Abstract
Tau and amyloid precursor protein (APP) are key proteins in the pathogenesis of sporadic and inherited Alzheimer’s disease. Thus, developing ways to inhibit production of these proteins is of great research and therapeutic interest. The selective silencing of mutant alleles, moreover, represents an attractive strategy for treating inherited dementias and other dominantly inherited disorders. Here, using tau and APP as model targets, we describe an efficient method for producing small interfering RNA (siRNA) against essentially any targeted region of a gene. We then use this approach to develop siRNAs that display optimal allele-specific silencing against a well-characterized tau mutation (V337M) and the most widely studied APP mutation (APPsw). The allele-specific RNA duplexes identified by this method then served as templates for constructing short hairpin RNA (shRNA) plasmids that successfully silenced mutant tau or APP alleles. These plasmids should prove useful in experimental and therapeutic studies of Alzheimer’s disease. Our results suggest guiding principles for the production of allele-specific siRNA, and the general method described here should facilitate the production of gene-specific siRNAs.
INTRODUCTION
RNA interference (RNAi) plays an important role in diverse aspects of biology (1). Techniques that exploit the power of RNAi to suppress target genes have already become indispensable tools in research and may soon prove to be therapeutically useful (1,2). In particular, the production of small interfering RNAs (siRNAs) that silence specific disease-related genes could have wide-ranging therapeutic applications.
One promising therapeutic role for siRNA is the silencing of genes that cause dominantly inherited disease. We and others recently established the feasibility of this approach, and demonstrated that it is possible to engineer siRNAs that selectively silence mutant alleles while retaining expression of normal alleles (3–7). Such allele-specific suppression may be important for disorders in which the defective gene normally plays an important or essential role.
Generating effective siRNAs for target genes is not always straightforward, however, particularly when designing siRNAs that selectively target mutant alleles (3,5). Here we describe a simple, novel approach for producing siRNAs that should facilitate the development of gene- and allele-specific siRNAs. Using this strategy, we created allele-specific siRNA for mutations in two important neurodegenerative disease genes, the genes encoding amyloid precursor protein (APP) and tau.
APP and tau were chosen as candidate RNAi targets because of their central role in inherited and acquired forms of age-related dementia, including Alzheimer’s disease (AD) (8–12). AD is characterized by two major pathological hallmarks: senile plaques, which contain beta-amyloid (Aβ) derived from cleavage of APP; and neurofibrillary tangles, which contain filamentous tau protein. Rare inherited forms of AD have revealed an essential role for Aβ production in the pathogenesis of all forms of AD, both sporadic and inherited (8). Mutations in the three genes known to cause familial AD—the genes encoding APP, presenilin 1 and presenilin 2—act dominantly to enhance the production of neurotoxic Aβ (8).
The best studied AD mutation is the Swedish double mutation in APP (APPsw), in which two consecutive missense changes alter adjacent amino acids near the β cleavage site (10). APPsw has been used to create several widely used transgenic mouse models of AD (13,14), thus we chose it as an ideal mutation against which to generate allele-specific siRNAs for AD research. Such siRNA might also have therapeutic value because RNAi-mediated silencing of APP should inhibit Aβ deposition.
Tau, the major component of neurofibrillary tangles, likewise plays a significant role in AD pathogenesis (9). Mutations in tau cause a similar dominantly inherited neurodegenerative disease, frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17). In FTDP-17, tau mutations either alter the tau protein sequence or lead to aberrant splicing (9,13,14). Abnormalites of tau expression also contribute to several other important neurodegenerative disorders, including progressive supranuclear palsy and cortical-basal ganglionic degeneration (15). Thus, efforts to reduce tau expression, either generally or in an allele-specific manner, may prove to be therapeutically useful in FTDP-17, AD or other tau-related diseases.
Recently we demonstrated allele-specific silencing for tau and two other dominant neurogenetic disease genes (3,4). But due to constraints imposed by the method of siRNA production, we could not systematically analyze the effect of positioning mutations at each point along the antisense guide strand that mediates siRNA silencing. Here, we have developed an efficient strategy to produce and screen siRNAs. Using this approach with APP and tau as model target genes, we demonstrate that allele specificity of siRNA targeting is optimal when mutations are placed centrally within the 21-nucleotide siRNA.
MATERIALS AND METHODS
siRNA synthesis
In vitro synthesis of siRNA was done using a previously described protocol (3,16). Desalted DNA oligonucleotides (Integrated DNA Technologies, Coralville, IA) encoding sense and antisense target sequences were used with the AmpliScribeT7 high-yield transcription kit (Epicentre Technologies, Madison, WI) to generate siRNA duplexes (Table 1). After measuring reaction yields through absorbance at 260 nm, double-stranded nature was confirmed by agarose gel (1% w/v) electrophoresis and ethidium bromide staining. Note that for all siRNAs used in this study the most 5′ nucleotide in the targeted cDNA sequence is referred to as position 1 and each subsequent nucleotide is numbered in ascending order from 5′ to 3′.
