Abstract
Objectives
Lower pill burden leads to improved antiretroviral therapy (ART) adherence among HIV patients. Simpler dosing regimens have not been widely explored in real-world populations. We retrospectively assessed ART adherence, all-cause hospitalisation risk and costs, and other healthcare utilisation and costs in Medicaid enrollees with HIV treated with ART as a once-daily single-tablet regimen (STR) or two or more pills per day (2+PPD).
Design
Patients with an HIV diagnosis from 2005 to 2009 receiving complete ART (ie, two nucleoside/nucleotide reverse transcriptase inhibitors plus a third agent) for ≥60 days as STR or 2+PPD were selected and followed until the first of (1) discontinuation of the complete ART, (2) loss of enrolment or (3) end of database. Adherence was measured using the medication possession ratio. Monthly all-cause healthcare utilisation and costs were observed from regimen initiation until follow-up end.
Results
Of the 7381 patients who met inclusion criteria, 1797 were treated with STR and 5584 with 2+PPD. STR patients were significantly more likely to reach 95% adherence and had fewer hospitalisations than 2+PPD patients (both p<0.01). STR patients had mean (SD) total monthly costs of $2959 ($4962); 2+PPD patients had $3544 ($5811; p<0.001). Hospital costs accounted for 53.8% and pharmacy costs accounted for 32.5% of this difference. Multivariate analyses found that STR led to a 23% reduction in hospitalisations and a 17% reduction in overall healthcare costs. ART adherence appears to be a key mechanism mediating hospitalisation risk, as patients with ≥95% adherence (regardless of regimen type) had a lower hospitalisation rate compared with <95% adherence.
Conclusions
While it was expected that STR patients would have lower pharmacy costs, we also found that STR patients had fewer hospitalisations and lower hospital costs than 2+PPD patients, resulting in significantly lower total healthcare costs for STR patients.
Keywords: Health Economics, Therapeutics
Article summary.
Article focus
To assess the association between a single-tablet-per-day antiretroviral therapy (ART) regimen (STR) and treatment adherence, all-cause hospitalisation risk and other all-cause healthcare utilisation and costs in a large population of Medicaid enrollees in the USA who received treatment for HIV infection.
Key messages
Patients who received ART as a single-pill per day were significantly more likely to be highly adherent (≥95%) to therapy than patients who received multiple-pill regimens.
Improved adherence among patients treated with STR conferred a lower risk of hospitalisation.
The use of an STR may reduce healthcare costs as well as patient morbidity by decreasing hospitalisation rates, which were higher in patients with less-than-complete medication adherence.
Strengths and limitations of this study
This retrospective analysis used pharmacy refill dates as the best available proxy for pill-taking behaviour; one advantage to this method is that we can identify those patients who may not have had all or some of their medications available on any given date based on an analysis of the timing in between refills, which also notes the amount of medication dispensed each time.
Rates of hospitalisation and correlates of hospitalisation also were assessed from these claims data and should be highly accurate, as should measures of overall monthly healthcare utilisation and costs.
While our prescription claims-based measure of adherence has been found to be a valid proxy for actual medication-taking behaviour, we had no measure of actual patient adherence (ie, daily ingestion/consumption) to the prescriptions they filled.
As we did not randomise patients to the two different treatment regimens, we cannot exclude unmeasured confounding factors that may have influenced our outcomes; although we attempted to control for some of these variables through the use of multivariable models that included some of these factors (substance abuse and psychiatric diagnoses), residual confounding may remain.
We had no laboratory results from patients and thus cannot confirm the degree of virological suppression obtained across the regimens.
Introduction
The 2012 Department of Health and Human Services (DHHS) guidelines state that there are four preferred regimens for initiating HIV treatment in adults. Furthermore, there are multiple alternatives to these four regimens.1
Patients and their treating physicians can choose from among these four preferred regimens, using the criteria of greatest efficacy, safety and simplicity. The latter category is important because regimen simplicity is associated with greater long-term adherence. For example, all four preferred regimens are constructed with a relatively low pill burden (ie, between one and four tablets per day), and three of the four regimens have once-daily dosing. While randomised trials have compared the components of some of these four regimens with each other, until now no studies have compared the four regimens with each other as they are prescribed (ie, in a real-world setting), given that these study trials have been blinded.2 3
Adherence to antiretroviral therapy (ART) is essential for achieving durable clinical outcomes in patients with HIV. Patients with inadequate adherence to ART are at an increased risk for incomplete viral suppression, and unless a new suppressive regimen is quickly constructed to re-establish virological suppression, viraemia is associated with an increased risk of disease progression and death.4–8 It has been suggested that an ART adherence rate of at least 95% is required to achieve a lower risk of virological failure, fewer hospital days and reduced morbidity and mortality in patients with HIV,8 9 although one previous study indicated that viral suppression may be possible at less than 95% adherence.10 In the past several years, the availability of fixed-dose combinations and agents with prolonged half-lives have simplified pill burden and thus increased regimen adherence.1 11 Several clinical trials and cohort studies support the conclusion that once-daily single-tablet regimens (STR) can lead to significantly improved adherence, patient satisfaction and virological outcomes.12–15 For example, among the homeless or marginally housed patients, those receiving an ART regimen composed of a single tablet per day had better virological outcomes and a 26% increase in adherence than patients receiving other multipill regimens.15 One recently published study analysing a claims database noted that compared with various multipill regimens, an STR was associated with increased adherence (as determined by pharmacy refill data). Furthermore, the increased likelihood of complete adherence was associated with a 25% decrease in the rate of hospitalisation.16
In this study, we sought to assess how robust these findings were by analysing similar metrics in a separate data set. The primary objective of this retrospective database analysis was to assess the association between a single-tablet-per-day ART regimen and treatment adherence, all-cause hospitalisation risk and total all-cause healthcare costs in a large population of Medicaid enrollees in the USA who received treatment for HIV infection. The secondary objective of this study was to examine the association between STR and other types of all-cause healthcare utilisation (emergency department, pharmacy, outpatient and other service types) and costs.
Methods
Data for this analysis were taken from the MarketScan Medicaid Multi-State Database, which contains healthcare claims from approximately 30 million Medicaid enrollees from 11 geographically dispersed states. The database includes patient-level demographics; periods of Medicaid enrolment; primary and secondary diagnoses and detailed information about hospitalisations and therapeutic procedures, inpatient and outpatient physician services and prescription drug use. Each medical and pharmacy claim in the database also includes original cost information, which represents direct paid amounts (in US dollars) from Medicaid to providers for each service or prescription. In compliance with the Health Insurance and Portability and Accountability Act of 1996, all data were de-identified to protect the privacy of individual patients, physicians and hospitals. As the data were retrospective, pre-existing and de-identified, RTI International's institutional review board (IRB) determined that this study met all criteria for exemption from requirements of patient consent.
