Abstract
Objective
The research involved three objectives: 1) determine the feasibility of using cell phone based ecological momentary assessment (EMA) to measure blood glucose monitoring and insulin administration in adolescent type 1 diabetes, 2) relate EMA to traditional self-report and glycemic control, and 3) identify patterns of adherence by time of day and over time using EMA.
Methods
Adolescents with type 1 diabetes (n=96) completed baseline measures of cell phone use and adherence. Glycemic control (HbA1c) was obtained from medical records. A subgroup of adolescents (n=50) completed 10 days of EMA to assess blood glucose monitoring frequency, timing of glucose monitoring, insulin administration, and insulin dosing.
Results
One-third of adolescents were not allowed to use their cell phones for diabetes at school. Parental restrictions on cell phone use at home were not prevalent. The EMA response rate (59%) remained stable over the 10-day calling period. Morning time was associated with worse monitoring and insulin administration, accounting for 59–74% of missed self-care tasks. EMA-reported missed glucose checks and missed insulin doses were correlated to traditional self-report data, but not correlated to HbA1c. Trajectory analyses identified two sub-groups: one with consistently adequate adherence, and one with more variable, and worse, adherence. The latter adherence style showed worse glycemic control.
Conclusion
Mobile phones provide a feasible method to measure glucose monitoring and insulin administration in adolescents, given a limited assessment duration. The method provided novel insights regarding patterns of adherence and should be explored in clinical settings for targeting or tailoring interventions
Keywords: diabetes, adolescent, adherence, assessment, mobile, ecological momentary assessment
INTRODUCTION
Type 1 diabetes (T1D) is one of the most common pediatric chronic illnesses (Centers for Disease Control and Prevention, 2008). The primary diabetes outcome is glycemic control, as measured by a blood test (glycosylated hemoglobin or HbA1c) that indicates average plasma glucose for the previous 2–3 months. Poor glycemic control has been related to short-term consequences such as hypo- or hyperglycemia and diabetic ketoacidosis, as well as serious health consequences later in life such as limb amputation, retinopathy, and renal failure (Centers for Disease Control and Prevention, 2008). Completion of recommended self-care tasks is critical to glycemic control (K. K. Hood, Peterson, Rohan, & Drotar, 2009). The primary self-care tasks that help to maintain glycemic control, such as monitoring blood glucose levels, injecting insulin, and dosing insulin according to meter results or other factors, must be carried out several times per day, often around meals, and in varied contexts such as school, home, restaurants, and sports. Given their frequency and nature, it is not surprising that their completion of these tasks in adolescents may be suboptimal (Anderson, Auslander, Jung, Miller, & Santiago, 1990; Greening, Stoppelbein, Konishi, Jordan, & Moll, 2007).
Because blood glucose monitoring and insulin administration are critical for diabetes outcomes, measurement of those behaviors is an important part of research and clinical care. However, challenges in the measurement of adherence may negatively impact research designed to understand and improve outcomes. Self-report questionnaires that assess frequencies of self-care tasks have generally shown small to moderate relationships with HbA1c (K. K. Hood, et al., 2009). This relationship may be influenced by a restricted range of values (e.g. ceiling effects), possibly due to social desirability and/or biased recall on traditional retrospective self-report measures (Shiffman, Stone, & Hufford, 2008). Retrospective questionnaire self-report data tend to result in higher adherence estimates compared to relatively more objective methods, such as ecological momentary assessment or the use of blood glucose values from meters (Guilfoyle, Crimmins, & Hood, In press; Shiffman, et al., 2008).
Ecological momentary assessment (EMA) is the sampling of behaviors and experiences in real time using mobile devices. Ideally, the behavior of interest is measured within close proximity in time and environment in which that behavior occurs (Shiffman, et al., 2008). Considerations for implementing EMA include ubiquitous access to a mobile device, and the timing and duration of assessments. This method has been used to improve the measurement of health behaviors in a number of areas including asthma, eating disorders, cancer, health promotion and diabetes among others (Anhoj & Moldrup, 2004; Bielli et al., 2004; Dunton, Atienza, Castro, & King, 2009; Helgeson, Lopez, & Kamarck, 2009; Hilbert, Rief, Tuschen-Caffier, de Zwaan, & Czaja, 2009). The promise and potential of EMA lies in minimizing response biases, accessing information that traditional retrospective self-report does not assess well, and assessing those variables over time in a more frequent and fine-grained manner. Insights into patterns of adherence behaviors over time within specific contexts could provide powerful feedback and inform clinical decision-making.
