Abstract
Objectives
Hypertension guidelines recommend screening for chronic kidney disease (CKD) using serum creatinine and urine dipstick; this strategy may lead to misclassification. Persons with occult CKD [i.e. missed by creatinine but detected by cystatin C or albumin-to-creatinine ratio (ACR)] have higher risks for death, cardiovascular events, and end-stage renal disease.
Methods
We studied occult CKD prevalence among nondiabetic, hypertensive adults in National Health and Nutrition Examination Survey 1988–1994 (N = 2088) and 1999–2002 (N = 737). We defined occult CKD as estimated glomerular filtration rate by cystatin C (eGFRcys) less than 60 ml/min per 1.73m2 and/or ACR at least 30 mg/g among persons with eGFRcreat more than 60 ml/min per 1.73m2. We studied occult CKD prevalence by either marker, stratified by age, race/ethnicity, and assessed clinical predictors associated with occult CKD presence.
Results
In 1988–1994, occult CKD was prevalent among 25% of nondiabetic hypertensive persons, and it was 22% in 1999–2002. Each marker’s ability to detect occult CKD varied by age and race. Cystatin C detected occult CKD among 8.9% of persons more than 65 years, and among 3.8% of whites. ACR detected occult CKD among 9.3% of persons less than 45 years, 16.6% of Blacks, and 20.6% of Mexican–Americans. In multivariate models, each decade of advancing age was associated with a higher occult CKD prevalence by cystatin C (OR 3.1, 95% CI 2.5–3.8) in 1988–1994 and 1999–2002 (OR 2.9, 1.8–4.6).
Conclusion
Current hypertension guidelines may fail to detect a large proportion of high-risk individuals with CKD who can be identified by cystatin C or ACR. Future studies are needed to evaluate targeted use of multimarker renal panels among hypertensives.
Keywords: albumin-to-creatinine ratio, chronic kidney disease, cystatin C, National Health and Nutrition Examination Survey
INTRODUCTION
The high prevalence of kidney disease among persons with hypertension is well established [1]. Chronic kidney disease (CKD) is defined as an estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73m2 or the presence of albuminuria [albumin-to-creatinine ratio (ACR) ≥30 mg/g] [2]. CKD is an independent risk factor for cardiovascular disease, heart failure, progression to end-stage renal disease (ESRD) and death [3]. In addition, the presence of CKD among persons with hypertension directly impacts choice of antihypertensive medication and may influence blood pressure targets. Therefore, hypertension guidelines recommend universal screening for CKD at the time of diagnosis [4,5]. The current strategy advocated by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII) includes measuring serum creatinine to estimate GFR and a urine dipstick test to assess for proteinuria [6]. However, these recommendations may result in important misclassification of CKD status, and potential missed opportunities for adequate intervention.
Serum creatinine is biased by age, sex, and race, and equations to estimate GFR have not been validated among all ethnic groups or the elderly [7]. Additionally, dipstick proteinuria may miss persons who have mild degrees of proteinuria [8,9]. Therefore, these two measures may be incomplete for screening. Albuminuria can be quantified from a spot urine sample, and even low levels of albuminuria well below the CKD threshold of 30 mg/g are associated with increased cardiovascular risk [10]. In fact, European guidelines recommend the use of ACR among persons with hypertension [5]. Cystatin C is an alternative filtration marker known to have stronger and more linear associations with adverse outcomes compared with creatinine; and cystatin C is also less biased by age, sex, and race [11]. We recently showed in a large national cohort that a triple marker approach using the combination of serum creatinine, serum cystatin C, and urine ACR improved detection, classification and risk stratification of CKD [12]. In that population-based study, we detected occult CKD (CKD that is missed by creatinine but detected by cystatin C or ACR) in 16% of adults, and persons with occult CKD had elevated risks for death, cardiovascular events, and heart failure, compared to persons with no CKD.
