Dev Biol. 1975;44:394–436. doi: 10.1016/0012-1606(75)90411-x. [DOI] [PubMed] [Google Scholar]
- Felix MA, Antony C, Wright M, Maro B. Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Nakamura K, Kato T, Watanabe N, Ishizaki T, Kimura K, Mizoguchi A, Narumiya S. Ropporin, a sperm-specific binding protein of rhophilin, that is localized in the fibrous sheath of sperm flagella. J Cell Sci. 2000;113:103–112. doi: 10.1242/jcs.113.1.103. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf R, Euteneuer U, Ueda M, Schliwa M. Isolation of nucleation-competent centrosomes from Dictyostelium discoideum. Eur J Cell Biol. 1998;76:167–175. doi: 10.1016/S0171-9335(98)80031-9. [DOI] [PubMed] [Google Scholar]
- Hinsch KD, Habermann B, Just I, Hinsch E, Pfisterer S, Schill WB, Aktories K. ADP-ribosylation of Rho proteins inhibits sperm motility. FEBS Lett. 1993;334:32–36. doi: 10.1016/0014-5793(93)81674-o. [DOI] [PubMed] [Google Scholar]
- Hoyer-Fender S, Petersen C, Brohmann H, Rhee K, Wolgemuth DJ. Mouse Odf2 cDNAs consist of evolutionary conserved as well as highly variable sequences and encode outer dense fiber proteins of the sperm tail. Mol Reprod Dev. 1998;51:167–175. doi: 10.1002/(SICI)1098-2795(199810)51:2<167::AID-MRD6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Itoh M, Morita K, Kubota K, Tsukita Sh. Direct binding of three tight junction-associated MAGUKs, Zo-1, Zo-2, and Zo-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi HC, Palacios MJ, McNamara L, Cleveland DW. γ-Tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Kellogg DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Kenney J, Karsenti E, Gowen B, Fuller SD. Three-dimensional reconstruction of the mammalian centriole from cryoelectron micrographs: the use of common lines for orientation and alignment. J Struct Biol. 1997;120:320–328. doi: 10.1006/jsbi.1997.3922. [DOI] [PubMed] [Google Scholar]
- Klotz C, Dabauvalle MC, Paintrand M, Weber T, Bornens M, Karsenti E. Parthenogenesis in Xenopus eggs requires centrosomal integrity. J Cell Biol. 1990;110:405–415. doi: 10.1083/jcb.110.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M, Schiebel E. Receptors determine the cellular localization of a gamma-tubulin complex and thereby the site of microtubule formation. EMBO J. 1998;17:3952–3967. doi: 10.1093/emboj/17.14.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange BMH, Gull K. A molecular marker for centriole maturation in the mammalian cell cycle. J Cell Biol. 1995;130:919–927. doi: 10.1083/jcb.130.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Matsui T, Imamura M, Tsukita Sh, Tsukita Sa. Expression level, subcellular distribution and rho-GDI binding affinity of merlin in comparison with ezrin/radixin/moesin proteins. Oncogene. 1999;18:4788–4797. doi: 10.1038/sj.onc.1202871. [DOI] [PubMed] [Google Scholar]
- Mariani BD, Slate DL, Schimke RT. S phase-specific synthesis of dihydrofolate reductase in Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1981;78:4985–4989. doi: 10.1073/pnas.78.8.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall LG, Stearns T. Cytoskeleton : anatomy of an organizing center. Curr Biol. 1997;7:R754–R756. doi: 10.1016/s0960-9822(06)00396-4. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984;312:232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner M. Isolation of mammalian centrosomes. Methods Enzymol. 1986;134:261–268. doi: 10.1016/0076-6879(86)34094-1. [DOI] [PubMed] [Google Scholar]
- Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci. 2000;113:3013–3023. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Moritz M, Zheng Y, Alberts BM, Oegema K. Recruitment of the gamma-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J Cell Biol. 1998;142:775–786. doi: 10.1083/jcb.142.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SM, Urbani L, Stearns T. The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J Cell Biol. 1998;141:663–674. doi: 10.1083/jcb.141.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley CE, Oakley BR. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Paintrand M, Moudjou M, Delacroix H, Bornens M. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J Struct Biol. 1992;108:107–128. doi: 10.1016/1047-8477(92)90011-x. [DOI] [PubMed] [Google Scholar]