Patients were selected for inclusion if they received at least one HIV or AIDS diagnosis (International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 042.xx) between 1 June 2006 and 31 December 2009. Patients also were required to have evidence of receipt of a complete ART regimen, defined as two nucleoside/nucleotide reverse transcriptase inhibitors plus a third agent (ie, another nucleoside/nucleotide reverse transcriptase inhibitor, a non-nucleoside/nucleotide reverse transcriptase inhibitor, a protease inhibitor (PI), a chemokine receptor R5 antagonist or an integrase inhibitor). The first date of receipt of a complete regimen was termed the index date. ART agents were identified in the claims database by using National Drug Codes associated with relevant generic and brand names. Patients also were required to remain on the complete ART regimen for at least 60 days following their index dates and to have evidence of continuous enrolment in Medicaid during this period. To assess treatment-naïve versus experienced status and baseline comorbidities, patients were required to have at least 6 months of preindex date Medicaid enrolment, with enrolment information available from 1 January 2006 (ie, 6 months before the earliest possible index date).
Patients were grouped into two mutually exclusive cohorts according to the daily pill count of their complete ART regimen. Patients were assigned to the STR cohort if they received an ART regimen consisting of a single tablet (ie, an STR) at any point during the selection window, regardless of prior or subsequent use of other regimens. At the time of this study, only coformulated tenofovir/emtricitabine/efavirenz was available as an STR. Patients were assigned to the two-or-more-pills-per-day (2+PPD) cohort if they received a regimen consisting of 2+PPD during the selection window and if they did not receive an STR at any point during that time.
Patients were followed from the start of their complete ART regimen (ie, after 1 June 2006, the study index date) until the earliest date of regimen discontinuation, disenrolment from the health plan or the end of the database (ie, 31 March 2009). Furthermore, patients receiving 2+PPD were allowed to change medications comprising the regimen, provided that the patients continued to receive a combination of agents that could still be classified as a complete 2+PPD regimen. Patients receiving STR were followed for as long as they remained on STR. Discontinuation was defined as 60 consecutive days in which no refills were observed for any component of the regimen. Women with an ICD-9-CM diagnosis code indicating a pregnancy during the follow-up period were excluded from the analysis because the one available STR is not recommended for pregnant women, and hospitalisations for labour and delivery may have biased results in favour of STR.
Patient characteristics measured at the index date included age, sex and ART classes received (ie, nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside/nucleotide reverse transcriptase inhibitors, PIs, ritonavir boosting therapy or other therapies). The presence of comorbid medical conditions other than HIV or AIDS were assessed during the 6-month preindex period using an established algorithm, the Charlson Comorbidity Index (CCI) score.17 This score is made up of 17 comorbidities (defined by the ICD-9-CM diagnosis and procedure codes), such as myocardial infarction and chronic pulmonary disease, which are weighted to correspond to the severity of the comorbid condition of interest. A higher comorbidity score represents a higher overall comorbidity burden during the preindex period. Additionally, the incidence of other concomitant mental disorders (ICD-9-CM codes 306.xx through 319.xx) and drug and alcohol abuse (ICD-9-CM codes 292.xx and 303.xx through 305.xx) during the 6-month preindex period was also assessed.
Medication adherence was assessed using the medication possession ratio (MPR), which has been shown to be the most widely adopted measure (57% of all studies) in published claims-based analyses of medication adherence18 and has been used in studies of ART adherence among individuals with HIV.19 MPR, which is a proxy for refill compliance, generally measures the proportion of the ART-exposure period in which supply was maintained for all ART components comprising the regimen. Specifically, MPR was calculated as the number of filled prescription days for all ART regimen components (using the days supplied in the pharmacy claims) divided by the number of days from the first observed prescription in the regimen through the earliest of either the exhaustion of the days supplied of the last observed prescription or the end of follow-up. For each patient in our study, MPR was calculated over the period in which the patient remained on his or her ART regimen. For patients in the 2+PPD cohort, late refills and resulting days of missing supply for one or more ART components were all factored against their adherence measurements. For example, patients in the 2+PPD cohort with a supply for only one of the ART components on a given day were considered to have zero adherence for that day. In addition to reporting the mean (SD) MPR achieved, we also reported the numbers and percentages of patients achieving various adherence thresholds (ie, MPRs of 1.0–0.95, 0.94–0.90, 0.89–0.85 and 0.84–0.80, corresponding to 100–95%, 94–90%, 89–85% and 84–80% adherence, respectively).
To further understand adherence to ART regimens, for each patient in the 2+PPD cohort, complete (ie, having a complete regimen), partial (ie, receiving some but not all components of a complete regimen) and no medication days also were assessed. Specifically, we reported the percentage of days that each patient had complete, partial and no medications available, along with the mean number of days that the patient had complete, partial and no medications. Additionally, we also reported the maximum number of consecutive days the patient had either an incomplete regimen or no medications available.
Hospitalisations were identified from the claims database using relevant place of service codes. Hospitalisations were observed from the index date until the earliest date of regimen discontinuation, end of enrolment in the health plan or end of the database. The number and percentage of patients with at least one hospitalisation were reported, along with the mean (SD) number of hospitalisations and the mean (SD) number of inpatient days. Furthermore, we compared and reported the number of hospitalisations per 100 patient-years, along with the rate ratios and 95% CIs, for both cohorts as well as by adherence status (at least 95% vs less than 95%).
For each patient, overall healthcare utilisation and associated costs were aggregated across all encounters, regardless of reason, that were observed during the follow-up period; we reported these costs by average and per-month amounts. The following categories of overall healthcare utilisation and costs were evaluated and reported: inpatient, emergency department, office visit, home health visit, laboratory service, pharmacy, other outpatient care and total. For each category of overall healthcare, the number and percentage of patients, the mean (SD) number of visits per month, and monthly per-patient costs were reported. Additionally, for patients with an inpatient visit, the average number of inpatient days per month among patients with at least one stay during follow-up also was reported. All cost data, which represented payments incurred by the Medicaid system, were standardised at the claim level to 2010 US dollars using the medical care component of the US Consumer Price Index.
All analyses were carried out using SAS (V.9; Cary, North Carolina, USA) statistical software. Descriptive analyses were conducted for all outcome measures and included means and SDs for continuous variables of interest (eg, MPR) and frequency distributions of categorical variables of interest (eg, geographic region). All descriptive analyses were stratified by cohort. Healthcare costs were updated to 2010 US dollars using the medical care component of the consumer price index.