Table 1. Primer sequences for in vitro generation of siRNA duplexes using T7 polymerase.
| Name | Primer sequence (5′-3′) |
|---|---|
| Miscellaneous | |
| siMiss |
ATGAACTTCATGCTCAGCTTGC |
| |
CGGCAAGCTGCGCATGAAGTTC |
| siMiss+G |
AACTTCACCCTGAGCTTGCC |
| |
CGGCAAGCTCAGGGTGAAGT |
| siGFP |
ATGAACTTCAGGGTCAGCTTGC |
| |
CGGCAAGCTGACCCTGAAGTTC |
| siGFP+G |
AACTTCAGGGTCAGCTTGCC |
| |
CGGCAAGCTGACCCTGAAGT |
| siLamin |
AACTGGACTTCCAGAAGAAC |
| |
TGTTCTTCTGGAAGTCCAGT |
| Tau | |
| siA9 |
GTGGCCAGATGGAAGTAAAA |
| |
ATTTTACTTCCATCTGGCCA |
| siA10 |
GGTGGCCAGATGGAAGTAAA |
| |
TTTTACTTCCATCTGGCCAC |
| siA11 |
AGGTGGCCAGATGGAAGTAA |
| |
TTTACTTCCATCTGGCCACC |
| siA12 |
GAGGTGGCCAGATGGAAGTA |
| |
TTACTTCCATCTGGCCACCT |
| APP | |
| siAPP |
AAGTGAAGATGGATGCAGAATTC |
| |
CGGAATTCTGCATCCATCTTCAC |
| siAPP+G |
TGAAGTGAAGATGGATGCAG |
| |
TCTGCATCCATCTTCACTTC |
| siT8/C9 |
AAGTGAATCTGGATGCAGAA |
| |
ATTCTGCATCCAGATTCACT |
| siT9/C10 |
GAAGTGAATCTGGATGCAGA |
| |
TTCTGCATCCAGATTCACTT |
| siT10/C11 |
TGAAGTGAATCTGGATGCAG |
| |
TCTGCATCCAGATTCACTTC |
| siT11/C12 |
CTGAAGTGAATCTGGATGCA |
| |
CTGCATCCAGATTCACTTCA |
| siT12/C13 |
TCTGAAGTGAATCTGGATGC |
| TGCATCCAGATTCACTTCAG | |
All primers used for T7 synthesis contain the following promoter sequence at their 3′ ends: 5′-CTATAGTGAGTCGTATTA-3′. The following primer was annealed to all templates to synthesize siRNA duplexes: 5′-TAATACGACTCACTATAG-3′.
Plasmids
The plasmid used for green fluorescent protein (GFP) expression was pEGFP-C1 (BD Biosciences Clontech, Palo Alto, CA). Gloria Lee (University of Iowa, Iowa City, IA) kindly provided the constructs encoding human flag-tagged tau and V337M-GFP tau (3). Constructs encoding APP and APPsw mutant proteins were kindly provided by R. Scott Turner (University of Michigan, Ann Arbor, MI).
shRNA plasmid construction
The tRNA-valine promoter was constructed by annealing two primers: (forward 5′-CAGGACTAGTCTTTTAGGTCAA AAAGAAGAAGCTTTGTAACCGTTGGTTTCCGTAGTGTA-3′ and reverse 5′-TTCGAACCGGGGACCTTTCGCG TGTTAGGCGAACGTGATAACCACTACACTACGGAA ACCAAC-3′), extending the primers with PCR, and cloning them into pCR 2.1-TOPO vector using the TOPO TA Cloning Kit (Invitrogen Life Technologies, Carlsbad, CA) (17,18). Head-to-head 21 bp shRNA fragments were PCR amplified using as a template the resulting tRNA-valine promoter in the Topo TA vector, the forward primer above and the reverse primers below. Each shRNA fragment was subsequently cloned into pCR 2.1-TOPO vector. Reverse primers used for generation of tRNA-valine driven shRNA are as follows:
Miscellaneous. tvMiss. AAAAAAATGAACTTCCCCGTCA GCTTGCAAGCTTCCAAGCTGACGGGGAAGTTCATCTTCGAACCGGGGACCTTTCG.
Tau. tvTau: AAAAAAGTGGCCAGGTGGAAGTAAAAT CCAAGCTTCGATTTTACTTCCACCTGGCCACCTTCG AACCGGGGACCTTTCG.
tvA10: AAAAAAGGTGGCCAGATGGAAGTAAACC AAGCTTCGTTTACTTCCATCTGGCCACCCTTCGAACCGGGGACCTTTCG.
APP. tvAPP: AAAAAATGAAGTGAAGATGGATGCAG CCAAGCTTCGCTGCATCCATCTTCACTTCACTTCGA ACCGGGGACCTTTCG.
tvT10/C11: AAAAAATGAAGTGAATCTGGATGCAG CCAAGCTTCGCTGCATCCAGATTCACTTCACTTCGA ACCGGGGACCTTTCG.