Due to the clinical importance of correct classification of CKD in persons with hypertension, we designed this study to evaluate the prevalence of occult CKD among nondiabetic hypertensive adults in two NHANES cohorts, from 1988–1994 to 1999–2002. We studied the overall prevalence of occult CKD by each marker, and then stratified by age and race/ethnicity. We also assessed clinical predictors associated with the presence of occult CKD.
METHODS
Participants
We included participants from three waves of the National Health and Nutrition Examination Survey (1988–1994 and 1999–2000, and 2001–2002, with the latter two waves combined, from here on referred to as 1999–2002). NHANES is a complex, stratified, multistage probability sample of noninstitutionalized US civilians. Details of survey design are available at the Centers for Disease Control and Prevention website [13]. Persons age 20 years and older who had a serum cystatin C measurement were eligible for these analyses. Among those eligible, we included those with hypertension but without diabetes. Hypertension was defined as participant report of being diagnosed with hypertension, using hypertension medications, or having a SBP at least 140 mmHg and/or DBP at least 90 mmHg. We excluded participants who were diagnosed with diabetes, taking diabetes medications or insulin, or with fasting serum glucose at least 126 mg/dl. We specifically exclude diabetes because guidelines already recommend yearly kidney function measures, including quantification of the ACR. After further excluding participants without serum creatinine measurements, we obtained subsamples of 2741 and 945 participants for NHANES 1988–1994 and NHANES 1999–2002, respectively.
The cystatin C sampling strategy has been described previously [14]. Briefly, both NHANES waves measured serum cystatin C using stored serum samples from participants at least 60 years of age, using a random 25% sample of all participants aged 12–59 years, as well as in all individuals with high serum creatinine (>1.2 mg/dl in men and >1.0 mg/dl in women). Because we are interested in the prevalence of occult CKD, we excluded persons with an eGFR less than 60 ml/min per 1.73m2 by the creatinine-based CKD-EPI equation (N = 546 and N = 214 for 1988–1994 and 1999–2002, respectively) (see below for measures of kidney function). We hypothesize that these persons would already be classified as having CKD, as most laboratories now report eGFR and alert the clinician to the presence of CKD based on this value [15]. After exclusion of persons without valid kidney function measures (urinary albumin-to-creatinine ratio, 107 and 14 excluded respectively), our total sample size was 2088 for NHANES 1988–1994 and 737 for NHANES 1999–2002.
Measures of kidney function
For these analyses, we used standardized serum creatinine based on published guidelines [16] to estimate eGFRcreat using the CKD-EPI equation [17]. Cystatin C was measured used an N Latex Cystatin C assay run on a BNII nephelometer [Dade Behring (now Siemens), www.siemens.com], which has an intra-assay coefficient of variation of 2.0–3.0% and an inter-assay coefficient of variation of 3.2–4.4% [18]. We estimated eGFR using the CKD-EPI equation: eGFRcys = 76.7 × cystatin C – 1.19 [19]. Urinary albumin and creatinine concentrations were measured from urine spot samples using the modified Jaffe method on a Synchron AS/Astra Analyzer (Beckman Coulter, www.beckmancoulter.com) [14]. We estimated ACR and defined albuminuria as an ACR at least 30 mg/g, as suggested by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [18].
Variables of interest
On the basis of self-report, race/ethnicity in NHANES 1988–1994 was classified as non-Hispanic white, non-Hispanic black, Mexican–American, and other race. For NHANES 1999–2002, race/ethnicity was classified as non-Hispanic white, non-Hispanic black, Mexican–American, other Hispanics, and other race. We grouped Mexican–American and other Hispanics into one category, due to the low numbers of other Hispanics in the 1999–2002 NHANES. Blood pressure was measured by a trained technician using a mercury sphygmomanometer (http://www.cdc.gov/nchs/nhanes.htm), and calculated as the average of three or four measurements, excluding the first measurement. Hypertension was defined by self-report, as was education. Self-reported cardiovascular disease from 1988 to 1994 included history of heart attack, congestive heart failure, or stroke, whereas data from 1999 to 2002 included coronary heart disease/angina, angina pectoris as well. Current smokers were identified by interview questions.