A generalised linear model with a log link and a Poisson distribution was estimated to assess the relationship between the number of pills per day and the number of hospitalisations observed during follow-up. The dependent variable was a count of hospitalisations during exposure to the ART regimen. Additionally, a generalised linear model with a log link and a negative binomial distribution were estimated to assess monthly healthcare costs, adjusted for the patient and treatment characteristics. The dependent variables were monthly total costs and monthly total costs excluding pharmacy costs. For both models, based on a previous work by Sax et al,16 independent variables included the following: treatment regimen received (ie, STR vs 2+PPD), age, sex, CCI score, treatment-naïve status, preindex presence of mental health disorders, preindex presence of alcohol or drug abuse disorders, length of follow-up (in days, hospital model only), and whether or not the patient met a 0.95 adherence threshold (cost model only). For the hospital model, incidence rate ratios (IRRs) were reported for all covariates, along with the mean predicted number of hospitalisations for patients receiving an STR versus patients receiving a 2+PPD. For the cost model, adjusted predicted mean costs were reported.
Results
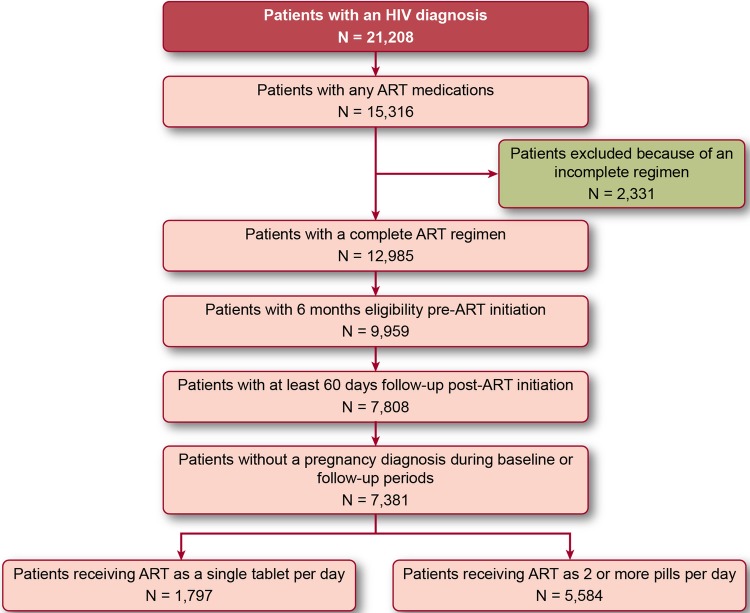
A total of 7381 patients met the selection criteria (figure 1). Of these, 5584 patients (75.7%) received their ART regimen as 2+PPD; 1797 patients (24.3%) received their ART regimen as an STR. On average, patients were approximately 42 years of age. Approximately 46% of patients were female (table 1). Across both cohorts, the average CCI score was approximately the same (mean (SD) 0.67 (1.38) among patients receiving an STR and 0.65 (1.36) among patients receiving 2+PPD). Furthermore, the incidence of concomitant mental disorders and drug and alcohol abuse diagnoses did not vary substantially by cohort. Patients receiving an STR had a mean regimen duration of 348 days; this was approximately 2.8 months shorter than the mean regimen duration of 433 days observed for patients receiving 2+PPD. Forty-seven per cent of patients receiving an STR were treatment naïve, compared with 24.5% of patients receiving 2+PPD.
Figure 1.
Sample selection flow chart.
Table 1.
Characteristics of the study sample, by cohort
| Characteristic | STR (n=1797) |
2+PPD (n=5584) |
p Value | ||
|---|---|---|---|---|---|
| Age (mean (SD)) | 41.6 | (10.56) | 42.32 | (11.37) | 0.0137 |
| Gender (N, %) | |||||
| Male | 945 | 52.59 | 3063 | 54.85 | 0.1123 |
| Female | 852 | 47.41 | 2521 | 45.15 | 0.1439 |
| Race (N, %) | |||||
| White | 387 | 21.54 | 1221 | 21.87 | 0.8893 |
| Black | 1187 | 66.05 | 3658 | 65.51 | 0.6877 |
| Hispanic | 18 | 1.00 | 82 | 1.47 | 0.7844 |
| Other | 204 | 11.35 | 621 | 11.12 | 0.7846 |
| Unknown | 1 | 0.06 | 2 | 0.04 | 0.8766 |
| Basis of medicaid eligibility (N, %) | |||||
| Aged | 1 | 0.06 | 8 | 0.14 | 0.5634 |
| Disabled | 1089 | 60.60 | 4071 | 72.90 | <0.0001 |
| Income | 583 | 32.44 | 1159 | 20.76 | <0.0001 |
| Other | 58 | 3.23 | 202 | 3.61 | 0.8710 |
| Unknown | 65 | 3.62 | 141 | 2.53 | 0.0487 |
| Medicare eligibility (N, %) | |||||
| Not dually eligible | 1791 | 99.67 | 5558 | 99.53 | 0.9987 |
| Dually eligible | 5 | 0.28 | 24 | 0.43 | 0.6523 |
| Unknown | 1 | 0.05 | 2 | 0.04 | 0.9014 |
| Charlson comorbidity index score, mean (SD) | 0.67 | (1.38) | 0.65 | (1.36) | 0.5919 |
| Concomitant mental health and substance abuse comorbidities (N, %) | |||||
| Mental disorders | 382 | 21.26 | 1340 | 24.00 | 0.0456 |
| Drug or alcohol abuse | 338 | 18.81 | 856 | 15.33 | 0.0323 |
| Treatment naïve at index | 853 | 47.47 | 1366 | 24.46 | <0.0001 |
| Regimen length, mean (SD) | 348.17 | (259.32) | 433.46 | (351.50) | <0.0001 |
| Index medications (N, %) | |||||
| NRTI | 1797 | 100.00 | 5584 | 100.00 | – |
| NNRTI | 1797 | 100.00 | 1500 | 26.86 | <0.0001 |
| PI | – | – | 4064 | 72.78 | – |
| Kaletra at index | – | – | 1633 | 40.18 | – |
| Boosted PI at index | – | – | 1664 | 40.94 | – |
| Non-boosted PI at index | – | – | 767 | 18.87 | – |
| PE | – | – | 1712 | 30.66 | – |
| Other | – | – | 87 | 1.56 | – |
2+PPD, two or more pills per day; NNRTI, non-nucleoside/nucleotide reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PE, pharmacokinetic enhancer; PI, protease inhibitor; STR, once-daily single-tablet regimen.