Cell culture and transfections
Methods for culturing Cos-7 and HeLa cells have been described previously (19). Plasmids and siRNAs were transiently transfected with Lipofectamine Plus (Invitrogen) in 12-well plates with cells plated at 70–90% confluency. Except where noted a 5:1 ratio of siRNA to expression plasmid was transfected into cells, while for tRNA-valine shRNA experiments, a 10:1 ratio of shRNA plasmid to expression plasmid was used (3). Transfection efficiency ranges from 50 to 70% under these conditions by visual counting of live fluorescent cells in random fields (data not shown).
Western blot analysis
Lysates from Cos-7 cells expressing GFP and tau constructs were harvested 24 h after transfection, while APP and APPsw expressing cell lysates were harvested at 48 h. Lysates from HeLa cells expressing endogenous lamin were harvested at 72 h after transfection of anti-lamin siRNA. Lysates were analyzed by western blot as reported previously (19). GFP and lamin were detected with anti-GFP mouse monoclonal antibody (1:1000 dilution; Medical and Biological Laboratories, Naka-ku Nagoya, Japan) and anti-lamin goat polyclonal antibody (1:25 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) respectively. Additional antibodies used in this study include anti-tau mouse monoclonal antibody at 1:500 dilution (Calbiochem, San Diego, CA), 22C11 anti-APP mouse monoclonal antibody at 1:500 dilution (Chemicon International, Temecula, CA), and as a loading control, mouse monoclonal antibody to α-tubulin at 1:20 000 dilution (Sigma, St Louis, MO). Secondary antibodies were peroxidase-conjugated donkey anti-goat or peroxidase-conjugated donkey anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA) at 1:15 000 dilution. Each blot shown in Figures 1–3 is representative of two to four independent experiments (see text and legends for details).
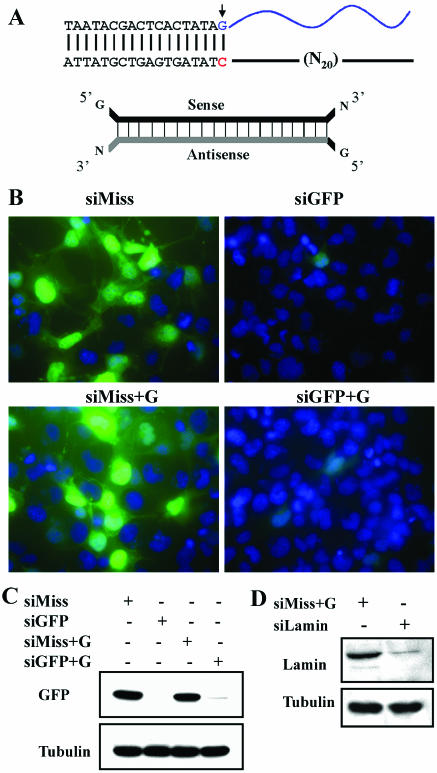
Figure 1.
siRNA+G duplexes silence endogenous and reporter genes. (A) Schematic of siRNA synthesis depicting DNA template and structure of synthesized duplexes. Blue indicates the RNA product synthesized from the DNA template (upper). For the siRNA duplex, gray indicates the region with perfect complementarity to the intended target while black depicts the sense sequence and additional non-complementary nucleotides added by the synthesis method. N represents any ribonucleotide. (B) Comparison of GFP silencing by perfectly complementary siRNA versus siRNA of the ‘+G’ design. Images depict Cos-7 cells transfected with a GFP expression construct and the indicated siRNA. Images of GFP fluorescence are merged with images of the same field showing DAPI-stained nuclei. Shown on the left are results with negative control, mistargeted siRNAs (siMiss and siMiss+G respectively), which fail to silence GFP expression. On the right, GFP expresssion is efficiently suppressed by siRNA of both configurations. (C) Western blot analysis of lysates from the same experiment as in (B). Tubulin staining is shown as a loading control. (D) Efficient silencing of endogenous lamin gene expression with siRNA+G duplexes. HeLa cells were transfected with the indicated siRNA and expression of lamin A/C was evaluated by western blot 72 h later. The siRNA+G against human lamin markedly decreased protein levels relative to the mistargeted control siRNA. All western blots shown are representative of two independent experiments.
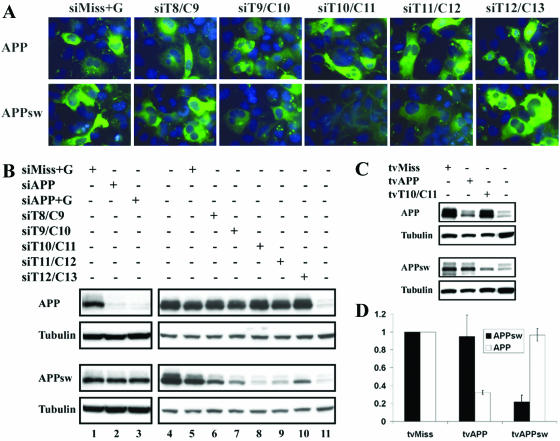
Figure 3.