Outcomes
Our primary outcome of interest was prevalence of occult CKD. Among persons with eGFRcreat (CKD-EPI equation) at least 60 ml/min per 1.73m2, we defined mutually exclusive outcome categories as follows: No CKD (eGFRcys ≥60 ml/min per 1.73m2 and ACR <30 mg/g); Occult CKD ACR only (eGFRcys ≥60 ml/min per 1.73m2 and ACR ≥30 mg/g); Occult CKD cysC only (eGFRcys <60 ml/min per 1.73m2 and ACR <30 mg/g); and Occult CKD both (eGFRcys <60 ml/min per 1.73m2 and ACR ≥30 mg/g). For initial descriptive analyses, we also considered persons with any occult CKD, defined as persons with occult CKD by either ACR or cysC.
Statistical analyses
We first estimated the prevalence of any occult CKD in the study sample. We then categorized each NHANES cohort into the four mutually exclusive occult CKD categories as described above, stratified by survey periods, and compared the characteristics of participants in each CKD category using ANOVA or χ2 as appropriate. For completeness, we also report the prevalence of CKD by cystatin C and ACR among persons with CKD by creatinine (eGFR-creat <60 ml/min per 1.73m2). We then estimated the age-adjusted prevalence of each category of occult CKD, by cohort and race/ethnic category, following recommended survey procedures based on NHANES analytical guidelines (NHANES III, NHANES 1999–2002) [13]. The 1980 Census population was used as the standard population for NHANES 1988–1994, and the 2000 Census population for NHANES 1999–2002. For these analyses, we categorized age into three broad groups of less than 45 years of age, 45–64, and at least 65 years.
We estimated the number needed to screen (NNS) to detect occult CKD by cystatin C and ACR. We present NNS estimates among persons age 65 years and older, as this group had complete selection for cystatin C testing. The NNS for cystatin C was estimated using the formula 1/(# with eGFRcys <60 ml/min per 1.73m2 AND ACR <30/# with eGFRcreat ≥60 ml/min per 1.73m2); NNS for ACR was estimated as 1/(# with ACR ≥30 but eGFRcys ≥60 ml/min per 1.73m2/# with eGFRcreat >60 ml/min per 1.73m2).
Finally, using multivariable logistic regression, we investigated demographic and clinical characteristics associated with occult CKD by cystatin C only and by ACR only.
RESULTS
The mean (standard error) age of our study population was 51.8 (1.0) for NHANES 1988–1994, and 51.9 (1.0) for NHANES 1999–2002. In NHANES 1988–1994, 76.9% were white, 13.4% were black, 3.7% identified as Mexican–American, and 6.0% were classified as other. The race/ethnicity composition of NHANES 1999–2002 was similar, with 72.9% of the cohort being white, 13.7% black, 5.6% Mexican–American, and 7.8% under the other category. Among the 2088 nondiabetic hypertensive persons in NHANES 1988–1994 in our analysis, 511 (25%) had any occult CKD, corresponding to 17% of the total US hypertensive population. In comparison, 161 (22%) individuals had any occult CKD for 1999–2002, corresponding to 16% of the population. We then categorized the sample into four mutually exclusive categories. In 1988–1999, 13% of nondiabetic hypertensives had occult CKD by ACR only; 8% had occult CKD by cysC only, and 3% had occult CKD by both markers (Table 1).
TABLE 1.