Patients receiving an STR had significantly better adherence than patients receiving 2+PPD (table 2). Approximately 25.3% of patients receiving an STR achieved 95% adherence or greater, compared with 17.4% of patients receiving 2+PPD (p≤0.0001). Mean (SD) MPR was 0.84 (0.14) among patients receiving an STR and 0.80 (0.15) among patients receiving 2+PPD (table 2). Patients in the 2+PPD cohort received a complete regimen for 80.3% of the follow-up period (mean (SD) 361.9 (315.0) days), a partial regimen for 5.6% of the follow-up period (mean (SD) 22.2 (45.6) days), and no available medications for 14.1% of the follow-up period (mean (SD) 49.4 (57.1) days; table 3). Alternatively, patients in the STR cohort received a complete regimen for 84.4% of the follow-up period (mean (SD) 299.4 (234.6) days) and no available medications for 15.6% of the follow-up period (mean (SD) 48.8 (54.2) days), which was a similar percentage of days to patients receiving 2+PPD. Patients receiving an STR had, on average, a maximum of 19.5 (SD 15.9) consecutive days without a complete regimen (ie, either a partial regimen or no medications available); patients receiving 2+PPD had, on average, a maximum of 23.9 (SD 16.7) consecutive days without a complete regimen.
Table 2.
Adherence to antiretroviral therapy, by cohort
| MPR/persistency ratio (N, %) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Number of patients | Mean (SD) MPR |
<0.8 |
0.8–<0.85 |
0.85–<0.9 |
0.9–<0.95 |
0.95–1 |
||||||
| STR | 1797 | 0.84 | (0.14) | 537 | 29.88% | 178 | 9.91 | 243 | 13.52 | 385 | 21.42 | 454 | 25.26 |
| 2+PPD | 5584 | 0.80 | (0.15) | 2255 | 40.38 | 621 | 11.12 | 779 | 13.95 | 957 | 17.14 | 972 | 17.41 |
| Overall | 7381 | 0.81 | (0.15) | 2792 | 37.83 | 799 | 10.83 | 1022 | 13.85 | 1342 | 18.18 | 1426 | 19.32 |
| p Value (1 vs 2) | <0.0001 | <0.0001 | 0.1491 | 0.6477 | <0.0001 | <0.0001 | |||||||
2+PPD, two or more pills per day; MPR, medication possession ratio; STR, once-daily single-tablet regimen.
Table 3.
Summary of incomplete adherence, by cohort
| Adherence characteristic | STR (n=1797) |
2+PPD (n=5584) |
p Value | ||
|---|---|---|---|---|---|
| Percentage of days with complete adherence | 84.42% | 80.37% | <0.0001 | ||
| Percentage of days with partial adherence | – | 5.56% | – | ||
| Percentage of days with no ART medications | 15.58% | 14.07% | 0.0356 | ||
| Complete adherence days, mean (SD) | 299.36 | (234.56) | 361.87 | (315.03) | <0.0001 |
| Partial adherence days, mean (SD) | – | 22.24 | (45.58) | – | |
| Days with no medication available, mean (SD) | 48.81 | (54.24) | 49.35 | (57.11) | 0.0356 |
| Total follow-up duration, mean (SD) | 348.17 | (259.31) | 433.46 | (351.50) | <0.0001 |
| Maximum consecutive gap in therapy mean (SD)* | 19.48 | (15.89) | 23.92 | (16.67) | <0.0001 |
*Represents either days with a partial regimen or days with no medications.
2+PPD, two or more pills per day; ART, antiretroviral therapy; STR, once-daily single-tablet regimen.
Among patients receiving an STR, 21% had at least one hospitalisation, compared with 24.4% of patients receiving 2+PPD (p=0.003; table 4). Among patients with a hospitalisation, those receiving an STR had numerically similar, although significantly fewer, hospitalisations over all available follow-up, when compared with patients receiving 2+PPD (mean (SD) 1.9 (1.6) among patients receiving an STR vs 2.1 (2.2) among patients receiving 2+PPD; p=0.001).
Table 4.
All-cause average per patient healthcare utilisation and costs, by cohort
| Resource used | STR (n=1797) |
2+PPD (n=5584) |
p Value | ||
|---|---|---|---|---|---|
| Hospitalisations | |||||
| Had ≥1 hospital admission (N, %)* | 378 | 21.04 | 1,365 | 24.44 | 0.0031 |
| Number of hospitalisations (overall follow-up) mean (SD)† | 1.88 | (1.59) | 2.1 | (2.23) | 0.0012 |
| Inpatient days (overall follow-up) mean (SD)† | 9.99 | (12.33) | 12.33 | (18.90) | 0.0228 |
| Costs per month, mean (SD) | $834 | ($4480) | $1152 | ($5212) | 0.0203 |
| Emergency room (ER) | |||||
| Had ≥1 ER visit (N, %)* | 903 | 50.25 | 2,749 | 49.23 | 0.4517 |
| Number of visits per month, mean (SD) | 0.97 | (3.00) | 1.01 | (2.99) | 0.6107 |
| Costs per month, mean (SD) | $45 | ($160) | $46 | ($135) | 0.873 |
| Office visits (primary care) (N, %) | |||||
| Had ≥1 office visit (N, %)* | 1509 | 83.97 | 4699 | 84.15 | 0.8576 |
| Number of visits per month, mean (SD) | 1.52 | (3.00) | 1.43 | (2.19) | 0.1669 |
| Costs per month, mean (SD) | $75 | ($229) | $70 | ($291) | 0.5087 |
| Home health (N, %) | |||||
| Had ≥1 home health visit (N, %)* | 504 | 28.05 | 1861 | 33.33 | <0.0001 |
| Number of visits per month, mean (SD) | 0.64 | (3.00) | 0.79 | (3.16) | 0.0625 |
| Costs per month, mean (SD) | $47 | ($198) | $88 | ($642) | 0.007 |
| Laboratory (N, %) | |||||
| Had ≥1 lab order (N, %)* | 1168 | 65.00 | 3530 | 63.22 | 0.1722 |
| Number of lab tests per month, mean (SD) | 1.24 | (2.00) | 1.19 | (1.69) | 0.2962 |
| Costs per month, mean (SD) | $52 | ($94) | $46 | ($120) | 0.0401 |
| Pharmacy (N, %) | |||||
| Had ≥1 pharmacy claim (N, %)* | 1797 | 100.00 | 5584 | 100.00 | – |
| Number of prescriptions per month, mean (SD) | 4.99 | (4.00) | 6.73 | (4.05) | <0.0001 |
| Costs per month, mean (SD) | $1593 | ($1105) | $1779 | ($1307) | <0.0001 |
| OP/ancillary (N, %) | |||||
| Had ≥1 other outpatient/ancillary visit (N, %)* | 1754 | 97.61 | 5469 | 97.94 | 0.3957 |
| Number of visits per month, mean (SD) | 0.15 | (0.00) | 0.14 | (0.13) | 0.0078 |
| Costs per month, mean (SD) | $313 | ($607) | $363 | ($733) | 0.0087 |
| Total healthcare utilisation and costs | |||||
| Had ≥1 medical visit/encounter (N, %)* | 1797 | 100.00 | 5584 | 100.00 | – |
| Number of total encounters per month, mean (SD) | 14.69 | (14.00) | 16.97 | (13.72) | <0.0001 |
| Costs per month, mean (SD) | $2959 | ($4962) | $3544 | ($5811) | 0.0001 |
*Estimated overall available follow-up.