Optimization of allele-specific silencing of mutant APP. Cos-7 cells were transfected with expression constructs encoding wild type APP (APP) or mutant (APPsw) and the indicated siRNAs or shRNA plasmids. Silencing was evaluated in separate transfections of the wild type and mutant constructs because the 22C11 antibody detects both APP and APPsw proteins. (A) Immunofluorescence of Cos-7 cells cotransfected with plasmids encoding APP or APPsw and the indicated siRNA. Representative images of fields (630×) reveals that allele specificity is optimal when the double mismatch is placed at the central position (siT10/C11) of the targeted sequence. APP proteins are visualized with APP antibody followed by secondary antibody labeled with FITC (green). Nuclei are stained with DAPI (blue). (B) Lanes 5–10 show a western blot of cells transfected as in (A), confirming preferential silencing of APPsw with siRNA containing central mismatches. Lane 4 is APP or APPsw transfected without siRNA. Lane 11 represents untransfected cells showing endogenous APP. Also shown in lanes 1–3 is comparable silencing of APP with siRNA or siRNA+G duplexes targeted to APP. Tubulin is shown as a loading control. (C) Western blot analysis of Cos-7 cells transfected with APP or APPsw and the indicated shRNA plasmids. tvAPP silences APP whereas tvT10/C11 selectively suppresses APPsw expression. Endogenous APP in untransfected cells is shown in the last lane. Tubulin loading control is also shown. Western blots and immunofluorescence images are representative of two (C and D) or four (A and B) independent experiments. (D) Quantitation of two independent western blot experiments performed as in (C). Bars depict mean signal intensity and standard deviations between experiments. Signal from cells co-transfected with tvMiss was set at 1.
Quantitation of western blots
Signal intensity was quantitated using NIH image as previously described (4). Signal intensity in every lane was expressed relative to tubulin staining of the same gel to control for transfection efficiency and small differences in loading of gels.
Immunofluorescence
Forty-eight hours after transfection, Cos-7 cells were fixed with 4% paraformaldehyde/PBS. APP and APPsw expression were detected with Ab 22C11 at 1:1000 dilution, followed by fluorescein (FITC)-conjugated donkey anti-mouse secondary antibody (Jackson Labs) at 1:2000 dilution. Nuclei were stained with 5 µg/ml 4′,6-diamidine-2-phenylindole HCl (DAPI) at room temperature for 10 min. Fluorescence was visualized with a Zeiss (Thornwood, NY) Axioplan fluorescence microscope. All images were captured digitally with a Zeiss MRM AxioCam camera and assembled in Photoshop 6.0 (Adobe Systems, Mountain View, CA).
RESULTS
An approach to in vitro transcription of siRNA that eliminates priming constraints of T7 RNA polymerase
An efficient way to create siRNAs against a gene of interest is to produce short RNA duplexes complementary to the target gene in in vitro transcription reactions employing T7 RNA polymerase. However, the priming requirements for T7 polymerase dictate that a G be the priming nucleotide initiating transcription (20). This limits the nucleotide positions in a target gene to which corresponding in vitro transcribed RNA duplexes can be generated. To overcome this restriction imposed by T7 RNA polymerase, we designed siRNAs that contained a non-complementary G nucleotide at the 5′ ends. The resulting siRNA contains 20 complementary nucleotides on the antisense strand with a single 5′ mismatch to the target (Table 1 and Fig. 1A). This incorporation of an initiating G should in principle allow dsRNAs to be generated in vitro against any 20-nucleotide segment of a targeted gene.
To determine whether adding this non-complementary G still produced effective siRNAs, we compared the silencing capability of this novel ‘+G’ configuration to in vitro synthesized siRNA that was perfectly complementary to the target. We assessed suppression of a reporter gene product, GFP and of an endogenous gene product, lamin (Fig. 1B–D). Cos-7 cells were co-transfected with a plasmid encoding GFP and siRNAs containing either a perfect match to the GFP mRNA or the single 5′ G mismatch. siRNAs containing multiple mismatches were used as negative controls for any non-specific effects of the transfection or siRNA. As assessed by fluorescence microscopy and western blot (Fig. 1B and C), the 5′ mismatched siRNA displayed silencing efficiency similar to that of the perfectly matched siRNA targeted to the same region of the GFP mRNA.
We next investigated the ability of these novel siRNAs to inhibit expression of an endogenous gene product, lamin. We transfected HeLa cells with a negative control siRNA (siMiss) or a siRNA directed against endogenous lamin (21), and assessed expression 72 h after transfection. Lamin expression was markedly reduced in cells transfected with siLamin+G, but remained robust in cells transfected with siMiss+G (Fig. 1D). Thus, we conclude that ‘+G’ siRNA remains an effective trigger of RNA interference.
Optimizing allele-specific inhibition of mutant tau
In our previous study of the FTDP-17 tau mutant (V337M), we succeeded in engineering siRNA duplexes that preferentially silenced the mutant allele (3). Placing the mismatch near the center of the siRNA was most effective for allele discrimination, but due to the constraints imposed by T7 polymerase we could not place the mutation precisely at the center of the siRNA. To enhance allele specificity in this earlier study, we thus needed to introduce additional mismatches into the siRNA such that it contained two mismatches versus wild type alleles but only a single mismatch versus the mutant tau allele (3). Although this improved preferential suppression of the mutant allele, recent data suggest that siRNAs with multiple internal mismatches may act by inhibiting translation (via a microRNA-like mechanism) rather than by cleaving the targeted mRNA (22,23). Accordingly, we took advantage of our new siRNA synthesis strategy in an effort to improve allele-specific silencing with the single mismatch.