Baseline characteristics of non-diabetic National Health and Nutrition Examination Survey Number Needed to Screen participants with hypertension from 1988–1994 (N=2088) and 1999–2002 (N=737) with estimated glomerular filtration rate creat more than 60 ml/min per 1.73m2
| Characteristics | No occult CKD | Occult CKD ACR only | Occult CKD CysC only | Occult CKD both |
|---|---|---|---|---|
| N (%) | ||||
| 1988–1994 | 1570 (75) | 277 (13) | 174 (8) | 67 (3) |
| 1999–2002 | 575 (78) | 115 (16) | 33 (4) | 14 (2) |
|
| ||||
| Age, years | ||||
| 1988–1994 | 50 (1) | 55 (2) | 70 (1) | 71 (2) |
| 1999–2002 | 51 (1) | 55 (3) | 67 (5) | 69 (3) |
|
| ||||
| Female (%) | ||||
| 1988–1994 | 53 | 53 | 47 | 47 |
| 1999–2002 | 49 | 58 | 68 | 50 |
|
| ||||
| Race (%) | ||||
| White | ||||
| 1988–1994 | 77 | 70 | 89 | 84 |
| 1999–2002 | 74 | 63 | 93 | 66 |
| Black | ||||
| 1988–1994 | 13 | 16 | 7 | 10 |
| 1999–2002 | 13 | 22 | 1 | 18 |
| Mexican–American | ||||
| 1988–1994 | 4 | 5 | 2 | 5 |
| 1999–2002 | 5 | 8 | 1 | 4 |
| Other | ||||
| 1988–1994 | 6 | 9 | 2 | 1 |
| 1999–2002 | 8 | 7 | 5 | 12 |
|
| ||||
| SBP, mmHg | ||||
| 1988–1994 | 135 (1) | 149 (3) | 146 (2) | 153 (3) |
| 1999–2002 | 135 (1) | 141 (4) | 140 (3) | 149 (6) |
|
| ||||
| Hypertension medication (current, %) | ||||
| 1988–1994 | 31 | 29 | 53 | 56 |
| 1999–2002 | 45 | 38 | 74 | 81 |
|
| ||||
| LDL | ||||
| 1988–1994 | 164 (12) | 139 (9) | 169 (21) | 124 (6) |
| 1999–2002 | 125 (2) | 125 (5) | 146 (13) | 104 (6) |
|
| ||||
| HDL | ||||
| 1988–1994 | 62 (7) | 55 (4) | 62 (11) | 49 (2) |
| 1999–2002 | 53 (1) | 51 (2) | 48 (1) | 47 (3) |
|
| ||||
| BMI, kg/m2 | ||||
| 1988–1994 | 29 (0) | 28 (1) | 29 (1) | 28 (1) |
| 1999–2002 | 30 (0) | 32 (2) | 34 (3) | 31 (2) |
|
| ||||
| Current smoker (%) | ||||
| 1988–1994 | 24 | 25 | 24 | 30 |
| 1999–2002 | 21 | 23 | 10 | 23 |
|
| ||||
| Cardiovascular diseasea (%) | ||||
| 1988–1994 | 5 | 7 | 19 | 26 |
| 1999–2002 | 9 | 21 | 15 | 17 |
|
| ||||
| ACR 30–299 mg/gb | ||||
| 1988–1994 | 0 (0) | 243 (92) | 0 (0) | 59 (89) |
| 1999–2002 | 0 (0) | 103 (84) | 0 (0) | 14 (100) |
|
| ||||
| ACR ≥300 mg/gb | ||||
| 1988–1994 | 0 (0) | 34 (8) | 0 (0) | 8 (11) |
| 1999–2002 | 0 (0) | 12 (16) | 0 (0) | 0 (0) |
|
| ||||
| eGFRcreat | ||||
| 1988–1994 | 96 (1) | 90 (2) | 75 (2) | 70 (1) |
| 1999–2002 | 93 (1) | 90 (3) | 70 (2) | 70 (3) |
|
| ||||
| eGFRcys | ||||
| 1988–1994 | 91 (1) | 84 (3) | 54 (1) | 50 (1) |
| 1999–2002 | 95 (1) | 88 (3) | 55 (1) | 53 (2) |
ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; cysC, cystatin C; eGFRcreat, estimated glomerular filtration rate by the CKD-EPI equation; eGFRcys, estimated glomerular filtration rate by the cystatin C equation; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Results reported as mean (standard error) for continuous variables and percentage for categorical variables.
Self-reported cardiovascular disease from 1988–1994 includes history of heart attack, congestive heart failure, or stroke. Self-reported cardiovascular disease from 1999–2002 includes coronary heart disease/angina, angina pectoris, heart attack, congestive heart failure, or stroke.
Reported as N (%) of participants.