†Among hospitalised patients.
The multivariate Poisson regression model showed that receiving an STR was associated with a significantly lower hospitalisation rate than receiving the 2+PPD regimen (IRR=0.8457; p<0.001; table 5). When the received regimen type was controlled for, we found that patients were significantly more likely to be hospitalised if they had the following characteristics: a concomitant mental disorder diagnosis (vs no concomitant mental disorder diagnosis; IRR=1.2917; p<0.001), a concomitant drug or alcohol abuse diagnosis (vs no concomitant drug or alcohol abuse diagnosis; IRR=2.0357; p<0.001), a CCI score greater than 1 (IRR increased with increasing CCI score, from 2.3779 among patients with a CCI between 1 and 2 to 2.6432 among patients with a CCI greater than 3; all p<0.001), were female (vs male; IRR=1.1069; p=0.003), or were older than 35 years (vs younger than 35 years; IRR increased with increasing age, up to 54 years, from 1.2482 among patients aged 35–44 years to 1.555 among patients aged 45–54 years; both p<0.1). Additionally, the likelihood of a hospitalisation increased slightly with each additional day of follow-up (IRR=1.0013; p<0.0001). Finally, being treatment naïve prior to the index date was predictive of an approximately 13% higher hospitalisation rate as compared with being treatment experienced (IRR=1.1270; p=0.0033).
Table 5.
Predictors of hospitalisation, using multivariate Poisson regression, and controlling for treatment cohort
| Poisson count model |
|||
|---|---|---|---|
| Specification: adherence covariate excluded | Parameter estimate | Incidence rate ratio | p Value |
| Received an STR (vs 2+PPD regimen) | −0.1654 | 0.8475 | 0.0001 |
| Female (vs male) | 0.1003 | 1.1069 | 0.003 |
| Age (vs less than 35) (years) | |||
| 35–44 | 0.1016 | 1.2482 | 0.0669 |
| 45–54 | 0.2217 | 1.5550 | <0.0001 |
| 55+ | 0.4415 | 1.1056 | <0.0001 |
| Charlson comorbidity index score (vs Charlson comorbidity index score less than 1) | |||
| Between 1 and 2 | 0.8662 | 2.3779 | <0.0001 |
| Greater than 2 | 0.972 | 2.6432 | <0.0001 |
| Treatment naïve (vs treatment experienced) | 0.1196 | 1.1270 | 0.0033 |
| Had a mental disorder diagnosis (vs no mental disorder diagnosis) | 0.256 | 1.2917 | <0.0001 |
| Had a drug or alcohol abuse diagnosis (vs no drug or alcohol abuse diagnosis) | 0.7109 | 2.0357 | <0.0001 |
| Length of follow-up (in days) | 0.0013 | 1.0013 | <0.0001 |
2+PPD; two or more pills per day; STR, once-daily single-tablet regimen.
From the Poisson regression analysis described above, we found the adjusted rate of hospitalisation to be significantly lower for patients receiving an STR than for patients receiving 2+PPD (ie, 39.5 hospitalisations per 100 patient-years for patients receiving STR vs 51.2 hospitalisations per 100 patient-years for those receiving 2+PPD; figure 2). These adjusted hospitalisation rates translated to a 23% lower risk of hospitalisation among patients receiving an STR, compared with patients receiving 2+PPD. As shown in figure 3, adherence status seems to be a key mechanism mediating hospitalisation risk as patients with at least 95% adherence (regardless of regimen type) had a statistically significantly lower hospitalisation rate compared with patients with less than 95% adherence. Improved adherence among patients treated with STR therefore appears to confer a lower risk of hospitalisation and associated costs.
Figure 2.
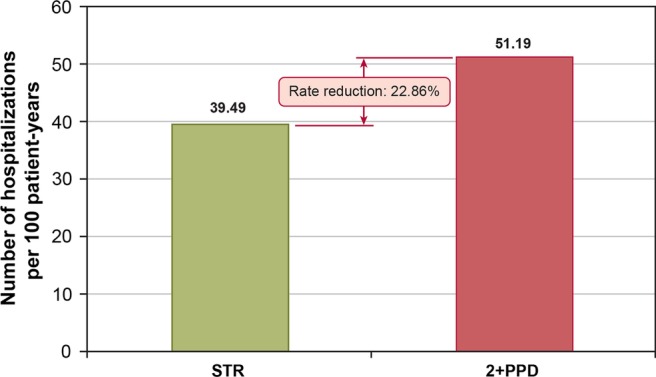
Adjusted rate of hospitalisations per 100 patient-years, by cohort.
Figure 3.
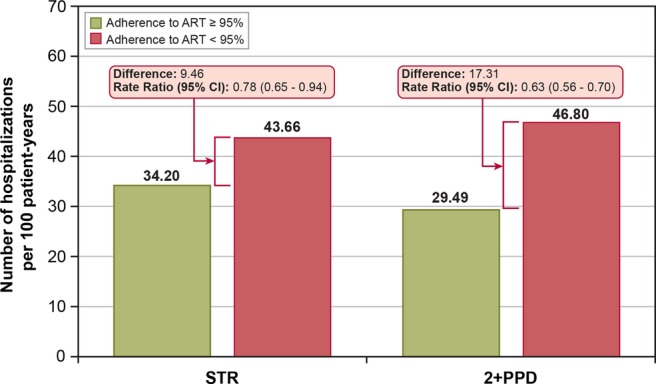
Hospitalisations per 100 patient-years, by cohort and adherence.
Examining other types of healthcare utilisation, the percentage of patients with at least one home health visit was significantly lower among patients receiving STR than for patients receiving 2+PPD (table 4). Between the two cohorts, no differences were observed in the percentage of patients with at least one emergency room, office visit or laboratory claim. Similarly, no significant differences were found in the number of emergency room visits, office visits, home health visits or laboratory claims per month. However, patients who received an STR had significantly lower costs per month associated with inpatient, home health, laboratory, pharmacy, other and total healthcare than patients receiving 2+PPD. Mean (SD) total healthcare costs per month were $2959 ($4962) among patients receiving an STR and $3544 ($5811) among patients receiving 2+PPD; thus, patients receiving an STR accrued, on average per month, $585 less than patients receiving 2+PPD (p<0.001). The largest difference in costs between the two cohorts was observed for inpatient admissions ($317 more for patients receiving 2+PPD), followed by pharmacy costs ($187 more for patients receiving 2+PPD).