We systematically tested the effect of placing the single nucleotide mismatch at each position near the predicted RISC cleavage site. Through this, we hoped to identify siRNAs that would maximize allele specificity for V337M tau. We co-transfected Cos-7 cells with flag epitope-tagged wild type tau, GFP-tagged mutant tau (V337M) and siRNAs in which the mutation had been placed at positions 9–12 of the targeted sequence. When the mismatch was placed at position 10 (siA10), the mutant allele was strongly suppressed (Fig. 2A). In contrast, placement of the mismatch more towards the 5′ or 3′ end of the target sequence resulted in siRNAs that poorly discriminated between alleles (Fig. 2A). It is important to note that although silencing of the mutant allele was strongly preferred with more centrally located mismatches, no siRNA was completely inactive against the wild type allele. Even with the mismatch optimally placed at position 10, some residual activity was still observed against the wild type allele. These results support our previous work (3,4) and results from other laboratories (5–7) indicating that central mismatches at or near the RISC cleavage site are best at discriminating between alleles. However, specificity will also be determined in part by the precise nucleotide change (5). For some mutations, introducing additional mismatches at other sites in the siRNA may be required to obtain optimal specificity.
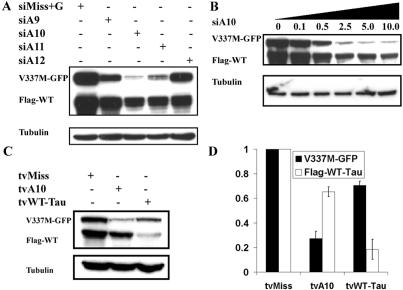
Figure 2.
Optimization of allele-specific silencing of mutant tau. Cos-7 cells were cotransfected with expression constructs encoding mutant (V337M-GFP) and WT (Flag-WT) tau and the indicated siRNAs or shRNA plasmids. (A) Western blot results showing the efficacy of allele-specific silencing when varying the placement of the point mutation (G to A) in the siRNA from positions 9–12. (B) Western blot analysis of cells cotransfected as in (A). Amounts of expression plasmids were held constant while the concentration of siA10 was varied from 0 to 10 µg per well. (C) Silencing tau with shRNA plasmid expressed from the tRNA-valine promoter. Shown is a western blot analysis of cells cotransfected with mutant and wild type tau and the indicated shRNA plasmids. Placing the mutation at position 10 (tvA10) of the hairpin results in strong preferential silencing of mutant tau. shRNA directed against wild type (mismatched at position 9 relative to mutant tau) tau inhibits expression from both alleles but shows a preference for the wild type sequence. (D) Quantitation of western blot signal from two independent experiments performed as in (C). Bars depict mean signal intensity and standard deviations between experiments. Signal from cells co-transfected with tvMiss was set at one. All western blots shown are representative of two independent experiments.
We also tested the effect of siRNA dosage on silencing preference with siA10. We found that concentrations as much as 10 times less than that required for maximal silencing still produced noticeable and preferential silencing of the mutant allele (Fig. 2B). No effective concentration abolished residual activity against wild type tau. It is difficult to directly predict the siRNA concentration required for therapeutic effects in vivo, where the disease protein is expressed at much lower levels than in our experimental system and siRNA delivery could be less efficient. However, these results do suggest that significant knockdown of mutant protein levels may be achievable with relatively modest intracellular concentrations of siRNA.
Therapeutic applications of siRNA to neurodegenerative diseases may require sustained intracellular production of siRNA. Accordingly, we next constructed and tested expression plasmids employing the tRNA-valine promoter to produce shRNA against tau (18). The shRNA consists of a 21-nucleotide-paired stem consisting of the annealed sense and antisense strands derived from our most effective in vitro synthesized duplexes. The stem is connected by an 8-nucleotide loop. An RNA polymerase III termination signal consisting of six consecutive Ts is provided immediately following the targeting sequences (for detailed protocol: http://www.cshl.org/public/SCIENCE/hannon.html).
We again co-transfected flag-WT-tau and V337M-GFP mutant tau together with shRNA plasmids designed to target either wild type or mutant tau. The tvA10 plasmid, based on the siA10 siRNA, showed strong silencing of the mutant allele with only slight inhibition of wild type expression. A shRNA directed against the wild type allele silenced wild type tau expression but also produced some suppression of the mutant allele (Fig. 2C and D). We conclude that multiple siRNA designs can rapidly be generated and screened by the method described here in order to identify the best target sequence with which to create successful shRNA expression vectors. Once validated, these shRNAs can be incorporated into recombinant viral vectors for in vivo testing (3,24).