Although mostly formative in nature, mobile health research has been growing rapidly. The prevalence of cell phones has improved the feasibility of using EMA. However, application of cell-phone based momentary assessment in pediatric diabetes has been limited (Franklin, Greene, Waller, Greene, & Pagliari, 2008; Hanauer, Wentzell, Laffel, & Laffel, 2009; Helgeson, et al., 2009). Although cell phone use amongst 13–17 year olds is estimated at 93% (Lenhart, Purcell, Smith, & Zickuhr, 2010), little is known how adolescents currently use cell phones for diabetes or how rules at home and school may impact the measurement of health behaviors. Thus, one important goal of this research was to identify factors that could limit adolescent access to cell phones.
Studies which examine patterns of diabetes adherence over time, subtypes of adherence, or contextual influences on adherence are also limited. One cross-sectional study identified three styles of adherence in adolescent type 1diabetes that they labeled methodical, adaptive, and inadequate (Schneider et al., 2007). Schlundt et al (1996) identified contexts which posed barriers to adequate nutritional decision-making in adolescent diabetes, such as social events and holidays. These and other similar studies (Jacobson, Hauser, Lavori, & Wolfsdorf, 1990; Johnson et al., 1992) contribute significantly to our understanding of adolescent adherence over time and in context, but have not measured adherence in near real-time or in close proximity to their actual occurrence.
The present study expands our understanding of adolescent diabetes by 1) identifying how adolescents use their phones for diabetes and barriers to cell phone based EMA, 2) relating EMA-reported blood glucose monitoring and insulin administration to traditional retrospective questionnaire-based data and glycemic control, and 3) identifying patterns of adherence as related to times of day and over time. It was hypothesized that the EMA system designed and tested here would provide a feasible method to assess specific self-care behaviors, the number of missed self-care tasks measured through EMA would positively relate to traditional self-report and HbA1c, and that unique information regarding sub-group patterns of adherence by time of day and over time would be identified. Results provide the basis for recommendations regarding expansion of this method for research and clinical settings in pediatric diabetes.
METHODS
Study Setting, Recruitment, and Procedures
Adolescents from a large academic diabetes clinic were invited to participate in a study regarding the use of cell phones to measure diabetes adherence. Inclusion criteria for the study included a diagnosis of type 1 diabetes for at least one year, age 12–17, adolescent ownership of a cell phone, and permission from a parent to be called on their phone twice per day for the research. Survey participants were invited to take part in the EMA portion of the study. In order to obtain a sub-sample of 50, we needed to ask 60 consecutive participants (an acceptance rate of 83%). The survey was administered online using REDCap survey (Harris et al., 2009). The research was approved by the Institutional Review Board, and parent consent and adolescent assent were obtained for all participants.
Measurement
Demographics, Cell Phone Use, and Metabolic Control
Demographic and diabetes clinical information was obtained from parents at baseline. At baseline, adolescents completed a 20-item cell phone use survey created for this study. Items assessed information such as access to the cell phone at home and in school, times when adolescents did not carry their phones, use of the phone for diabetes self-care, and preferred methods of communication. Responses were either dichotomous (yes/no), short answer, check all that apply, or Likert-type options (Items available upon request). HbA1c testing was completed in the clinic on the day of baseline measures, and obtained through medical record review.
Baseline Adherence
Adolescents completed the Diabetes Behavior Rating Scale (DBRS) (Iannotti et al., 2006) at baseline. This 37-item scale assesses a broad range of diabetes self-care tasks over the previous week related to frequency of blood glucose testing, insulin administration, diet, exercise, and other behaviors, such as keeping records of blood glucose levels, wearing a diabetes bracelet, keeping clinic appointments, carrying fast sugar, and responding appropriately to hypo- and hyperglycemia. Each item is rated on a scale from 1 (never) to 5 (always). Because the DBRS measures many different adherence behaviors that were not relevant for the EMA assessment, the six items that corresponded to the self-care behaviors assessed via EMA were analyzed: blood glucose monitoring and timing, insulin administration, and insulin dosing (e.g., “How often were blood sugar levels tested as recommended by the doctor?”). The six items from the DBRS demonstrated adequate reliability in this sample (α =.73).
Ecological Momentary Assessment of Adherence
For the EMA portion of the research, an automated interactive touch tone telephone response system using Telesage® was implemented (www.telesage.com). Telesage is a commercially available interactive voice response (IVR) system that uses recorded questions. Participants were called twice per day for 10 days. Calls were always initiated on a weekday and continued for 10 days in order to include at least one weekend. Participants identified their own preferred and/or feasible call times within each of the morning, afternoon, and evening periods that would allow them to answer calls after taking care of diabetes. Although adolescents determined the actual time they were called, the call schedule rotated by day to sample two time periods (e.g., morning/afternoon, afternoon/evening, morning/evening).
Self-care behaviors measured via EMA included completion of blood glucose monitoring, timing of blood glucose monitoring, administering insulin, and timing and dosing of insulin according to a blood glucose meter reading. The following questions were administered through the cell phone: 1) “Just thinking about today, enter the time when you most recently should have checked your sugar,” 2) “Enter the time when you most recently did check your sugar. If you did not check your sugar, hit zero, “ 3) “Enter the time you most recently took insulin today. If you did not take insulin, hit zero,” and 4)”The last time you took insulin, did you dose it according to your meter?” A data coding error precluded using data related to timing of insulin administration in analyses. Timing of blood glucose monitoring is presented below.