Persons with occult CKD by ACR only, cysC only, or both were more likely to be older and have higher SBP levels (Table 1). The race distribution differed by occult CKD category; Blacks and Mexican–Americans were more likely to have occult CKD detected by ACR only or by both markers compared with whites.
The overall prevalence of occult CKD varied by age, with approximately one-third of persons age 65 years and older having occult CKD in 1988–1994 and over one-quarter in 1999–2002. Conversely, less than 10% of persons under 45 had occult CKD (Fig. 1a and b). The proportion of persons whose CKD was detected by cysC, ACR, or both varied substantially by age. For example, occult CKD was almost fully detected by ACR among persons younger than 45 years in both survey waves. In contrast, 9 and 13% of persons age 65 years and older in NHANES 1988–1994 and 1999–2002, respectively, had occult CKD detected by cysC only (Fig. 1a and b). Among persons age more than 65 years with eGFRcreat more than 60 ml/min per 1.73m2 in NHANES III, most persons with CKD by cystatin C had eGFRcys of 45–59 ml/min per 1.73m2 (N = 178, 89%).
FIGURE 1.
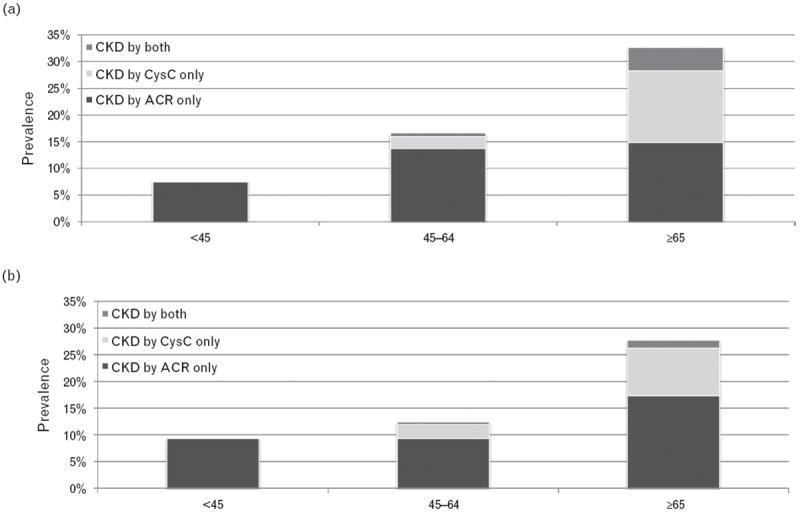
Age-adjusted prevalence of occult CKD among hypertensive adults of all races stratified by age for NHANES 1988–1994 (a) and 1999–2002 (b)*. *Age adjustment is conducted based on NHANES III analytical guidelines. The 1980 Census population was used as the standard population.
The prevalence of each category of occult CKD also varied by race (Fig. 2a and b). During both eras, whites had the highest prevalence of occult CKD by cysC only, but lowest prevalence of CKD by ACR only. The prevalence of CKD by ACR only was highest for Mexican–Americans, closely followed by Blacks. Among white participants with occult CKD by either marker in 1988–1994, approximately 3% had occult CKD detected by cysC alone.
FIGURE 2.
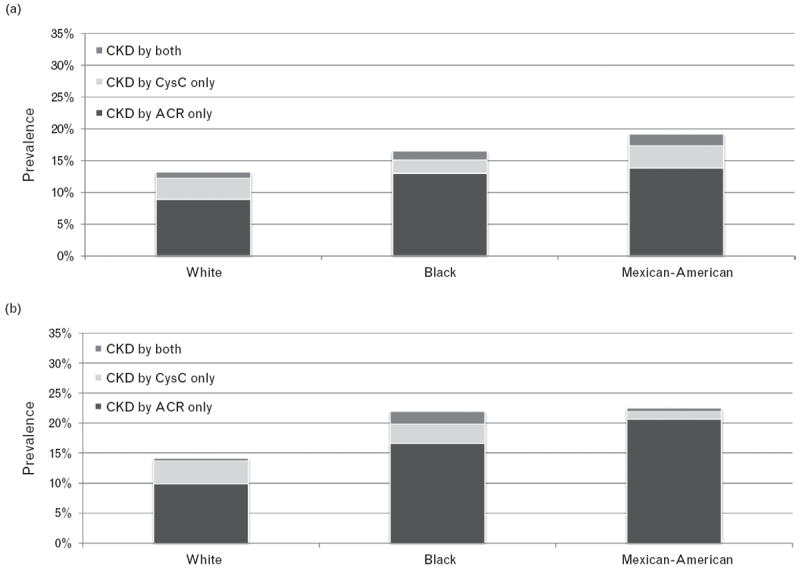
Age-adjusted prevalence of occult CKD stratified by race for NHANES 1988–1994 (a) and 1999–2002 (b).