When monthly healthcare costs were adjusted for demographic, clinical and treatment characteristics, patients receiving an STR had monthly total costs averaging $2947; patients receiving 2+PPD had monthly total costs averaging $3549 (figure 4). Thus, patients receiving 2+PPD had $602 more in monthly healthcare costs, which corresponded to a 17% reduction in costs associated with STR. Additionally, when monthly healthcare costs, excluding pharmacy costs, were adjusted for demographic, clinical and treatment characteristics, patients receiving an STR had monthly total costs averaging $1370; patients receiving 2+PPD had monthly total costs averaging $1797. Thus, patients receiving 2+PPD had $427 more in adjusted monthly healthcare costs, which corresponded to a 23.8% reduction in costs associated with STR.
Figure 4.
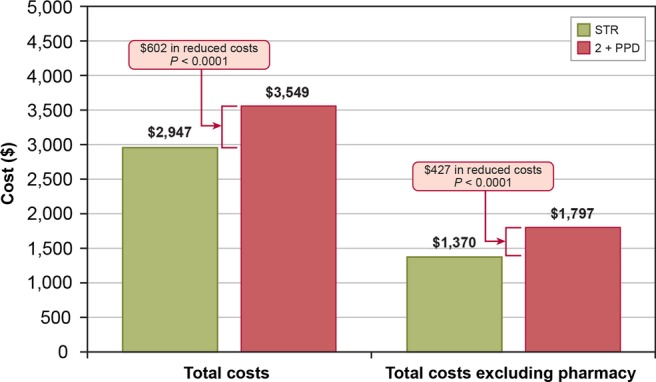
Adjusted monthly healthcare costs, by cohort.
Discussion
This retrospective database analysis examined adherence to ART regimens among patients with HIV infection, using pharmacy refill dates as the best available proxy for pill-taking behaviour. One advantage to this method is that we can identify those patients who may not have had all or some of their medications available on any given date based on an analysis of the timing in between refills, which also notes the amount of medication dispensed each time. The rate of hospitalisation and correlates of hospitalisation also were assessed from these claims data and should be highly accurate, as should the overall monthly healthcare utilisation and costs.
This analysis largely confirms the previous report from Sax et al16: we found that patients receiving an STR had significantly better adherence rates than patients receiving multiple pills per day. Our other finding was that higher rates of adherence were associated with similar or lower rates of hospitalisation, regardless of the regimen; less-than-complete adherence was associated with higher rates of hospitalisation and overall costs. Thus, multiple-pill regimens were associated with lower rates of complete adherence and correspondingly higher overall healthcare costs. We observed a significantly higher rate of hospitalisations occurring in patients receiving multiple-pill regimens (p<0.001) than in patients receiving an STR. The greater total healthcare costs were due to differences in the pharmacy costs of the regimen components as well as the costs of hospitalisations and associated care. Therefore, one implication of our findings is that choosing a multiple-pill regimen for its cost alone might inadvertently result in little to no total healthcare cost-savings for a payer, given the potential risk of more frequent hospitalisations in patients receiving multiple-pill regimens.
Similar to previous studies,4 20 we found that patients who were adherent to therapy were less likely to be hospitalised. Our data demonstrated similar rates of hospitalisations among patients with the highest levels of complete adherence—at least 95%. This was consistent across both treatment cohorts. This finding suggests that the differences observed in the rates of hospitalisations across regimens are primarily due to differences in adherence rates between the STR and 2+PPD regimens rather than any concerns for toxicities. This finding also may partially address the potential contribution of channelling bias, a concern with any observational data set. We found that adherent patients on any regimen have similar rates of hospitalisation, which suggests that there may not have been a consistent bias to prescribe to more clinically immunosuppressed patients or to patients who were at greater risk for hospitalisation due to other factors than a multiple-pill regimen. Furthermore, we found that the outcome of fewer hospitalisations for patients receiving an STR was consistent when we compared hospitalisation risks for treatment-naïve patients with hospitalisation risks for treatment-experienced patients. In the latter group, the impact of stage of illness prior to treatment would be lessened, given the impact of prior treatment on improving pretreatment immunosuppression, with an STR regimen. Of final note regarding channelling bias, previous analyses of Medicaid beneficiaries with HIV have shown that patients receiving ART are completely non-adherent (ie, days with no ART supply/coverage on hand) for approximately 14% of their regimen duration regardless of the number of pills in the regimen.21 This finding suggests that clinicians are not channelling more adherent patients to STRs. Together, these data support the observation that facilitating greater adherence to ART at any stage of illness may result in reducing hospitalisation risk.
One follow-up question our study findings raises is whether the observed reduction in hospitalisation risk and costs with STR was also due to less prevalent chronic comorbidities in patients prescribed STR. To assess this possibility, we replicated key descriptive analyses on hospitalisation rates for patients with no baseline comorbidities as reported by CCI. We found that the majority (∼70%) of STR and 2+PPD patients had no other CCI comorbidities. Among STR patients with no other comorbidities from CCI, 13.9% had a hospitalisation compared with 18.3% of 2+PPD patients with no other comorbidities. Further, among STR patients with no comorbidities, 11.4% of adherent patients had a hospitalisation compared with 14.7% of non-adherent patients. Similarly, among 2+PPD patients with no comorbidities, 12.4% of adherent patients had a hospitalisation compared with 19.7% of non-adherent patients. The results of this sensitivity analysis, combined with the observation that the vast majority of patients in our study had no major comorbidities (from the CCI) requiring other chronic treatment, suggest that the observed association between poorer adherence and higher hospitalisation was most likely due to reduced ART adherence and not due to reduced adherence with other medications that the patients were taking.
There were several measurable differences present in the study population at baseline. Our study attempted to control for effects these differences may have had on rates of hospitalisation between STR and 2+PPD patients. We used multivariate regressions to control for patient demographics, treatment characteristics (ie, treatment naïve vs experienced, type of ART received) and clinical characteristics (ie, CCI score, concomitant mental disorder, drug and alcohol abuse diagnoses). We found that a number of factors were associated with an increased risk of hospitalisation independent of the treatment regimen, including having a CCI score greater than 1; having a concomitant drug or alcohol abuse diagnosis; having a concomitant mental health disorder; being femaleand of older age; and being treatment naïve.