Allele-specific silencing of APP
Next we chose to test this approach with a second gene implicated in age-related dementia, the APP gene. Many mutations have been identified in APP that cause early onset, dominantly inherited AD (Alzheimer Disease Mutations Database: http://molgen-www.uia.ac.be/ADMutations/ and references therein). We sought to suppress expression of wild type APP and the Swedish double APP mutation (K670N/M671L), or APPsw, a tandem nucleotide missense mutation that is widely employed in mouse models of AD (10,13,14). We systematically placed the tandem mismatch at each point in the central region of the siRNA duplexes to define the optimal placement for allele-specific suppression. APP silencing was assessed in Cos-7 cells cotransfected with constructs encoding wild type APP or APPsw with in vitro synthesized siRNAs. Similar to the results with tau, allelic discrimination was conferred only when the mismatches were placed centrally, as shown by APP immunofluorescence 48 h after transfection (Fig. 3A). We confirmed these results by western blot analysis, which revealed highly specific silencing of APPsw with siT10/C11, the siRNA in which the double mismatch is placed immediately across from the presumed RISC cleavage site (Fig. 3B, lanes 5–10). The corresponding wild type-specific siRNA led to robust suppression of wild type APP (Fig. 3B, lanes 2 and 3).
Next, we engineered plasmids expressing anti-APP shRNAs based on our most effective in vitro duplex sequences. As shown in Figure 3C and D, shRNA designed to target the wild type sequence silenced only wild type APP expression, whereas shRNA designed to target APPsw specifically suppressed the mutant allele. These results describe novel and important reagents for functional studies of APP, the physiological function of which is still not fully understood (8). The siRNAs and shRNAs developed here should also permit the in vivo testing of siRNA as a therapeutic strategy to reduce amyloid deposition in AD mouse models (13,14).
DISCUSSION
Efficient siRNA design for any target sequence
RNAi holds promise as a potential therapy for human diseases. Yet a limitation to successfully developing gene-specific or allele-specific siRNAs is the selection and design of siRNAs with the desired silencing characteristics. Individual siRNAs targeted to different regions of a transcript often display striking differences in efficacy and specificity (3,5). Typically, several target sites and designs need to be tested before optimal silencing is achieved (3). Here we have described a simple method that not only circumvents the time and cost disadvantages of chemically synthesizing siRNA duplexes but also removes the sequence restrictions imposed by in vitro transcription with T7 polymerase.
The insertion of a single G mismatch at the 5′ of the siRNA duplex permitted efficient priming by T7 polymerase without compromising the silencing efficacy of the resultant siRNA. Such ‘+G’ siRNAs can rapidly be generated to essentially any point in a targeted gene and tested for efficacy. This approach to siRNA design should facilitate the in vitro generation of effective siRNAs. As demonstrated here for two important disease targets, tau and APP, these in vitro transcribed duplexes can then serve as guides for producing shRNA plasmids that retain silencing capability and allele specificity. This approach represents an improved, stepwise method for optimized silencing of essentially any gene of interest.
Indeed, based on new insights into RISC assembly, manipulating the 5′ terminal nucleotide of the guide strand in this way may be highly advantageous. Schwarz et al. (25) recently discovered marked asymmetry in the rate at which each strand of an RNA duplex enters the RISC complex. Preferential entry of the guide, or antisense, strand into RISC can be achieved by introducing 5′ mismatches in the antisense strand while maintaining perfect base pairing at the 5′ terminus of the sense strand. This maximizes entry of the antisense strand into the RISC complex while also reducing potential off-target inhibition by the sense strand. The ‘+G’ approach to siRNA design is perfectly suited to engineering dsRNAs based on this principle that should display preferred RISC entry of the guide strand.
Central placement of mismatches are required for allelic discrimination
Using our approach to in vitro siRNA production, we were able to systematically test the effect of placing mismatches at each point along the guide strand of the siRNA. For tau and APP, central placement of mismatches resulted in optimal allele-specific silencing of mutant alleles. With the APPsw double mutation, for example, we found that placing the two mismatches immediately across from the predicted RISC cleavage site resulted in highly specific allele discrimination. Together with recent findings by others (3–7), these results demonstrate the importance of central placement of mutations for successful allele-specific silencing.
For tau, however, siRNAs with centrally placed mismatches still retained some activity against the wild type allele. This suggests that both the position of the mismatch along the guide strand and the chemical nature of the mismatch are important for determining whether RISC associated nucleases will cleave a given mRNA. This is consistent with results suggesting that disruption of the predicted structure between mRNA and the RISC-associated guide strand will prevent cleavage (26). Systematic testing of RISC-mediated cleavage in vitro strongly suggests that cleavage occurs across from the central nucleotides of the guide strand, most likely 9–11 nucleotides from the 5′ end (5). While perfect base pairing is optimal, some mismatches are less disruptive and still permit partial cleavage activity. For example, in RNAi studies targeting a single nucleotide change in the polyglutamine disease gene MJD1, a G-G clash between the antisense strand of the siRNA and the target mRNA resulted in a complete inability to silence the wild type allele while the mutant allele was strongly suppressed (3). In contrast, even with the tau (V337M) mutation optimally placed centrally in the siRNA, we continued to observe some silencing of wild type tau (3). This suggests that the less disruptive G-U clash in the case of the tau mutation does not allow for complete allelic discrimination by siRNA. In such cases additional mismatches may need to be incorporated into the siRNA.