Adolescents were called via “outbound” calls but, if they missed a call, they could call back into the system via “inbound” calls. Data obtained through inbound calls were used if the incoming call was made before the next scheduled outbound call. This insured that there were not two call records referring to the same adherence behaviors.
Analytic Approach
Descriptive statistics were used to summarize the sample characteristics and key study variables. If data distributions were skewed, we report both the mean and median values. To describe variability we report the inter-quartile range (IQR) representing the middle 50% of the sample. Spearman correlations were used for bivariate associations. Because each participant had a variable number of calls reporting adherence, EMA variables were calculated as a percentage of calls that reported missed glucose monitoring, insulin administration, or insulin dosing. Timing of blood glucose monitoring was calculated as the difference between the two EMA questions assessing when adolescents last should have completed a task and when they reported completing it.
A group-based, log-likelihood, trajectory modeling approach (Nagin et al., 1993) was used to detect subgroups of participants with similar longitudinal patterns of missed glucose checks and insulin administration reports. For a participant to be included in the trajectory analyses, they must have responded to at least 4 calls. At least one of the 4 responses had to be within the first two days, and one within the last two days. The Bayesian information criterion (BIC) statistic, as well as cluster size, were used as criteria for trajectory model selection (Nagin et al., 2001). The BIC is a measure of the relative goodness of fit of a statistical model. The BIC introduces a penalty term for the number of parameters in the model, thus reducing the likelihood of improved fit simply by adding parameters. Insulin dosing was not analyzed in this manner due to the low frequency of reported incorrect dosing.
RESULTS
Sample
Table 1 shows demographic and clinical data for the adolescents (N=96) who completed the baseline cell phone survey and the sub-sample of 50 adolescents who completed the EMA portion of the study.
Table 1.
Description of demographic and clinical characteristics of the total sample and EMA sub-sample.
| Mean (SD) or % | ||
|---|---|---|
|
| ||
| Total Sample N=96 | EMA Sub- Sample N=50 | |
| Age | 14.96 (1.62) | 15.11 (1.60) |
| Male (%) | 53.2 | 50.1 |
| Ethnicity (%) | ||
| White | 91.7 | 97.8 |
| African American | 6.0 | 2.2 |
| Pacific Islander | 1.2 | 0 |
| Hispanic | 1.2 | 0 |
| Household Income (%) | ||
| Less than $20,000 | 6.4 | 4.8 |
| $20,001–$40,000 | 15.4 | 16.7 |
| $40,001–$70,000 | 30.8 | 26.2 |
| More than $70,000 | 42.3 | 47.6 |
| Decline to Answer | 5.1 | 4.8 |
| Type of School (%) | ||
| Public | 76.2 | 75.6 |
| Private | 10.7 | 6.7 |
| Home Schooled | 13.1 | 17.8 |
| Duration of Diabetes (years) | 8.12 (3.76) | 6.41 (3.77) |
| Using Insulin Pump (%) | 53.6 | 53.3 |
| HbA1c | 8.78 (1.93) | 9.04 (1.95) |
Adolescent Cell Phone Access and Use
When asked about rules regarding cell phones at home, 17.7% of adolescents reported restrictions on when they were allowed to use their phone, and 8.3% were required to at least partially pay for, or complete tasks to earn, their cell phone. The majority (76%) said that they always carry their cell phone with them. Only 20.0% of adolescents had Internet access through their phones. The majority (60%) reported they prefer to use text messages to talking on the phone for communication.
Table 2 shows how adolescents reported using their cell phones for diabetes. Cell phones were most frequently used to send parents blood glucose values in a text message, or to call a parent if they forgot diabetes supplies. Of those adolescents who attended public or private schools (n = 84), 35.4% reported that they were allowed to use their cell phone at school for diabetes, 32.3% were not allowed, and 32.2% did not know. Twenty-five percent believed that limited cell phone access at school negatively impacted how they take care of diabetes.
Table 2.
How teens use cell phones for diabetes (N=96).
| How often do you use your cell phone to: | Mean (SD) |
|---|---|
| Access online information about diabetes* | 1.40 (0.84) |
| Send a text message about diabetes | 2.03 (1.11) |
| Set a reminder to do something for diabetes | 1.95 (1.35) |
| Call a family member about diabetes | 2.55 (1.18) |
| Talk to a nurse or doctor | 1.94 (1.23) |
1 = Never, 2 = Once or twice a month, 3 = Once or twice a week, 4 = Almost every day, 5 = Every day;
Although all adolescents were asked this question, only 20% reported having Internet access through their phones.