Even after controlling for the factors noted above, we still detected an independent association of regimen type with hospitalisation rates and, in fact, observed an increase in the apparent protective effect of STR based on the predicted, adjusted hospitalisation rate derived from the Poisson model (39.5/100 patients in the STR group vs 51.2/100 patients in the 2+PPD group; see figure 2). One possible explanation for this difference is that the Poisson model corrected a substantial imbalance in the proportion of patients who were treatment naive at index (47.5% of STR patients vs 24.5% of 2+PPD patients). Lack of naivety to ART exposure has been shown in some studies to be a positive predictor of hospitalisation in HIV patients,22 perhaps because approximately one-third of the HIV patients wait to seek care until their disease has progressed to the point that they need acute treatment.23 24 As noted in a recent study by Metsch et al,25 these patients often obtain initial care in emergency departments and hospital inpatient wards, and they tend not to persist with follow-up outpatient care. This pattern of treatment induction may further increase their risk of infection and rehospitalisation in the short term. As being treatment naïve was shown in our data to be predictive of hospitalisation, the Poisson model's adjustment for the over-representation of treatment naivety in the STR group may therefore have resulted in the larger difference between STR and 2+PPD in hospitalisations than observed in the crude, unadjusted comparison.
One hypothesis for a plausible mechanism by which the outcomes observed in our study could occur stems from observations in the SMART study.26 That study, comparing continuous antiviral treatment versus periodic treatment interruptions, demonstrated that HIV treatment interruptions that were of sufficient length of time to lead to recurrent HIV viraemia were associated with a significantly higher risk of all-cause morbidity and mortality. Our analysis was consistent with those findings: the mean maximum duration of non-adherence was about 3 weeks, which is a sufficient length of time to expect a return of HIV viraemia. The SMART study noted that the higher risk of illness was not necessarily proximal to the time of the interruption but was observed for months afterwards. While there are differences between the SMART study design and population and our study population, our findings are consistent with SMART and with what might be expected in a population who periodically are without antivirals for an average time of more than 3 weeks. Of note, short-cycle interruptions of 2 days were not associated with virological rebound in patients receiving the STR that was used in the SMART study.27 Therefore, our finding that the typical interruptions were much longer than this is supportive of a mechanism that could have resulted in increased patient morbidity.
It is also important to note that patients in this study generally were reasonably adherent to ART, with a mean adherence of just over 80% regardless of the number of pills received per day. This rate of adherence is consistent with other published reports of adherence, although other reports found even higher adherence rates to an STR.13 14 Furthermore, the difference observed in our study between the STR and 2+PPD regimens (approximately 4%) is consistent with what was observed by Sax et al16 of 2.2%. This difference is also consistent with the differences in adherence rates reported when comparing average improvement between once-daily and twice-daily regimens (2.9%).28 It is important to note that there also were highly non-adherent patients to both the STR and the 2+PPD regimes in this study population, supporting the generalisability of this population.
Of further note, the differences observed in our study were associated with factors that typically are not present during randomised clinical trials. Randomised trials typically actively work for patient adherence to study medications and use study coordinators to regularly monitor patients to minimise missed doses. In our observational study, these typical adherence supports are not in place; thus, our data may reflect real-world lapses in patient behaviour in refilling prescriptions, including partial regimen refills, which would not be observed in clinical trials. While there are concerns about the interpretation of observational data and the determination of causal relationships, it is not clear if a randomised study comparing an STR with a multiple-pill regimen would be able to detect the observed differences unless there was less patient support than is standard in clinical trials.
Our data do not suggest that all patients should be on an STR. There are many factors that weigh in the decision of which regimen is best for any given patient, including pre-existing virological resistance and tolerability. In our study, the anticipated adherence benefits observed in association with a lower pill burden is relevant but should not be construed as a suggestion that an STR is the ideal choice for the entire population of patients with HIV. Nevertheless, our data do support the continued development of additional STR options, to broaden the number of patients for whom this is an option and the number of subsequent beneficial outcomes.
Our study has several limitations common to observational claims database analyses. Adherence was calculated by using pharmacy refill dates, and we have no measure of actual patient adherence to the prescriptions they filled. However, this measure has been found to be a useful proxy for actual medication adherence.29 As we did not randomise patients to the two different treatment regimens, we cannot exclude unmeasured confounding factors that may have influenced our outcomes. Among the most important of these factors in this study was that multiple trials have shown that medication resistance at the time of virological failure is significantly less common in boosted PI treatments than on other regimens, including non-nucleoside/nucleotide reverse transcriptase inhibitor-based treatments.30 31 Clinicians could have chosen to prescribe a boosted PI-containing regimen (all of which contain three or more pills per day) to their less-adherent patients. It cannot be determined from this data set that these patients would have been more adherent on an STR. Although we attempted to control for some of these variables through the use of multivariable models that included some of these factors (substance abuse and psychiatric diagnoses), residual confounding may remain. In addition, we had no laboratory results from patients and thus cannot confirm the degree of virological suppression obtained across the regimens. Finally, although our data include information from the Medicaid programmes in 11 states, the authors were blinded (as per data privacy rules) as to which specific states are captured. Although the database's documentation suggests that the states are geographically dispersed, we cannot assert that our findings would be fully representative of the general Medicaid population in the USA.
In our study, a large proportion of HIV-treated individuals (15% of the total HIV-treated population) were excluded from the analysis due to their having received incomplete ART regimens. We did not have sufficient data on these patients to explain why their regimens were incomplete. However, a previous study found that the physician medication errors were somewhat common in individuals with HIV, with the most common error occurring with boosted PIs (estimated at 5.3% of patients); such errors may explain some of the incomplete regimens observed in our analysis.32 Increased adoption of fixed-dose combinations as part of HIV treatment may help to alleviate the issue of incomplete regimens.
During our study period, the only available single-pill ART regimen was coformulated efavirenz/emtricitabine/tenofovir disoproxil fumarate. It is possible that these results would not be generalisable to other one-pill and multipill regimens if other treatments have different efficacy and toxicity profiles. With the recent approval by the Food and Drug Administration of two other STRs (ie, tenofovir, emtricitabine and rilpivirine and tenofovir, emtricitabine, elvitegravir and cobicistat), it may eventually be possible to explore the applicability of our observations to other STRs.
In summary, this study supported the results as reported by Sax et al16 We found that patients who received ART as a single pill per day were significantly more likely to be highly adherent to therapy than patients who received multiple-pill regimens. This difference in adherence was associated with a lower risk of hospitalisations: patients with less-than-complete adherence were more likely to be hospitalised. While we acknowledge the limitations associated with any observational study, our data support our finding that the use of an STR may reduce healthcare costs as well as patient morbidity by decreasing hospitalisation rates, which are higher in patients with less-than-complete medication adherence.