Experimental and therapeutic implications
The RNAi reagents developed here against tau and APP constitute an experimental and potential therapeutic advance for AD and related dementias. Although abnormal deposition of tau and the APP cleavage product Aβ are central to AD pathogenesis, the precise roles of these proteins in the brain remain to be elucidated (8,9). These siRNA reagents, which can be used to selectively silence expression of mutant or wild type tau and APP, should facilitate loss of function experiments aimed at identifying the neuronal functions of these proteins.
For potential therapeutic applications of siRNA, we have established expression vectors that silence mutant or wild type forms of tau and APP. For individuals with dominantly inherited AD or tauopathy, selective removal of the mutant protein might ameloriate or even prevent disease. The demonstration of specific silencing of mutant alleles extends the potential utility of the approach to genes with important or essential functions. For APP we achieved specific silencing of either the widely studied Swedish double mutant or wild type APP. Reagents that suppress APPsw should be useful in testing RNAi therapy in mouse models of AD, and reduction of wild type APP may also have therapeutic potential for the common, sporadic form of AD. Based on the amyloid cascade hypothesis of AD, the most selective intervention would be a reagent that suppresses APP protein production with minimal effects on unintended targets (8). Aβ production requires cleavage of APP by two proteases, the β site APP-cleaving enzyme BACE and the γ-secretase complex, which contains presenilin (27). Thus, additional gene targets in AD include BACE and, for most familial AD, dominantly acting presenilin mutations.
A major challenge in applying siRNA therapy to the nervous system is achieving sustained, effective delivery of siRNA to the correct target cells in the brain. These data, combined with in vivo results from other groups (24,28), suggest that siRNA will effectively suppress expression of the targeted gene, provided that it can be delivered efficiently to the appropriate neurons. Hope is offered by the observation here and elsewhere that sustained intracellular production of siRNA can be achieved with expression plasmids. These plasmids retain their silencing characteristics when incorporated into viral vectors that are known to transduce CNS neurons (29). If brain delivery can be achieved, questions still remain concerning the long-term safety of chronically co-opting the RNAi pathway to target a specific gene. RNAi studies in transgenic animal models of AD and other diseases should help to answer these questions.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Gloria Lee and R. Scott Turner for providing constructs. We also thank members of the Paulson laboratory for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health Predoctoral Training Grant T32 GM08629 (to the University of Iowa program in genetics and V.M.M.) and a Paul Beeson Physician Faculty Scholar Award (to H.L.P.) funded by the American Federation for Aging Research, Atlantic Philanthropies Inc., The John A. Hartford Foundation and The Starr Foundation.
REFERENCES
- 1.McManus M.T. and Sharp,P.A. (2002) Gene silencing in mammals by small interfering RNAs. Nature Rev. Genet., 3, 737–747. [DOI] [PubMed] [Google Scholar]
- 2.Song E., Lee,S.K., Wang,J., Ince,N., Ouyang,N., Min,J., Chen,J., Shankar,P. and Lieberman,J. (2003) RNA interference targeting Fas protects mice from fulminant hepatitis. Nature Med., 9, 347–351. [DOI] [PubMed] [Google Scholar]
- 3.Miller V.M., Xia,H., Marrs,G.L., Gouvion,C.M., Lee,G., Davidson,B.L. and Paulson,H.L. (2003) Allele-specific silencing of dominant disease genes. Proc. Natl Acad. Sci. USA, 100, 7195–7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Alegre P., Miller,V.M., Davidson,B.L. and Paulson,H.L. (2003) Toward therapy for DYT1 dystonia: allele-specific silencing of mutant TorsinA. Ann. Neurol., 53, 781–787. [DOI] [PubMed] [Google Scholar]
- 5.Ding H., Schwarz,D.S., Keene,A., Affar,B., Fenton,L., Xia,X., Shi,Y., Zamore,P.D. and Xu,Z. (2003) Selective silencing by RNAi of a dominant allele that causes amyotrophic lateral sclerosis. Aging Cell, 2, 209–217. [DOI] [PubMed] [Google Scholar]
- 6.Abdelgany A., Wood,M. and Beeson,D. (2003) Allele-specific silencing of a pathogenic mutant acetylcholine receptor subunit by RNA interference. Hum. Mol. Genet., 12, 2637–2644. [DOI] [PubMed] [Google Scholar]