Feasibility of EMA Method: Missing Data and Response Bias
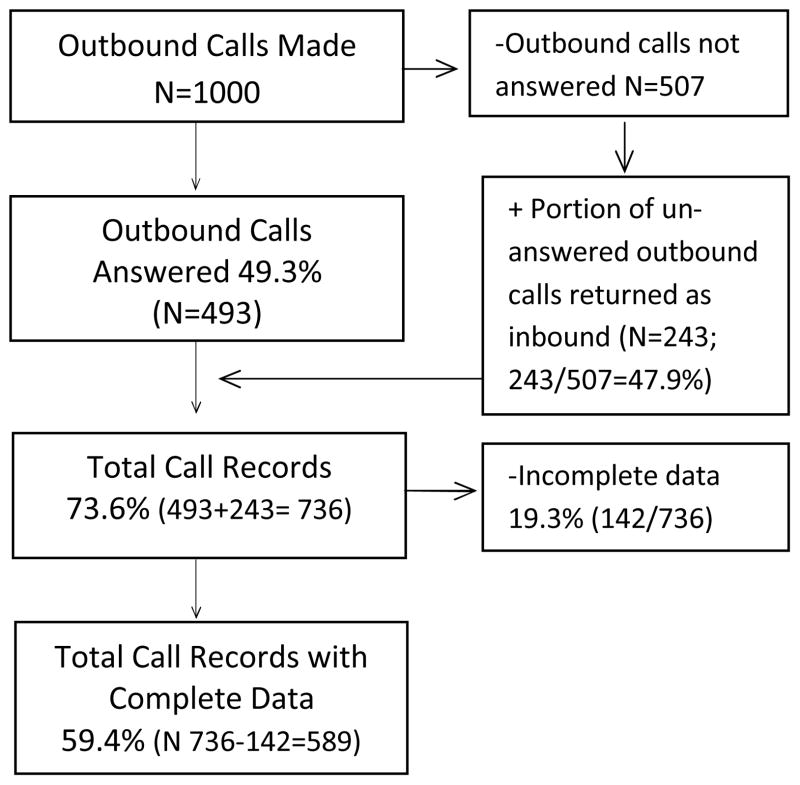
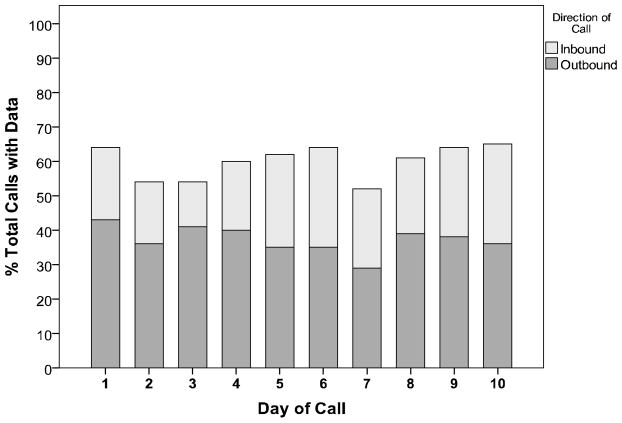
A total of 1000 calls were made to participants (50 subjects * 2 calls /day * 10 days). Each adolescent was called 20 times to report adherence behaviors. Figure 1 shows the disposition of the 1000 outbound calls made. Approximately 50% of outbound calls to participants were answered, and half (49.7%) of the missed calls were returned as inbound calls. Approximately 19% of all calls had incomplete or missing data due to either dropped calls or hang-ups, or Telesage interpreted voicemail as a human voice and so created a call record with no data. There was a resulting total of 58.9% of calls with data (n = 589), and an average of 12.04 (SD 6.13, range 1–21) calls per subject with data for analyses. Figure 2 shows total outbound and inbound calls with data obtained over the 10-day calling period. The percent of calls with data averaged 55%, and remained stable over the study period (Likelihood χ2=8.94, p = .443). When examined by time of day, there were 27.7% (163/589) of calls with complete data for the morning period, 41.6% (245/589) for the afternoon period, and 30.7 % (181/589) for the evening period.
Figure 1.
Disposition of EMA calls (N=50).
Figure 2.
Percent of calls with data by day (N=50).
To explore possible response bias in missing data, the percent of calls with data per participant was correlated with EMA-reported adherence and glycemic control (A1C). There were no statistically significant associations between percent of calls with data and level of adherence for any of the EMA questions. There was a statistically significant association between the number of calls with data and A1C (rs = −.451, p = .001). Therefore, reported associations between the EMA data and A1C were adjusted for the number of calls with data available for each participant.
Analysis of adherence by type of call indicated similar levels of missed blood glucose checks reported through outbound calls (12.5% of outbound calls) versus inbound calls (10.1% of inbound calls; χ2 .788, p=.375). Similar levels of missed insulin doses were also reported through outbound calls (20.0%) compared to inbound calls (17.3%; χ2 .684, p=.408).