Administrative statements
Protection of human subjects
The research organisation that conducted this study, RTI Health Solutions, a business unit of RTI International (RTI), holds a Federal-Wide Assurance (FWA #3331 effective until 17 June 2014) from the DHHS Office for Human Research Protections that allows us to review and approve human subjects protocols through our IRB committees. Since pre-existing, retrospective, de-identified patient data were analysed for this study, which involved no patient contact or medical interventions and therefore no patient consent forms, the RTI IRB committee approved this study as exempt.
Supplementary Material
Acknowledgments
The authors would like to thank Francois Everhard (Gilead Sciences) for his support and input on the study design and manuscript.
Footnotes
Contributors: CC, JLM and KLD conceived the idea of the study and were responsible for the design of the study; undertaking the data analysis and producing the tables and graphs; they also provided input into the data analysis and interpretation of the results. The initial draft of the manuscript was prepared by JLM and KLD and then circulated repeatedly among all authors (CC, JLM and KLD) for critical revision. JLM was responsible for the acquisition of the data. All authors helped plan the study, evolve the analysis plans, interpret the data and critically revise successive drafts of the manuscript.
Funding: This study was funded by Gilead Sciences, which is conducting clinical research in and markets current treatments for HIV/AIDS.
Competing interests: The authors report no competing interests beyond previous disclosure of funding.
Ethics approval: RTI International IRB (Federal-Wide Assurance #3331).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Raw data used for this study are unavailable for public sharing (per terms of the private data use agreement governing original data acquisition).
References
- 1.Thompson MA, Aberg JA, Cahn P, et al. Recommendations of the IAS-USA Panel. JAMA 2010;304:321–33 [DOI] [PubMed] [Google Scholar]
- 2.Henry K. Report from the 17th Conference on Retroviruses and Opportunistic Infections. More data and answers from ACTG 5202. J Watch AIDS Clin Care 2010;22:30. [PubMed] [Google Scholar]
- 3.Rockstroh JK, Lennox JL, Dejesus E, et al. STARTMRK Investigators Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naïve human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis 2011;53:807–16 [DOI] [PubMed] [Google Scholar]
- 4.Fielden SJ, Rusch ML, Yip B, et al. Nonadherence increases the risk of hospitalization among HIV-infected antiretroviral naïve patients started on HAART. J Int Assoc Phys AIDS Care 2008;7:238–44 [DOI] [PubMed] [Google Scholar]
- 5.Kitahata MM, Reed SD, Dillingham PW, et al. Pharmacy-based assessment of adherence to HAART predicts virological and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS 2004;15:803–10 [DOI] [PubMed] [Google Scholar]
- 6.Wood E, Hogg RS, Yip B, et al. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 109 cells/L. Ann Intern Med 2003;139:810–16 [DOI] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Perry S, Charlebois ED, et al. Nonadherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001;15:1181–3 [DOI] [PubMed] [Google Scholar]
- 8.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30 [DOI] [PubMed] [Google Scholar]
- 9.Arnsten J, Demas P, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis 2001;33:1417–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis 2006;43:939–41 [DOI] [PubMed] [Google Scholar]
- 11.Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services. 2009. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescnets. http://www.aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL001419.pdf (accessed 25 Aug 2012)
- 12.DeJesus E, Young B, Morales-Ramirez JO, et al. Simplification of antiretroviral therapy to a single tablet regimen consisting of efavirenz, emtricitabine and tenofovir disoproxil fumarate versus unmodified antiretroviral therapy in virologically suppressed HIV-1-infected patients. J Acquir Immune Defic Syndr 2009;51:163–74 [DOI] [PubMed] [Google Scholar]
- 13.Hodder SL, Mounzer K, DeJesus E, et al. Patient-reported outcomes in virologically suppressed, HIV-1-infected subjects after switching to a simplified, single tablet regimen of efavirenz, emtricitabine and tenofovir DF. AIDS Patient Care STDs 2010;24:115–25 [DOI] [PubMed] [Google Scholar]
- 14.Airoldi M, Zaccarelli M, Bisi L, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence 2010;4:115–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bangsberg DR, Ragland K, Monk A, et al. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. AIDS 24:2835–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sax PE, Meyers JL, Mugavero M, et al. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS ONE 7:e31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 40:373–83 [DOI] [PubMed] [Google Scholar]
- 18.Andrade SE, Kristijan KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 15:565–74 [DOI] [PubMed] [Google Scholar]
- 19.Legorreta A, Yu A, Chernicoff H, et al. Adherence to combined lamivudine + zidovudine versus individual components: a community-based retrospective Medicaid claims analysis. AIDS Care 2005;17:938–48 [DOI] [PubMed] [Google Scholar]
- 20.Juday T, Gupta S, Grimm K, et al. Factors associated with complete adherence to HIV combination antiretroviral therapy. HIV Clin Trials 12:71–8 [DOI] [PubMed] [Google Scholar]
- 21.Cohen CJ, Davis KL, Meyers J. Association of partial adherence to antiretroviral therapy with hospitalizations and healthcare costs in an HIV population. Poster presented at the 11th International Congress on Drug Therapy in HIV Infection. Glasgow, UK, 2012 [Google Scholar]
- 22.Gebo KA, Diener-West M, Moore RD. Hospitalization rates in an urban cohort after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2006;27:143–52 [DOI] [PubMed] [Google Scholar]
- 23.Fleming PL, Byers RH, Sweeney PA, et al. HIV prevalence in the United States, 2000. Abstract presented at: 9th Conference on Retroviruses and Opportunistic Infections. Seattle, WA, 2002 [Google Scholar]
- 24.Samet JH, Freedberg KA, Savetsky JB, et al. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS 2001;15:77–85 [DOI] [PubMed] [Google Scholar]
- 25.Metsch LR, Bell C, Pereyra M, et al. Hospitalized HIV-infected patients in the era of highly active antiretroviral therapy. Am J Public Health 2009;99:1045–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burman W, Clumeck N, Cohen CJ, et al. CD4+ count-guided interruption of antiretroviral treatment. N Eng J Med 2006;355:2283–96 [DOI] [PubMed] [Google Scholar]
- 27.Cohen CJ, Colson AE, Pierone G, et al. The FOTO study: 24-week results support the safety of a 2-day break on efavirenz-based antiretroviral therapy. J Int AIDS Soc 2008;11(Suppl 1):019 [Google Scholar]
- 28.Parienti J-J, Bangsberg DR, Verdon R, et al. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis 48:484–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon JH, Jordan MR, Kelley K, et al. Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis 2011;52:493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daar ES, Tierney C, Fischi MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011;154:445–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008; 358:2095–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellinger FJ, Encinosa WE. The cost and incidence of prescribing errors among privately insured HIV patients. Pharmacoeconomics 2010;28:23–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.