- Petersen C, Fuzesi L, Hoyer-Fender S. Outer dense fiber proteins from human sperm tail: molecular cloning and expression analyses of two cDNA transcripts encoding proteins of approximately 70 kDa. Mol Hum Reprod. 1999;5:627–635. doi: 10.1093/molehr/5.7.627. [DOI] [PubMed] [Google Scholar]
- Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles centrosome activity and behavior in vertebrate cells. J Cell Biol. 2000;149:317–329. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Borisy GG. The centrosome cycle in PtK2 cells: asymmetric distribution and structural change in the pericentriolar material. Biol Cell. 1982;44:117–132. [Google Scholar]
- Schalles U, Shao X, van der Hoorn FA, Oko R. Developmental expression of the 84-kDa ODF sperm protein: localization to both the cortex and medulla of outer dense fibers and to the connecting piece. Dev Biol. 1998;199:250–260. doi: 10.1006/dbio.1998.8931. [DOI] [PubMed] [Google Scholar]
- Schiebel E. Gamma-tubulin complexes: binding to the centrosome, regulation and microtubule nucleation. Curr Opin Cell Biol. 2000;12:113–118. doi: 10.1016/s0955-0674(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Schnackenberg BJ, Hull DR, Balczon RD, Palazzo RE. Reconstitution of microtubule nucleation potential in centrosomes isolated from Spisula solidissima oocytes. J Cell Sci. 2000;113:943–953. doi: 10.1242/jcs.113.6.943. [DOI] [PubMed] [Google Scholar]
- Schnackenberg BJ, Khodjakov A, Rieder CL, Palazzo RE. The disassembly and reassembly of functional centrosomes in vitro. Proc Natl Acad Sci USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Tarnasky HA, Lee JP, Oko R, van der Hoorn FA. Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev Biol. 1999;211:109–123. doi: 10.1006/dbio.1999.9297. [DOI] [PubMed] [Google Scholar]
- Shao X, Tarnasky HA, Schalles U, Oko R, van der Hoorn FA. Interactional cloning of the 84-kDa major outer dense fiber protein Odf84. J Biol Chem. 1997;272:6105–6113. doi: 10.1074/jbc.272.10.6105. [DOI] [PubMed] [Google Scholar]
- Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Stearns T, Winey M. The cell center at 100. Cell. 1997;91:303–309. doi: 10.1016/s0092-8674(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Tassin AM, Celati C, Moudjou M, Bornens M. Characterization of the human homologue of the yeast spc98p and its association with gamma-tubulin. J Cell Biol. 1998;141:689–701. doi: 10.1083/jcb.141.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sa, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T, Tsukita Sh. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol. 1991;113:867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sa, Oishi K, Sato N, Sagara J, Kawai A, Tsukita Sh. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sh, Tsukita Sa. Isolation of cell-to-cell adherens junctions from rat liver. J Cell Biol. 1989;108:31–41. doi: 10.1083/jcb.108.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JM, Stearns T, Rieder CL, Palazzo RE. Centrosomes isolated from Spisula solidissima oocytes contain rings and an unusual stoichiometric ratio of α/β-tubulin. J Cell Biol. 1997;137:193–202. doi: 10.1083/jcb.137.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev IA, Nadezhdina ES. The centrosome and its role in the organization of Mts. Int Rev Cytol. 1987;106:227–293. doi: 10.1016/s0074-7696(08)61714-3. [DOI] [PubMed] [Google Scholar]
- Whitehead CM, Salisbury JL. Regulation and regulatory activities of centrosomes. J Cell Biochem Suppl. 1999;32–33:192–199. doi: 10.1002/(sici)1097-4644(1999)75:32+<192::aid-jcb23>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman W, Sparks CA, Doxsey SJ. Amorphous no longer: the centrosome comes into focus. Curr Opin Cell Biol. 1999;11:122–128. doi: 10.1016/s0955-0674(99)80015-5. [DOI] [PubMed] [Google Scholar]