Blood Glucose Monitoring
Table 3 shows mean and median adherence levels for blood glucose monitoring and timing. On average, adolescents reported missing 14% of blood glucose checks. This distribution was skewed (Median 2.5%), with 50% of participants reporting perfect adherence (e.g. no checks missed) to blood glucose monitoring over the 10 day calling period.
Table 3.
Descriptive summaries of EMA-reported adherence over 10 days (N=50).
| Mean, Median (IQR) a | |
|---|---|
| Blood Glucose Monitoring | |
| Missed Checks (%) | 14.2, 2.5 (0.0 – 18.8) |
| Timing (minutes late) | 40.44, 5.0 (0.0 – 60.0) |
|
| |
| Insulin Administration | |
| Missed Doses (%) | 20.4, 15.8 (0.0 – 27.1) |
| Incorrect Doses (%) | 4.6, 0.0 (0.0 – 0.0) |
IQR: 25th and 75th inter-quartile boundaries
Patterns of Adherence
When missed blood glucose checks were examined by time of day, morning accounted for 59.40% of the missed checks, afternoon for 27.52%, and evening for 13.15% (χ2 44.61 p<.001).
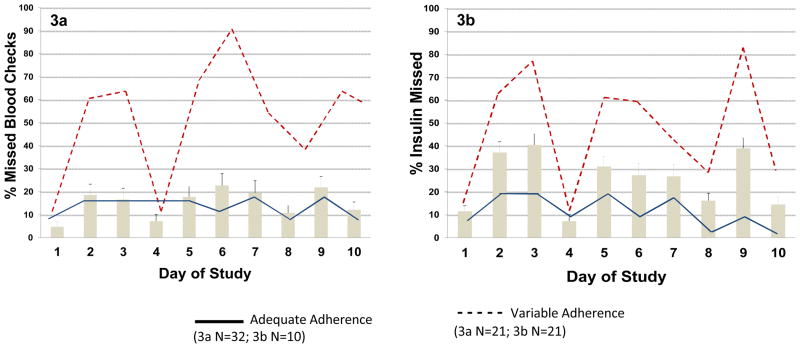
Figure 3a shows the average blood glucose monitoring adherence by day for the whole sample, as well as the two adherence sub-groups identified through trajectory analyses. The two-group trajectory solution produced a BIC that demonstrated minimal change with an increase to three groups (−151.09 vs. −150.74). As shown by the solid line, one subgroup (the “adequate” group; n = 32) reported consistently fewer missed blood checks over the 10 days, while the other group (the “variable” group, shown by the dashed line; n=10), reported a generally higher, but also more variable, number of missed blood checks. The percent of missed blood glucose checks for the variable adherence group was higher than that of the adequate adherence group (36.50% vs. 16.81%; Mann-Whitney U = 10.00, Z-statistic=-4.65, p <.001). There was no statistically significant difference between the number of calls with data per participant between the two trajectory pattern groups (Mann-Whitney U = 134.00, Z-statistic=0.77, p =.441).
Figure 3.
Average adherence and trajectories for missed blood checks (3a), and missed insulin doses (3b). Average adherence for the entire sample is represented by the bars (N=42).
Insulin Administration and Dosing
Descriptive summaries of insulin administration and dosing are shown in Table 3. On average, adolescents reported missing 20% of their insulin doses. This distribution was skewed (Median 16%) with thirty percent of subjects reporting perfect adherence (e.g. no missed doses) for insulin administration. Table 3 also shows that the average rate of reported incorrect insulin doses was very low 4.6% (Median 0%).
Patterns of Adherence
When missed insulin injections were examined by time of day, morning calls were associated with 74.1% of the missed injections, afternoon calls accounted for 17.9% of the missed injections, and evening calls 8.0% (χ2 75.26, p<.001).
Figure 3b shows the average insulin administration adherence by day for the whole sample and the two adherence sub-groups identified through trajectory analyses. Again, the two group trajectory solution produced a BIC that demonstrated minimal change with an increase to three groups (−216.58 vs. −216.72). As shown by the solid line, the ”adequate” subgroup (n=21) consistently reported few missed insulin doses, and the “variable” group (shown by the dashed line; n=21) reported a generally higher, but more highly variable, number of missed insulin doses. Percent of missed insulin doses for the variable adherence trajectory group was greater than that of the adequate adherence group (30.74% vs.12.26%; Mann-Whitney U = 26.50, Z-statistic=−4.91, p <.001). There was no statistically significant difference between the number of calls with data per participant between the two trajectory pattern groups (Mann-Whitney U = 134.00, Z-statistic=0.77, p =.441).