The NNS to detect a person with occult CKD among persons age 65 years and older was low for both survey waves and for both tests. For cysC, we found that eight would be required, and seven for ACR in 1988–1994. For 1999–2002, 12 would be needed for cysC and six for ACR.
Finally, we evaluated demographic and clinical characteristics associated with occult CKD by each marker. After adjustment, each decade of older age was associated with three-fold odds of having occult CKD by cysC only in both NHANES surveys (Table 2). Additionally, smoking and having a history of CVD was associated with occult CKD by cysC only in the 1988–1994 survey. Self-identified Blacks had lower odds of CKD by cysC only in both surveys (Table 2). In sharp contrast, age was not independently associated with occult CKD by ACR only in either NHANES survey. Black race and Mexican–American origin were associated with higher odds of occult CKD by ACR only in the 1999–2002 survey.
TABLE 2.
Association of baseline characteristics and occult chronic kidney disease
| Characteristic | Odds ratio (95% confidence interval)
|
|
|---|---|---|
| 1988–1994 | 1999–2002 | |
| Occult CKD ACR only | ||
| Age (per 10 years) | 0.9 (0.7–1.1) | 1.3 (0.9–1.7) |
| Female | 1.3 (0.8–2.1) | 1.3 (0.5–3.8) |
| Race | ||
| White | Reference | Reference |
| Black | 1.2 (0.7–2.1) | 2.3 (1.1–4.7) |
| Mexican–American | 1.4 (0.6–3.4) | 3.0 (1.4–6.7) |
| Other Race | 1.3 (0.5–3.2) | 1.7 (0.4–7.5) |
| BMI (kg/m2) | 1.0 (0.9–1.0) | 1.0 (1.0–1.1) |
| SBP (per 10 mmHg) | 1.6 (1.3–1.9) | 1.1 (0.9–1.3) |
| History of cardiovascular diseasea | 1.0 (0.5–1.9) | 2.6 (0.7–10.5) |
| Current smoker | 0.9 (0.4–1.8) | 1.2 (0.3–4.2) |
|
| ||
| Occult CKD cysC only | ||
| Age (per 10 years) | 3.1 (2.5–3.8) | 2.9 (1.8–4.6) |
| Female | 0.5 (0.3–0.8) | 0.8 (0.3–2.3) |
| Race | ||
| White | Reference | Reference |
| Black | 0.5 (0.3–1.0) | 0.05 (0.003–0.8) |
| Mexican–American | 0.7 (0.5–1.2) | 0.2 (0.03–1.5) |
| Other Race | 0.4 (0.1–1.4) | 0.8 (0.1–5.4) |
| BMI (kg/m2) | 1.1 (1.0–1.1) | 1.2 (1.0–1.4) |
| SBP (per 10 mmHg) | 0.9 (0.8–1.1) | 0.9 (0.7–1.2) |
| History of cardiovascular diseasea | 1.7 (0.9–3.1) | 0.9 (0.2–4.3) |
| Current smoker | 3.2 (1.7–6.2) | 0.5 (0.04–6.9) |
ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; cysC, Cystatin C. Each variable adjusted for others listed.
History of CVD includes heart attack, congestive heart failure, stroke, coronary heart disease, and angina.