- 7.Martinez L.A., Naguibneva,I., Lehrmann,H., Vervisch,A., Tchenio,T., Lozano,G. and Harel-Bellan,A. (2002) Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc. Natl Acad. Sci. USA, 99, 14849–14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy J. and Selkoe,D.J. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science, 297, 353–356. [DOI] [PubMed] [Google Scholar]
- 9.Lee V.M., Goedert,M. and Trojanowski,J.Q. (2001) Neurodegenerative tauopathies. Annu. Rev. Neurosci., 24, 1121–1159. [DOI] [PubMed] [Google Scholar]
- 10.Mullan M., Crawford,F., Axelman,K., Houlden,H., Lilius,L., Winblad,B. and Lannfelt,L. (1992) A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nature Genet., 1, 345–347. [DOI] [PubMed] [Google Scholar]
- 11.Poorkaj P., Bird,T.D., Wijsman,E., Nemens,E., Garruto,R.M., Anderson,L., Andreadis,A., Wiederholt,W.C., Raskind,M. and Schellenberg,G. (1998) Ann. Neurol., 43, 815–825. [DOI] [PubMed] [Google Scholar]
- 12.Hutton M., Lendon,C.L., Rizzu,P., Baker,M., Froelich,S., Houlden,H., Pickering-Brown,S., Chakraverty,S., Isaacs,A. et al. (1998) Nature, 393, 702–705. [DOI] [PubMed] [Google Scholar]
- 13.Lewis J., Dickson,D.W., Lin,W.L., Chisholm,L., Corral,A., Jones,G., Yen,S.H., Sahara,N., Skipper,L., Yager,D., Eckman,C., Hardy,J., Hutton,M. and McGowan,E. (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science, 293, 1487–1491. [DOI] [PubMed] [Google Scholar]
- 14.Oddo S., Caccamo,A., Shepherd,J.D., Murphy,M.P., Golde,T.E., Kayed,R., Metherate,R., Mattson,M.P., Akbari,Y. and LaFerla,F.M. (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron, 39, 409–421. [DOI] [PubMed] [Google Scholar]
- 15.Houlden H., Baker,M., Morris,H.R., MacDonald,N., Pickering-Brown,S., Adamson,J., Lees,A.J., Rossor,M.N., Quinn,N.P., Kertesz,A., Khan,M.N., Hardy,J., Lantos,P.L., St George-Hyslop,P., Munoz,D.G., Mann,D., Lang,A.E., Bergeron,C., Bigio,E.H., Litvan,I., Bhatia,K.P., Dickson,D., Wood,N.W. and Hutton,M. (2001) Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology, 56, 1702–1706. [DOI] [PubMed] [Google Scholar]
- 16.Donze O. and Picard,D. (2002) RNA interference in mammalian cells using siRNAs synthesized with T7 RNA polymerase. Nucleic Acids Res., 30, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koseki S., Tanabe,T., Tani,K., Asano,S., Shioda,T., Nagai,Y., Shimada,T., Ohkawa,J. and Taira,K. (1999) Factors governing the activity in vivo of ribozymes transcribed by RNA polymerase III. J. Virol., 73, 1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki H. and Taira,K. (2003) Short hairpin type of dsRNAs that are controlled by tRNA(Val) promoter significantly induce RNAi-mediated gene silencing in the cytoplasm of human cells. Nucleic Acids Res., 31, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai Y., Koppenhafer,S.L., Shoesmith,S.J., Perez,M.K. and Paulson,H.L. (1999) Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum. Mol. Genet., 8, 673–682. [DOI] [PubMed] [Google Scholar]
- 20.Kato M., Frick,D.N., Lee,J., Tabor,S., Richardson,C.C. and Ellenberger,T. (2001) A complex of the bacteriophage T7 primase-helicase and DNA polymerase directs primer utilization. J. Biol. Chem., 276, 21809–21820. [DOI] [PubMed] [Google Scholar]
- 21.Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. [DOI] [PubMed] [Google Scholar]
- 22.Zeng Y., Yi,R. and Cullen,B.R. (2003) MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl Acad. Sci. USA, 100, 9779–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doench J.G., Petersen,C.P. and Sharp,P.A. (2003) siRNAs can function as miRNAs. Genes Dev., 17, 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia H., Mao,Q., Paulson,H.L. and Davidson,B.L. (2002) siRNA-mediated gene silencing in vitro and in vivo. Nat. Biotechnol., 20, 1006–1010. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz D.S., Hutvagner,G., Du,T., Xu,Z., Aronin,N. and Zamore,P.D. (2003) Asymmetry in the assembly of the RNAi enzyme complex. Cell, 115, 199–208. [DOI] [PubMed] [Google Scholar]
- 26.Chiu Y.L. and Rana,T.M. (2002) RNAi in human cells: basic structural and functional features of small interfering RNA. Mol. Cell., 10, 549–561. [DOI] [PubMed] [Google Scholar]
- 27.Sisodia S.S. and St George-Hyslop,P.H. (2002) gamma-Secretase, Notch, Abeta and Alzheimer’s disease: where do the presenilins fit in? Nature Rev. Neurosci., 3, 281–290 [DOI] [PubMed] [Google Scholar]
- 28.Rubinson D.A., Dillon,C.P., Kwiatkowski,A.V., Sievers,C., Yang,L., Kopinja,J., Rooney,D.L., Ihrig,M.M., McManus,M.T., Gertler,F.B., Scott,M.L. and Van Parijs,L. (2003) A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nature Genet., 33, 401–406. [DOI] [PubMed] [Google Scholar]
- 29.Davidson B.L. and Breakefield,X.O. (2003) Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci., 4, 353–364. [DOI] [PubMed] [Google Scholar]