Relation of EMA to Baseline Self-Report of Adherence
Self-report of adherence at baseline was related to EMA-reported missed blood glucose checks (rs = −.32, p=.023) and missed insulin doses (rs = −.29, p=.045), but not timing of glucose monitoring (rs = .27, p= .072) or incorrect insulin dosing (rs = −.18, p= .216).
Relation of EMA to HbA1c
Overall, EMA-reported adherence was not related to A1C for % missed blood checks (rs = .035, p=.813), % missed insulin doses (rs = .014, p=.924), or % correct insulin dosing (rs =.091, p=.542). For the blood glucose monitoring trajectory analyses, the “variable” adherence group mean HbA1c was 9.06 (SD 1.99) and the “adequate” adherence group mean HbA1c was 8.85 (SD 2.51). The difference in HbA1c between the two blood glucose monitoring trajectory groups was 0.21% (Mann-Whitney U =137.5, Z-statistic=.665, p =.512; Cohen’s d statistic .09). For the insulin administration trajectory analyses, the variable adherence group mean HbA1c was 9.39 (SD 2.29) and the adequate adherence group mean HbA1c was 8.62 (SD 1.87). The difference in HbA1c between the two insulin administration trajectory groups was 0.77% (Mann-Whitney U =177.50, Z-statistic=1.08, p =.279; Cohen’s d statistic .34).
DISCUSSION
The purpose of this research was to identify barriers to adolescent cell phone use, determine the feasibility of using cell phones to measure adherence in type 1 diabetes, describe how adolescents use the cell phone for diabetes, relate ecological momentary assessment (EMA) to traditional self-report and glycemic control, and identify patterns of adherence by time of day and over time. The study is novel because it is the first to measure pediatric diabetes adherence using EMA.
Overall, adolescents reported limited use of their phones to support diabetes self-care. Simple functions, such as setting reminders, were infrequent, but may also be limited by cell phone functionality, and/or school cell phone policies. There were few times when adolescents were not allowed to use their phones at home. However, one-third of adolescents were not allowed to use their phones for diabetes at school, and another one-third was not sure about school policies. School cell phone policies most likely did not impact this study for several reasons: adolescents were asked to schedule call times when they were available to answer the phone; the afternoon calling period did not have a greater portion of missed calls; and the majority of afternoon calls were scheduled after school. This restriction at school likely resulted in reporting afternoon adherence behaviors several hours after they occurred. While the timing of the present EMA study allowed for a delay between behavior and reporting of it, school policies may impact the use of cell phones to measure self-care behaviors. Given that school-based barriers to adherence are so relevant for adolescents (Nabors, Troillett, Nash, & Masiulis, 2005), it will be useful to work with school systems and professionals to adapt policies for students with chronic illnesses.
The duration and timing of the calls used in this study appear to provide a useful template for assessment of type 1 adherence. Data were obtained for just over half of the EMA assessments, and response rates remained stable over the 10-day calling period. Technical issues resulted in approximately 19% data loss defined as call records with incomplete data. The rate of call records with partially missing data included dropped calls or hang ups. The rates of those specific events could not be quantified. Comparison of this level of incomplete data to previous research is difficult. Many previous studies have successfully utilized such calling systems for health assessment in adult and adolescent populations (Estabrooks & Smith-Ray, 2008; Piette, 2000; Reidel, Tamblyn, Patel, & Huang, 2008), but fewer have specifically addressed adherence or used the systems with cell phones (Reidel, et al., 2008; Winland-Brown & Valiante, 2000). Allowing adolescents to call back in to the system resulted in a greater number of assessments. Adherence rates, as reported through outgoing calls, were equal to adherence reported through incoming calls. Although on average, there were an adequate number of observations per participant, the number varied considerably across participants. Some analyses were limited to those adolescents with at least 4 assessments over different days. However, it is unclear how many assessments are needed to provide maximal sensitivity and adequate sampling of type 1 diabetes adherence behaviors.
Mornings were the most challenging time of day for blood glucose monitoring and insulin administration. This insight regarding a vulnerable time of day provides a critical leverage point for the clinical improvement glycemic control in adolescents. Evening times were associated with the highest levels of adherence. Improved adherence may be related to predictable family and home routines in this population (Greening, et al., 2007). Momentary and mobile interventions have great potential to be tailored to individually relevant times of day, and provide situational prompts and skills needed to address barriers to adherence (e.g., time management and planning).
Trajectory analyses replicated previous cross-sectional research identifying an “inadequate” self-management style (Schneider, et al., 2007). Glycemic control for both of the “variable” groups was worse compared to the “adequate” groups. The proportion of adolescents reporting adequate blood glucose monitoring frequency was higher than that for insulin administration. Adolescents appeared more willing to complete blood glucose monitoring, which did not always appropriately lead to insulin administration. The difference in glycemic control between the insulin administration trajectory groups was clinically significant. Missed insulin doses may be one of the most impactful of self-care behaviors on glycemic control (Olinder, Kernell, & Smide, 2009). Each trajectory group had similar EMA response rates, so the trajectory group representing “variable” adherence does not appear to be biased by more missing (and possibly less adherent) data. The patterns of adherence found here indicate that diabetes researchers and clinicians could more efficiently and effectively direct intervention resources by identifying patterns of adherence in subgroups over time.