In an analysis including only nondiabetic, hypertensive persons with CKD by creatinine (eGFRcreat <60 ml/min per 1.73m2) in NHANES III, 441 (82%) had ACR at least 30 mg/g or eGFRcys <60 ml/min per 1.73m2. Approximately, 18% had CKD by creatinine that was not confirmed by either cystatin C or ACR.
DISCUSSION
In this nationally representative sample of nondiabetic adults with hypertension, we found that cystatin C and ACR could detect occult CKD in up to 25% of US adults whose CKD was otherwise missed by creatinine. We found that the yield of each marker for detecting occult CKD varied by age and race. Cystatin C detected a large proportion of the occult CKD among the elderly, whereas ACR detected a large proportion of occult CKD among younger persons and among Blacks and Mexican–Americans. In multivariate models, we showed that older age was independently associated with having occult CKD detected by cystatin C in both waves, whereas Black race and Mexican–American origin were associated with having occult CKD detected by ACR in 99–02. Taken together, our findings suggest that current CKD screening guidelines among hypertensive may miss persons at risk. Moreover, evaluation of improvement in CKD screening guidelines among hypertensives may require a targeted approach that incorporates demographic and clinical characteristics.
Our report is supported by prior data from our group on the high prevalence of occult CKD among middle-aged and elderly persons [12,20]. We have also previously shown that persons with occult CKD detected by cystatin C, ACR, or both are at high risk for death, cardiovascular disease, heart failure, and progression to ESRD. Notably, although persons who had occult CKD by cystatin C and ACR but missed by creatinine were at higher risks for adverse outcomes, persons with CKD by creatinine only had risks comparable to persons without CKD by any marker [12]. Our findings in NHANES suggest that the current guidelines to screen for renal end organ damage among persons with hypertension may misclassify individuals at high risk, resulting in missed opportunities for prevention. Future research is needed to understand characteristics, including family history, of persons likely to have occult CKD.
Our results that the utility of each marker in detecting CKD varies by age and race are noteworthy. These findings are in accordance with histopathological evidence that hypertensive nephrosclerosis typically manifests with two distinct renal lesions: ischemic, obsolete glomeruli, typically not associated with proteinuria; and hypertrophied sclerotic glomeruli associated with large amounts of proteinuria [21]. Studies have also shown that although ischemic lesions may be more common in the elderly, sclerosis may be more common among Blacks [22]. This is of clinical importance, because decreased eGFR in the absence of proteinuria is commonly seen in clinical practice in the elderly. As creatinine is biased by age and muscle mass, cystatin C may be an important adjunct to risk classification of CKD in hypertensive elderly patients. In addition, higher prevalence of proteinuria among Blacks compared with Whites has been documented in many studies [23]. Accurate and timely diagnosis of CKD in hypertensive Blacks may reduce observed disparities in CKD progression.
This is the first study to assess the prevalence of CKD among hypertensives using a triple marker approach. NHANES has the advantage of having all three measures of kidney function, as well as sampling strategies that allow for nationally representative estimates. Our design is in accordance with the new KDIGO guidelines that recommend reporting the ACR at all eGFR levels [18]. In addition, European guidelines recommend assessment of ACR [5]. However, we must consider several limitations to this study. Due to a small sample size and the current sampling strategy for cystatin C, we did not have reliable population estimates, particularly for the latter NHANES survey. Furthermore, we do not have direct measures of GFR, but this is impractical in large epidemiological studies. Although cystatin C may not be universally available, it is FDA approved, and the cost of the assay has dropped dramatically to less than US$ 2 per test. We also recognize that current US guidelines recommend a dipstick for assessment of proteinuria, which is a cheap test that may identify some of the persons with target organ damage and preserved GFR. However, urinary dipstick tests have very low sensitivity, and are not designed to detect lower levels of proteinuria [8,9]. In fact, levels as low 10mg/g have been associated with adverse outcomes, and risk of outcomes increases with increasing ACR levels [10]. Moreover, the upcoming KDIGO guidelines will recommend assessment of the ACR at all levels of eGFR. In our sample, macroalbuminuria was relatively rare, but many persons were in the microalbuminuria range. In addition, NHANES does not have repeated measures of ACR in all participants, thus, limiting our ability to establish persistent albuminuria. As ACR measurements are known to vary with time, some misclassification is likely in this group. A further limitation of our study was our inability to calculate NNS in younger age groups because of the sampling strategy of cystatin C.