EMA-reported adherence was not correlated with glycemic control. Trajectory analyses did indicate worse glycemic control for the “variable” adherence groups. It is important to note that the number of calls per participant with data was related to glycemic control. This finding confirms related research that has found the number of values in blood glucose meters is a valuable indicator of adherence and is related to glycemic control (Guilfoyle, et al., In press). The EMA method paradoxically provides greater opportunity to opt out of measurement and, thus, potentially greater bias toward reporting positive adherence behaviors (Shiffman, et al., 2008). There are several indications that did not occur in this study: there was no difference in reported adherence through inbound versus outbound calls; there were equal numbers of calls per participant between the trajectory subgroups; and, although missing call data was greater for the morning period, reports of missed blood checks and insulin doses were also worse for that time period, as opposed to being higher, and representing a response bias.
The EMA-reported adherence was moderately related to a traditional questionnaire report. Comparison between traditional self-report measures and EMA-reported adherence is complicated by the fact that established measures are typically multi-dimensional, and assess a broad range of behaviors. Items were selected from a standardized adherence questionnaire to more closely match the behaviors assessed in the EMA portion of the study. However, time frames used for responses on questionnaires, wording of items, and metrics for the response formats often do not lend themselves to direct comparison of specific behaviors. Future research that incorporates an EMA system for adherence will need to weigh the potential benefits of this method against traditional self-report. Participants needed ubiquitous access to a cell phone and a reliable connection to avoid dropped calls. At times, circumstances may have caused delays in response, lack of response, or hang ups. While the 10-day calling period tested here showed no decline in response rates, patient engagement over longer periods of time with EMA systems may be limited (Hanauer, et al., 2009).
This research has several limitations. The smaller sample for the EMA limited the size of trajectory groups, and although type 1 diabetes is primarily a Caucasian disease, there was a somewhat higher rate of Caucasian adolescents in this sample than in previous studies (Hood, Butler, Anderson, & Laffel, 2007; Nansel et al., 2009; Schneider, et al., 2007). Response rates might have been higher for simple text-based assessments. Text messaging was not used here because of limited flexibility in assessing multiple behaviors through one communication. Additional insights could have been gained by coding day of the week, coding intentional versus unintentional missed adherence tasks, and including carbohydrate counting in the assessment calls. At times, call scheduling required a time lag between the actual adherence behavior and report of it. That time lag was unavoidable, given the need to use outbound calls to collect data during relevant time intervals, but that lag was not quantified in this study. Finally, an assumption incorporated into the EMA assessment was that adolescents knew that they should check their blood glucose several times per day around meals.
Ecological momentary assessment provides unique opportunities for improved measurement and understanding of adherence. Results support the feasibility and utility of this method. New insights were gained into patterns of adolescent adherence that provide leverage points for targeting or tailoring interventions to improve self-care. Results should be confirmed in a larger sample and expanded to identify situational variables that influence adherence, and additional research conducted to determine the feasibility of implementing EMA in clinical practice.
Acknowledgments
This research was supported by a Pilot and Feasibility grant to Dr. Mulvaney from the Vanderbilt Diabetes Research and Training Center (P60DK020593) and by the Vanderbilt Institute for Clinical and Translational Research (1 UL1 RR024975 NCRR).
References
- Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology. 1990;15(4):477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- Anhoj J, Moldrup C. Feasibility of collecting diary data from asthma patients through mobile phones and sms (short message service): Response rate analysis and focus group evaluation from a pilot study. Journal of Medical Internet Research. 2004;6(4):e42. doi: 10.2196/jmir.6.4.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielli E, Carminati F, La Capra S, Lina M, Brunelli C, Tamburini M. A wireless health outcomes monitoring system (whoms): Development and field testing with cancer patients using mobile phones. BMC Medical Informatics and Decision Making. 2004;4(1):7. doi: 10.1186/1472-6947-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: General information and national estimates on diabetes in the united states, 2007. Centers for Disease Control and Prevention; Atlanta, GA: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- Dunton GF, Atienza AA, Castro CM, King AC. Using ecological momentary assessment to examine antecedents and correlates of physical activity bouts in adults age 50+ years: A pilot study. [Research Support, N.I.H., Extramural] Annals of Behavioral Medicine. 2009;38(3):249–255. doi: 10.1007/s12160-009-9141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooks PA, Smith-Ray RL. Piloting a behavioral intervention delivered through interactive voice response telephone messages to promote weight loss in a pre-diabetic population. Patient Education and Counseling. 2008;72(1):34–41. doi: 10.1016/j.pec.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Franklin V, Greene A, Waller A, Greene S, Pagliari C. Patients’engagement with “sweet talk”–a text messaging support system for young people with diabetes. Journal of Medical Internet Research. 2008;10(2) doi: 10.2196/jmir.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening L, Stoppelbein L, Konishi C, Jordan SS, Moll G. Child routines and youths’ adherence to treatment for type 1 diabetes. Journal of Pediatric Psychology. 2007;32(4):437–447. doi: 10.1093/jpepsy/jsl029. [DOI] [PubMed] [Google Scholar]
- Guilfoyle SM, Crimmins NA, Hood KK. Blood glucose monitoring and glycemic control in adolescents with type 1 diabetes: Meter downloads versus self-report. Pediatric Diabetes. doi: 10.1111/j.1399-5448.2010.00735.x. In press. [DOI] [PubMed] [Google Scholar]
- Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized automated reminder diabetes system (cards): E-mail and sms cell phone text messaging reminders to support diabetes management. Diabetes Technology & Therapeutics. 2009;11(2):99–106. doi: 10.1089/dia.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson VS, Lopez LC, Kamarck T. Peer relationships and diabetes: Retrospective and ecological momentary assessment approaches. Health Psychology. 2009;28(3):273–282. doi: 10.1037/a0013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert A, Rief W, Tuschen-Caffier B, de Zwaan M, Czaja J. Loss of control eating and psychological maintenance in children: An ecological momentary assessment study. Behaviour Research and Therapy. 2009;47(1):26–33. doi: 10.1016/j.brat.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Hood KK, Butler DA, Anderson BJ, Laffel LMB. Updated and revised diabetes family conflict scale. Diabetes Care. 2007;30(7):1764–1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: A meta-analysis. Pediatrics. 2009;124(6):e1171–1179. doi: 10.1542/peds.2009-0207. [DOI] [PubMed] [Google Scholar]
- Iannotti RJ, Nansel TR, Schneider S, Haynie DL, Simons-Morton B, Sobel DO, Clark L. Assessing regimen adherence of adolescents with type 1 diabetes. Diabetes Care. 2006;29(10):2263–2267. doi: 10.2337/dc06-0685. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Hauser ST, Lavori P, Wolfsdorf JI. Adherence among children and adolescents with insulin-dependent diabetes mellitus over a four-year longitudinal follow-up: 1. The influence of patient coping and adjustment. Journal of Pediatric Psychology. 1990;15(4):511–526. doi: 10.1093/jpepsy/15.4.511. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Kelly M, Henretta JC, Cunningham WR, Tomer A, Silverstein JH. A longitudinal analysis of adherence and health status in childhood diabetes. Journal of Pediatric Psychology. 1992;17(5):537–553. doi: 10.1093/jpepsy/17.5.537. [DOI] [PubMed] [Google Scholar]
- Lenhart A, Purcell K, Smith A, Zickuhr K. Social media & mobile internet use among teens and young adults. Washington, DC: Pew, Internet & American Life Project; 2010. [Google Scholar]
- Nabors L, Troillett A, Nash T, Masiulis B. School nurse perceptions of barriers and supports for children with diabetes. The Journal of School Health. 2005;75(4):119–124. [PubMed] [Google Scholar]
- Nansel TR, Rovner AJ, Haynie D, Iannotti RJ, Simons-Morton B, Wysocki T, Laffel L. Development and validation of the collaborative parent involvement scale for youths with type 1 diabetes. Journal of Pediatric Psychology. 2009;34(1):30–40. doi: 10.1093/jpepsy/jsn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinder AL, Kernell A, Smide B. Missed bolus doses: Devastating for metabolic control in csii-treated adolescents with type 1 diabetes. Pediatric Diabetes. 2009;10(2):142–148. doi: 10.1111/j.1399-5448.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- Piette JD. Interactive voice response systems in the diagnosis and management of chronic disease. American Journal of Managed Care. 2000;6(7):817–827. [PubMed] [Google Scholar]
- Reidel K, Tamblyn R, Patel V, Huang A. Pilot study of an interactive voice response system to improve medication refill compliance. BMC Medical Informatics and Decision Making. 2008;8(1):46. doi: 10.1186/1472-6947-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Iannotti RJ, Nansel TR, Haynie DL, Simons-Morton B, Sobel DO, Plotnick LP. Identification of distinct self-management styles of adolescents with type 1 diabetes. Diabetes Care. 2007;30(5):1107–1112. doi: 10.2337/dc06-2197. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone A, Hufford M. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Winland-Brown JE, Valiante J. Effectiveness of different medication management approaches on elders’ medication adherence. [Clinical Trial, Controlled Clinical Trial] Outcomes Management for Nursing Practice. 2000;4(4):172–176. [PubMed] [Google Scholar]