Perspectives
In this study, we found that cystatin C and ACR can detect occult CKD missed by creatinine in a large proportion of nondiabetic adults with hypertension. Our findings suggest that the current strategy for CKD screening recommended by JNC guidelines may miss a large proportion of persons at high risk. We also found that the ability of ACR and cystatin C to detect occult CKD varied by both age and race. Future studies are needed to understand whether targeted addition of cystatin C and ACR as screening tools for kidney end organ damage among hypertensives may be cost-effective in clinical practice.
Acknowledgments
C.A.P. is funded by the National Institutes of Diabetes and Digestive and Kidney Diseases 1K23SK082793–01 and a Robert Wood Johnson Harold Amos award. These funding sources had no involvement in the design or execution of this study.
Abbreviations
- ACR
albumin-to-creatinine ratio
- CKD
chronic kidney disease
- eGFRcreat
estimated glomerular filtration rate by creatinine
- eGFRcys
estimated glomerular filtration rate by cystatin C
- ESRD
end-stage renal disease
- KDIGO
kidney disease improving global outcomes
- NHANES
National Health and Nutrition Examination Survey
- NNS
number needed to screen
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 2.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 5.British Hypertension Society: Hypertension Guidelines. [20 September 2012];2011 http://www.bhsoc.org/latest-guidelines/sub-page-11/
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Panesar A, Lewis RR. Dipstick proteinuria: can it guide hypertension management? Am J Kidney Dis. 2002;39:1190–1195. doi: 10.1053/ajkd.2002.33389. [DOI] [PubMed] [Google Scholar]
- 9.Zeller A, Sigle JP, Battegay E, Martina B. Value of a standard urinary dipstick test for detecting microalbuminuria in patients with newly diagnosed hypertension. Swiss Med Wkly. 2005;135:57–61. doi: 10.4414/smw.2005.10859. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 12.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics and Centers for Disease Control and Prevention: Analytic and reporting guidelines: The third National Health and Nutrition Examination Survey, NHANES III (1988–1994) [September 20, 2012]; http://www.cdc.gov/nchs/nhanes/nh3rrm.htm#ag.
- 14.Foley RN, Wang C, Snyder JJ, Collins AJ. Cystatin C levels in U.S. adults, 1988–1994 versus 1999–2002: NHANES. Clin J Am Soc Nephrol. 2009;4:965–972. doi: 10.2215/CJN.05281008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmelgarn BR, Zhang J, Manns BJ, James MT, Quinn RR, Ravani P, et al. Nephrology visits and healthcare resource use before and after reporting estimated glomerular filtration rate. JAMA. 2010;303:1151–1158. doi: 10.1001/jama.2010.303. [DOI] [PubMed] [Google Scholar]
- 16.Selvin E, Manzi J, Stevens LA, van Lente F, Lacher DA, Levey AS, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.National Health and Nutrition Examination Survey (NHANES) Documentation, Codebook, and Frequencies: Surplus Specimen Laboratory Component: Cystatin C (Surplus Sera) [September 20, 2012];2007 http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/sscyst_b.pdf.
- 19.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peralta CA, Katz R, Sarnak MJ, Ix J, Fried IF, de Boer I, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22:147–155. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266–270. doi: 10.1097/MNH.0b013e3282f88a1f. [DOI] [PubMed] [Google Scholar]
- 22.Marcantoni C, Ma LJ, Federspiel C, Fogo AB. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int. 2002;62:172–180. doi: 10.1046/j.1523-1755.2002.00420.x. [DOI] [PubMed] [Google Scholar]
- 23.Bryson CL, Ross HJ, Boyko EJ, Young BA. Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006;48:720–726. doi: 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]


